Abstract
Most gastrointestinal stromal tumors (GISTs) harbor a gain-of-function mutation in the Kit receptor. GIST patients treated with the tyrosine kinase inhibitor imatinib frequently develop imatinib resistance as a result of second-site Kit mutations. To investigate the consequences of second-site Kit mutations on GIST development and imatinib sensitivity, we engineered a mouse model carrying in the endogenous Kit locus both the KitV558Δ mutation found in a familial case of GIST and the KitT669I (human KITT670I) “gatekeeper” mutation found in imatinib-resistant GIST patients. Similar to KitV558∆/+ mice, KitV558∆;T669I/+ mice developed gastric and colonic interstitial cell of Cajal hyperplasia as well as cecal GIST. In contrast to the single-mutant KitV558∆/+ control mice, treatment of the KitV558∆;T669I/+ mice with either imatinib or dasatinib failed to inhibit oncogenic Kit signaling and GIST growth. However, this resistance could be overcome by treatment of KitV558∆;T669I/+ mice with sunitinib or sorafenib. Although tumor lesions were smaller in KitV558∆;T669I/+ mice than in single-mutant mice, both interstitial cell of Cajal hyperplasia and mast cell hyperplasia were exacerbated in KitV558∆;T669I/+ mice. Strikingly, the KitV558∆;T669I/+ mice developed a pronounced polycythemia vera-like erythrocytosis in conjunction with microcytosis. This mouse model should be useful for preclinical studies of drug candidates designed to overcome imatinib resistance in GIST and to investigate the consequences of oncogenic KIT signaling in hematopoietic as well as other cell lineages.
Keywords: soft tissue sarcoma, hematopoiesis, erythropoiesis, drug resistance
Gastrointestinal stromal tumor (GIST) is the most common mesenchymal tumor of the gastrointestinal tract. GISTs express receptor tyrosine kinase KIT and are thought to derive from a KIT+ or KITlow interstitial cell of Cajal (ICC) progenitor or from ICCs themselves (1). The principal genetic events responsible for the pathogenesis of GIST are thought to be gain-of-function mutations in the KIT gene or in a small subset in the PDGFR-alpha gene (2, 3). KIT-activating mutations in GIST are found predominantly in the juxtamembrane domain of the KIT receptor (exon 11) (4), but mutations in the extracellular (exon 9) and kinase domains of KIT have been described as well (5, 6). The KIT juxtamembrane domain has an autoinhibitory role and stabilizes an inactive conformation of the KIT kinase; mutation of this domain disrupts the conformational integrity and thus diminishes autoinhibition (7). KIT activation-loop mutations found in acute myeloid leukemias, mast cell neoplasms, and seminomas stabilize an active conformation of the KIT kinase.
Imatinib mesylate, an inhibitor of the KIT, PDGFR, and BCR-ABL tyrosine kinases, is the first-line therapy in patients with chronic myelogenous leukemia (CML) and metastatic GIST. Imatinib is most effective in GISTs with KIT-activating mutations in the juxtamembrane domain, some kinase domain mutations, or extracellular domain mutations. However, KIT mutations that destabilize the inactive form of the kinase are resistant to inhibition by imatinib. Imatinib binds to the inactive conformation of the ABL and KIT kinases and not to the active conformation and inhibits juxtamembrane domain KIT mutants but not activation-loop KIT mutants (7).
That oncogenic KIT mutations have a critical role in the development of human neoplasias was strengthened by the observation of familial GIST and familial mastocytosis (8). Patients with familial GIST also may have cutaneous mastocytosis and hyperpigmentation. The observation of germ-line KIT gain-of-function mutations provided us with a rationale for developing a mouse model for familial GIST. The KIT-V558 deletion mutation found in the first familial GIST case was introduced into the mouse genome using a knockin strategy (9). The mutant animals developed ICC hyperplasia and neoplastic lesions in the cecum indistinguishable from human GIST with complete penetrance (10, 11).
Long-term imatinib treatment of GIST and of patients who have CML is associated with the development of drug resistance. In GIST most cases of resistance appear to derive from second-site mutations in the kinase domain of the KIT receptor (12, 13). In patients who have CML, second-site mutations in BCR-ABL are the predominant mechanism of drug resistance (14, 15). The second-site mutations in acquired imatinib-resistant GIST tend to be single amino acid substitutions in KIT, located on the allele with the primary mutation (12, 16). Second-site mutations in GIST occur in catalytic domain II of KIT, exons 17 and 18, as well as in the N-terminal kinase domain, exon 13 (V654A) and exon 14 (T670I) (12). In the gatekeeper T670I mutation, the isoleucine methyl group protrudes into the imatinib binding site and disrupts an important hydrogen bond formation between imatinib and the kinase, precluding proper binding of imatinib (7). Second-site mutations in the activation loop within the kinase domain stabilize the active conformation of KIT and maintain it constitutively activated at a high level, thereby preventing imatinib binding. Currently, several drugs, including sunitinib, dasatinib, and sorafenib, are being evaluated for efficacy in the treatment of imatinib-resistant GIST. Previous in vitro studies indicated that both sunitinib and sorafenib inhibit the T670I gatekeeper mutation, but imatinib, dasatinib, and nilotinib failed to do so (17, 18).
Because of the clinical importance of imatinib resistance, the development of new strategies for the treatment of GIST is highly relevant. Such strategies may be based on the development of KIT kinase inhibitors that show efficacy with the resistant forms of KIT; targeting of downstream signaling components critical for oncogenic KIT function could provide a second approach. However, models to examine these approaches and their possible side effects in vivo have not been reported. Here we describe the derivation of a mouse model for imatinib-resistant GIST that includes both the juxtamembrane domain KitV558Δ and KitT669I gatekeeper mutations as a tool to develop therapeutic strategies for imatinib-resistant GIST and to investigate the consequences of KIT oncogenic signaling in other KIT-dependent cell lineages in particular in hematopoiesis.
Results
Derivation and Phenotypic Characterization of KitV558Δ;T669I/+ Gatekeeper Mice.
To investigate the consequences of second-site KIT mutations on imatinib susceptibility and GIST development in vivo, we generated a mouse model introducing both the KitV558Δ and the KitT669I gatekeeper mutation, corresponding to human KITT670I and found in cases of imatinib-resistant GIST, into the endogenous Kit locus. To facilitate simultaneous introduction of the two point mutations into the mouse Kit gene, the targeting vector included a floxed neomycin-resistance gene (NEO) cassette in Kit intron 11 for positive selection of recombinant ES cells containing both the V558Δ (exon 11) and T669I (exon 14) mutations (Fig. 1A). After successful integration and germ-line transmission of the KitV558Δ;T669I-NEO allele, the intronic NEO cassette was removed by crossing to Tg(EIIa-cre) mice (19). The resulting KitV558Δ;T669I allele retains a single loxP site in intron 11 (Fig. 1A3). A KitV558Δ allele with a loxP site in intron 11 was generated as a control for the KitV558∆;T669I/+ allele (Fig. S1A).
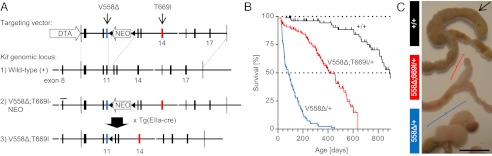
Fig. 1.
Derivation and phenotypic characterization of Kit-gatekeeper mice. (A) Targeting strategy for the simultaneous knock-in of V558Δ (exon 11) and T669I (exon 14) into the 129/Sv Kit locus. Blue and red bars denote the exons with the respective point mutations. A similar targeting vector with a shorter 3′ homology arm was used to generate the single-mutant KitV558Δ/+ mice (Fig. S1A). Triangles (not drawn to scale) indicate loxP sites; white gaps indicate BamHI restriction sites; bar indicates 518-bp Southern blot probe. DTA, diphtheria toxin A gene; NEO, neomycin resistance gene. (B) Kaplan–Meier survival plot showing increased survival of gatekeeper-mutant KitV558Δ;T669I/+ mice in comparison with KitV558Δ/+ mice (n ≥ 43 each; ticks indicate censored subjects). (C) Photographs of ileocecal junctions showing reduced length and diameter of tumor alongside the cecum in KitV558Δ;T669I/+ mice (Middle; red bracket indicates straight cecal GIST) in comparison with KitV558Δ/+ mice (Bottom; blue bracket indicates twisted cecal GIST). Of note, the cecum is significantly shorter in KitV558∆;T669I/+ mice than in wild-type mice (Top; black arrow) and KitV558∆/+ mice. Representative pictures of 3-mo-old animals are shown with colon facing down left and ileum facing down right (n ≥ 59 each). (Scale bar, 1 cm.)
Double-mutant KitV558Δ;T669I/+ mice are viable and fertile but, in contrast to KitV558Δ/+ mice, were born at sub-Mendelian ratios when crossed to wild-type mice (35% instead of 50% heterozygous offspring). In comparison with single-mutant KitV558Δ/+ mice, double-mutant KitV558Δ;T669I/+ mice had a prolonged lifespan with a median survival of 14 mo (n > 43 each, P < 0.0001) (Fig. 1B).
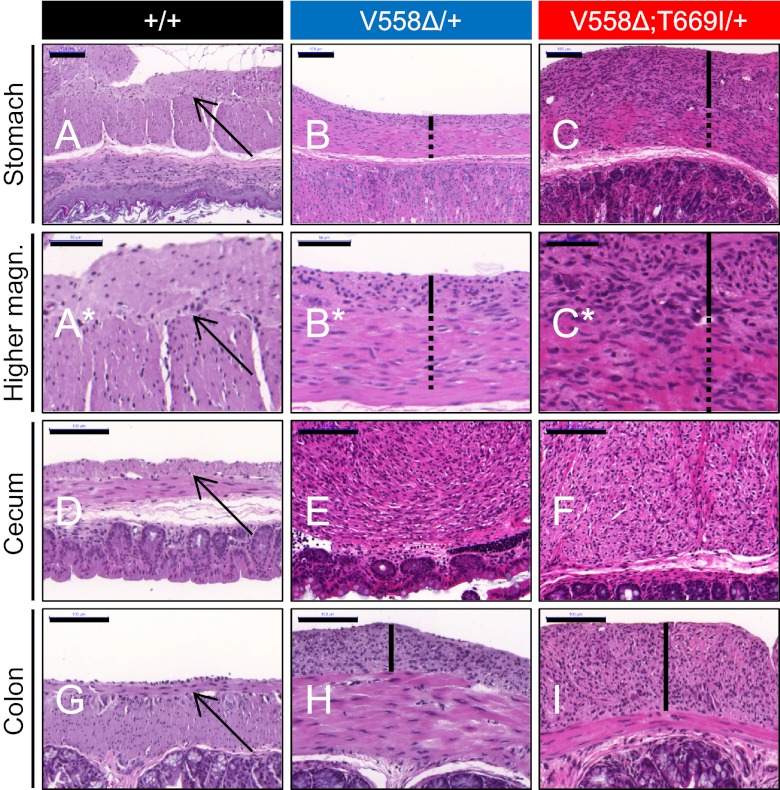
Invariably, KitV558Δ;T669I/+ mice developed cecal tumors. These tumors were smaller than in KitV558Δ/+ mice, perhaps explaining the improved survival by a decreased chance of intestinal obstruction (Fig. 1C). The average tumor diameter in 3-mo-old animals was fivefold smaller in KitV558Δ;T669I/+ than in KitV558Δ/+ mice (1.4 ± 0.1 mm vs. 7.0 ± 0.3 mm, P < 0.001). Interestingly, not only were the cecal tumors smaller, but the length of the cecum was significantly shorter in KitV558Δ;T669I/+ mice compared with KitV558Δ/+ and wild-type mice (13 ± 2 mm vs. 24 ± 2 mm, P = 0.003) (Fig. 1C). Histological analysis of the shortened cecum of the KitV558Δ;T669I/+ mice revealed an intact lumen and mucosa similar to those in KitV558Δ/+ mice. In contrast to the overall reduction in tumor size in the cecum, KitV558∆;T669I/+ mice developed more pronounced ICC hyperplasia in the stomach (Fig. 2 C and I; also see Fig. S3A a–d) and colon than KitV558Δ/+ mice (Fig. 2 B and H; also see Fig. S3B a–d). Immunohistochemical analysis of gastric and colonic sections revealed KIT staining as well as phosphorylation of S6, MAPK, and STAT3 in the ICC hyperplasia of KitV558Δ/+ as well as KitV558Δ;T669I/+ mice (see Fig. S3 A e–n and B e–n), but no significant differences in signal transduction were apparent in the two strains that could explain the exacerbated ICC hyperplasia in KitV558Δ;T669I/+ mice. H&E-stained sections of cecal tumor lesions in both KitV558Δ/+ and KitV558∆;T669I/+ mice showed a histology indistinguishable from human GIST (Fig. 2 E and F).
Fig. 2.
Cecal GIST and pronounced gastric and colonic ICC hyperplasia in KitV558Δ;T669I/+ mice. Cross-sections of stomach (A–C; higher magnification is shown in A*–C*), cecum (D–F), and colon (G–I) of 3- to 4-mo-old wild-type, KitV558Δ/+, and KitV558Δ;T669I/+ mice. Arrows indicate normal thin layer of myenteric ICC in wild-type samples. ICC hyperplasia in stomach and colon samples is indicated by black bars. Note the extensive hyperplasia involving the circular muscle layer (dotted lines) in the stomach of KitV558Δ;T669I/+ mice. Photographs show representative H&E staining; n ≥ 3 each. (Scale bars: 50 μm in A*–C*; 100 μm in A–I.)
KitV558Δ;T669I/+ Gatekeeper Mutation Confers Resistance to Imatinib and Dasatinib in Vivo.
To investigate the in vivo sensitivity of the KitV558Δ;T669I gatekeeper mutation to tyrosine kinase inhibitors (TKIs) with KIT inhibitory potential, we treated cohorts of 3- to 4-mo-old KitV558∆;T669I/+ mice with imatinib, dasatinib, sunitinib, or sorafenib. First, we analyzed signaling cascades known to be affected by imatinib after short-term (6-h) drug treatments (10, 11). Second, proliferation, apoptosis, and histological changes within tumors and adjacent mucosa were assessed after long-term (7-d) treatment with Kit inhibitors. In addition, changes in the phosphorylation status of proteins in GIST following long-term treatment were evaluated. Age-matched single-mutant KitV558∆/+ mice were treated analogously to serve as controls.
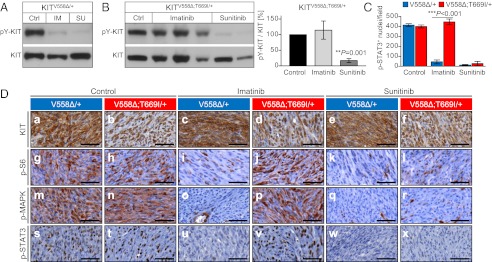
To examine if the KIT kinase in KitV558∆;T669I/+ mice is sensitive to inhibition by imatinib, tumor lysates of individual mice were subjected to Western blotting with phospho-Y719-KIT and KIT antibodies. In tumors of imatinib-treated KitV558∆/+ control mice, KIT phosphorylation was inhibited, as reported earlier (Fig. 3A) (10, 11). In contrast, in tumors of KitV558∆;T669I/+ mice, KIT phosphorylation was unchanged after treatment with imatinib (Fig. 3B). KIT inhibition could be restored by treatment with sunitinib, which diminished KIT phosphorylation to similarly low levels in both KitV558∆/+ and KitV558∆;T669I/+ mice (Fig. 3 A and B). Because of the small size of neoplastic lesions in KitV558∆;T669I/+ mice, the effect of drug treatment on downstream signaling networks was examined primarily by using an immunohistochemical (IHC) approach. As is characteristic for most human GIST, tumors stained positive for KIT independent of the drug used or treatment duration (Figs. 3D a–f and 4D a–d and Fig. S2 A a–f and B a–f). Tumors of control (vehicle-treated) animals of both genotypes showed strong phosphorylation of ribosomal protein S6, MAPK, and STAT3 (Fig. 3 C and D g, h, m, n, s, and t). After 6 h of treatment with imatinib, phosphorylation of these signaling components was strongly reduced in tumors of KitV558Δ/+ animals (Fig. 3 C and D i, o, and u) but not in tumors of animals with the KitV558∆;T669I mutation (Fig. 3 C and D j, p, and v). The latter result is of particular note, because imatinib has the potential to inhibit multiple kinases (e.g., ABL and PDGFR) that might be expressed and activate these targets in GIST parallel to or downstream of KIT. Furthermore, in vitro assays with the intracellular kinase domain of KIT had demonstrated that imatinib inhibits wild-type KIT as efficiently as KITV558Δ (20). It is not known whether KIT heterozygosity (i.e., the coexpression of one mutant and one wild-type KIT allele), as detected in the GISTs of most patients, results in the formation of functional KIT heterodimers driving oncogenic signaling. Assuming that imatinib inhibits wild-type KIT and possibly other kinases expressed in our heterozygous KitV558∆;T669I/+ mice, we deduce that wild-type KIT and off-target kinase inhibition is not sufficient to affect GIST signal transduction. This apparent KITV558∆;T669I-isoform dependence of phosphorylation of S6, MAPK, and STAT3 also was confirmed when Kit-mutant mice were treated with dasatinib. Treatment with dasatinib down-regulated phosphorylation of these components of the signal transduction network in GISTs of single-mutant KitV558∆/+ mice (Fig. 4D e, i, and m) but not in GISTs of double-mutant KitV558∆;T669I/+ mice (Fig. 4D f, j, and n). These results were confirmed by Western blotting and quantified by densitometry as the ratio of phosphorylated (pY-)S6 to total S6 protein as well as pY-MAPK to total MAPK protein (Fig. 4 B and C). Importantly, the observed resistance at the histochemical and biochemical levels was mirrored in results obtained by long-term treatments: Twice daily treatment for 7 d with imatinib or dasatinib significantly reduced cell proliferation, as determined by Ki67 staining, in GISTs of single-mutant KitV558∆/+ mice (Fig. 4E). In contrast, treatment of double-mutant KitV558∆;T669I/+ mice with imatinib or dasatinib did not inhibit GIST proliferation, nor did it elicit a histological response (Fig. 4E and Table 1). Together, these results demonstrate that the sole addition of the gatekeeper mutation in an in vivo model of GIST can cause resistance, as postulated for patients who have imatinib-refractory GIST with a correlating gatekeeper mutation.
Fig. 3.
The KitV558Δ;T669I double mutation confers resistance to imatinib in vivo. Sunitinib overcomes resistance. (A and B) Tumor protein lysates from individual KitV558∆/+ (A) and KitV558∆;T669I/+ (B) (Left) animals treated for 6 h with vehicle (Ctrl), imatinib (IM), or sunitinib (SU) were subjected to Western blotting with phospho-Y719-KIT and KIT antibodies. Representative blots are shown. (Right) The ratio of phosphorylated to total KIT in GISTs of KitV558∆;T669I/+ mice after imatinib and sunitinib treatment was quantified by densitometry (n = 3–5 animals per treatment group; error bars indicate means ± SD). (C) Quantification of phospho-STAT3+ nuclei per field (cf. D s–x and corresponding explanations). n = 3 each; error bars indicate means ± SD. (D) Representative results of IHC analysis with antibodies specific for KIT (a–f), phospho-S6 ribosomal protein (g–l), phospho-MAPK (m–r), and phospho-STAT3 (s–x) on sections of GISTs from single- or double-mutant mice treated for 6 h with vehicle, imatinib, or sunitinib. Tumor sections from different treatment groups and genotypes (n ≥ 3 each) were placed next to each other on the same microscopy slide to enable cross-comparison of staining intensities. (Scale bars: 50 μm.)
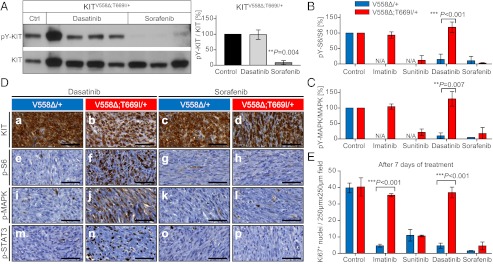
Fig. 4.
Resistance to dasatinib and sensitivity to sorafenib treatment in KitV558∆;T669I/+ mice. (A–C) Tumor lysates from individual KitV558Δ/+ and KitV558∆;T669I/+ animals treated with vehicle, imatinib, sunitinib, dasatinib, or sorafenib were subjected to Western blotting to detect the abundance of phosphorylated and total KIT, S6, and MAPK proteins. Representative blots for phospho-Y719-KIT and KIT are shown. The ratio of phosphorylated to total protein was quantified by densitometry. n = 3–4 animals per treatment group; error bars indicate means ± SD. (D) Representative results after short-term treatment with dasatinib and sorafenib; IHC on GIST sections with antibodies as specified in Fig. 3D. (E) Cell proliferation in GIST of KitV558Δ;T669I/+ mice is unaffected by long-term treatment with imatinib and dasatinib. Sunitinib and sorafenib overcome resistance and attenuate cell proliferation. Ki67+ nuclei per 250 × 250 μm field; n ≥ 3 each; error bars indicate means ± SD.
Table 1.
Histological response of GIST lesions in KitV558Δ/+ and KitV558Δ;T669I/+ mice to 7-d treatment with imatinib, dasatinib, sunitinib, or sorafenib
| Strain | Vehicle | Imatinib | Dasatinib | Sunitinib | Sorafenib |
| KitV558Δ/+ | None | Minimal | Minimal | Minimal | Moderate |
| None | None | Minimal | None | Mild | |
| None | None | Minimal | Minimal | Mild | |
| None | |||||
| None | |||||
| KitV558Δ;T669I/+ | None | None | None | None | Minimal |
| None | None | None | None | Minimal | |
| None | None | None | None | Minimal | |
| Minimal | Minimal | Minimal-mild | |||
| None | Minimal | Mild | |||
| None | Minimal |
Sunitinib and Sorafenib Overcome Resistance.
Next, we determined the response of the imatinib-resistant KitV558∆;T669I/+ mice to second-generation TKIs, namely sunitinib and sorafenib, which had been shown in vitro to inhibit cells expressing the KITV558∆;T669I mutation (17, 18). Short-term treatment with sunitinib and sorafenib reduced phosphorylation of KIT, S6, MAPK1/3, and STAT3 to similarly low levels in tumors of KitV558Δ/+ and KitV558Δ;T669I/+ mice as assessed by IHC (Figs. 3 C and D k, l, q, r, w, and x and 4D g, h, k, l, o, and p) and Western blotting (Figs. 3 A and B and 4 A–C). After long-term treatment with sunitinib and sorafenib, KIT-mediated signal transduction and proliferation was diminished significantly in GISTs of KitV558∆;T669I/+ mice to the levels achieved by all four inhibitors (imatinib, sunitinib, dasatinib, and sorafenib) in single-mutant KitV558∆/+ mice (Fig. 4E and Fig. S2 A g–x and B g–x). Cell proliferation in tumor- and ICC hyperplasia-adjacent gastrointestinal epithelial cells was not impaired after long-term treatment, indicating no overt toxic side effects of these TKIs in mice at the concentrations used (Fig. S3C a–i). These experiments demonstrated that the resistance mediated by the KitV558Δ;T669I mutation could be overcome by treatment with sunitinib and sorafenib.
To investigate whether the ICC hyperplasia in the KitV558Δ;T669I/+ mice recapitulated the resistance/susceptibility pattern observed in the cecal neoplastic lesions, gastric cross-sections were examined after 7 d of treatment with imatinib or sunitinib. In concordance with our results in cecal tumor lesions, the ICC hyperplasia in KitV558Δ;T669I/+ mice exhibited resistance to imatinib and susceptibility to sunitinib inhibition of Kit signaling and ICC proliferation (Fig. S3 C g–x and D), implying that second-generation TKIs also could inhibit the early stages of imatinib-resistant GIST development in KitV558Δ;T669I/+ mice.
Hyperproliferation in Hematopoietic Cell Lineages in KitV558Δ;T669I/+ Mice.
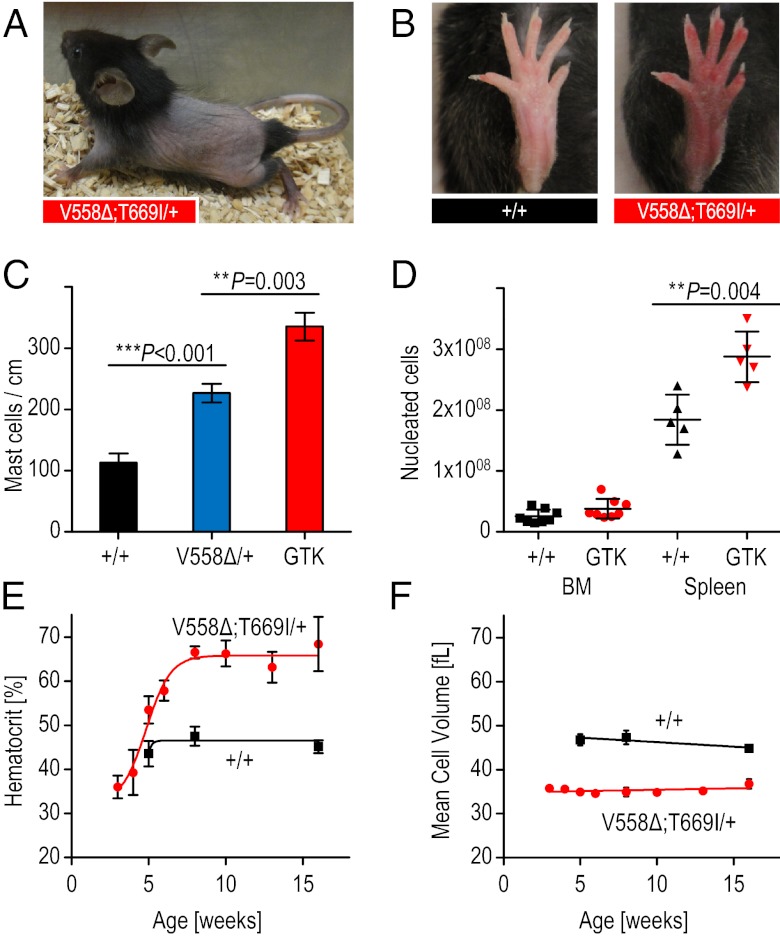
In addition to the pronounced ICC hyperplasia in KitV558Δ;T669I/+ mice in comparison with KitV558Δ/+ mice, we observed hyperproliferation in other KIT-dependent lineages. The mast cell hyperplasia we had observed previously in the dorsal skin of KitV558Δ/+ mice (9) was exacerbated in the KitV558Δ;T669I/+ mice (Fig. 5C and Fig. S4G a and b). In approximately half the male KitV558Δ;T669I/+ mice we observed mast cell accumulation around Leydig cells in the interstitial space of the testes. Interestingly, KitV558∆;T669I/+ mice exhibited intermittent partial alopecia of the trunk between postnatal day (P)15 and P40 (Fig. 5A), a phenotype reported for Il10−/− mice and shown to be associated with increased numbers of mast cells (21).
Fig. 5.
Increased mast cell and red blood cell numbers in KitV558∆;T669I/+ mice. (A) Representative photograph of alopecia of truncal hair in 4-wk-old KitV558Δ;T669I/+ mice. (B) Representative photographs of wild-type hind-paw in 3-mo-old wild-type mice and “red paw” phenotype in 3-mo-old KitV558Δ;T669I/+ mice. (C) Increased mast cell numbers in dorsal skin of KitV558Δ;T669I/+ (GTK) mice in comparison with wild-type and KitV558Δ/+ mice (n = 3). (D) BM cellularity of KitV558Δ;T669I/+ mice measured as total nucleated cells per bone is similar to that in wild-type mice. Spleen cellularity is significantly increased in the mutants compared with wild-type mice. n = 6. (E) Time course of hematocrit and (F) MCV showing development of erythrocytosis and microcytosis phenotype in KitV558Δ;T669I/+ mice (red curves) in and wild-type mice (black curves). n = 5–17. Error bars indicate means ± SD.
Importantly, the KitV558Δ;T669I/+ mice developed a pronounced microcytic erythrocytosis. Manifestation of erythrocytosis first was detected phenotypically by reddening of the paws, distinguishable from wild-type littermates from P40 onwards (Fig. 5B). Analysis of peripheral blood values of 8-wk-old animals revealed a substantial increase in erythrocyte counts and hematocrit values in comparison with wild-type and KitV558Δ/+ mice (Fig. S4 A and B). Furthermore, the mean corpuscular volume (MCV) was decreased significantly, and the mean platelet volume was increased (Fig. S4 C and D). Scanning electron microscopy confirmed that erythrocytes were smaller in diameter in KitV558Δ;T669I/+ mice than wild-type mice but otherwise were morphologically normal (Fig. S4 E and F). To gain insight into the kinetics of erythrocytosis and microcytosis development, we assessed hematocrit and MCV values in bleeding-naive (never before bled) KitV558∆;T669I/+ mice at weeks 3, 4, 5, 6, 8, 10, 13, and 16. The hematocrit showed a steep increase between postnatal weeks 3 and 8 (Fig. 5E). In contrast, the MCV already was reduced in 3-wk-old animals and did not change significantly over time (Fig. 5F). These results indicate that the development of the microcytosis is independent of the systemic erythrocyte overload (i.e., congestion of blood vessels in liver, spleen, and other organs), which becomes apparent only after 5 wk (Fig. S4G g and h). Of note, the microcytic erythrocytosis of the KitV558Δ;T669I/+ mice is the opposite phenotype of the macrocytic anemia associated with Kit and Kitl loss-of-function mutations (22–24).
Cytokine Dependence and Pharmacological Inhibition of Erythroid Colony Growth in KitV558Δ;T669I/+ Mice.
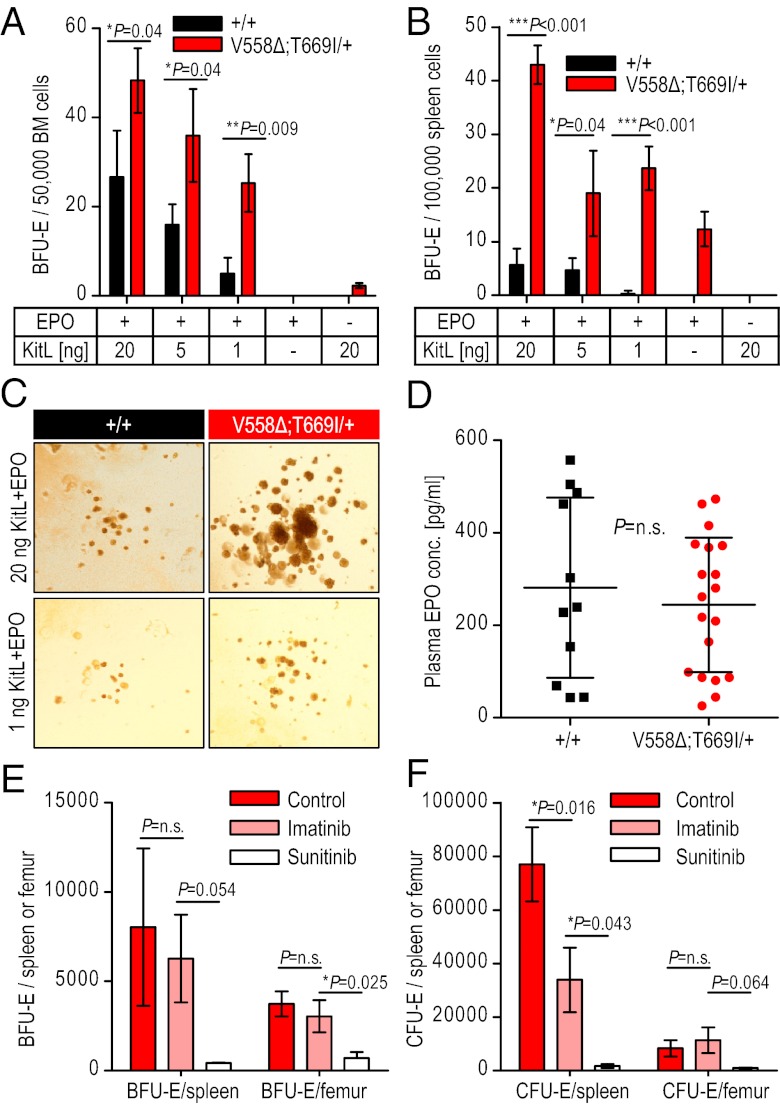
In accordance with the elevated hematocrit values, spleens in the KitV558Δ;T669I/+ mice were enlarged compared with wild-type mice, and spleen cellularity of KitV558Δ;T669I/+ mice was increased 1.5-fold without gross alterations in splenic architecture (Fig. 5D and Fig. S4G c and d). Bone marrow (BM) cellularity and histology was unchanged (Fig. 5D and Fig. S4G e and f). Although the erythrocytosis was reminiscent of myeloproliferative neoplasms, we noted several marked differences: The platelet counts were not elevated in KitV558Δ;T669I/+ mice (Kit+/+: 672 ± 248 × 103/μL and KitV558Δ;T669I/+: 681 ± 231 × 103/μL), and there was no myelofibrosis in either the BM or spleen, even in 1-y-old KITV558Δ;T669I/+ mice. Kit has important functions in erythroid progenitors that can be revealed by the formation of in vitro burst-forming unit erythroid (BFU-E) colonies in semisolid medium. The number of BFU-Es obtained from the KitV558Δ;T669I/+ BM and spleen in the presence of KitL, IL-3, and erythropoietin (EPO) was higher than in wild-type mice, and a concomitant increase in cfu erythroid (CFU-E) numbers also was observed in the mutant BM and spleen (Table 2). KitL and EPO play a synergistic role in erythroid colony growth and survival (25). To test whether wild-type and KitV558Δ;T669I/+ BFU-E formation differed in the presence of variable concentrations of cytokines, BM and spleen cells were cultured in the presence of decreasing concentrations of KitL and a fixed concentration (6 U/mL) of EPO (Fig. 6 A and B). BFU-Es from KitV558Δ;T669I/+ mice showed significantly reduced dependence on KitL compared with those from wild-type mice. The hypersensitivity of KitV558Δ;T669I/+ erythroid colonies to KitL was emphasized by the observation that the BFU-Es from KitV558Δ;T669I/+ BM and spleen were phenotypically larger than wild-type colonies at each concentration of KitL tested (Fig. 6C). Furthermore, KitV558Δ;T669I/+ BFU-E growth was dependent on EPO, because no colonies were observed in presence of KitL alone without EPO (Fig. 6 A and B). Importantly, EPO levels in the peripheral blood were not significantly different (Kit+/+: 281.3 ± 195 pg/mL; KitV558Δ;T669I/+: 244.5.5 ± 146 pg/mL) (Fig. 6D), indicating that the erythrocytosis in the KitV558Δ;T669I/+ mice was caused by increased KIT signaling in early erythroid progenitors.
Table 2.
Increased erythroid progenitors in spleen of KitV558Δ;T669I/+ mice
| BM |
Spleen |
|||
| Colony type | KIT+/+ | KitV558∆;T669I/+ | KIT+/+ | KitV558Δ;T669I/+ |
| BFU-E | 3,938 ± 1,042 | 6,386 ± 3,193 | 3,317 ± 1,436 | 16,198 ± 8,439 |
| CFU-E | 1,707 ± 910 | 4,562 ± 1,645 | 3,731 ± 1,759 | 25,575 ± 9,645* |
BM and spleen cells from wild-type and KitV558∆;T669I/+ mice were cultured in the presence of KitL (100 ng/mL), EPO (6 U/mL), and IL-3 (100 ng/mL). CFU-E and BFU-E colonies were scored at the end of 2 and 10 d, respectively. n = 3 mice per group.
*P < 0.05 in comparison with wild type.
Fig. 6.
KitV558∆;T669I/+ erythroid progenitor growth is hypersensitive to KitL and is susceptible to sunitinib inhibition. (A and B) BM (A) and spleen (B) cells were cultured in colony assays in the presence of EPO and decreasing levels of KitL. KitV558∆;T669I/+ BM gives rise to a significantly higher number of BFU-Es for each KitL+EPO combination. KitV558∆;T669I/+ spleen also gives rise to significantly greater number of BFU-Es at all the KitL+EPO combinations tested compared with wild-type spleen. Error bars indicate means ± SD; n = 3. (C) Representative images depict size of BFU-E colonies obtained from wild-type and KitV558∆;T669I/+ BM. (Magnification: 10 ×.) (D) EPO levels from sera of wild-type and KitV558∆;T669I/+ mice did not differ statistically. Error bars indicate means ± SD. (E and F) KitV558Δ;T669I/+ mice were treated with vehicle, imatinib, or sunitinib for 7 d. Femoral BM and spleen cells were cultured in the presence of KitL (100 ng/mL), EPO (6 U/mL), and IL3 (100 ng/mL). BFU-E (E) and CFU-E (F) colonies were scored after 10 d and 3 d, respectively. Error bars indicate means ± SD; n = 3.
To investigate the effect of pharmacologic intervention on the hematopoietic phenotype in the KitV558Δ;T669I/+ mice, we analyzed erythroid progenitor numbers in the BM and the spleen of KitV558Δ;T669I/+ mice after 7 d of treatment with either imatinib or sunitinib. Imatinib treatment did not change the number of BFU-Es from BM and spleen compared with control vehicle-treated animals (Fig. 6 E and F); in contrast, sunitinib treatment significantly reduced BFU-E numbers from both BM and spleen and concomitantly reduced CFU-E numbers from both organs (Fig. 6 E and F), suggesting that, in addition to the ICC hyperplasia and GIST development, the erythrocytosis phenotype of the KitV558Δ;T669I/+ mice is dependent largely on abnormal KIT kinase activity.
Discussion
By generating KITV558∆,T669I/+ mice we have produced an in vivo model for imatinib-resistant GIST. It was unknown if the introduction of imatinib-resistance mutations found in human GIST patients into the germ line of mice would produce viable offspring or would be lethal. The heterozygous double-mutant KitV558∆;T669I/+ mice we have generated not only are viable; their lifespan is extended significantly compared with that of KitV558∆/+ mice, presumably because of the reduced death rate from intestinal obstruction by GIST. Although the gastric and colonic ICC hyperplasia is more extensive in KitV558∆;T669I/+ mice, suggesting that the double-mutant Kit receptor is a stronger oncogene, the GIST lesions in the cecum were significantly smaller, and the cecum appeared to be severely truncated. We speculate that the reduced size of the cecum may result from a secondary effect of ICC progenitors expressing the KITV558∆;T669I mutation on embryonic cecum development at the time of budding of the cecal pouch (26). It is of interest that the KITT670I mutation is found only in imatinib-resistant GIST patients in combination with a primary KIT mutation. There are no reports to indicate that the KITT670I mutation may be found alone as a single-site mutation and on its own may have a role in GIST tumorigenesis, although in vitro experiments in COS cells with single-mutant KITT670I in the absence of KitL indicated KIT autophosphorylation (27).
Although GISTs of KitV558∆/+ and KitV558∆;T669I/+ mice were similar in histology and oncogenic signaling, the KitV558∆;T669I/+ mice were resistant to imatinib and dasatinib therapy. In agreement with previous in vitro studies (17, 18), these drugs failed to inhibit KITV558Δ;T669I/+ autophosphorylation and downstream signaling in GIST lesions of the KitV558∆;T669I/+ mice. This resistance could be overcome by sunitinib and sorafenib, supporting a rationale for using sunitinib as second-line therapy for imatinib-refractory GIST. Moreover, because imatinib and dasatinib inhibit wild-type KIT and other kinases in GIST, their lack of any significant impact on GIST signal transduction and proliferation in heterozygous KitV558∆;T669I/+ mice underlines the dependence of tumorigenesis on the oncogenic KitV558∆;T669I allele. Similarly, the ability of imatinib and dasatinib to inhibit the KIT receptor and KIT downstream signaling in KitV558∆/+ but not in KitV558∆;T669I/+ mice confirms that the response to imatinib and dasatinib treatment in KitV558∆/+ mice is mostly the result of direct inhibition of oncogenic KIT signaling rather than off-target effects. These observations underline the usefulness of the KitV558∆/+ and KitV558∆;T669I/+ mice in investigating oncogenic KIT signaling in GIST and their utility for preclinical studies of drug candidates.
The Kit receptor has a critical role in erythropoiesis and mast cells, and Kit loss-of-function mutations result in macrocytic anemia and mast cell deficiency (22, 28). Mice with the oncogenic KITV558∆ mutation, in addition to GIST, develop mast cell hyperplasia, but peripheral blood values are normal (9). In the KitV558∆;T669I/+ mice the mast cell hyperplasia is increased, but, fascinatingly, these mice develop a highly penetrant erythrocytosis similar to that seen in myeloproliferative neoplasms and polycythemia vera (PV) in addition to GIST. KIT gain-of-function mutations have been identified in mastocytosis and in acute myelogenous leukemia but not in PV, although KIT mutations of unclear significance have been reported in PV previously (29). Therefore it was somewhat surprising that KitV558Δ;T669I/+ mice developed erythrocytosis. Because KIT is known to have a role in early erythroid progenitors, the hypersensitivity observed in BFU-E assays and the lack of an effect on later progenitors and on serum erythropoietin levels confirms the role of KIT in early erythropoiesis (25). These observations also serve to distinguish PV (the molecular basis of which most often is an activating mutation in JAK2) from the microcytic erythrocytosis in the KitV558∆;T669I/+ mice (30). The erythroid phenotype in the KitV558∆;T669I/+ mice becomes evident between 3 and 5 wk of age. Interestingly, the earliest manifestation of erythrocytosis in the KitV558∆;T669I/+ mice coincides with the switch from fetal to adult hematopoiesis at 3 wk of age, and this timing may reflect the distinct cellular contexts in fetal and adult hematopoiesis (31).
In comparison with KitV558∆, the KitV558Δ;T669I mutation in vivo produces increased ICC hyperplasia and more pronounced, as well as distinct, hematopoietic phenotypes. Although in vitro the autoactivation of the KITV558Δ kinase is unchanged compared with the KITV558Δ;T669I kinase, the kinase activity of the KITV558Δ;T669I kinase is nearly doubled and could explain the KIT hyperactivity observed in the ICC, mast cell, and erythrocyte lineages in KitV558Δ;T669I/+ mice (32). However, at this time we cannot rule out the possibility that the T669I second-site mutation alters oncogenic Kit signaling in a qualitative fashion. Our results highlight the importance of a combination of factors, including the type of activation mutation and the cellular context, in determining mutant/oncogenic phenotypes in vivo.
Materials and Methods
Generation of Mouse Strains.
The V558∆ mutation was introduced by site-directed mutagenesis into a 2.1-kb EcoRI/MluI fragment across Kit exons 9–11 from a 129/SvJ mouse library (19) serving as the 5′ homology arm of the targeting vector (Fig.1A). It was linked 3′ to a floxed neomycin-resistance (NEO) gene-expression cassette. The 3′ homology arm for the KitV558∆ allele was a 1.3-kb MluI/BsrGI-NcoI fragment across exons 12–13 (Fig. S1A). For the KitV558∆;T669I allele this arm was elongated by a 2.1-kb BsrGI-NcoI/BamHI fragment including the T669I mutation in exon 14. To improve homologous recombination, a 3.8-kb BamHI fragment across exons 15–17 was added 3′ of T669I. The final targeting vector was sequenced completely before linearization and electroporation into CJ7 ES cells (33). Screening of 192 clones by Southern blot with a 5′ external probe across Kit exon 8 yielded four positive clones (Fig. 1A and Fig. S1B). One carried both the V558Δ mutation and the T669I mutation as assessed by sequencing and showed a normal karyotype. In C57BL/6J blastocyst injections this clone gave rise to 11 high-grade chimeras (more than 90% agouti coat color). After crossing to C57BL/6J mice, in all agouti F1 animals heterozygous for the NEO allele, the presence of the V558Δ and T669I mutations and the integrity of both loxP sites were confirmed by sequencing. To remove the floxed NEO cassette, F1 KitV558∆;T669I-NEO/+ males were bred to B6.FVB-Tg(EIIa-cre)C5379Lmgd/J females (Jackson Laboratory). Genotyping PCR was performed across the original intron 11 MluI site, which was replaced by a 134-bp loxP scar in the case of the targeted alleles. Of the two resulting fragments [wild-type allele (643 bp) and targeted allele (777 bp)], the longer was isolated by gel purification, and the integrity of the V558Δ mutation and of the remaining loxP site was confirmed by sequencing (Fig. S1C). Only KitV558Δ;T669I animals that genotyped negative for the Cre allele were used for subsequent backcrosses to the C57BL/6J background. A PCR strategy bracketing the 134-bp loxP scar was used for routine genotyping thereafter (wild-type allele, 291 bp; targeted allele, 425 bp) with the primers mKitEx11F, 5′-CATAGACCCGACGCAACTTC-3′; mKitIn11R, 5′-GGTTCCCAAATCAACAAGGC-3′. Initial experiments were done with backcross generation N4, and repetitions were done with N6–N13 animals. No change in phenotype was observed in different backcross generations. All animal procedures were approved by the Institutional Animal Care and Use Committee of Memorial Sloan Kettering Cancer Center.
Drug Treatments.
Cohorts of 3- to 4-mo-old animals were injected i.p. twice daily with imatinib mesylate (45 mg/kg body mass) (Novartis), dasatinib salt (25 mg/kg; synthesized in house), or vehicle control (water). Sunitinib malate (40 mg/kg) or sorafenib tosylate (60 mg/kg) (both from LC Laboratories) were solubilized freshly in 30% (vol/vol) Cremophor EL (Sigma Aldrich), 30% (vol/vol) polyethylene glycol 400, 10% (vol/vol) ethanol, and 10% (wt/vol) glucose every 4 d and were administered once daily by gavage.
Histologic and IHC Analyses.
Microscopic and IHC analyses were performed as described previously (10). Animals were dissected 6 h after injection [sorafenib-treated animals were dissected 3 h after injection (34)], and tumors were frozen immediately in liquid nitrogen and/or were fixed in fresh 4% paraformaldehyde at 4 °C overnight. After paraffin embedding, 5-μm sections of the tumors of at least three different animals per treatment group and of at least three different treatments were placed on microscopic slides next to one another to enable cross-comparison within a slide after IHC staining with the antibodies indicated. Antibodies used were Phospho (P)-ribosomal protein S6 (S235/236) (D57.2.2E), P-MAPK (ERK-1/2) (T202/204) (20G11), and P-STAT3 (Y705) (D3A7) (Cell Signaling); Ki67 (Vector Laboratories); and KIT (Oncogene Science). Stained slides were scanned with MIRAX Scan (Carl Zeiss), and Ki67 and STAT3 phosphorylation were quantified by counting stained nuclei in nine 250 × 250 μm fields of at least three different tumors per genotype and treatment.
Tissues for H&E staining were fixed in 10% neutral buffered formalin, and bones were decalcified in 0.5 M EDTA. Tissue samples were subjected to routine histological procedures and embedded in paraffin. Five-micrometer sections were used for histological staining with H&E.
Western Blotting.
Tumor lysates and Western blotting with KIT (D13A2), P-KIT (Y719), S6 (5G10), P-S6 (S235/236) (D57.2.2E), MAPK (9102), and P-MAPK (ERK-1/2) (T202/204) (20G11) antibodies (all from Cell Signaling) were done as described (9) with the following modifications. Liquid nitrogen-frozen samples were minced in 50 μL lysis buffer in Petri dishes on solid CO2 with scalpels, sonicated twice for 10 s with a Sonifier S-250A with double-stepped microtip (Branson Ultrasonics), and cleared by centrifugation. Densitometry of Western blotting bands was done with Multi Gauge V3.1 (Fujifilm) by setting the ratio of the intensities of control-treated phospho-KIT bands and their corresponding total KIT bands to 100% and normalizing the ratio of the treated phospho-KIT/total KIT band intensities to that standard.
Colony-Forming Assay.
Unfractionated BM cells (50,000 cells per plate) or spleen cells (100,000 cells per plate) from at least three mice were plated in 1 mL Iscove's modified Dulbecco's medium containing 1.2% (wt/vol) methylcellulose, 30% FCS, 2 mmol/L glutamine, 0.1 mmol/L 2-mercaptoethanol, and 4 mmol/L hemin with cytokines (EPO 6 U/mL; IL-3 100 ng/mL, and KitL 100 ng/mL). Cultures were maintained in triplicate at 37 °C in humidified 5% CO2. CFU-Es were scored after 2 d, and BFU-E colonies were scored after 7–10 d of culture. BFU-E growth also was assessed in the presence of 6 U/mL EPO and 1, 5, or 20 ng/mL KitL. Colonies were scored using Nikon Eclipse TE 200, and images were taken Nikon Eclipse Ti-S.
Erythropoietin Measurement.
Sera from wild-type or KitV558Δ;T669I/+ mice obtained by retroorbital bleeding were used to measure EPO levels using the Milliplex assay system (Millipore) according to the manufacturer’s instructions.
Statistical Analysis.
Comparison between two groups was done by unpaired t test analysis using GraphPad Prism (version 5.0). Statistical significance was achieved when P < 0.05.
Supplementary Material
Acknowledgments
We thank Maureen Sullivan, LingBo Shen, Yasemin Yozgat, and Fabienne Brenet for expert assistance; Aleksander M. Baldys, Afsar Barlas, Ning Fan, Volodia Gueorguiev, and Mesruh Turkekul from the Molecular Cytology, Huiyong Zhao from the Antitumor Assessment, and Willie H. Mark and Antoinette Rookard from the Mouse Genetics Core Facilities at Memorial Sloan-Kettering Cancer Center, and Chingwen Yang from the Rockefeller University Gene Targeting Resource Center for expert help; and John Burrowes and Zachary Oberzan for secretarial assistance. This work was supported by National Institutes of Health Grants R01-CA102774, RO1-HL55748, and P50-CA140146 (to P.B.) and by grants from the Starr Cancer Consortium (to P.B., C.R.A., and J.M.S.) and the LifeRaft Group (to P.B. and C.R.A.).
Footnotes
Conflict of interest statement: P.B. received funding from Novartis for research on GIST.
This article is a PNAS Direct Submission.
See Author Summary on page 13480 (volume 109, number 34).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1115240109/-/DCSupplemental.
References
- 1.Kwon JG, et al. Changes in the structure and function of ICC networks in ICC hyperplasia and gastrointestinal stromal tumors. Gastroenterology. 2009;136:630–639. doi: 10.1053/j.gastro.2008.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heinrich MC, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708–710. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 3.Hirota S, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 4.Antonescu CR, et al. Association of KIT exon 9 mutations with nongastric primary site and aggressive behavior: KIT mutation analysis and clinical correlates of 120 gastrointestinal stromal tumors. Clin Cancer Res. 2003;9:3329–3337. [PubMed] [Google Scholar]
- 5.Lasota J, et al. Mutations in exons 9 and 13 of KIT gene are rare events in gastrointestinal stromal tumors. A study of 200 cases. Am J Pathol. 2000;157:1091–1095. doi: 10.1016/S0002-9440(10)64623-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubin BP, et al. KIT activation is a ubiquitous feature of gastrointestinal stromal tumors. Cancer Res. 2001;61:8118–8121. [PubMed] [Google Scholar]
- 7.Mol CD, et al. Structural basis for the autoinhibition and STI-571 inhibition of c-Kit tyrosine kinase. J Biol Chem. 2004;279:31655–31663. doi: 10.1074/jbc.M403319200. [DOI] [PubMed] [Google Scholar]
- 8.Nishida T, et al. Familial gastrointestinal stromal tumours with germline mutation of the KIT gene. Nat Genet. 1998;19:323–324. doi: 10.1038/1209. [DOI] [PubMed] [Google Scholar]
- 9.Sommer G, et al. Gastrointestinal stromal tumors in a mouse model by targeted mutation of the Kit receptor tyrosine kinase. Proc Natl Acad Sci USA. 2003;100:6706–6711. doi: 10.1073/pnas.1037763100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rossi F, et al. Oncogenic Kit signaling and therapeutic intervention in a mouse model of gastrointestinal stromal tumor. Proc Natl Acad Sci USA. 2006;103:12843–12848. doi: 10.1073/pnas.0511076103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rossi F, et al. Imatinib upregulates compensatory integrin signaling in a mouse model of gastrointestinal stromal tumor and is more effective when combined with dasatinib. Mol Cancer Res. 2010;8:1271–1283. doi: 10.1158/1541-7786.MCR-10-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antonescu CR, et al. Acquired resistance to imatinib in gastrointestinal stromal tumor occurs through secondary gene mutation. Clin Cancer Res. 2005;11:4182–4190. doi: 10.1158/1078-0432.CCR-04-2245. [DOI] [PubMed] [Google Scholar]
- 13.Debiec-Rychter M, et al. Mechanisms of resistance to imatinib mesylate in gastrointestinal stromal tumors and activity of the PKC412 inhibitor against imatinib-resistant mutants. Gastroenterology. 2005;128:270–279. doi: 10.1053/j.gastro.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 14.Gorre ME, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293:876–880. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- 15.Shah NP, et al. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell. 2002;2:117–125. doi: 10.1016/s1535-6108(02)00096-x. [DOI] [PubMed] [Google Scholar]
- 16.Tamborini E, et al. A new mutation in the KIT ATP pocket causes acquired resistance to imatinib in a gastrointestinal stromal tumor patient. Gastroenterology. 2004;127:294–299. doi: 10.1053/j.gastro.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 17.Carter TA, et al. Inhibition of drug-resistant mutants of ABL, KIT, and EGF receptor kinases. Proc Natl Acad Sci USA. 2005;102:11011–11016. doi: 10.1073/pnas.0504952102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo T, et al. Sorafenib inhibits the imatinib-resistant KITT670I gatekeeper mutation in gastrointestinal stromal tumor. Clin Cancer Res. 2007;13:4874–4881. doi: 10.1158/1078-0432.CCR-07-0484. [DOI] [PubMed] [Google Scholar]
- 19.Kissel H, et al. Point mutation in kit receptor tyrosine kinase reveals essential roles for kit signaling in spermatogenesis and oogenesis without affecting other kit responses. EMBO J. 2000;19:1312–1326. doi: 10.1093/emboj/19.6.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karaman MW, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 21.Vanderford DA, et al. Alopecia in IL-10-deficient mouse pups is c-kit-dependent and can be triggered by iron deficiency. Exp Dermatol. 2010;19:518–526. doi: 10.1111/j.1600-0625.2009.01032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russell ES. Hereditary anemias of the mouse: A review for geneticists. Adv Genet. 1979;20:357–459. [PubMed] [Google Scholar]
- 23.Bernstein A, et al. The mouse W/c-kit locus. Ciba Found Symp. 1990;148:158–166, discussion 166–172. [PubMed] [Google Scholar]
- 24.Besmer P. Kit-Ligand-Stem Cell Factor. New York, NY: Marcel Dekker; 1997. pp. 369–403. [Google Scholar]
- 25.Nocka K, Buck J, Levi E, Besmer P. Candidate ligand for the c-kit transmembrane kinase receptor: KL, a fibroblast derived growth factor stimulates mast cells and erythroid progenitors. EMBO J. 1990;9:3287–3294. doi: 10.1002/j.1460-2075.1990.tb07528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X, et al. Reciprocal epithelial-mesenchymal FGF signaling is required for cecal development. Development. 2006;133:173–180. doi: 10.1242/dev.02175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamborini E, et al. Functional analyses and molecular modeling of two c-Kit mutations responsible for imatinib secondary resistance in GIST patients. Oncogene. 2006;25:6140–6146. doi: 10.1038/sj.onc.1209639. [DOI] [PubMed] [Google Scholar]
- 28.Kitamura Y, Go S, Hatanaka K. Decrease of mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood. 1978;52:447–452. [PubMed] [Google Scholar]
- 29.Fontalba A, et al. Identification of c-Kit gene mutations in patients with polycythemia vera. Leuk Res. 2006;30:1325–1326. doi: 10.1016/j.leukres.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 30.Levine RL, Pardanani A, Tefferi A, Gilliland DG. Role of JAK2 in the pathogenesis and therapy of myeloproliferative disorders. Nat Rev Cancer. 2007;7:673–683. doi: 10.1038/nrc2210. [DOI] [PubMed] [Google Scholar]
- 31.Bowie MB, et al. Identification of a new intrinsically timed developmental checkpoint that reprograms key hematopoietic stem cell properties. Proc Natl Acad Sci USA. 2007;104:5878–5882. doi: 10.1073/pnas.0700460104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gajiwala KS, et al. KIT kinase mutants show unique mechanisms of drug resistance to imatinib and sunitinib in gastrointestinal stromal tumor patients. Proc Natl Acad Sci USA. 2009;106:1542–1547. doi: 10.1073/pnas.0812413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swiatek PJ, Gridley T. Perinatal lethality and defects in hindbrain development in mice homozygous for a targeted mutation of the zinc finger gene Krox20. Genes Dev. 1993;7:2071–2084. doi: 10.1101/gad.7.11.2071. [DOI] [PubMed] [Google Scholar]
- 34.Liu L, et al. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66:11851–11858. doi: 10.1158/0008-5472.CAN-06-1377. [DOI] [PubMed] [Google Scholar]