Abstract
Original antigenic sin is a phenomenon wherein sequential exposure to closely related influenza virus variants reduces antibody (Ab) response to novel antigenic determinants in the second strain and, consequently, impairs the development of immune memory. This could pose a risk to the development of immune memory in persons previously infected with or vaccinated against influenza. Here, we explored strategies to overcome original antigenic sin responses in mice sequentially exposed to two closely related hemagglutinin 1 neuraminidase 1 (H1N1) influenza strains A/PR/8/34 and A/FM/1/47. We found that dendritic cell–activating adjuvants [Bordetella pertussis toxin (PT) or CpG ODN or a squalene-based oil-in-water nanoemulsion (NE)], upon administration during the second viral exposure, completely protected mice from a lethal challenge and enhanced neutralizing-Ab titers against the second virus. Interestingly, PT and NE adjuvants when administered during the first immunization even prevented original antigenic sin in subsequent immunization without any adjuvants. As an alternative to using adjuvants, we also found that repeated immunization with the second viral strain relieved the effects of original antigenic sin. Taken together, our studies provide at least three ways of overcoming original antigenic sin.
Keywords: cross-reactivity, antigen presentation, memory T-cell activation
Original antigenic sin, first described in 1953 by Thomas Francis (1), is the phenomenon in which sequential exposure to viral variants induces preferential Ab response to a virus strain encountered in the past. As a result, the response to the current strain is diminished. Over the past five decades, original antigenic sin has been observed in humans, as well as other mammals such as mice, ferrets, and rabbits (2–5). This phenomenon could pose a cause for concern in the context of human immune responses to influenza vaccination programs. Hence, there is a need to develop strategies to overcome original antigenic sin.
Interestingly, original antigenic sin is reminiscent of an immunological phenomenon called carrier-mediated hapten suppression, first described by Herzenberg and colleagues in 1980 (6). They showed that in mice previously exposed to the carrier protein, keyhole limpet hemocyanin (KLH), a second immunization with hapten (dinitrophenyl)-conjugated KLH leads to selective suppression of Ab response to the hapten (6). This selective suppression is not unique to murine models of hapten-carrier systems. Human volunteers previously vaccinated with tetanus and later receiving a vaccine consisting of malaria sporozoite peptide conjugated to tetanus showed suppression of the antimalaria response (7). The precise mechanism of this epitopic suppression remained enigmatic until studies in 1998, Moser and colleagues provided significant insight into the cellular basis of carrier-mediated hapten suppression (8). They showed that the hapten-specific Ab response improved upon injection with dendritic cells (DCs) pulsed with hapten-carrier plus IL-12 during secondary immunization. They concluded that activating DCs and forcing a T-helper (Th)1 response could overcome carrier-mediated hapten-specific suppression.
Mature activated DCs serve as a link between the innate and adaptive arms of the immune system, and this process can be facilitated by several bacterial components (9, 10). Of these bacterial products, killed Bordetella pertussis is a potent adjuvant (11), and its components including B. pertussis toxin (PT) and B. pertussis endotoxin (LPS) have been shown to block or reverse carrier-mediated hapten suppression (12, 13). Specifically, PT stimulates DCs to up-regulate MHC class II and costimulatory molecules [cluster of differentiation (CD)80, CD86, CD40, and dendritic and epithelial cell (DEC)205] to produce IL-12 and TNF-α and to up-regulate phosphorylation of ERK (14). This overall DC activation by PT elicits a Th1 response by promoting T cells to produce IFN-γ. Bacterial DNA containing unmethylated CpG-dinucleotide is also a strong adjuvant for Th1 response and cytotoxic T-cell responses (15, 16). Similar to PT, the effect of CpG in promoting Th1-like immune responses (17, 18) is a result of DC maturation leading to up-regulation of MHC class II, B7, and CD40 molecules on the DC surface and production of IL-12, IL-6, and TNF-α (19). Therefore, the mechanism of action for both adjuvants is attributed to potent activation of DCs.
For human application of adjuvants, proven record of safety and efficacy is critical. Despite potent DC activation and the ability to relieve hapten suppression, PT or CpG is not suitable for the clinical application because of safety concerns. For this reason, squalene-based oil-in-water nanoemulsion (NE) represents more suitable adjuvants for human application. So far, two NE adjuvants, MF59 and AS03, have been approved for human use in Europe but not in the United States. Although the exact mechanisms of the action remain unclear, studies show that NE similarly enhances innate immunity by recruiting DCs to the site of injection and by promoting cytokine production (20, 21). Several clinical studies have shown that NE enhances immunogenicity of inactivated influenza vaccines while being well tolerated by recipients (22–25). Because NE also induces cross-reactivity against heterosubtypic strains of influenza virus (23, 24), it is of clinical importance to test whether this adjuvant can overcome/reduce original antigenic sin.
The purpose of this study was to develop strategies to overcome original antigenic sin responses to influenza viruses. Noting the similarities between original antigenic sin and carrier-mediated hapten suppression, we hypothesized that administration of DC-activating adjuvants could relieve original antigenic sin. Using the mouse model that we have established recently (26), here, we show that coadministration of adjuvants PT or CpG or a squalene-based NE during sequential exposure to two closely related hemagglutinin 1 neuraminidase 1 (H1N1) influenza strains, A/Puerto Rico/8/34 (PR8) and A/Fort Monmouth/1/47 (FM1), overcame original antigenic sin. This was evidenced by either undetectable or reduced lung viral titers following a lethal challenge with FM1. This was attributable to the activation of IFN-γ-producing memory CD4 and CD8 T cells and higher neutralizing-Ab titers against FM1. Interestingly, both PT and NE, but not CpG, when administered during the primary exposure, prevented the induction of original antigenic sin to the variant virus. Finally, we show that original antigenic sin can be overcome with a booster immunization with the same dose of the second influenza virus, FM1, without any adjuvant.
Results
Recently, we have shown that sequential exposure to antigenically related viruses induces varying degrees of original antigenic sin (26). As a result, the development of memory responses in the host is compromised, and, consequentially, the host fails to efficiently clear the virus upon a lethal challenge (26). In this study, we tested whether adjuvants could reduce or prevent the induction of original antigenic sin. Because PT is known to prevent the induction of carrier-mediated hapten suppression by activating DCs (12, 13), we administered PT during sequential viral exposure and then assessed the development of protective immunity. We also included CpG, which induces DC maturation in a manner similar to PT (19).
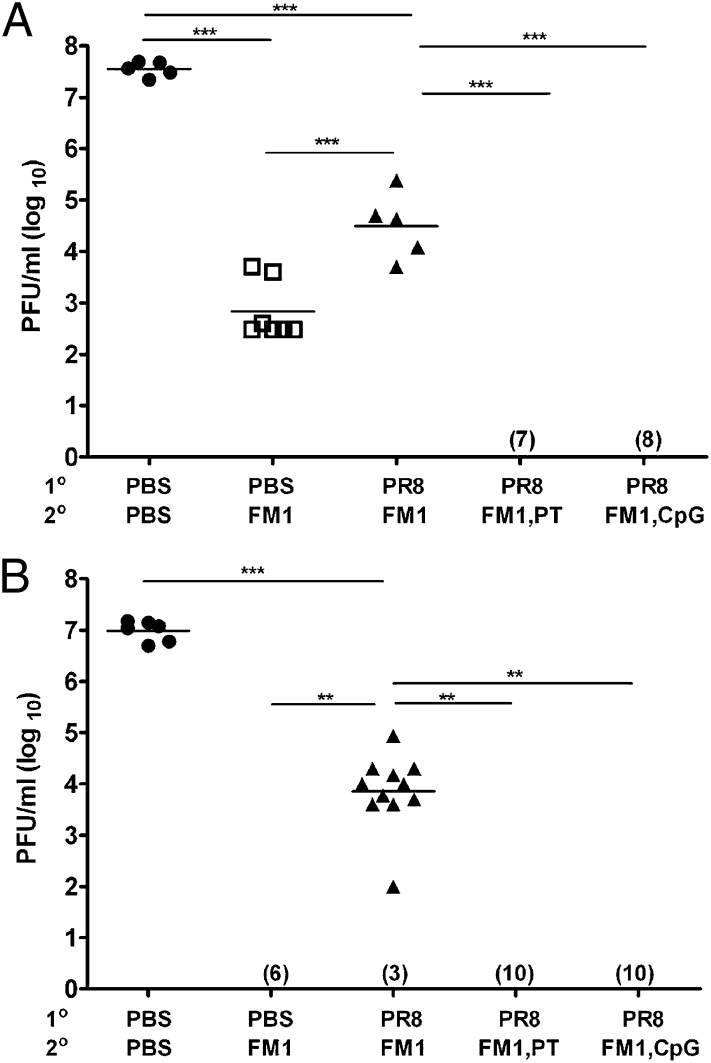
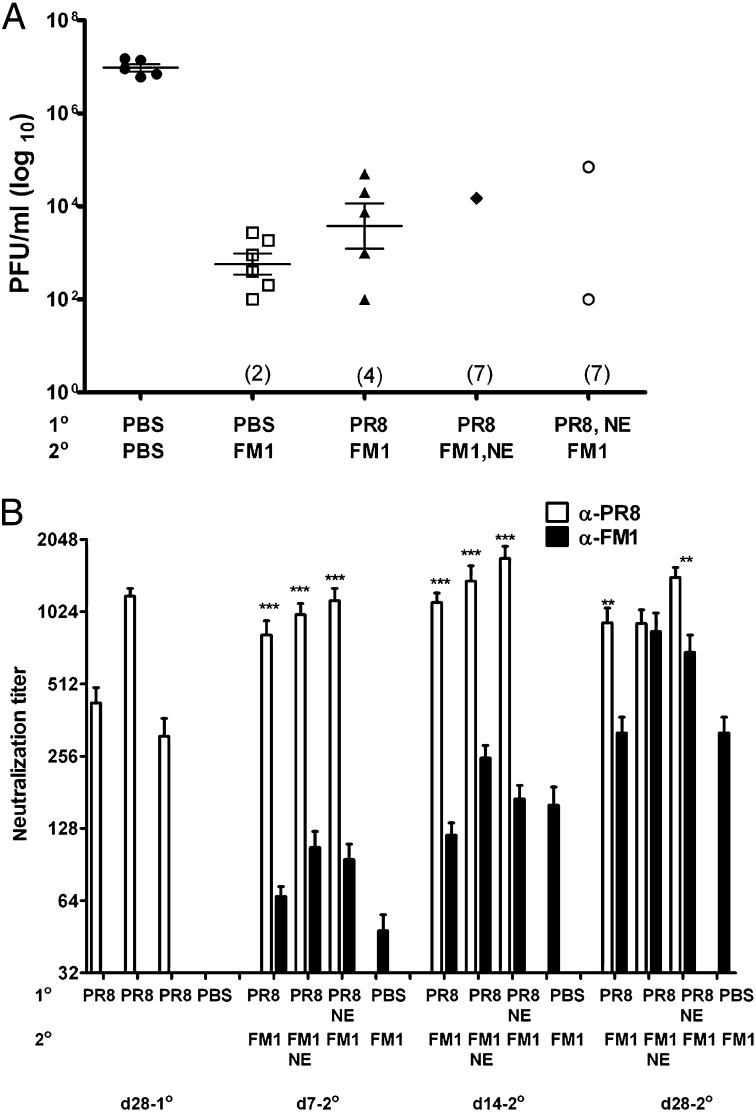
First, we tested the effect of PT and CpG in sequentially immunized mice. Mice were first immunized with 1,400 HA units (HAU) of whole-inactivated PR8 and then with 1,400 HAU FM1 a month later. A control group of mice received PBS first and, a month later, were immunized with FM1 (immune control). Adjuvant groups received 250 ng of PT or 50 μg of CpG during FM1 immunization. A month after FM1 immunization, we challenged the mice with 100× LD50 of live mouse-adapted FM1. We also set up naïve control that received PBS only. Four days following challenge, we harvested their lungs and assessed the lung viral titers by plaque assay (Fig. 1A). Whereas FM1 immunization (immune control) significantly reduced lung viral titers (7 × 102 pfu/mL) compared with naïve mice (5 × 107 pfu/mL), sequentially immunized mice had significantly higher lung viral titers (3 × 104 pfu/mL) than the immune control mice. This suggests that original antigenic sin compromised the establishment of immune memory. In sharp contrast, adjuvant groups had undetectable lung viral titers, indicating complete protection on day 4 from lethal challenge. These data demonstrate that PT or CpG overcomes original antigenic sin during sequential immunization with variant influenza viruses.
Fig. 1.
Coadministration of PT or CpG during second exposure confers complete protection against a lethal viral challenge. Cohorts of BALB/c mice (5–10 mice/group) were sequentially immunized with 1,400 HAU PR8 and FM1 viruses (A) or infected with 0.1× LD50 PR8 and FM1 viruses (B). Some mice received either 250 ng of PT i.p. or 50 μg of CpG s.c. during FM1 exposure. A month later, the mice were lethally challenged with 100× LD50 FM1 virus. Four days later, lung viral titers were measured using plaque assay on MDCK cells, shown as plaque forming units (pfu/mL). Each data point represents an individual animal. Numbers indicate the number of mice with undetectable level of lung viral titers. Error bars represent SEM. **P < 0.02; ***P < 0.001. Data are representative of two separate experiments. A portion of this figure was previously published as figures 1c and 3c in ref. 26. Copyright 2009. The American Association of Immunologists, Inc.
Next, we tested the effect of PT and CpG during sequentially infection (Fig. 1B). We sequentially infected the mice with 0.1× LD50 of mouse-adapted PR8 and then FM1 virus with or without adjuvants. We then challenged the mice and assessed the development of immune memory as described in Fig. 1A. A single FM1 infection provided a sterilizing immunity (immune control), whereas sequentially infected mice had significantly higher level of lung viral titers (104 pfu/mL). This indicates that sequential infection severely diminishes the memory development against the second virus. In striking contrast, administration of PT or CpG during FM1 infection resulted in undetectable lung viral titers. This demonstrates that adjuvants aided in conferring a sterilizing immunity to sequentially infected mice. Taken together, these data suggest that the both PT and CpG adjuvants improved the protective immunity in mice sequentially immunized or infected with antigenically related viruses. Therefore, a deficit in memory development caused by original antigenic sin can be overcome using adjuvants.
Preexisting neutralizing Abs provide immediate protection against an infection. We, therefore, tested whether the adjuvants improved the protection (Fig.1) by increasing the neutralizing-Ab titers in these mice using sera collected at different time points (Fig. 2 A and B). Following sequential immunization, the induction of original antigenic sin was minimal as reported previously (26): PR8 vs. FM1 titers were generally comparable except on day 14, and FM1 titers in these mice were comparable to those of the control group (Fig.2A). However, administration of PT or CpG gradually enhanced the FM1 titers through day 14, resulting in comparable PR8 vs. FM1 titers at day 28 after FM1 immunization. In addition, the FM1 titers in these mice were even higher than those of the control group. Adjuvants also significantly increased hemagglutination inhibition (HAI) titers against FM1 (Fig. S1A). Following sequential infection, the induction of original antigenic sin was profound, as reported previously (Fig. 2B) (26): FM1 titers were significantly lower than the PR8 titers, as well as FM1 titers of the control group, at all time points (Fig. 2B). Injection of adjuvants during FM1 infection increased the FM1 titers marginally, but, overall, these titers did not reach the level of the FM1 titers of the control group. Adjuvants also marginally increased FM1-HAI titers but not at a comparable level to the control group (Fig. S1B). Collectively, our data show that adjuvants can enhance the neutralizing-Ab titers against the second virus following sequential immunization or infection.
Fig. 2.
Coadministration of adjuvants PT or CpG during second exposure enhances neutralizing-Ab response against second virus. Serum samples were collected from BALB/c mice described in Fig.1 and neutralizing-Ab titers were measured using freshly grown PR8 and FM1 viruses in MDCK cells. Open vs. filled bars represent PR8 vs. FM titers. Error bars represent SEM. Data are representative of two to three separate experiments. Asterisks indicate significance between anti-PR8 vs. anti-FM1 titers. *P < 0.05; **P < 0.02; ***P < 0.001.
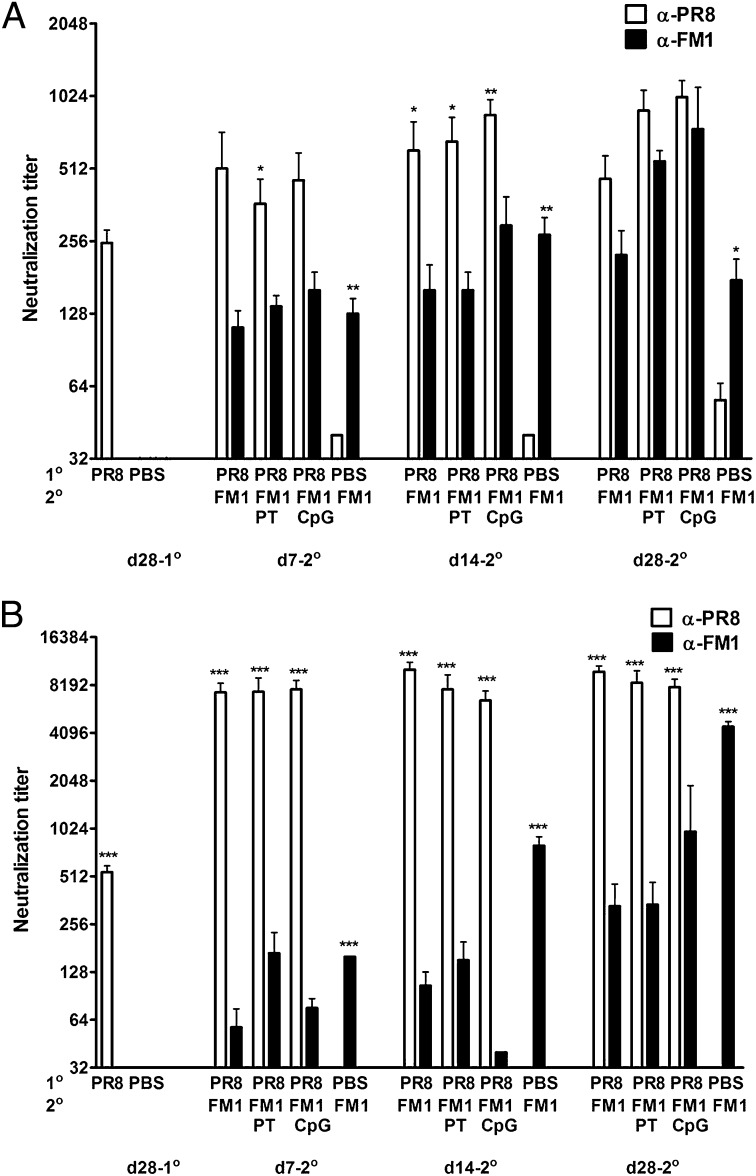
Our data demonstrate that adjuvants administered during the secondary viral exposure reduces original antigenic sin and enhances immune memory (Figs. 1 and 2). Next, we tested whether original antigenic sin can be prevented by administering adjuvants during the primary exposure. Mice were immunized with 1,400 HAU PR8 with or without adjuvants and then with FM1 a month later. We then challenged the mice and assessed the protective immunity a month later (Fig. 3A). Sequentially immunized mice had significantly higher viral titers (3 × 104 pfu/mL) than the FM1-immune control group (7 × 102 pfu/mL) following challenge. However, PT-injected mice during PR8 immunization had no detectable level of virus, indicating an establishment of cross-reactivity during the primary immunization. The reduction in viral load by CpG was not statistically significant (P = 0.09). Altogether, these data indicate that original antigenic sin can be prevented by administering PT but not CpG during the first immunization.
Fig. 3.
Coadministration of PT but not CpG during primary immunization prevents the induction of original antigenic sin. BALB/c mice (5-7 mice/group) were sequentially immunized with 1,400 HAU PR8 or PBS and then with 1,400 HAU FM1 a month later. Adjuvants (same as Fig. 1) were administered during PR8 immunization. Mice were challenged and lung viral titers were measured as described in Fig. 1A. Each data point represents an individual animal. Numbers indicate the number of mice with undetectable level of lung viral titers. Neutralization titers were measured using serum samples collected as shown (B). Open and filled bars represent PR8 and FM1 titers. Error bars represent SEM. Data are representative of three separate experiments. Asterisks indicate significance between anti-PR8 vs. anti-FM1 titers. *P < 0.05; **P < 0.02. A portion of A was previously published as figure 1c in ref. 26. Copyright 2009. The American Association of Immunologists, Inc.
We also measured the serum neutralizing-Ab titers from these mice as described in Fig. 2 (Fig. 3B). Administration of adjuvants with PR8 did not significantly increase PR8 titers before FM1 immunization (Fig. 3B). Upon FM1 immunization, PT-injected mice enhanced the FM1 titer both at days 7 and 14, resulting in higher FM1 titers than the control group. On the other hand, CpG-injected mice enhanced the FM1 titer only at day 14, while decreasing it at other time points. This may explain the reduced protection in CpG-adjuvanted mice following a lethal challenge (Fig. 3A). We observed similar response in HAI titers, such that PT injection, but not CpG, during the PR8 immunization enhanced FM1-HAI titers (Fig. S2). Taken together, our observations suggest that certain adjuvants can be administered during primary immunization to enhance the Ab titers against the second virus without further need for adjuvants.
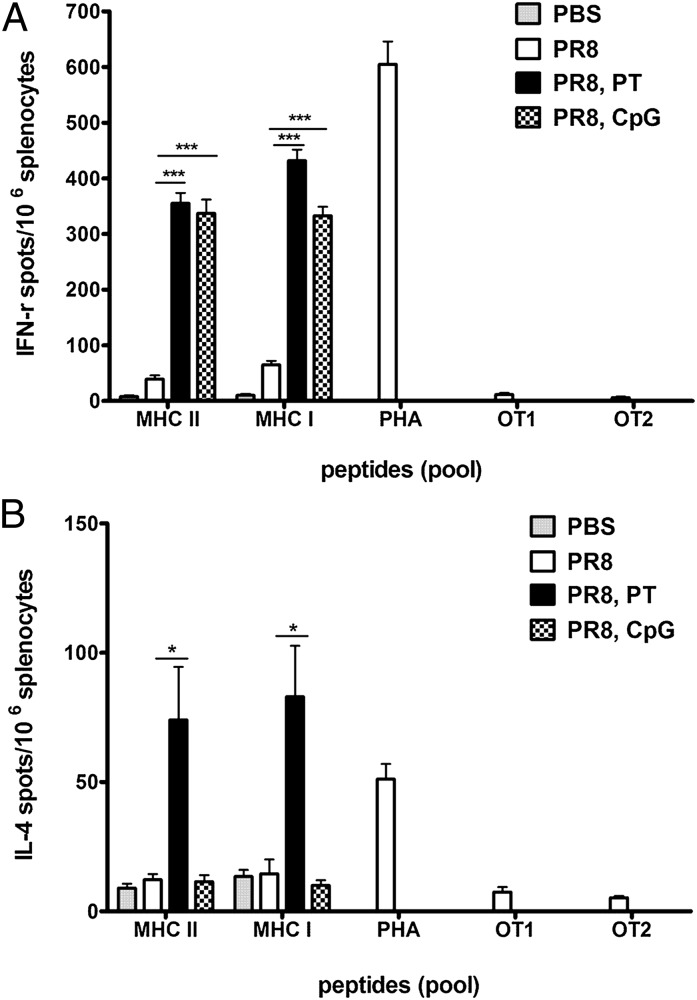
Our observations indicate that PT induced better protective immunity in sequentially immunized mice. Whereas high levels of neutralizing Abs are critical for preventing infection, cellular immunity is important for vial clearance. Here, we tested the extent to which PT and CpG induced antigen (Ag)-specific memory T-cell activation. Briefly, we immunized BALB/c mice with PR8 with or without adjuvants and, a month later, harvested the spleens and stimulated cells with a pool of PR8-specific MHC class I or MHC class II–restricted immunodominant peptides. We then assessed the frequency of IFN-γ or IL-4–secreting T cells by ELISPOT assay (Fig. 4 A and B). Positive and negative control cells were stimulated with phytohemagglutinin (PHA) and ovalbumin peptides (OTI or OTII) respectively. Both adjuvants significantly increased the frequency of IFN-γ–producing CD4 and CD8 T cells compared with PR8-immune mice, whereas PBS control mice had a negligible level of IFN-γ–producing T cells (Fig. 4A). In contrast, only PT significantly increased the numbers of IL-4–producing CD4 and CD8 T cells (Fig. 4B). Therefore, PT activated virus-specific IFN-γ– and IL-4–producing T cells, whereas CpG activated only IFN-γ–producing T cells.
Fig. 4.
Adjuvants enhance the IFN-γ–producing CD4 and CD8 T cells specific to PR8. BALB/c mice (three to four mice/group) were immunized with 10 μg of formalin-inactivated PR8 virus or PBS. Some mice received either 250 ng of PT i.p. or 50 μg of CpG s.c. A month later, spleens of these mice were harvested for analysis of Ag-specific T cells directly ex vivo by ELISPOT assay using immunodominant MHC class I– or class II–restricted peptides. The numbers of IFN-γ–secreting (A) or IL-4–secreting (B) PR8-specific MHC class II–restricted CD4 or MHC class I–restricted CD8 T cells per 106 total splenocytes are shown. Error bars represent SEM. Data are representative of three separate experiments. Adjuvant groups were compared against PR8 group. *P < 0.05; ***P < 0.001.
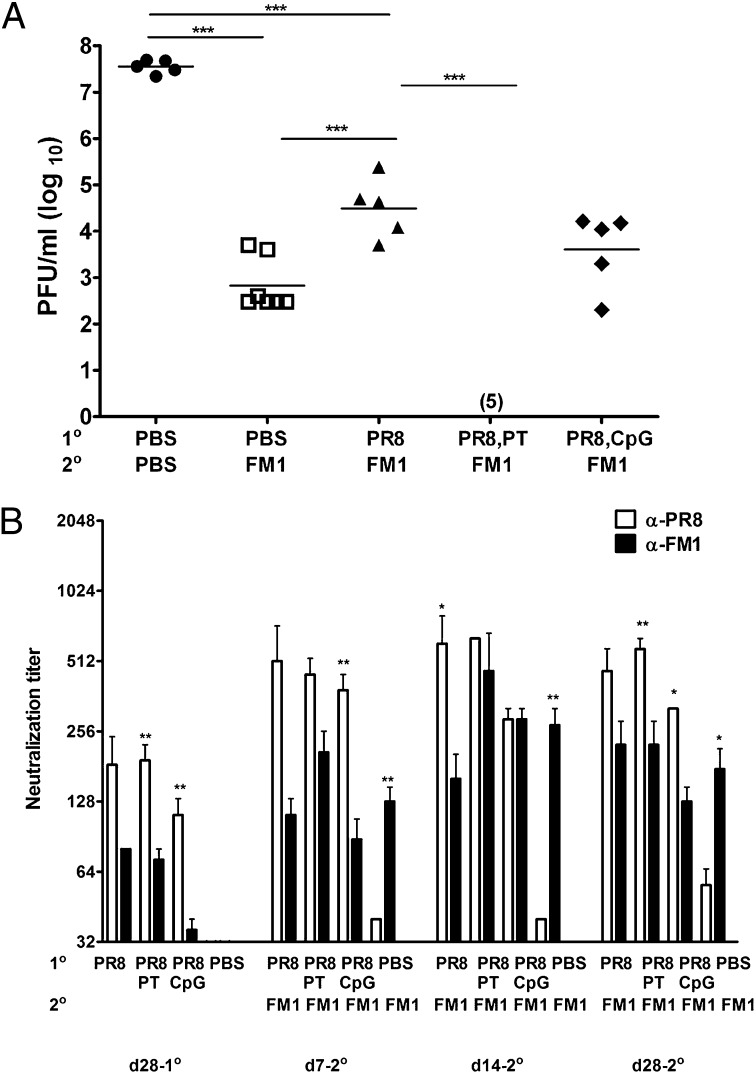
Our data so far show that original antigenic sin can be overcome using DC-activating adjuvants either during the primary or secondary viral exposure. However, these adjuvants are not applicable to human influenza vaccination. Therefore, we tested whether the squalene-based NE can reduce original antigenic sin in our mouse model. First, we tested whether the NE can improve the memory development during sequential immunization in mice. Whole inactivated viruses were mixed with the NE at a 1:1 ratio before administration into mice. We then lethally challenged these mice and assessed the protective immunity as described previously (Fig. 5A). Sequentially immunized mice had an elevated level of viral titers compared with the immune control mice, although this difference was not statistically significant (P = 0.1). On the other hand, seven of eight mice that received adjuvanted FM1 and seven of nine mice that received adjuvanted PR8 completely cleared FM1 virus. This shows that addition of NE adjuvant to whole influenza virus enhances the memory response against the second virus and that the adjuvant can be added into either primary or secondary exposure. Next, we determined whether the enhanced recall response was mediated by increased FM1 neutralizing-Ab titers (Fig. 5B). Consistent with the reduced viral titers (Fig. 5A), addition of the NE during either primary or secondary vaccination increased FM1 titers up to day 28 following FM1 vaccination. Collectively, our data show that the NE can be used with influenza vaccine to overcome original antigenic sin.
Fig. 5.
Oil-in-water emulsion adjuvant diminishes the induction of original antigenic sin. BALB/c mice (5-10 mice/group) were sequentially immunized (i.m.) with 1,400 HAU PR8 or PBS, and then with FM1. For adjuvantation of vaccine, inactivated PR8 or FM1 virus was mixed with oil-in-water emulsion at a 1:1 ratio immediately before injection. The mice were lethally challenged and lung viral titers were measured as in Fig.1A. The neutralization titers were measured using serum samples collected at time points shown (B). Open and filled bars represent PR8 vs. FM1 titers. NE stands for nanoemulsion. Asterisks indicate significance between anti-PR8 vs. anti-FM1 titers. *P < 0.05; **P < 0.02; ***P < 0.001.
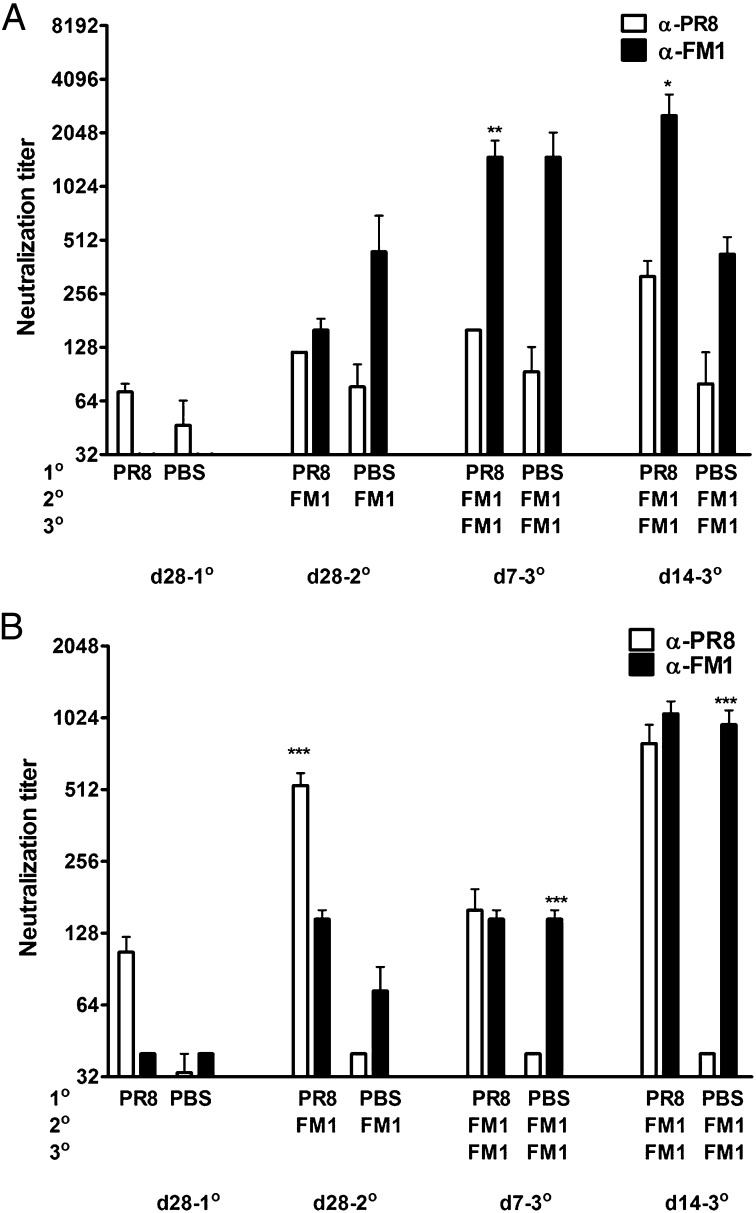
Next, as an alternative to the use of adjuvants, we determined whether original antigenic sin could be minimized by booster immunization with second virus. We sequentially immunized mice with: (i) HA-encoding DNA vaccines (2 μg PR8-HA then FM1-HA) using a gene gun; or (ii) inactivated influenza viruses (1,400 HAU PR8 then FM1). We then boosted these mice with the same dose of FM1 Ag one month later. We collected serum samples at different time points and determined the neutralization titers (Fig. 6). The FM1 boost significantly increased FM1 titers at days 7 and 14 (Fig. 6 A and B), resulting in either higher (Fig. 6A) or comparable (Fig. 6B) FM1 titers than PR8 titers. In addition, this increase in FM1 titers resulted in higher level of FM1 titers than the control group at day 28 following booster immunization. We also observed a similar response by HAI titer estimation (Fig. S3 A and B). Collectively, these data demonstrate that expanding memory cells by repeated exposure can overcome the effects of original antigenic sin in this mouse model.
Fig. 6.
Booster immunization with the second virus relieves original antigenic sin. BALB/c mice (five to six mice/group) were sequentially immunized with 2 μg of DNA vaccine encoding full-length HA from PR8 (PR8-HA) (A) or 1,400 HAU PR8 virus (B), and then with FM1-HA or 1,400 HAU FM1 virus a month later. Mice were booster immunized again with FM1 Ag a month later. Neutralizing-Ab titers were measured using serum samples collected at time points as shown. Open and filled bars represent PR8 and FM1 titers. Error bars represent SEM. Data are representative of two separate experiments. Asterisks indicate significance between anti-PR8 vs. anti-FM1 titers. *P < 0.05; **P < 0.02; ***P < 0.001.
Discussion
The phenomenon of original antigenic sin in immune responses to influenza has been documented in humans, as well as in animal models (1–5, 27–29). Although the risk of original antigenic sin associated with annual influenza vaccination remains, the development of strategies to prevent or minimize original antigenic sin has not been widely studied. In this paper, we show that original antigenic sin responses to influenza viruses can be minimized or prevented. This was accomplished by administering adjuvants during secondary or primary exposure or by booster immunization with the second virus.
Adjuvants enhance immune responses. In mice sequentially exposed to PR8 and FM1, we observed that administering PT or CpG or NE during FM1 exposure improved the protective immunity against FM1 and augmented the FM1 neutralizing-Ab titers (Figs. 1, 2, and 5). Surprisingly, we could achieve the same results by administering PT or NE once during PR8 immunization with no further need for adjuvants in subsequent immunizations (Figs. 3 and 5). Similar observations were documented in carrier-mediated hapten suppression model, wherein injection of killed B. pertussis during KLH priming enhanced NP-specific Ab production (12). Similarly, injection of pertussis vaccine or purified B. pertussis toxin or endotoxin during priming with tetanus toxoid blocked the epitopic suppression in a synthetic vaccine model (13). These studies indicate that injection of certain adjuvants during first immunization enhances cross-reactivity, leading to increased Ab production against the second related Ag.
Our current findings demonstrate that adjuvants relieve original antigenic sin by inducing cross-reactive memory B cells. Clinical studies using NE adjuvants for influenza vaccines support this idea. Individuals primed with MF59-adjuvanted H5N3 vaccine, compared with unprimed subjects, induce more Abs against distant heterologous strain, H5N1 (23). In addition, infant and young children vaccinated with MF59-adjuvanted seasonal influenza vaccine induce more seroprotective Ab titers against heterologous H1 and H3 Ags compared with unadjuvanted groups (24). The duration of Ab response following MF59-adjuvanted vaccine is also longer than that of unadjuvanted vaccine (24, 25). Therefore, NE-adjuvanted vaccines can be used to potentiate the vaccine efficacy and to broaden the spectrum of Ab responses. This feature is important for immunocompromised population, such as patients with chronic infections, older adults and infants/young children who are at risk for postinfluenza complications (30). Collectively, our findings imply that original antigenic sin could potentially be prevented in the naive human population, especially children, by administering adjuvants with the first influenza vaccine. Alternatively, in the older population with prior influenza virus exposure or vaccinations, original antigenic sin can be minimized by using adjuvants.
The exact mechanisms of original antigenic sin remain elusive. It is possible that original antigenic sin occurs because of Ag presentation by preexisting memory B cells instead of DCs. In the context of sequential exposure with PR8 and FM1, primary exposure induces proliferation of PR8 epitope-specific B cells and cross-reactive B cells. Upon exposure to FM1, selective activation of cross-reactive memory B cells may occur at the expense of FM1 novel epitope-specific naïve B cells, because of the higher frequency and the lower activation threshold of cross-reactive memory B cells compared with naïve B cells. In addition, binding of memory B cells to HA can be facilitated by sialic acid binding (31). All of these factors can lead to Ag uptake and presentation by memory B cells. This redirection of Ag presentation by B cells instead of DCs may lead to suboptimal activation signals that favor memory over naïve B-cell activation.
The administration of adjuvants may shift Ag presentation from memory B cells to DCs and enhance cellular immune response. Both PT and CpG promote maturation of DCs, induce them to produce cytokines including IL-12 and IFN-γ, and enhance Ag presentation (14, 17, 19, 32). The mechanism by which NE enhances immunogenicity is not completely understood, but available data suggest that MF59 triggers a local inflammatory environment by engaging muscle cells at the injection site, which then indirectly activates DCs (20). Regardless of the direct effect on DCs, activation of DCs could shift Ag presentation away from memory B cell to DCs, resulting in more recruitment of FM1-specific naïve B cells into the response. Thus, DC participation may be crucial in overcoming antigenic sin. Experiments to test this hypothesis are currently in progress.
Immediate neutralization of virus is mediated by preexisting neutralizing Abs (33). However, cellular immunity including Ag-specific CD8 T cells is critical in viral clearance (34, 35). In accordance with this, we observed that adjuvants significantly enhanced virus-specific CD8 and CD4 T-cell responses (Fig. 4). Our data demonstrate that mice receiving PT or CpG either during primary or secondary exposure showed enhanced or complete protection from a lethal challenge (Figs. 1 and 3A). This is attributable not only to enhanced Ab titers against FM1 (Figs. 2 and 3B) but also to activation of IFN-γ– and IL-4–producing memory CD8 T cells (Fig. 4). Thus, it is conceivable that adjuvants, through the activation of DCs, may enhance cytotoxic effects of memory CD8 T cells to accelerate the clearance of virus, while activating IFN-γ–producing CD4 T cells to aid in the neutralizing-Ab responses. Interestingly, PT yielded higher numbers of IL-4–producing CD4 T cells than CpG (Fig.4B), and this may explain why mice given PT during the first exposure developed better protective responses than the CpG group (Fig. 3A).
Finally, our findings present a third strategy to overcome original antigenic sin. Repeated immunization with the second viral strain induced robust responses to the second virus (Fig. 6). In mice sequentially immunized with either DNA vaccines or whole inactivated viruses, boosting animals with same dose of FM1 at the memory phase significantly enhanced the Ab titers against FM1 (Fig. 6). Thus, reexposure facilitates the development of the memory pool and protective immunity against the variant strain. This may occur through the selective activation of FM1-specific B cells generated during the secondary exposure.
With annual influenza vaccinations, the threat of original antigenic sin looms especially with related influenza viruses. Our current study provides strategies to prevent or minimize original antigenic sin by either using adjuvants or by booster immunizations. Although these approaches offer some clues into the mechanisms of original antigenic sin, the integrated molecular mechanisms that induce original antigenic sin await much more elaborate studies.
Methods
Mice, Immunizations, Infection, and Serum Collection.
BALB/c mice were used for the study. Housing, anesthetization, immunization, or infection of mice, serum collection, and treatment were performed as described (26). All animal studies were performed with the approval of the Emory University Institutional Animal Care and Use Committee.
Cells, Viruses, and HA-Encoding DNA Vaccines.
Madin–Darby canine kidney (MDCK) cells, DNA vaccines, and PR8 and FM1 viruses were described previously (26).
Adjuvants.
B. pertussis toxin and DNA containing unmethylated CpG-dinucleotide were purchased from List Biological and Operon, respectively. NE was made at the Centers for Disease Control and Prevention (CDC) in Atlanta, GA. This adjuvant is composed of a water phase (PBS, Tween 80) and oil phase (Squalene with or without α-tocopherol) at a 4:1 ratio. The nanoemulsion was prepared by passing the mixture through M-110 PMicrofluidizer (Microfluidics) under a pressure of 18,000 psi. The size of nanoemulsion particles was determined by dynamic light scattering technique (DynaPro Plate Reader; Wyatt Technology). The average particle size of the emulsion ranges from 100–110 nM. For adjuvanted vaccine, whole inactivated influenza virus was mixed with the NE at a 1:1 ratio before immunization of mice.
Influenza Virus Microneutralization, HAI Assay.
Treatment of sera followed by microneutralization or HAI assay were done as described previously (26).
Plaque Assay.
Viral titers in lung lysates were assessed using plaque assay as described (26).
T-Cell ELISPOT.
ELISPOT for IFN-γ, IL-4 was performed as described (36). For T-cell stimulation, a pool of H-2d–restricted class I HA and NA peptides (IYSTVASSL, TYQRTRALVRTGMDP) or a pool of eight class II HA peptides (SFERFEIFPKE, HNTNGVTAACSHE, CPKYVRSAKLRM, KLKNSYVNKKGK, NAYVSVVTSNYNRRF, ASMHECNTKCQT, EIAERPKVRDQAG, VLWGIHHPPNSK) were added into cells. Class I ovalbumin (OVA)-specific T cell epitope (OT) peptide (SINFEKL) and class II OT peptide derived from OVA protein were used as negative control. PHA was used as a positive control.
Statistics.
Student t test was used to generate all statistical values stated. For statistical designations, *P < 0.05; **P < 0.02; and ***P < 0.001.
Supplementary Material
Acknowledgments
We thank members of the J.J. laboratory for helpful discussions and Mrs. Leela Thomas for excellent mouse colony management. This research was supported by National Institutes of Health/National Institute of Allergy and Infectious Diseases Contract HHSN266 200700006C. J.J. is a research scholar of the American Cancer Society.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0912458109/-/DCSupplemental.
References
- 1.Davenport FM, Hennessy AV, Francis T., Jr Epidemiologic and immunologic significance of age distribution of antibody to antigenic variants of influenza virus. J Exp Med. 1953;98:641–656. doi: 10.1084/jem.98.6.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Virelizier JL, Allison AC, Schild GC. Antibody responses to antigenic determinants of influenza virus hemagglutinin. II. Original antigenic sin: A bone marrow-derived lymphocyte memory phenomenon modulated by thymus-derived lymphocytes. J Exp Med. 1974;140:1571–1578. doi: 10.1084/jem.140.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Virelizier JL, Postlethwaite R, Schild GC, Allison AC. Antibody responses to antigenic determinants of influenza virus hemagglutinin. I. Thymus dependence of antibody formation and thymus independence of immunological memory. J Exp Med. 1974;140:1559–1570. doi: 10.1084/jem.140.6.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Webster RG. Original antigenic sin in ferrets: The response to sequential infections with influenza viruses. J Immunol. 1966;97:177–183. [PubMed] [Google Scholar]
- 5.Fazekas de St Groth S, Webster RG. Disquisitions on Original Antigenic Sin. II. Proof in lower creatures. J Exp Med. 1966;124:347–361. doi: 10.1084/jem.124.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herzenberg LA, Tokuhisa T, Herzenberg LA. Carrier-priming leads to hapten-specific suppression. Nature. 1980;285:664–667. doi: 10.1038/285664a0. [DOI] [PubMed] [Google Scholar]
- 7.Di John D, et al. Effect of priming with carrier on response to conjugate vaccine. Lancet. 1989;2:1415–1418. doi: 10.1016/s0140-6736(89)92033-3. [DOI] [PubMed] [Google Scholar]
- 8.Renjifo X, et al. Carrier-induced, hapten-specific suppression: A problem of antigen presentation? J Immunol. 1998;161:702–706. [PubMed] [Google Scholar]
- 9.Roake JA, et al. Dendritic cell loss from nonlymphoid tissues after systemic administration of lipopolysaccharide, tumor necrosis factor, and interleukin 1. J Exp Med. 1995;181:2237–2247. doi: 10.1084/jem.181.6.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Smedt T, et al. Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo. J Exp Med. 1996;184:1413–1424. doi: 10.1084/jem.184.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finger H, Emmerling P, Schmidt H. Accelerated and prolongated multiplication of antibody-forming spleen cells by Bordetella pertussis in mice immunized with sheep red blood cells. Experientia. 1967;23:591–592. doi: 10.1007/BF02137991. [DOI] [PubMed] [Google Scholar]
- 12.Herzenberg LA, Tokuhisa T. Epitope-specific regulation. I. Carrier-specific induction of suppression for IgG anti-hapten antibody responses. J Exp Med. 1982;155:1730–1740. doi: 10.1084/jem.155.6.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vogel FR, et al. Modulation of carrier-induced epitopic suppression by Bordetella pertussis components and muramyl peptide. Cell Immunol. 1987;107:40–51. doi: 10.1016/0008-8749(87)90264-4. [DOI] [PubMed] [Google Scholar]
- 14.Hou W, et al. Pertussis toxin enhances Th1 responses by stimulation of dendritic cells. J Immunol. 2003;170:1728–1736. doi: 10.4049/jimmunol.170.4.1728. [DOI] [PubMed] [Google Scholar]
- 15.Lipford GB, et al. CpG-containing synthetic oligonucleotides promote B and cytotoxic T cell responses to protein antigen: A new class of vaccine adjuvants. Eur J Immunol. 1997;27:2340–2344. doi: 10.1002/eji.1830270931. [DOI] [PubMed] [Google Scholar]
- 16.Roman M, et al. Immunostimulatory DNA sequences function as T helper-1-promoting adjuvants. Nat Med. 1997;3:849–854. doi: 10.1038/nm0897-849. [DOI] [PubMed] [Google Scholar]
- 17.Hartmann G, Weiner GJ, Krieg AM. CpG DNA: A potent signal for growth, activation, and maturation of human dendritic cells. Proc Natl Acad Sci USA. 1999;96:9305–9310. doi: 10.1073/pnas.96.16.9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chu RS, Targoni OS, Krieg AM, Lehmann PV, Harding CV. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J Exp Med. 1997;186:1623–1631. doi: 10.1084/jem.186.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sparwasser T, et al. Bacterial DNA and immunostimulatory CpG oligonucleotides trigger maturation and activation of murine dendritic cells. Eur J Immunol. 1998;28:2045–2054. doi: 10.1002/(SICI)1521-4141(199806)28:06<2045::AID-IMMU2045>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 20.Mosca F, et al. Molecular and cellular signatures of human vaccine adjuvants. Proc Natl Acad Sci USA. 2008;105:10501–10506. doi: 10.1073/pnas.0804699105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tritto E, Mosca F, De Gregorio E. Mechanism of action of licensed vaccine adjuvants. Vaccine. 2009;27:3331–3334. doi: 10.1016/j.vaccine.2009.01.084. [DOI] [PubMed] [Google Scholar]
- 22.Podda A. The adjuvanted influenza vaccines with novel adjuvants: Experience with the MF59-adjuvanted vaccine. Vaccine. 2001;19:2673–2680. doi: 10.1016/s0264-410x(00)00499-0. [DOI] [PubMed] [Google Scholar]
- 23.Galli G, et al. Fast rise of broadly cross-reactive antibodies after boosting long-lived human memory B cells primed by an MF59 adjuvanted prepandemic vaccine. Proc Natl Acad Sci USA. 2009;106:7962–7967. doi: 10.1073/pnas.0903181106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vesikari T, et al. Oil-in-water emulsion adjuvant with influenza vaccine in young children. N Engl J Med. 2011;365:1406–1416. doi: 10.1056/NEJMoa1010331. [DOI] [PubMed] [Google Scholar]
- 25.Clark TW, et al. Trial of 2009 influenza A (H1N1) monovalent MF59-adjuvanted vaccine. N Engl J Med. 2009;361:2424–2435. doi: 10.1056/NEJMoa0907650. [DOI] [PubMed] [Google Scholar]
- 26.Kim JH, Skountzou I, Compans R, Jacob J. Original antigenic sin responses to influenza viruses. J Immunol. 2009;183:3294–3301. doi: 10.4049/jimmunol.0900398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jensen KE, Davenport FM, Hennessy AV, Francis T., Jr Characterization of influenza antibodies by serum absorption. J Exp Med. 1956;104:199–209. doi: 10.1084/jem.104.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davenport FM, Hennessy AV. A serologic recapitulation of past experiences with influenza A; antibody response to monovalent vaccine. J Exp Med. 1956;104:85–97. doi: 10.1084/jem.104.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fazekas de St Groth S, Webster RG. Disquisitions of Original Antigenic Sin. I. Evidence in man. J Exp Med. 1966;124:331–345. doi: 10.1084/jem.124.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baldo V, Baldovin T, Floreani A, Carraro AM, Trivello R. Family Medicine Group of Pianiga MF59-adjuvanted influenza vaccine confers superior immunogenicity in adult subjects (18-60 years of age) with chronic diseases who are at risk of post-influenza complications. Vaccine. 2007;25:3955–3961. doi: 10.1016/j.vaccine.2007.02.045. [DOI] [PubMed] [Google Scholar]
- 31.Doucett VP, et al. Enumeration and characterization of virus-specific B cells by multicolor flow cytometry. J Immunol Methods. 2005;303:40–52. doi: 10.1016/j.jim.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 32.Van Uden JH, Tran CH, Carson DA, Raz E. Type I interferon is required to mount an adaptive response to immunostimulatory DNA. Eur J Immunol. 2001;31:3281–3290. doi: 10.1002/1521-4141(200111)31:11<3281::aid-immu3281>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 33.Gerhard W. The role of the antibody response in influenza virus infection. Curr Top Microbiol Immunol. 2001;260:171–190. doi: 10.1007/978-3-662-05783-4_9. [DOI] [PubMed] [Google Scholar]
- 34.Topham DJ, Tripp RA, Doherty PC. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J Immunol. 1997;159:5197–5200. [PubMed] [Google Scholar]
- 35.Doherty PC, Turner SJ, Webby RG, Thomas PG. Influenza and the challenge for immunology. Nat Immunol. 2006;7:449–455. doi: 10.1038/ni1343. [DOI] [PubMed] [Google Scholar]
- 36.Garg S, Oran AE, Hon H, Jacob J. The hybrid cytomegalovirus enhancer/chicken beta-actin promoter along with woodchuck hepatitis virus posttranscriptional regulatory element enhances the protective efficacy of DNA vaccines. J Immunol. 2004;173:550–558. doi: 10.4049/jimmunol.173.1.550. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.