Abstract
From microbes to humans, the success of many organisms is achieved by dividing tasks among specialized group members. The evolution of such division of labor strategies is an important aspect of the major transitions in evolution. As such, identifying specific evolutionary pressures that give rise to group-level division of labor has become a topic of major interest among biologists. To overcome the challenges associated with studying this topic in natural systems, we use actively evolving populations of digital organisms, which provide a unique perspective on the de novo evolution of division of labor in an open-ended system. We provide experimental results that address a fundamental question regarding these selective pressures: Does the ability to improve group efficiency through the reduction of task-switching costs promote the evolution of division of labor? Our results demonstrate that as task-switching costs rise, groups increasingly evolve division of labor strategies. We analyze the mechanisms by which organisms coordinate their roles and discover strategies with striking biological parallels, including communication, spatial patterning, and task-partitioning behaviors. In many cases, under high task-switching costs, individuals cease to be able to perform tasks in isolation, instead requiring the context of other group members. The simultaneous loss of functionality at a lower level and emergence of new functionality at a higher level indicates that task-switching costs may drive both the evolution of division of labor and also the loss of lower-level autonomy, which are both key components of major transitions in evolution.
Keywords: digital evolution, problem decomposition, specialization, task partitioning, fraternal transition
Division of labor is a strategy used by a diverse set of biological groups, ranging in size and complexity from microorganisms to humans (1–13). Within human economies, Adam Smith considered the avoidance of task-switching costs to be a significant benefit resulting from division of labor (14). However, task-switching costs, such as cognitive overhead (12), travel time to a new location (9, 10), and costs associated with morphological alterations (15), are also present within other organic systems. As proposed by Dornhaus (16), we explore whether the avoidance of task-switching costs promotes the evolution of division of labor. This is a challenging topic to study in natural settings, owing to sparse phylogenetic data with missing intermediate states, as well as the inherent difficulty of inferring nonmorphological forms of division of labor from the fossil record (refs. 16 and 17; but see ref. 18). Although there have been pioneering laboratory selection experiments involving the propagation of large collections of groups of organisms (19–21), even microbes with short generation times are still difficult to track over long evolutionary periods.
Here, we perform experimental evolution on digital organisms, which compose a model system that exhibits open-ended evolutionary dynamics with rapid generations. Specifically, we use the Avida digital-evolution platform (22), previously used to study topics including the evolutionary origin of complex features (23), adaptive radiation (24), and the evolution of altruism (25). Within Avida, organisms are fully functional computer programs that must self-replicate to survive in a user-defined environment where they are subject to mutations and natural selection. A digital organism executes its genome on a virtual central processing unit (CPU), allowing it to perform computations, self-replicate, and interact with its neighbors or environment in a variety of ways. Digital evolution enables us to start with a set of groups of organisms, impose task-switching costs upon individuals, and observe in real-time whether the groups evolve to exhibit more or less division of labor. Using Avida, we can also investigate how groups that perform division of labor evolved to coordinate tasks. Although we provide several potential coordination mechanisms, including spatial information and communication capabilities, the ways in which the organisms evolve to make use of these mechanisms, either individually or in concert, is open-ended.
We created worlds consisting of 400 competing “colonies,” each containing up to 25 clonal organisms. Colony fecundity is based on the speed at which its members accumulate resources. Nine types of resources are available, each associated with a distinct Boolean logic function (Table 1) (23) that the organism must export to uptake the resource. The resources are set up in a virtual chemostat. Each resource has a constant inflow rate of one unit per update (an update is the standard unit of time in Avida; organisms receive, on average, 30 CPU cycles per update and live for 5–20 updates), while at the same time 1% of the available resources flow out, limiting total accumulation to 100 units. When an organism exports the result of a function, it uptakes 5% of the available resource associated with that function. A colony that collects a designated number of units of resources (of any type) divides into two colonies, replacing a random competing colony. As a result of resource scarcity, colony performance is improved if, collectively, its members target multiple resource types. Organisms can evolve to accomplish this objective anywhere along the continuum from generalists to specialists. A perfect generalist organism could sequentially export each logic function, collecting multiple types of resources in series, whereas a perfect specialist organism repeatedly targets a small subset of available resources, relying on other colony members to acquire additional resource types. The specialist dynamic is analogous to honey bee colonies where bees specialize on collecting nectar from one type of flower but collectively gather nectar from all flowers in their habitat (9, 26). Experimental runs are seeded with organisms that grow into colonies capable of collecting just the resource associated with the NOT function, eventually gathering enough of it for the colony to replicate. Organisms within a colony are clonal; mutations occur only during colony division. Over time, colonies evolve organisms that perform different types of logic functions, potentially engaging in strategies to coordinate task allocation and thus perform division of labor. Because of the clonal nature of the group, evolved division of labor strategies cannot rely on genetic heterogeneity. Instead, their polyphenism must arise from stochasticity or plasticity to environmental heterogeneity. Because organisms can send messages to one another, this environmental heterogeneity may be created by the organisms themselves.
Table 1.
Logic functions that can be exported by organisms to accrue resources
| Function name | Logic operation | Example |
| A: 1001 | ||
| B: 1010 | ||
| NOT | ¬A; ¬B | 0110; 0101 |
| NAND | ¬(A and B) | 0111 |
| AND | A and B | 1000 |
| ORNOT | (A or ¬ B); (¬ A or B) | 1101; 1110 |
| OR | A or B | 1011 |
| ANDNOT | (A and ¬ B); (¬ A and B) | 0001; 0010 |
| NOR | ¬(A or B) | 0100 |
| XOR | (A and ¬ B) or (¬ A and B) | 0011 |
| EQU | (A and B) or (¬ A and ¬ B) | 1100 |
Organisms have only NAND gates (a universal logic gate) from which to build the other logic operations. The logic operations are ordered in terms of the number of NAND operations required to complete them. More complex logic operations can be built using the results for simpler logic operations (e.g., XOR can be performed by ORing the results of two ANDNOT operations together). Although this example uses 4-bit numbers, organisms perform logic operations on 32-bit numbers.
Results and Discussion
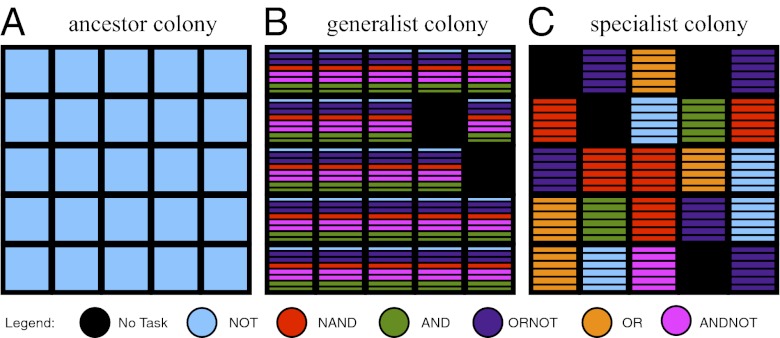
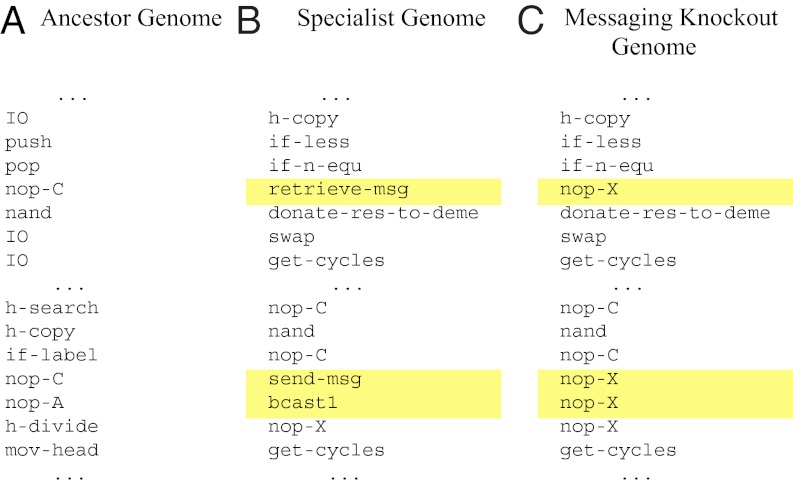
We created three treatments that vary the penalty organisms pay for changing types of tasks (i.e., exporting solutions to different logic functions): a control (with no costs), a moderate-cost treatment (requiring organisms to wait 25 CPU cycles each time a different type of task is exported), and a high-cost treatment (requiring a delay of 50 CPU cycles). We replicated each treatment 50 times and used Shannon mutual information [as proposed by Gorelick et al. (27); see Materials and Methods] to measure the amount of division of labor that evolved within the colonies. Table 2 summarizes our results. For our initial treatments, we required colonies to collect 500 units of resource to replicate. Trials with higher task-switching costs evolved more division of labor (Kruskal-Wallis multiple comparison, P = 0.005). Fig. 1 depicts the phenotypes of three colonies: an ancestral colony, a typical colony that evolved a generalist strategy in the control treatment, and a typical colony that evolved specialist organisms exhibiting division of labor under high task-switching costs (hereafter referred to as our case-study colony). Fig. 2 depicts segments of the genomes of the organisms within the ancestor colony (Fig. 2A) and the specialist case-study colony (Fig. 2B). We verified that the number of types of resources and the types of tasks did not drive our results in a second environment that used 25 resources associated with simpler tasks (SI Results and Discussion, Twenty-Five-Role-Environment Experiments).
Table 2.
Observed amounts of division of labor
| Task-switch treatment | Task-switch cost | 250 units required | 500 units required | 1,000 units required |
| Control | 0 | 0.027 ± 0.01 | 0.400 ± 0.04 | 0.735 ± 0.05 |
| Moderate | 25 | 0.322 ± 0.04 | 0.813 ± 0.04 | 0.899 ± 0.05 |
| High | 50 | 0.639 ± 0.04 | 1.066 ± 0.04 | 0.915 ± 0.06 |
Level of division of labor observed for various individual-level task switching costs (rows) and colony-level resource requirements for replication (columns). Division of labor is gauged as the Shannon mutual information between the tasks exported and the individuals exporting them, measured across 50 trials for each experimental configuration. High task-switching costs or higher resource requirements were observed to increase evolved division of labor.
Fig. 1.
A snapshot of the tasks exported (and thus the task-specific resources used) for three colonies. Each square represents the phenotype of an organism. Squares divided into segments represent multiple tasks exported; colors denote which tasks were exported. (A) An ancestral colony in which all organisms export the NOT task exactly once. (B) A colony that evolved a generalist strategy in which all organisms export five distinct tasks a total of eight times. (C) A colony that evolved a division of labor strategy in which each organism specializes on one of seven possible tasks that it exports a total of six times. (At the instant depicted, the organisms are not exporting NOR, which other colony members export at other times).
Fig. 2.
Segments of code across a genome. (A) Portions of the ancestral genome for performing task NOT and self-replicating. (B) An evolved specialist genome from our case-study colony, with the messaging instructions highlighted in yellow. (C) The knockout version of the specialist genome described in B, where messaging instructions have been replaced with a neutral instruction (nop-X), highlighted in yellow. These knockout organisms cease to be able to perform any task at all.
Intrinsic Task-Switching Costs.
To further confirm the robustness of these results, we performed two additional treatments in which the amount of resources required for the colony to replicate was set to 250 units (half the original amount) and 1,000 units (double the original amount). For the 250-requirement experiment, as task-switching costs increased, the colonies increasingly evolved division of labor strategies, which is consistent with our hypothesis (Kruskal-Wallis multiple comparison, P = 0.005). For the 1,000-requirement experiment, however, the levels of division of labor in the colonies evolved under treatments with distinct task-switching costs are not significantly different from one another. Instead, the control colonies (no cost) evolved to exhibit a high degree of division of labor that was almost equal to that exhibited by the higher-cost treatments. This behavior results from intrinsic task-switching costs (further details in SI Results and Discussion, Intrinsic Task-Switching Costs). As the resource requirements rose, colonies evolved to export more tasks that had greater complexity (e.g., control colonies performed 6.600 ± 0.211 different types of tasks in the 1,000-requirement environment, compared with 4.182 ± 0.073 and 5.397 ± 0.095 in the 250 and 500 requirements, respectively). These more-complex tasks entailed a greater intrinsic task-switching cost and made a division of labor strategy increasingly beneficial.
Mechanisms Used to Perform Division of Labor.
We investigated how the organisms performed division of labor. Organisms could evolve to use stochastic information, communication via messaging, or location awareness to divide up tasks. These mechanisms are each used by organisms in nature (6, 10, 28, 29). Within Avida, we provided instructions enabling organisms to send a message containing two numbers (the specific values were determined by the organisms), receive a message, and sense their x- and y-coordinates. The genome of the ancestor organism did not contain these instructions, and thus organisms had to incorporate them into their genomes by mutation. We isolated the best-performing colony from each of the trials for our central experiment in which colonies were required to amass 500 units of resource to replicate.
To understand the evolved genomes, we took inspiration from molecular genetics studies and conducted knockout experiments, whereby we replaced specific instructions in a genome with a neutral substitute and then observed the behavior of the colony. Fig. 2B depicts a portion of the evolved genome from our case study, and Fig. 2C shows a knockout version of the same genome. We tested each colony with its location-sensing instructions knocked out, and again with its messaging capabilities removed. We found that colonies evolved to make use of stochastic information, spatial location, and communication (knockout data in SI Results and Discussion, Division of Labor Knockout Data). Communication via messaging was the preferred method of task coordination for colonies evolved with higher task-switching costs; colonies evolved with low intrinsic and explicit task-switching costs made only limited use of this mechanism.
Colony Case Study.
To understand how colonies used messaging to perform division of labor, we analyzed our case-study colony in detail. This colony exported seven tasks (NOT, NAND, AND, ORNOT, OR, ANDNOT, and NOR) in its evolved form but was sterile at both the individual and colony level when messaging capabilities were removed. Its genotype used messaging to send a variety of information, including task results that, when received by neighboring organisms, were used to compute additional logic functions (Fig. 3).
Fig. 3.
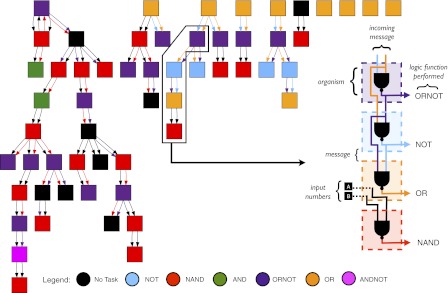
Depiction of the complex system of task partitioning evolved by our case-study colony. Organisms (squares) export tasks and exchange messages (pairs of arrows) that may include the results of tasks, input values, constants, or previously received messages. Although colonies are limited to 25 organisms at a time, offspring can replace previous organisms; for this case study colony, there are 57 organisms between colony replication events. Each organism sends seven messages and receives one; only successfully received messages are depicted. Organism colors represent tasks exported and thus resources targeted by an organism; black represents organisms that did not export any task. Each message consists of two numbers and is represented by a pair of arrows whose color denotes the contents of the message. Black arrows represent messages that are not the result of a task. Inset highlights four of these organisms: the top organism exporting ORNOT (purple) sends a message containing the solutions to the OR (orange) and ORNOT (purple) tasks to a neighboring organism, which NANDs these results together to export NOT (blue) [i.e., ([A ORNOT B] NAND [A OR B] = NOT A)].
Over the course of its life, each organism in the colony produced seven different messages and attempted to receive one message. Fig. 4 depicts the internal circuitry used by the organisms to create their messages. Each message consisted of a pair of numbers. The organisms evolved to send messages containing (i) input values, (ii), constant values, and (iii) the results of a logic operation. This information was either exogenously supplied (e.g., input numbers generated by executing the input/output instruction), generated by the organism (e.g., constants or new task results), or was relayed information received from another organism (e.g., input numbers, constants, or task results received as messages).
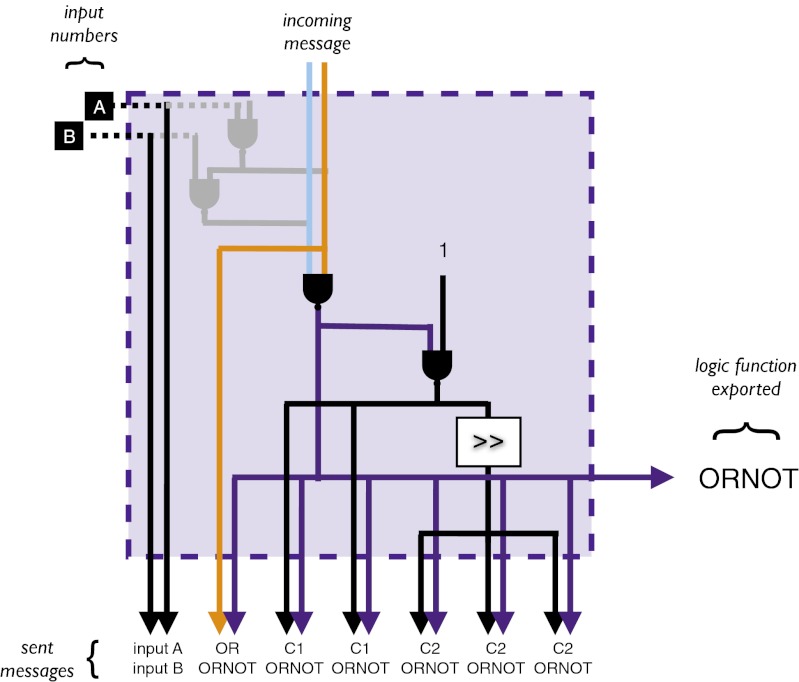
Fig. 4.
Internal circuitry used by the organisms in the case-study colony to send seven different messages (depicted as pairs of arrows). Each organism has the same internal circuitry. However, the messages that an organism receives and thus the task it exports may be different from other organisms. The first sent message contains the input values (32-bit numbers available to each organism). The second sent message contains the result of the task exported by the organism (ORNOT) and part of the contents of a message received by the organism—in this case, the result of a task exported by the other organism (OR). The remaining five sent messages contain the result of the task exported by the organism (ORNOT) and one of two constants created by the organism. One of the possible constants involves a bit shift operation (indicated by > >), which essentially makes that component of the message meaningless. Because each organism sends seven messages, but receives only one message, the contents of most messages will not be used by the group.
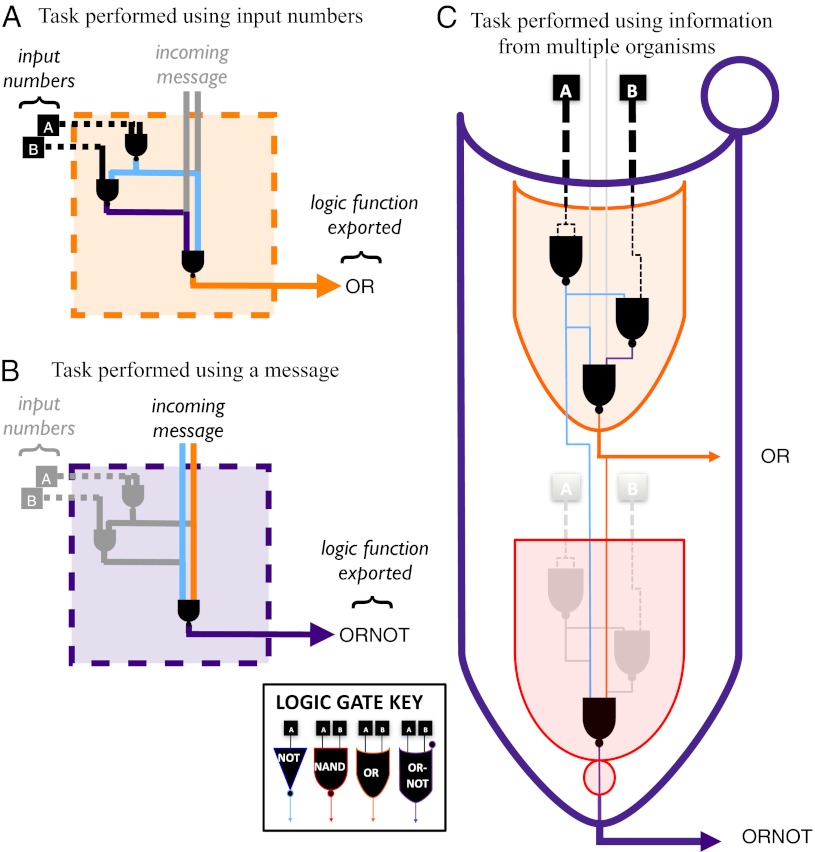
The task exported by an organism depended on whether it had received a message (Fig. 5 A and B). If an organism did not receive a message, then it exported task OR. Otherwise, the organism may have exported one of the other logic operations. Fig. 5C provides a hierarchical perspective on how multiple organisms participate in exporting a more-complex logic operation. We examined the organisms and discovered that they have the internal circuitry to perform only a subset of the logic operations (i.e., NOT, NAND, ORNOT, and OR) that were exported by the group. To export the other tasks (i.e., AND, ANDNOT, and NOR), the organisms relied on messages from other organisms. This reliance upon other organisms to perform tasks that cannot be done by an organism in isolation is the emergence of functionality only accessible to the organisms living in groups.
Fig. 5.
Internal circuitry used by organisms in the case-study colony to export logic operations varies depending on whether they have successfully received a message. (A) An organism that exports operation OR on the input values. This individual does not receive a message (grayed-out lines) and processes inputs A and B through its logic circuitry of three NAND gates. Note {[(A NAND A)] NAND [(A NAND A) NAND B]} = (A OR B). Thus, this individual performs and then exports the OR task. (B) An organism that exports the results of an ORNOT operation using the contents of a received message. This organism performs the same initial steps as the organism depicted in A. However, it successfully receives a message that overwrites the partially processed input values. It NANDs together the received values to produce the result for ORNOT, which it exports. (C) A hierarchical perspective on how multiple organisms participate via messaging in performing a more-complex logic operation. Within this figure, we depict the same two organisms as in A and B. However, we vary their shape and color to represent the internal logic operations performed, rather than the task exported. The first organism highlighted in orange is the organism in part A. This individual ORs inputs A and B together and as such is represented by an OR gate. Additionally, it passes a message with two components to the second individual: (NOT A) and (A OR B). The second organism (from B) is highlighted in red and is represented by a NAND gate, because it receives the message (represented as blue and orange lines) sent by the first organism and performs a NAND operation on the components of the message. Because of the message contents, which were created by organism A, the resulting operation is (A ORNOT B), which is exported by the organism. Note that [(NOT A) NAND (A OR B)] = (A ORNOT B). Combined, these two individuals serve as an ORNOT gate, as depicted by the large purple gate surrounding the pair of individuals.
Division of labor is a hallmark of advanced societies. Its emergence in digital organisms, including task-allocation systems based on communication and other mechanisms, shows that only a few specific conditions are necessary for its evolution. Effectively, colony members decomposed problems by breaking logic tasks into simpler components, solving those components, sharing the solutions, and assembling them into the results of more-complex tasks. This strategy reflects the task-partitioning approach commonly adopted by organisms that perform division of labor (30, 31). For example, leafcutter ants (Atta vollenweideri) decompose the task of tending to fungi into majors that cut leaves, mediae that move leaves from the tree to the colony, and minims that tend to the fungal gardens (5). The leaves are passed from one worker to the next as they are processed. Like this division of labor in the leafcutter ants, the strategy evolved by this digital organism colony exhibits problem decomposition and assembly line processing of task material. Our results suggest that the efficiency advantages afforded by task partitioning are sufficient to favor the evolution of division of labor.
Shifts in Individuality.
Major transitions in evolution occur when formerly individual autonomous units that are coexisting within a group shift to a state in which they are intrinsically dependent upon one another (11, 32, 33). These transitions can be fraternal, whereby genetically similar individuals (i.e., close kin) differentiate to create a superorganism [e.g., the origins of multicellularity (21, 34–36)], or egalitarian, whereby formerly distinct organisms come together to create a superorganism that replicates all of its genetic material [e.g., formation of the eukaryotic cell (37)] (32, 38). Two key challenges for fraternal transitions addressed by this study are (i) how genetically identical individuals evolve to exhibit division of labor; and (ii) whether the way in which individuals accomplish this division of labor also results in a loss of lower-level autonomy.
With regard to the first challenge, within our study, colonies placed under high task-switching costs evolved to exhibit division of labor. The colonies used different mechanisms, including stochasticity, spatial location, and communication, depending on experimental conditions. For the second challenge, many of the organisms in colonies under high task-switching costs exhibited a loss of autonomy and specific dependence upon one another. Organisms within these colonies evolved to be reliant upon communication to the extent that individuals were able to perform tasks within the context of their colony that they could not perform alone (SI Results and Discussion, Loss of Task Diversity Resulting from Communication Knockouts). For example, within the case study, an individual in isolation only ever performed task OR; however, a group of these organisms synergistically interacted to perform up to seven different logic tasks. While these organisms contained internal subcircuitry necessary to perform four of the logic operations (i.e., NOT, NAND, ORNOT, OR), the other three logic operations (i.e., AND, ANDNOT, NOR) are emergent functionality requiring computation and communication by two or more organisms. In contrast, most of the colonies evolved without task-switching costs maintained their ability to perform all of the different types of tasks, even when communication capabilities were removed (SI Results and Discussion, Loss of Task Diversity Resulting from Communication Knockouts).
Moreover, when the starting composition of a specialist colony was perturbed to include an individual from a different lineage, the ability of the colony to rapidly perform logic operations to consume resources diminished (SI Results and Discussion, Perturbation of Colony Starting Conditions). However, when the same perturbation was performed on different lineages evolved under low task-switching costs, fitness did not diminish suggesting that these low-level individuals maintained their individuality. These data serve as preliminary evidence that making it costly for individuals to switch tasks not only favors division of labor but also favors a shift in individuality to a higher level.
Materials and Methods
Avida Digital Evolution Platform.
An Avida population consists of a set colonies. Each colony is a 5 × 5 toroidal grid that can contain up to 25 clonal digital organisms at one time. Organisms may replicate over one another, thus the colony may contain more than 25 organisms over time. The series of events that take place as part of colony replication are depicted in Fig. 6.
Fig. 6.
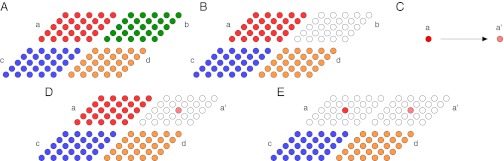
Colony replication process. (A) Colony a (depicted in red) amasses sufficient resources to replicate. (B) A random colony from the population (b, depicted in green) is selected as the target of the replication, and the organisms within the target colony are removed. (C) The genotype of the source colony, a, is used to produce the genotype of the new colony, a', possibly with mutations. (D) One individual from the new genotype is placed into the target colony. (E) The original colony is also reset to a single organism.
Each digital organism is defined by a circular list of instructions (its genome), a virtual CPU, and its position in the colony. We used genetically identical organisms for this study, to focus on our central question of understanding which evolutionary situations favor division of labor in the absence of competition within the colony. (Further details on how violations of this assumption affect division of labor are given in SI Results and Discussion, Exploring the Conditions Under Which Division of Labor Evolves.) Organisms execute the instructions in their genomes sequentially unless an instruction alters this order. The particular instructions that are executed determine the organism’s behavior, including the ability to sense and change properties of its environment.
We provide the standard set of Avida instructions (detailed in ref. 23) to enable organisms to perform basic computational tasks (addition, subtraction, bit-shifts, etc.), control execution flow, and allow for replication. Our instruction set also included communication and location-sensing instructions (summarized in Table 3).
Table 3.
Coordination instructions for this study
| Instruction | Description |
| send-msg | Send a message to a neighbor of the caller. |
| retrieve-msg | Load the contents of a received message into the caller’s virtual CPU. |
| rotate-left-one | Rotate this organism counterclockwise one step. |
| rotate-right-one | Rotate this organism clockwise one step. |
| get-role-id | Set register BX to the value of the caller’s role-id register. |
| set-role-id | Set the caller’s role-id register to the value in register BX. |
| bcast1 | Send a message to all neighboring organisms. |
| get-cell-xy | Set register BX and CX to the (x, y) coordinates of the caller. |
Organisms can perform tasks that enable them to accumulate resources from their environment and contribute to colony replication. Resources within this environment are limited. (Further details on how violations of this assumption affect division of labor are given in SI Results and Discussion, Exploring the Conditions Under Which Division of Labor Evolves.) For the majority of experiments, we required the organisms to perform bitwise Boolean logic operations on 32-bit integers. [Lenski et al. (23) provide detailed examples of these operations.]
To study how the presence and magnitude of task-switching costs affect the evolution of division of labor, we created a configurable task-switching penalty. Specifically, if an organism changes the type of task it is performing, then it incurs a time penalty that is applied before the resources for the second task are collected. We implement this time penalty as wasted CPU cycles, whereby a CPU cycle is the amount of time it takes an organism to execute one instruction.
For each experiment, we conducted 50 trials to account for the stochastic nature of evolution. Within each trial, the Avida world consists of 400 colonies. All genotypes are fixed at a length of 100 instructions. Mutations occur to a genotype when the colony replicates; the mutation rate is set to an average of one mutation per genome per replication event. The trials last for 201,000 updates. After the first 200,000 updates, the colonies go through a 1,000-update ecological period, in which the mutation rate is set to zero. The ecological period prunes dysfunctional colonies that occur as the result of deleterious mutations that are not able to fix in the population. In this case, the ecological phase enables us to better analyze and assess the behavior of the colonies.
Measuring Division of Labor.
To measure the amount of division of labor present within a colony, we use Shannon mutual information as proposed by Gorelick et al. (27). Shannon mutual information is defined as:
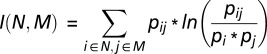 |
where i is an organism, N is the set of organisms that performed a task within the colony, j is a type of task, and M is the set of types of tasks. pi is the probability of picking individual i at random. For this study, we treat the probability of all individuals (pi) as equal. pij is the joint probability of a random unit of work being individual i working on task type j. For this measurement, we normalize by individual productivity, to determine the percentage of time an individual spends on a specific type of task. Thus, we set pij equal to the percentage of time each individual spent on task type j divided by the total number of organisms. pj is the probability that individuals are working on task j. To compute pj, we sum pij across all organisms.
Intuitively, Shannon mutual information captures two reciprocal pieces of information: given an individual, how much information do we have about the type of task it spends its time performing, and given a type of task, how much information do we have about the individual that is most likely to be working on performing it? Information will be high when individuals specialize on performing one type of task but the group as a whole contains specialists that focus on performing a diverse set of tasks. Specifically, Shannon mutual information (and division of labor) will be maximized for a given population size and number of tasks performed when each organism is a perfect specialist and the organisms within the colony are evenly divided among the tasks. If all members of a colony are performing the same set of tasks with the same proportions, then information is zero.
Supplementary Material
Acknowledgments
We thank F. C. Dyer, D. B. Knoester, N. Serra, and members of the Devolab for their insights during discussions of this work. This work has been supported in part by National Science Foundation Grants CCF-0643952, DBI-0939454, CCF-0820220, OCI-1122620, DEB-0952825, and R3D381.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.E.S. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1202233109/-/DCSupplemental.
References
- 1.Bonner JT, Raper KB. A theory of the control of differentiation in the cellular slime molds. Q Rev Biol. 1976;51:296–312. [Google Scholar]
- 2.Crespi BJ. The evolution of social behavior in microorganisms. Trends Ecol Evol. 2001;16:178–183. doi: 10.1016/s0169-5347(01)02115-2. [DOI] [PubMed] [Google Scholar]
- 3.Duffy JE. The Ecology and Evolution of Eusociality in Sponge-Dwelling Shrimp. Hokkaido Univ Press, Sapporo, Japan; 2003. [Google Scholar]
- 4.Gintis H, Bowles S, Boyd R, Fehr E. Moral Sentiments and Material Interests: On the Foundations of Cooperation in Economic Life. Cambridge, MA: MIT Press; 2005. [Google Scholar]
- 5.Hölldobler B, Wilson EO. The Superorganism: The Beauty, Elegance, and Strangeness of Insect Societies. New York, NY: WW Norton & Company; 2009. [Google Scholar]
- 6.Jandt JM, Dornhaus A. Spatial organization and division of labour in the bumblebee bombus impatiens. Anim Behav. 2009;77:641–651. [Google Scholar]
- 7.Michod RE. The group covariance effect and fitness trade-offs during evolutionary transitions in individuality. Proc Natl Acad Sci USA. 2006;103:9113–9117. doi: 10.1073/pnas.0601080103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Queller DC, Strassmann JE. Eusociality. Curr Biol. 2003;13:R861–R863. doi: 10.1016/j.cub.2003.10.043. [DOI] [PubMed] [Google Scholar]
- 9.Seeley T. The Wisdom of the Hive. Cambridge, MA: Harvard Univ Press; 1995. [Google Scholar]
- 10.Sendova-Franks A, Franks N. Spatial relationships within nests of the ant Leptothorax unifasciatus (Latr.) and their implications for the division of labour. Anim Behav. 1995;50:121–136. [Google Scholar]
- 11.Maynard Smith J, Szathmáry E. The Major Transitions in Evolution. New York: Oxford Univ Press; 1997. [Google Scholar]
- 12.Waser N, Chittka L, Price M, Williams N, Ollerton J. Generalization in pollination systems, and why it matters. Ecology. 1996;77:1043–1060. [Google Scholar]
- 13.Wilson EO. Caste and division of labor in leaf-cutter ants (Hymenoptera: Formicidae: Atta): I. The overall pattern in A. sexdens. Behav Ecol Sociobiol. 1980;7:143–156. [Google Scholar]
- 14.Smith A. The Wealth of Nations. London: W. Strahan and T. Cadell; 1776. [Google Scholar]
- 15.Oster GF, Wilson EO. Caste and Ecology in the Social Insects. Princeton: Princeton Univ Press; 1979. [PubMed] [Google Scholar]
- 16.Dornhaus A. Specialization does not predict individual efficiency in an ant. PLoS Biol. 2008;6:e285. doi: 10.1371/journal.pbio.0060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeanson R, et al. Division of labour and socially induced changes in response thresholds in associations of solitary halictine bees. Anim Behav. 2008;76:593–602. [Google Scholar]
- 18.Simpson C. The evolutionary history of division of labour. Proc Biol Sci. 2012;279:116–121. doi: 10.1098/rspb.2011.0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wade M. An experimental study of group selection. Evolution. 1977;31:134–153. doi: 10.1111/j.1558-5646.1977.tb00991.x. [DOI] [PubMed] [Google Scholar]
- 20.Swenson W, Wilson DS, Elias R. Artificial ecosystem selection. Proc Natl Acad Sci USA. 2000;97:9110–9114. doi: 10.1073/pnas.150237597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ratcliff WC, Denison RF, Borrello M, Travisano M. Experimental evolution of multicellularity. Proc Natl Acad Sci USA. 2012;109:1595–1600. doi: 10.1073/pnas.1115323109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ofria C, Wilke CO. Avida: A software platform for research in computational evolutionary biology. Artif Life. 2004;10:191–229. doi: 10.1162/106454604773563612. [DOI] [PubMed] [Google Scholar]
- 23.Lenski RE, Ofria C, Pennock RT, Adami C. The evolutionary origin of complex features. Nature. 2003;423:139–144. doi: 10.1038/nature01568. [DOI] [PubMed] [Google Scholar]
- 24.Chow SS, Wilke CO, Ofria C, Lenski RE, Adami C. Adaptive radiation from resource competition in digital organisms. Science. 2004;305:84–86. doi: 10.1126/science.1096307. [DOI] [PubMed] [Google Scholar]
- 25.Clune J, Goldsby HJ, Ofria C, Pennock RT. Selective pressures for accurate altruism targeting: Evidence from digital evolution for difficult-to-test aspects of inclusive fitness theory. Proc Biol Sci. 2011;278:666–674. doi: 10.1098/rspb.2010.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heinrich B. Bumblebee Economics. Cambridge, MA: Harvard Univ Press; 1979. [Google Scholar]
- 27.Gorelick R, Bertram SM. Quantifying division of labor: Borrowing tools from sociology, sociobiology, information theory, landscape ecology, and biogeography. Insectes Soc. 2007;54:105–112. [Google Scholar]
- 28.Greene MJ, Gordon DM. Social insects: Cuticular hydrocarbons inform task decisions. Nature. 2003;423:32. doi: 10.1038/423032a. [DOI] [PubMed] [Google Scholar]
- 29.Powell S, Tschinkel WR. Ritualized conflict in Odontomachus brunneus and the generation of interaction-based task allocation: A new organizational mechanism in ants. Anim Behav. 1999;58:965–972. doi: 10.1006/anbe.1999.1238. [DOI] [PubMed] [Google Scholar]
- 30.Hirsh A, Gordon D. Distributed problem solving in social insects. Ann Math Artif Intell. 2001;31:199–221. [Google Scholar]
- 31.Ratnieks F, Anderson C. Task partitioning in insect societies. Insectes Soc. 1999;46:95–108. [Google Scholar]
- 32.Queller DC. Cooperators since life began. Q Rev Biol. 1997;72:184–188. [Google Scholar]
- 33.Buss L. The Evolution of Individuality. Princeton: Princeton Univ Press; 1987. [Google Scholar]
- 34.Koschwanez JH, Foster KR, Murray AW. Sucrose utilization in budding yeast as a model for the origin of undifferentiated multicellularity. PLoS Biol. 2011;9:e1001122. doi: 10.1371/journal.pbio.1001122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuzdzal-Fick JJ, Fox SA, Strassmann JE, Queller DC. High relatedness is necessary and sufficient to maintain multicellularity in Dictyostelium. Science. 2011;334:1548–1551. doi: 10.1126/science.1213272. [DOI] [PubMed] [Google Scholar]
- 36.Michod RE, Roze D. Cooperation and conflict in the evolution of multicellularity. Heredity (Edinb) 2001;86:1–7. doi: 10.1046/j.1365-2540.2001.00808.x. [DOI] [PubMed] [Google Scholar]
- 37.Martin W, Müller M. The hydrogen hypothesis for the first eukaryote. Nature. 1998;392:37–41. doi: 10.1038/32096. [DOI] [PubMed] [Google Scholar]
- 38.Queller DC. Relatedness and the fraternal major transitions. Philos Trans R Soc Lond B Biol Sci. 2000;355:1647–1655. doi: 10.1098/rstb.2000.0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.