Abstract
Expression of functional breast cancer susceptibility gene 1 (BRCA1) in human breast and ovarian cancers is associated with resistance to platinum-based chemotherapeutics and poly(ADP ribose) polymerase (PARP) inhibitors. BRCA1 is a nuclear tumor suppressor that is critical for resolving double-strand DNA breaks (DSBs) and interstrand crosslinks (ICLs) by homologous recombination (HR). In vitro, animal and human clinical data have demonstrated that BRCA1-deficient cancers are highly sensitive to ICL-inducing chemotherapeutic agents, are amenable to synthetic lethal approaches that exploit defects in DSB/ICL repair, and may be associated with improved survival. Conversely, high or restored expression of BRCA1 in breast and ovarian cancer is associated with therapeutic resistance and poor prognosis. There has been much interest in identifying agents that interfere with BRCA1-dependent DSB/ICL repair to restore or enhance sensitivity to cancer therapeutics. We demonstrate that the heat-shock protein 90 (HSP90) inhibitor 17-allylamino-17-demethoxygeldanamycin [17-AAG (Tanespimycin)], currently in Phase II/III clinical evaluation for several cancers, induces BRCA1 ubiquitination and proteasomal degradation, resulting in compromised repair of ionizing radiation- and platinum-induced DNA damage. We show that loss of HSP90 function abolishes BRCA1-dependent DSB repair and that BRCA1-deficient cells are hypersensitive to 17-AAG due to impaired Gap 2/Mitosis (G2/M) checkpoint activation and resultant mitotic catastrophe. In summary, we document an upstream HSP90-dependent regulatory point in the Fanconi anemia/BRCA DSB/ICL repair pathway, illuminate the role of BRCA1 in regulating damage-associated checkpoint and repair responses to HSP90 inhibitors, and identify BRCA1 as a clinically relevant target for enhancing sensitivity in refractory and/or resistant malignancies.
Keywords: chemosensitivity, DNA repair
Inherited mutations in the breast cancer susceptibility gene 1 (BRCA1) predispose the development of breast, ovarian, and other malignancies (1–5). BRCA1 is a nuclear tumor suppressor critical for repair of double-strand DNA breaks (DSBs) and interstrand crosslinks (ICLs) by homologous recombination (HR) (6). BRCA1 is phosphorylated by ataxia telangiectasia mutated (ATM), ataxia telangiectasia and Rad3 related (ATR), and checkpoint kinase 2 (CHK2) kinases in response to DNA damage and recruits and organizes multiple distinct protein complexes that recognize and repair damaged DNA and activate cell cycle checkpoints (7, 8). In vitro and in vivo studies have shown that tumor cells expressing high levels of BRCA1 are resistant to both ionizing radiation (IR) and several classes of chemotherapeutic agents, and that ablation of BRCA1 expression can restore sensitivity to these agents (9, 10). Indeed, cancers arising in BRCA1 mutation carriers are relatively hypersensitive to platinum-based therapies, and high BRCA1 mRNA expression in sporadic cancers is a biomarker for poor response to these same agents (11–13).
DSB-repair-deficient cancers arising in BRCA1/2 mutation carriers are highly sensitive to inhibitors of poly(ADP ribose) polymerase (PARP), an enzyme critical in base excision repair (14). Clinical trials using PARP inhibitors are currently ongoing and these agents show promise in the treatment of BRCA1- and BRCA2-associated breast, ovarian, and prostate cancers, as well as sporadic basal-like breast cancers, which are thought to have dysfunction of the BRCA1 pathway in the absence of mutations at its genetic locus (3, 15, 16). Therapy-induced secondary mutations in ovarian cancers that restore the BRCA1 or BRCA2 reading frames occur with significant frequency and are directly responsible for resistance to platinum-based therapies and PARP inhibitors (17, 18). Targeting BRCA1 and/or its associated protein complexes in cancer chemo- and radiotherapy may induce hypersensitivity to agents that induce DSBs and prevent the development or recurrence of resistant disease (19). Here we identify BRCA1 as a client protein of heat-shock protein 90 (HSP90) and demonstrate that inhibition of HSP90 using 17-allylamino-17-demethoxygeldanamycin (17-AAG) results in profound loss of BRCA1 expression and function.
HSP90 chaperones “client” proteins into their native conformations, regulating multiple aspects of protein function (20). Natural compounds including geldanamycin, radicicol, and novobiocin have been identified as disrupting HSP90 chaperone function, leading to client protein degradation via the ubiquitin–proteasome pathway (21). HSP90 inhibitors, including 17-AAG, are undergoing clinical trials for the treatment of a variety of human malignancies (22). These inhibitors have shown promise in sensitizing tumor cells to numerous genotoxic agents that are commonly used in cancer therapy, including DNA alkylating agents, ionizing radiation, DNA replication inhibitors, and PARP inhibitors (23, 24).
Multiple components of the HR/ICLR and non-homologous end joining (NHEJ) DSB repair machinery, including checkpoint kinase 1 (CHK1), breast cancer susceptibility 2 (BRCA2), RecA homolog (RAD51), Fanconi anemia complementation group A (FANCA), and the catalytic subunit of the DNA-dependent protein kinase (DNA-PKcs) have been described to be clients of HSP90 (25–27). Except for DNA-PKcs, the activity and/or recruitment of these molecules to sites of DNA damage is dependent on BRCA1 function (8, 11, 28–32). We report here that pharmacologic inhibition of HSP90 results in rapid loss of BRCA1 through the ubiquitin–proteasome pathway, subsequent failure of BRCA1 to assemble at ionizing radiation-induced foci (IRIF) and ensuing functional defects in both HR and NHEJ. Moreover, we demonstrate that BRCA1 is a critical mediator of 17-AAG-induced arrest at the G2/M checkpoint, and that consequently, BRCA1-deficient cells progress into catastrophic mitosis. In summary, we show that loss of BRCA1 following HSP90 inhibition is a key upstream event leading to defective DSB repair, failure of G2/M checkpoint activation, and potentiation to DNA damaging agents.
Results
HSP90 Interacts with BRCA1 and Is Necessary for Its Stability.
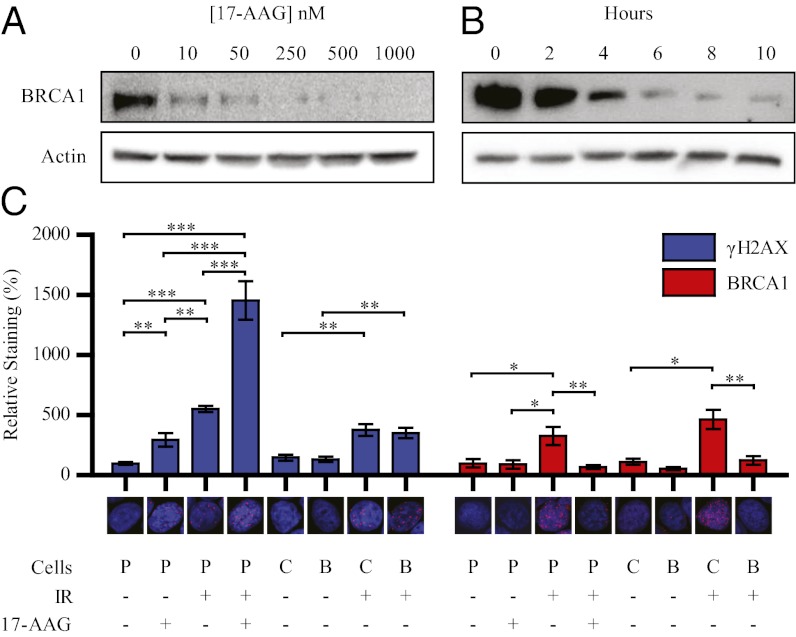
To understand the regulatory properties of HSP90 on BRCA1 expression, we examined the effects of 17-AAG treatment on BRCA1 in MCF7 (Michigan Cancer Foundation-7) breast cancer cells. 17-AAG down-regulated BRCA1 in a dose- and time-dependent manner (Fig. 1 A and B), and when combined with cycloheximide, increased the rate of BRCA1 decay beyond that observed with cycloheximide alone (Fig. S1A). Transcriptional activation of the BRCA1 promoter and levels of BRCA1 mRNA showed delayed effects, with a modest increase in expression of BRCA1 promoter-driven luciferase at relatively low doses up to 72 h post 17-AAG treatment (Fig. S2). BRCA1 protein degradation preceded loss of BRCA1 mRNA (Fig. S3). These data suggest that destabilization of BRCA1 protein is likely the major mechanism for BRCA1 loss in response to 17-AAG, although transcriptional alterations may contribute to loss of BRCA1 at later time points. 17-AAG treatment also increased expression of HSP90 mRNA (Fig. S3), reflecting a previously described feedback stimulation of stress-responsive genes (33). 17-AAG treatment did not simultaneously alter expression of the constitutively associated BRCA1-associated ring domain 1 (BARD1) protein, which is necessary for BRCA1 stability and function (Fig. S1B) (34). Kinetic studies in SK-BR-3 (Sloan-Kettering, Breast-3), MDA-MB-231 (MD Anderson, Metastatic Breast-231), SK-OV-3 (Sloan-Kettering, Ovarian-3), and HT29 (human colorectal cancer cells) cells revealed that 17-AAG induced degradation of BRCA1 expression in all cell lines tested (Fig. S1C). Treatment of MCF7 cells with radicicol and novobiocin, two additional inhibitors of HSP90 chaperone function, also resulted in loss of BRCA1 (Fig. S1D).
Fig. 1.
Inhibition of HSP90 induces degradation of BRCA1. (A) Western blots of MCF7 cells treated with indicated concentrations of 17-AAG for 8 h. (B) Western blots of MCF7 cells treated with 250 nM 17-AAG for indicated duration. (C) MCF7 cells were treated with DMSO or 250 nM 17-AAG for 24 h. Cells were exposed to 0 or 10 Gy of IR and then were fixed and immunostained for γ-H2AX or BRCA1 4 h post IR. Graphs represent the fluorescence intensity of the γ-H2AX or BRCA1 channel normalized to the DAPI channel in five random fields from one representative experiment. “Cells” designations: P, parental MCF7, C; control shRNA; B, BRCA1 shRNA1. Error bars represent SEM; *P < 0.05, **P < 0.01, and ***P < 0.001 (Student’s t test).
These data suggest that BRCA1 may be a client protein of HSP90. Coimmunoprecipitation studies revealed that BRCA1 and HSP90 interact at basal levels, that irradiation of cells increases the association between BRCA1 and HSP90, and that treatment with 17-AAG can abolish this interaction (Fig. S1E). We were unable to reproducibly demonstrate the interaction in the opposite direction, implying that the bulk of cellular BRCA1 may not be constitutively associated with HSP90. 17-AAG treatment of MCF7 cells infected with wild-type and BRCA1-deletion mutant expressing adenoviruses suggest that amino acids 775–1292 are likely important in mediating 17-AAG-induced BRCA1 degradation, as exogenously expressed protein lacking this domain was unaffected by 17-AAG (Fig. S1F). Assessment of the effect of 17-AAG on a large N-terminal truncation (Δ1–302) was not possible, as this mutant is unable to bind to BARD1 and would be expected to be highly unstable (Fig. S1F) (35). Inhibition of HSP90 induces polyubiquitination (pUb) and proteasomal degradation of proteins that are dependent on HSP90 chaperone function (21). To examine ubiquitination of BRCA1 in response to 17-AAG, we treated MCF7 cells with 10 μM MG132 (carbobenzoxy-L-leucyl-L-leucyl-L-leucinal), 250 nM 17-AAG, or both for 2 h and immunoprecipitated BRCA1. This duration of 17-AAG treatment alone had no detectable effect on BRCA1 levels, and treatment with MG132 alone resulted in mild accumulation of pUb BRCA1, supporting previous observations that the ubiquitin–proteasome pathway is involved in regulating basal levels of BRCA1 (Fig. S1G) (36). Simultaneous treatment with 17-AAG and MG132 led to robust accumulation of pUb BRCA1 (Fig. S1G). We confirmed that pre-treatment with MG132 for 1 h followed by 250 nM 17-AAG for an additional 8 h significantly rescued BRCA1 from degradation (Fig. S1H). Taken together, these results suggest that the chaperone activity of HSP90 is required to maintain expression of BRCA1 and that inhibition of HSP90 activity induces the polyubiquitination and subsequent proteasomal degradation of BRCA1.
Inhibition of HSP90 Impairs Assembly of BRCA1 to IRIF.
Phosphorylation of histone H2AX (γ-H2AX) initiates the recruitment of a number of proteins required for the stability and assembly of BRCA1 (6, 37). To confirm that 17-AAG abolishes BRCA1 localization to sites of DNA damage, we treated MCF7 cells with DMSO or 250 nM 17-AAG for 24 h, exposed them to 10 Gy of IR, and immunostained for γ-H2AX and BRCA1. MCF7 cells infected with lentiviruses expressing control or BRCA1-targeting shRNA (Fig. S4A) were included as a control. Treatment with 17-AAG before IR augmented γ-H2AX foci formation, but significantly interfered with assembly of BRCA1 to IRIF (Fig. 1C). Treatment with 17-AAG in the absence of IR also induced γ-H2AX foci formation, suggesting that 17-AAG leads to accumulation of spontaneous DSBs by inhibiting basal DSB repair (Fig. 1C). Although the expression or localization of other components of the HR DSB-repair complex are known to be affected by inhibiting HSP90 (25–27), BRCA1 is required for the recruitment and function of all of these molecules (8, 11, 28–31). We propose that loss of BRCA1 is a key upstream event leading to failure of DSB-repair processes following inhibition of HSP90.
Inhibition of HSP90 Impairs Both Homologous Recombination and Nonhomologous End Joining.
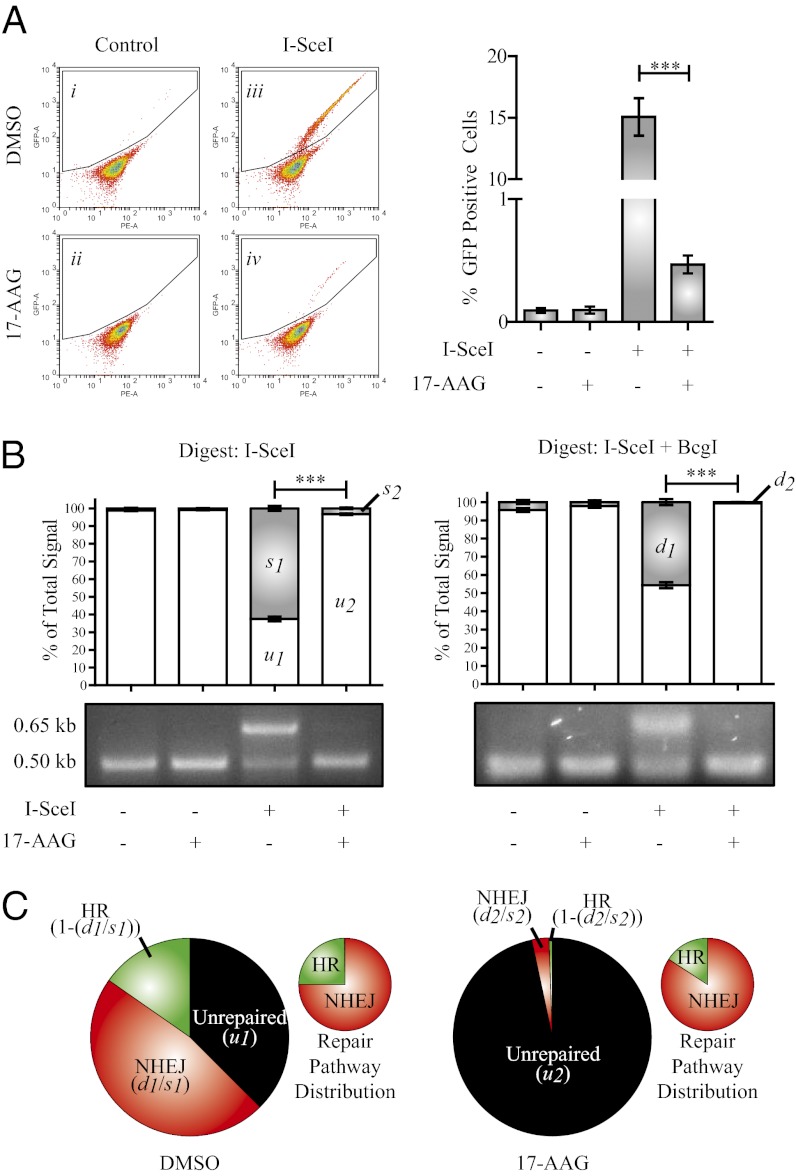
To functionally assess DSB repair capacity following treatment with 17-AAG, we used the Direct Repeat (DR)-GFP DSB repair reporter assay (Fig. S5A) (38). HeLa cells were used for this assay as they demonstrate more robust capacity for HR than do MCF7 cells, express higher levels of BRCA1, and have been used to examine BRCA1-associated defects in HR (39, 40). HeLa–DR–GFP stable transfectants were electroporated with a control vector or a vector encoding I-SceI and were immediately plated into media containing DMSO or 250 nM 17-AAG (Fig. 2A). Approximately 14.8% of the vehicle-treated cells expressing I-SceI-exhibited HR, and 0.4% of those treated with 17-AAG completed HR (Fig. 2A). The 17-AAG treatment had no effect on expression of an HA-tagged I-SceI, excluding the possibility that the observed difference in HR efficiency is due to impaired induction of DSBs in this assay (Fig. S5B). To confirm these data and to assess NHEJ capacity, we performed PCR amplification and enzymatic digestion of repair products from genomic DNA isolated from the HeLa–DR–GFP cells (arrows in Fig. S5A). Vehicle-treated HeLa cells expressing I-SceI demonstrated robust DSB repair (62.5%), and those treated with 17-AAG had a profoundly lower total repair product (3.3%) (Fig. 2 B and C). We calculated that, in vehicle-treated cells, 75% of total repair product was the result of NHEJ, and 25% was repaired by HR (Fig. 2C, Left). Distribution of repair pathway choice was only marginally skewed by 17-AAG treatment, with 86% and 14% repaired by NHEJ and HR, respectively (Fig. 2C). These values are consistent with the flow-cytometric assay of HR, as 25% of 62.5%, and 14% of 3.3% for DMSO- and 17-AAG-treated cells, respectively, would generate expected GFP+ frequencies of 15.6% and 0.5% (compared with the observed 14.8% and 0.4%). These data provide functional support of our finding that 17-AAG impairs BRCA1 expression and function and supports previous studies suggesting that HSP90 is required for both HR and NHEJ (26, 27, 41).
Fig. 2.
Inhibition of HSP90 impairs both HR and NHEJ. (A) HeLa cells stably expressing the DR–GFP reporter were electroporated with either control empty vector or a vector expressing I-SceI and were immediately plated into either DMSO or 250 nM 17-AAG for 24 h. Media was replaced after 24 h (no drug included) and cells were incubated for an additional 24 h before flow-cytometric analysis. Gated cells express GFP, indicating successful HR. Graph depicts number of GFP+ cells in three independent experiments. (B) I-SceI and I-SceI+BcgI digested PCR products. Gray bars in graph represent uncut (0.65 kb) fragment. (C) Summary of total repair capacity and repair pathway distribution in I-SceI transfected cells. Small graphs represent distribution of total repair product (HR+NHEJ). n = 3. Error bars represent SEM; *P < 0.05, **P < 0.01, and ***P < 0.001 (Student’s t test).
BRCA1 Expression Regulates Sensitivity to 17-AAG and Mediates HSP90 Inhibitor-Induced Sensitivity to Ionizing Radiation.
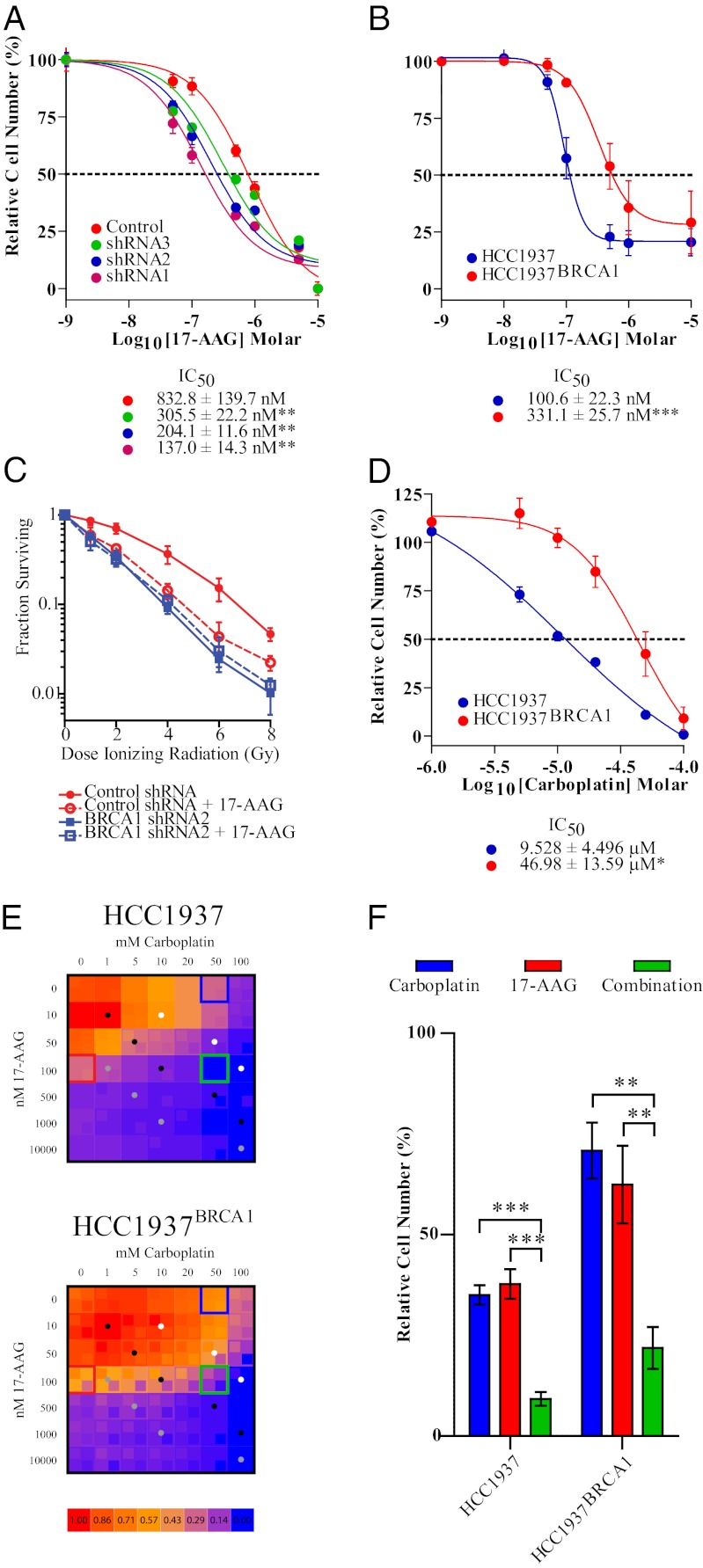
Because 17-AAG enhances accumulation of DSBs (Fig. 1C), we hypothesized that BRCA1-deficient cells would be intrinsically sensitive to 17-AAG. The ability of MCF7 cells with variable expression of BRCA1 to proliferate in the presence of 17-AAG closely parallels the level of BRCA1 expression (Fig. 3A and Fig. S4A). We confirmed this finding in the BRCA1 mutant breast cancer cell line Hamon Cancer Center-1937 (HCC1937) and the BRCA1 wild-type complemented clone HCC1937BRCA1 (Fig. 3B and Fig. S6). To address whether loss of BRCA1 is epistatic to the ability of 17-AAG to potentiate the effects of IR, the MCF7–shRNA2 and MCF7-control populations were treated with DMSO or 10 nM 17-AAG for two days and then were irradiated and assessed for clonogenic growth capacity. This low dose and shorter duration of 17-AAG was selected because it effectively ablates BRCA1 expression (Fig. 1 A and B) and has insignificant effects on the proliferation of both control and BRCA1-ablated MCF7 cells (Fig. 3A and Fig. S4B). The 17-AAG treatment or loss of BRCA1 alone significantly potentiated MCF7-control cells to clinically relevant doses of IR, although 17-AAG treatment of BRCA1-deficient MCF7 cells revealed no synergy between 17-AAG and IR (Fig. 3C). These data suggest that a major sensitizing effect of 17-AAG is due to loss of BRCA1.
Fig. 3.
BRCA1 expression mediates sensitivity to 17-AAG and is associated with the ability of 17-AAG to sensitize cells to DSB-inducing agents. (A and B) MTS assay of BRCA1–shRNA-lentivirus-infected MCF7 cells (A) or HCC1937 and HCC1937BRCA1 cells (B) treated with increasing concentrations of 17-AAG. (C) Clonogenicity assay of control shRNA and shRNA2 cells pretreated with vehicle or 10 nM 17-AAG for two days and exposed to 0–6 Gy IR. Percent surviving at each dose of IR is relative to colonies formed at 0 Gy for each particular clone. (D) MTS assay of HCC1937 and HCC1937BRCA1 cells treated with increasing concentrations of carboplatin. (E) Heat maps of HCC1937 and HCC1937BRCA1 proliferation in response to various combinations of 17-AAG and carboplatin. Inlayed boxes denote mean plus SD (Upper Left) or mean minus SD (Lower Right). Colored dots denote equal ratio of carboplatin:17-AAG [10:1 (gray), 100:1 (black), 1,000:1 (white)]. (F) Proliferation of HCC1937 and HCC1937BRCA1 cells in response to 50 μM carboplatin, 100 nM 17-AAG, or both (denoted by boxes in E).
Because mutation-associated resistance to platinum-based agents and PARP inhibitors arises from intragenic alterations in mutant BRCA1/2 alleles, we also documented the ability of 17-AAG to destabilize mutant forms of BRCA1 protein in HCC1937 breast cancer cells (homozygous BRCA1 5382 insertion C) and UWB1.289 (University of Washington BRCA1 family 289) ovarian cancer cells (homozygous BRCA1 2594 deletion C) (Fig. S1I). To functionally assess whether restoring wild-type BRCA1 could abolish repair-mediated resistance, we evaluated the ability of 17-AAG to sensitize HCC1937 and HCC1937BRCA1 cells to carboplatin. Complementation of wild-type BRCA1 increased resistance to carboplatin by approximately fivefold (Fig. 3D). HCC1937 and HCC1937BRCA1 cells were treated with combinations of 17-AAG and carboplatin and assessed by MTS assay to evaluate synergy. 17-AAG increased sensitivity to carboplatin in both cell lines (Fig. 3 E and F). Viability data at all equal-ratio combinations (dots in heat maps) enabled calculation of the combination indices (42, 43). Although 17-AAG and carboplatin were synergistic in both cell lines at all effect levels, synergy was generally more robust (i.e., closer to zero) in the wild-type complemented cell line (Fig. S7A). Moreover, dose-effect studies of carboplatin alone or in combination with 17-AAG suggest that that in combination, these drugs can sensitize BRCA1-complemented cells to a carboplatin concentration similar to that seen in BRCA1-mutant cells (Fig. 3 E and F and Fig. S7B).
Wild-Type BRCA1 Prevents 17-AAG-Mediated Mitotic Catastrophe.
In addition to coordinating the repair of DSBs, BRCA1 also induces the G2/M checkpoint to prevent perpetuation of genetic damage through mitosis. Accordingly, we sought to examine whether BRCA1 status affects 17-AAG-induced changes in DNA synthesis, cell cycle progression, and apoptosis.
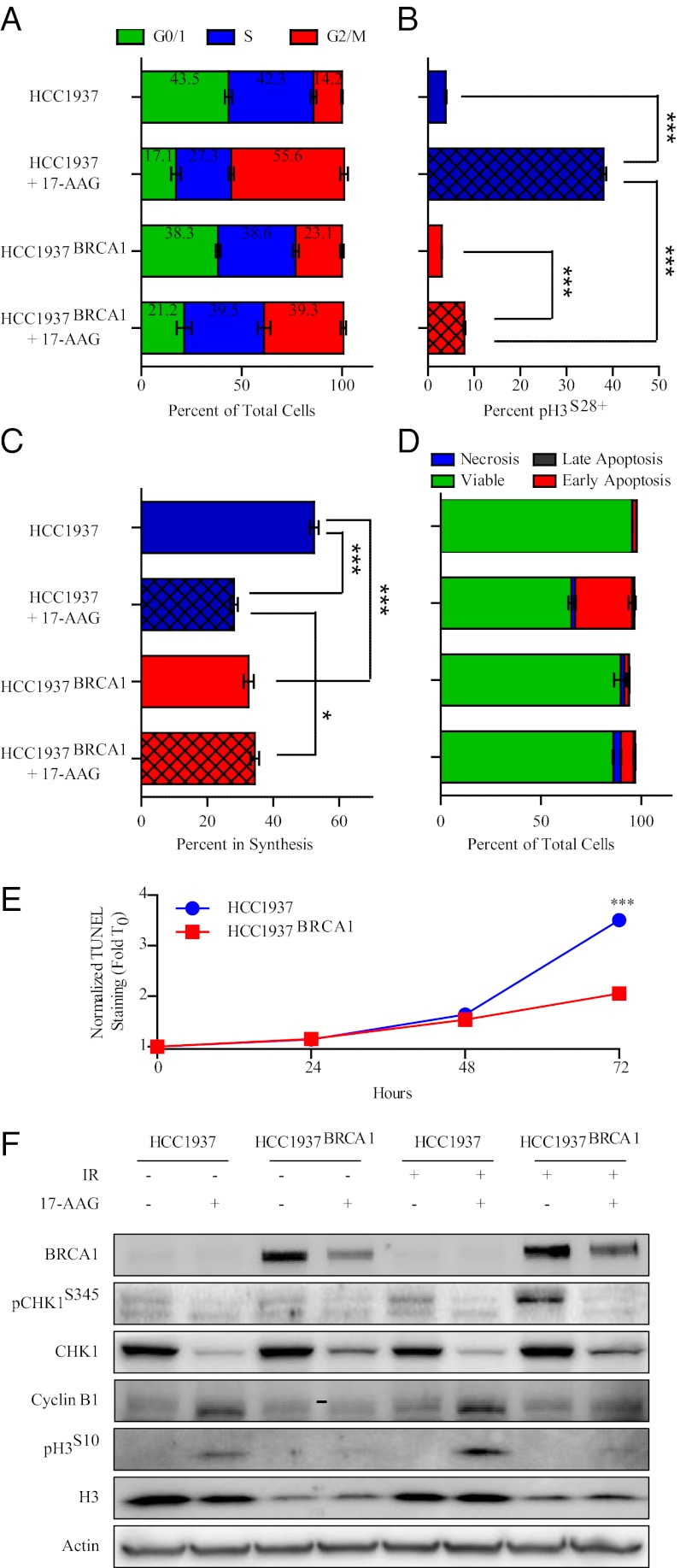
In both HCC1937 and HCC1937BRCA1 cells, treatment with 17-AAG induced a pronounced G2/M arrest at 24 h (Fig. 4A and Fig. S8A). Cell cycle analysis with costaining for pH3S28 revealed that the BRCA1-mutant HCC1937 cell line failed to arrest in G2 in response to 17-AAG, and the wild-type complemented cells arrested before entering mitosis (Fig. 4B and Fig. S8B). We also performed 5-ethinyl-2′-deoxyuridine (EdU) incorporation assays following 17-AAG treatment, and observed selective reduction in DNA synthesis in the mutant HCC1937 cell lines (Fig. 4C and Fig. S8C). To evaluate the contribution of apoptosis to the differential sensitivity observed in HCC1937 and HCC1937BRCA1 cells, we performed Annexin V staining at 24 h and TUNEL staining at 24, 48, and 72 h post 17-AAG treatment. Annexin V staining increased dramatically after 24 h of 17-AAG treatment in HCC1937 cells, but only marginally in HCC1937BRCA1 cells (Fig. 4D and Fig. S8D). No difference in TUNEL staining was evident at 24 or 48 h, but by 72 h, the HCC1937 cells exhibited significantly more DNA fragmentation than the wild-type HCC1937BRCA1 cells (Fig. 4E and Fig. S8E). In addition to preventing 17-AAG-induced entry into mitosis in the presence of damage, BRCA1 may also prevent apoptosis through expression of anti-apoptotic HSP27, which was markedly higher in HCC1937BRCA1 cells, although the rest of the heat-shock response appeared to be unaffected (Fig. S6A). Changes in checkpoint and mitosis-associated protein expression are consistent with our flow-cytometric findings, as both pH3S10 and cyclin B1 expression were induced after 17-AAG treatment only in BRCA1-mutant cells (Fig. 4F). We also observed that 17-AAG-induced CHK1 degradation may be partially rescued by wild-type BRCA1 complementation and may cooperate in preventing 17-AAG-induced mitotic catastrophe (Fig. 4F and Fig. S6B). Our data demonstrate that aberrant mitotic entry, reduced DNA synthesis, and exaggerated apoptosis in response to 17-AAG all contribute to the hypersensitivity of BRCA1 mutant cells.
Fig. 4.
BRCA1 status regulates cell cycle progression, DNA synthesis and apoptosis in response to 17-AAG. (A) Flow-cytometric evaluation of cell cycle distribution in HCC1937 and HCC1937BRCA1 cells after 24 h of exposure to 250 nM 17-AAG. (B) pH3S28 staining in HCC1937 and HCC1937BRCA1 cells after 24 h of exposure to 250 nM 17-AAG. (C) EdU incorporation in HCC1937 and HCC1937BRCA1 cells after 24 h of exposure to 250 nM 17-AAG. (D) Annexin V staining in HCC1937 and HCC1937BRCA1 cells after 24 h of exposure to 250 nM 17-AAG. (E) TUNEL staining in HCC1937 and HCC1937BRCA1 cells after 24, 48, or 72 h of exposure to 250 nM 17-AAG. (F) Western blots for cycle checkpoint and mitosis-associated proteins in HCC1937 and HCC1937BRCA1 cells after 24 h of exposure to 250 nM 17-AAG, 10 Gy IR, or both.
Discussion
Before our study, a single report suggested a link between BRCA1 and the heat-shock response (44). This study showed that BRCA1 plays a protective role in heat toxicity and that thermal stress induces BRCA1 degradation. This study observed profound destabilization of BRCA1 in breast and prostate cancer cells in response to incubation at 42 °C, although they were unable to rescue BRCA1 expression by inhibiting any of the well-characterized protein degradation pathways. Our results, which document that the ubiquitin–proteasome pathway is responsible for BRCA1 degradation, suggest that the mechanism of BRCA1 degradation in response to heat shock may be quite distinct from the mechanism of BRCA1 degradation in response to HSP90 inhibitors.
Although previous studies have described failed homology-directed repair of DSBs after inhibition of HSP90, these studies have focused on the effects of HSP90 inhibitors on BRCA2, RAD51, FANCA, CHK1, and the MRN (meiotic recombination 11 homolog A (MRE11)/RAD50 homolog (RAD50)/Nijmegan breakage syndrome 1 (NBS1)) complex (8, 11, 25–27, 31, 32, 45). Our report suggests that, of the HR/ICLR repair proteins that have been identified to be sensitive to HSP90 inhibitors, BRCA1 appears to be the most upstream. We support this model by demonstrating that BRCA1 depletion completely abolishes the ability of 17-AAG to further potentiate cells to ionizing radiation. Although there is no defect in γ-H2AX phosphorylation, we cannot currently exclude the possibility that other upstream factors necessary for BRCA1 assembly at DSBs are regulated by HSP90. Limited data supports a direct role for BRCA1 in NHEJ, although this function remains poorly described and is complicated by conflicting reports suggesting that BRCA1 mutant cancer cells may rejoin DSBs efficiently (46–49). Our data thus suggest that the potentiating effect of HSP90 inhibitors is due to inhibiting HR alone or that BRCA1 indeed participates in NHEJ.
With the emergence of synthetic lethal approaches for the treatment of cancer, our data suggest that combinatorial use of HSP90 inhibitors with PARP inhibitors or DNA damaging agents may be particularly effective. Because therapy-induced reversion mutations in the tumors of BRCA1/2 mutation carriers have been demonstrated to confer resistance to platinum-based drugs and PARP inhibitors (17, 18), inhibiting HSP90 may be a useful strategy to combat this mechanism of resistance or to treat recurrent or refractory disease. Moreover, the intrinsic sensitivity of BRCA1-mutant or -deficient cells to 17-AAG suggests that these agents might also show efficacy in both primary BRCA1 mutant tumors as well as sporadic tumors that have lost BRCA1 expression by non-mutational means.
Methods
Cell Culture.
HCC1937 and HCC1937BRCA1 cells were a gift from Junjie Chen (MD Anderson Cancer Center, Houston, TX). All other cell lines were obtained from American Type Culture Collection (ATCC). Culture of all cell lines was performed per ATCC recommendations.
Drug Treatment.
17-AAG was purchased from InvivoGen; radicicol was purchased from Tocris; and novobiocin, cycloheximide, carboplatin, and MG132 were purchased from Sigma-Aldrich. Carboplatin was dissolved in PBS, all other drugs were dissolved in DMSO.
Irradiation.
Cells were irradiated using a 137Cs source irradiator at a dose rate of 4.97 Gy·min−1.
DNA Foci Formation Assay.
Cells were grown in 8-well chamber slides, treated and exposed to 0 or 10 Gy ionizing radiation. Immunostaining protocol is detailed in the SI Methods.
Homologous Recombination and Nonhomologous End Joining.
The DR–GFP reporter construct, pCBASce (I-SceI) and pCAGGS (empty control) vectors were kindly provided by Maria Jasin (Memorial Sloan-Kettering Cancer Center, New York, NY). This assay was performed essentially as previously described (38). A detailed protocol is provided in SI Methods.
Adenoviruses and Adenoviral Production.
The HA-tagged wild-type and deletion mutant adenoviruses (50) were a kind gift from Jeffrey Parvin (The Ohio State University Medical Center, Columbus, OH) and were produced as describe above. Details of the construction of the HA-tagged I-SceI adenovirus as well as adenoviral production and titering are provided in SI Methods.
Clonogenicity Assay.
MCF7 cells infected with control or BRCA1-targeting lentivirus were pretreated for 48 h with 10 nM 17-AAG or DMSO control. Cells were trypsinized, counted, and 3,000 cells were plated in 60-mm dishes with fresh 17-AAG or DMSO. After resting overnight, plates were radiated with 0, 1, 2, 4, or 6 Gy and were then incubated for an additional 10 d. Plates were fixed with 100% methanol, stained with 1% crystal violet, and colonies were imaged and counted using the UVP BioImaging System and ImageJ, respectively.
MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) Assay.
Cells were plated at 3–5 × 103 cells per well in a 96-well plate and were treated for 4–5 d with indicated doses of 17-AAG or carboplatin. Assay was performed using the CellTiter 96 AQueous MTS kit (Promega) according to the manufacturer’s instructions.
Cell Cycle, DNA Synthesis, and Apoptosis Assays.
DNA synthesis, apoptosis, and cell cycle distribution experiments in HCC1937 and HCC1937BRCA1 cells were performed on an LSR II flow cytometer (BD Biosciences) or a Celigo adherent cell cytometer (Cyntellect). Assay details and protocols are provided in SI Methods.
Antibodies.
Antibodies used in this study are detailed in SI Methods.
Immunoprecipitation and Western Blot.
Protein was harvested using a modified radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris base, 150 mM NaCl, 2 mM EDTA, 1% Triton X-100, 0.5% Na- deoxycholate, 0.1% SDS, 50 mM NaF, 5 mM Na3VO4, plus 1× protease inhibitor mixture (Sigma-Aldrich), quantified using the DC protein assay (Bio-Rad), electrophoresed on 8% or 4–12% Tris-glycine gels (Invitrogen) and then transferred onto PVDF membranes. All blocking steps and antibody dilutions were in 3% nonfat dairy milk in PBS + 0.1% Tween-20. Densitometry calculations were made using the UVP BioImaging System. For IP, cells were lysed in IP buffer [20 mM Tris-HCl (pH 7.5–7.9), 100 mM NaCl, 0.05% Nonidet P-40, 1 mM PMSF], immunoprecipitated with 10 μg antibody overnight at 4 °C on a rotating platform. Complexes were bound to Protein-A agarose beads (15918–014; Invitrogen), washed and eluted according to manufacturer’s protocol.
Supplementary Material
Acknowledgments
We thank Drs. Shrikant Anant, George Vielhauer, Bruce Kimler, David Albertini, and Kapil Bhalla for critical review of this manuscript. This work was supported by the University of Kansas Cancer Center. Flow-cytometry experiments were performed in the University of Kansas Medical Center Flow Cytometry Shared Resource. The University of Kansas Medical Center Flow-Cytometry Shared Resource is supported in part by the University of Kansas Cancer Center and the National Institutes of Health Grant P20 RR016443 from the Centers of Biomedical Research Excellence program of the National Center for Research Resources. S.R.S. is supported by US Department of Defense Breast Cancer Research Fellowship W81XWH-11-1-0025.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1203326109/-/DCSupplemental.
References
- 1.Ford D, Easton DF, Bishop DT, Narod SA, Goldgar DE. Breast Cancer Linkage Consortium Risks of cancer in BRCA1-mutation carriers. Lancet. 1994;343:692–695. doi: 10.1016/s0140-6736(94)91578-4. [DOI] [PubMed] [Google Scholar]
- 2.King MC, Marks JH, Mandell JB. New York Breast Cancer Study Group Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302:643–646. doi: 10.1126/science.1088759. [DOI] [PubMed] [Google Scholar]
- 3.Thompson ME, Jensen RA, Obermiller PS, Page DL, Holt JT. Decreased expression of BRCA1 accelerates growth and is often present during sporadic breast cancer progression. Nat Genet. 1995;9:444–450. doi: 10.1038/ng0495-444. [DOI] [PubMed] [Google Scholar]
- 4.Hall JM, et al. Linkage of early-onset familial breast cancer to chromosome 17q21. Science. 1990;250:1684–1689. doi: 10.1126/science.2270482. [DOI] [PubMed] [Google Scholar]
- 5.Friedman LS, et al. Confirmation of BRCA1 by analysis of germline mutations linked to breast and ovarian cancer in ten families. Nat Genet. 1994;8:399–404. doi: 10.1038/ng1294-399. [DOI] [PubMed] [Google Scholar]
- 6.Huen MS, Sy SM, Chen J. BRCA1 and its toolbox for the maintenance of genome integrity. Nat Rev Mol Cell Biol. 2010;11:138–148. doi: 10.1038/nrm2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cortez D, Wang Y, Qin J, Elledge SJ. Requirement of ATM-dependent phosphorylation of brca1 in the DNA damage response to double-strand breaks. Science. 1999;286:1162–1166. doi: 10.1126/science.286.5442.1162. [DOI] [PubMed] [Google Scholar]
- 8.Yarden RI, Pardo-Reoyo S, Sgagias M, Cowan KH, Brody LC. BRCA1 regulates the G2/M checkpoint by activating Chk1 kinase upon DNA damage. Nat Genet. 2002;30:285–289. doi: 10.1038/ng837. [DOI] [PubMed] [Google Scholar]
- 9.Abbott DW, et al. BRCA1 expression restores radiation resistance in BRCA1-defective cancer cells through enhancement of transcription-coupled DNA repair. J Biol Chem. 1999;274:18808–18812. doi: 10.1074/jbc.274.26.18808. [DOI] [PubMed] [Google Scholar]
- 10.Scata KA, El-Deiry WS. p53, BRCA1 and breast Cancer chemoresistance. Adv Exp Med Biol. 2007;608:70–86. doi: 10.1007/978-0-387-74039-3_5. [DOI] [PubMed] [Google Scholar]
- 11.Bhattacharyya A, Ear US, Koller BH, Weichselbaum RR, Bishop DK. The breast cancer susceptibility gene BRCA1 is required for subnuclear assembly of Rad51 and survival following treatment with the DNA cross-linking agent cisplatin. J Biol Chem. 2000;275:23899–23903. doi: 10.1074/jbc.C000276200. [DOI] [PubMed] [Google Scholar]
- 12.Husain A, He G, Venkatraman ES, Spriggs DR. BRCA1 up-regulation is associated with repair-mediated resistance to cis-diamminedichloroplatinum(II) Cancer Res. 1998;58:1120–1123. [PubMed] [Google Scholar]
- 13.Silver DP, et al. Efficacy of neoadjuvant Cisplatin in triple-negative breast cancer. J Clin Oncol. 2010;28:1145–1153. doi: 10.1200/JCO.2009.22.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adhikari S, et al. Targeting base excision repair for chemosensitization. Anticancer Agents Med Chem. 2008;8:351–357. doi: 10.2174/187152008784220366. [DOI] [PubMed] [Google Scholar]
- 15.Fong PC, et al. Poly(adp)-ribose polymerase inhibition: Frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J Clin Oncol. 2010;28:2512–2519. doi: 10.1200/JCO.2009.26.9589. [DOI] [PubMed] [Google Scholar]
- 16.Turner NC, et al. BRCA1 dysfunction in sporadic basal-like breast cancer. Oncogene. 2007;26:2126–2132. doi: 10.1038/sj.onc.1210014. [DOI] [PubMed] [Google Scholar]
- 17.Swisher EM, et al. Secondary BRCA1 mutations in BRCA1-mutated ovarian carcinomas with platinum resistance. Cancer Res. 2008;68:2581–2586. doi: 10.1158/0008-5472.CAN-08-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ashworth A. Drug resistance caused by reversion mutation. Cancer Res. 2008;68:10021–10023. doi: 10.1158/0008-5472.CAN-08-2287. [DOI] [PubMed] [Google Scholar]
- 19.Chen X, Arciero CA, Godwin AK. BRCA1-associated complexes: New targets to overcome breast cancer radiation resistance. Expert Rev Anticancer Ther. 2006;6:187–196. doi: 10.1586/14737140.6.2.187. [DOI] [PubMed] [Google Scholar]
- 20.Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5:761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- 21.Blagg BS, Kerr TD. Hsp90 inhibitors: Small molecules that transform the Hsp90 protein folding machinery into a catalyst for protein degradation. Med Res Rev. 2006;26:310–338. doi: 10.1002/med.20052. [DOI] [PubMed] [Google Scholar]
- 22.Kim YS, et al. Update on Hsp90 inhibitors in clinical trial. Curr Top Med Chem. 2009;9:1479–1492. doi: 10.2174/156802609789895728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Camphausen K, Tofilon PJ. Inhibition of Hsp90: A multitarget approach to radiosensitization. Clin Cancer Res. 2007;13:4326–4330. doi: 10.1158/1078-0432.CCR-07-0632. [DOI] [PubMed] [Google Scholar]
- 24.Dungey FA, Caldecott KW, Chalmers AJ. Enhanced radiosensitization of human glioma cells by combining inhibition of poly(ADP-ribose) polymerase with inhibition of heat shock protein 90. Mol Cancer Ther. 2009;8:2243–2254. doi: 10.1158/1535-7163.MCT-09-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arlander SJ, et al. Hsp90 inhibition depletes Chk1 and sensitizes tumor cells to replication stress. J Biol Chem. 2003;278:52572–52577. doi: 10.1074/jbc.M309054200. [DOI] [PubMed] [Google Scholar]
- 26.Dote H, Burgan WE, Camphausen K, Tofilon PJ. Inhibition of hsp90 compromises the DNA damage response to radiation. Cancer Res. 2006;66:9211–9220. doi: 10.1158/0008-5472.CAN-06-2181. [DOI] [PubMed] [Google Scholar]
- 27.Oda T, Hayano T, Miyaso H, Takahashi N, Yamashita T. Hsp90 regulates the Fanconi anemia DNA damage response pathway. Blood. 2007;109:5016–5026. doi: 10.1182/blood-2006-08-038638. [DOI] [PubMed] [Google Scholar]
- 28.Chen L, Nievera CJ, Lee AY, Wu X. Cell cycle-dependent complex formation of BRCA1.CtIP.MRN is important for DNA double-strand break repair. J Biol Chem. 2008;283:7713–7720. doi: 10.1074/jbc.M710245200. [DOI] [PubMed] [Google Scholar]
- 29.D’Andrea AD, Grompe M. The Fanconi anaemia/BRCA pathway. Nat Rev Cancer. 2003;3:23–34. doi: 10.1038/nrc970. [DOI] [PubMed] [Google Scholar]
- 30.Folias A, et al. BRCA1 interacts directly with the Fanconi anemia protein FANCA. Hum Mol Genet. 2002;11:2591–2597. doi: 10.1093/hmg/11.21.2591. [DOI] [PubMed] [Google Scholar]
- 31.Zhang F, Fan Q, Ren K, Andreassen PR. PALB2 functionally connects the breast cancer susceptibility proteins BRCA1 and BRCA2. Mol Cancer Res. 2009;7:1110–1118. doi: 10.1158/1541-7786.MCR-09-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhong Q, et al. Association of BRCA1 with the hRad50-hMre11-p95 complex and the DNA damage response. Science. 1999;285:747–750. doi: 10.1126/science.285.5428.747. [DOI] [PubMed] [Google Scholar]
- 33.McCollum AK, et al. Cisplatin abrogates the geldanamycin-induced heat shock response. Mol Cancer Ther. 2008;7:3256–3264. doi: 10.1158/1535-7163.MCT-08-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joukov V, Chen J, Fox EA, Green JB, Livingston DM. Functional communication between endogenous BRCA1 and its partner, BARD1, during Xenopus laevis development. Proc Natl Acad Sci USA. 2001;98:12078–12083. doi: 10.1073/pnas.211427098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCarthy EE, Celebi JT, Baer R, Ludwig T. Loss of Bard1, the heterodimeric partner of the Brca1 tumor suppressor, results in early embryonic lethality and chromosomal instability. Mol Cell Biol. 2003;23:5056–5063. doi: 10.1128/MCB.23.14.5056-5063.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choudhury AD, Xu H, Baer R. Ubiquitination and proteasomal degradation of the BRCA1 tumor suppressor is regulated during cell cycle progression. J Biol Chem. 2004;279:33909–33918. doi: 10.1074/jbc.M403646200. [DOI] [PubMed] [Google Scholar]
- 37.Shrivastav M, De Haro LP, Nickoloff JA. Regulation of DNA double-strand break repair pathway choice. Cell Res. 2008;18:134–147. doi: 10.1038/cr.2007.111. [DOI] [PubMed] [Google Scholar]
- 38.Nakanishi K, et al. Human Fanconi anemia monoubiquitination pathway promotes homologous DNA repair. Proc Natl Acad Sci USA. 2005;102:1110–1115. doi: 10.1073/pnas.0407796102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kachhap SK, Vetale SP, Dange P, Ghosh SN. Reduced expression of the BRCA1 gene and increased chromosomal instability in MCF-7 cell line. Cell Biol Int. 2001;25:547–551. doi: 10.1006/cbir.2000.0657. [DOI] [PubMed] [Google Scholar]
- 40.Ransburgh DJ, Chiba N, Ishioka C, Toland AE, Parvin JD. Identification of breast tumor mutations in BRCA1 that abolish its function in homologous DNA recombination. Cancer Res. 2010;70:988–995. doi: 10.1158/0008-5472.CAN-09-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noguchi M, et al. Inhibition of homologous recombination repair in irradiated tumor cells pretreated with Hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin. Biochem Biophys Res Commun. 2006;351:658–663. doi: 10.1016/j.bbrc.2006.10.094. [DOI] [PubMed] [Google Scholar]
- 42.Chou TC, Talalay P. Analysis of combined drug effects: A new look at a very old problem. Trends Pharmacol Sci. 1983;4:450–454. [Google Scholar]
- 43.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 44.Xian Ma Y, et al. Role of BRCA1 in heat shock response. Oncogene. 2003;22:10–27. doi: 10.1038/sj.onc.1206061. [DOI] [PubMed] [Google Scholar]
- 45.Guervilly JH, Macé-Aimé G, Rosselli F. Loss of CHK1 function impedes DNA damage-induced FANCD2 monoubiquitination but normalizes the abnormal G2 arrest in Fanconi anemia. Hum Mol Genet. 2008;17:679–689. doi: 10.1093/hmg/ddm340. [DOI] [PubMed] [Google Scholar]
- 46.Zhuang J, et al. Checkpoint kinase 2-mediated phosphorylation of BRCA1 regulates the fidelity of nonhomologous end-joining. Cancer Res. 2006;66:1401–1408. doi: 10.1158/0008-5472.CAN-05-3278. [DOI] [PubMed] [Google Scholar]
- 47.Wei L, et al. Rapid recruitment of BRCA1 to DNA double-strand breaks is dependent on its association with Ku80. Mol Cell Biol. 2008;28:7380–7393. doi: 10.1128/MCB.01075-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bau DT, et al. Breast cancer risk and the DNA double-strand break end-joining capacity of nonhomologous end-joining genes are affected by BRCA1. Cancer Res. 2004;64:5013–5019. doi: 10.1158/0008-5472.CAN-04-0403. [DOI] [PubMed] [Google Scholar]
- 49.Mérel P, Prieur A, Pfeiffer P, Delattre O. Absence of major defects in non-homologous DNA end joining in human breast cancer cell lines. Oncogene. 2002;21:5654–5659. doi: 10.1038/sj.onc.1205742. [DOI] [PubMed] [Google Scholar]
- 50.Chiba N, Parvin JD. The BRCA1 and BARD1 association with the RNA polymerase II holoenzyme. Cancer Res. 2002;62:4222–4228. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.