Abstract
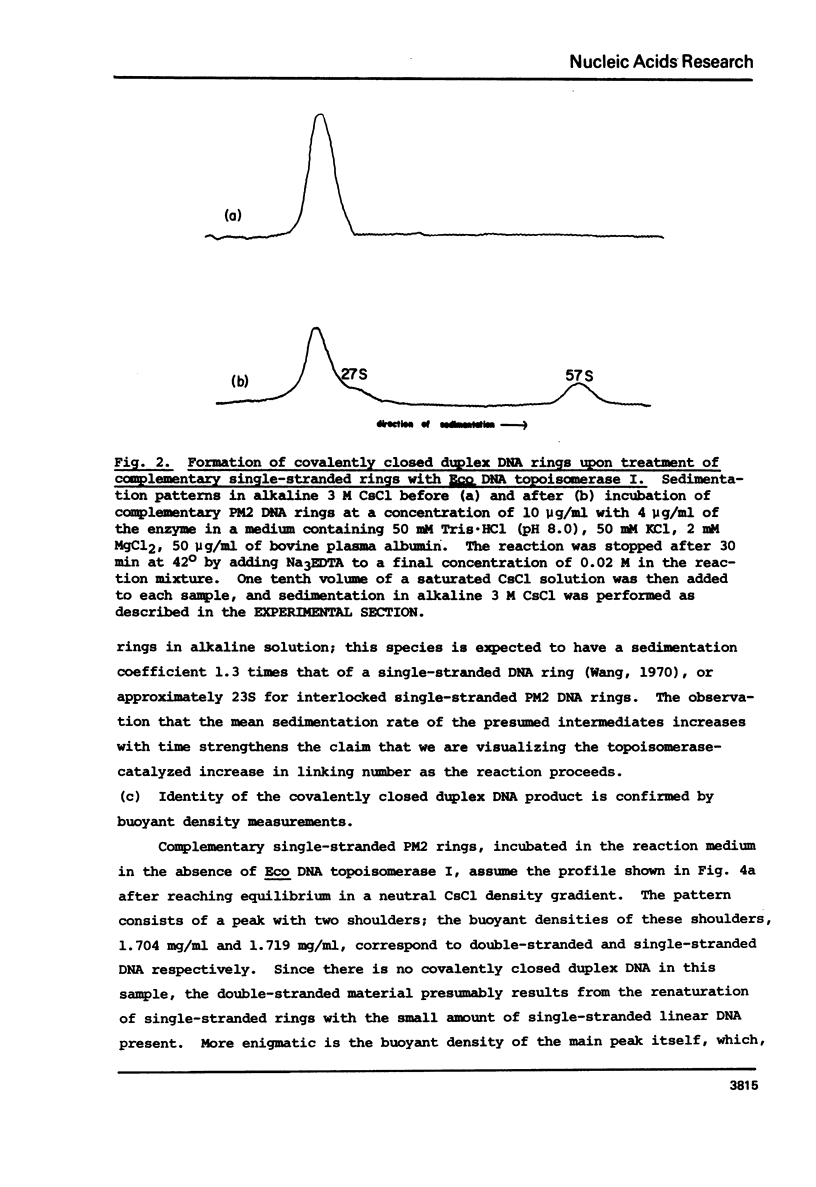
Eco DNA topoisomerase I (E. coli ω protein) has been observed to catalyze the formation of double-stranded, covalently closed DNA from complementary single-stranded DNA rings, a novel reaction which is topologically forbidden without the enzyme-catalyzed breakage and rejoining of DNA backbone bonds. Incubation of a mixture of single-stranded PM2 DNA rings of complementary base sequences with ω yields a species with a sedimentation coefficient in an alkaline medium characteristic of a covalently closed circular double-stranded DNA. Buoyant density measurements in CsCl at alkaline pH also identify the product as a covalently closed duplex ring. If the ω-catalyzed reaction is stopped short of completion, highly negatively supercoiled molecules are formed which sediment more slowly in an alkaline medium than the final duplex product. As the reaction proceeds the mean sedimentation rate of the intermediates increases. This is in agreement with the expectation that the linking number between the two complementary rings increases gradually during the course of the reaction from zero to that of a relaxed covalently closed circular DNA duplex. The possible role of DNA topoisomerases in genetic recombination is discussed.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer W. R., Ressner E. C., Kates J., Patzke J. V. A DNA nicking-closing enzyme encapsidated in vaccinia virus: partial purification and properties. Proc Natl Acad Sci U S A. 1977 May;74(5):1841–1845. doi: 10.1073/pnas.74.5.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. E., Davies J., Allet B., Rochaix J. D. Transposition of R factor genes to bacteriophage lambda. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3628–3632. doi: 10.1073/pnas.72.9.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broker T. R., Soll L., Chow L. T. Underwound loops in self-renatured DNA can be diagnostic of inverted duplications and translocated sequences. J Mol Biol. 1977 Jul 15;113(4):579–589. doi: 10.1016/0022-2836(77)90223-6. [DOI] [PubMed] [Google Scholar]
- Burgner J. W., 2nd, Ray W. J., Jr A study of pyruvate-induced inhibition in the dogfish lactate dehydrogenase system. Mechanistic comparison with the iodination of pyruvate. Biochemistry. 1974 Sep 24;13(20):4229–4237. doi: 10.1021/bi00717a025. [DOI] [PubMed] [Google Scholar]
- Champoux J. J., McConaughy B. L. Purification and characterization of the DNA untwisting enzyme from rat liver. Biochemistry. 1976 Oct 19;15(21):4638–4642. doi: 10.1021/bi00666a014. [DOI] [PubMed] [Google Scholar]
- Champoux J. J. Renaturation of complementary single-stranded DNA circles: complete rewinding facilitated by the DNA untwisting enzyme. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5328–5332. doi: 10.1073/pnas.74.12.5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depew R. E., Liu L. F., Wang J. C. Interaction between DNA and Escherichia coli protein omega. Formation of a complex between single-stranded DNA and omega protein. J Biol Chem. 1978 Jan 25;253(2):511–518. [PubMed] [Google Scholar]
- Espejo R. T., Canelo E. S., Sinsheimer R. L. DNA of bacteriophage PM2: a closed circular double-stranded molecule. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1164–1168. doi: 10.1073/pnas.63.4.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandini Attardi D., Mattoccia E., Tocchini-Valentini G. P. Formation of branched DNA structures by Xenopus laevis oocyte extract. Nature. 1977 Dec 22;270(5639):754–756. doi: 10.1038/270754a0. [DOI] [PubMed] [Google Scholar]
- Hotchkiss R. D. Models of genetic recombination. Annu Rev Microbiol. 1974;28(0):445–468. doi: 10.1146/annurev.mi.28.100174.002305. [DOI] [PubMed] [Google Scholar]
- Hsieh T. S., Wang J. C. Thermodynamic properties of superhelical DNAs. Biochemistry. 1975 Feb 11;14(3):527–535. doi: 10.1021/bi00674a011. [DOI] [PubMed] [Google Scholar]
- Kasamatsu H., Wu M. Structure of a nicked DNA-protein complex isolated from simian virus 40: covalent attachment of the protein to DNA and nick specificity. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1945–1949. doi: 10.1073/pnas.73.6.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller W. Characterization of purified DNA-relaxing enzyme from human tissue culture cells. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2550–2554. doi: 10.1073/pnas.72.7.2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N., Chan R. K., Tye B. K., Botstein D. Mutagenesis by insertion of a drug-resistance element carrying an inverted repetition. J Mol Biol. 1975 Oct 5;97(4):561–575. doi: 10.1016/s0022-2836(75)80059-3. [DOI] [PubMed] [Google Scholar]
- Kung V. T., Wang J. C. Purification and characterization of an omega protein from Micrococcus luteus. J Biol Chem. 1977 Aug 10;252(15):5398–5402. [PubMed] [Google Scholar]
- Liu L. F., Depew R. E., Wang J. C. Knotted single-stranded DNA rings: a novel topological isomer of circular single-stranded DNA formed by treatment with Escherichia coli omega protein. J Mol Biol. 1976 Sep 15;106(2):439–452. doi: 10.1016/0022-2836(76)90095-4. [DOI] [PubMed] [Google Scholar]
- Malamy M. H., Fiandt M., Szybalski W. Electron microscopy of polar insertions in the lac operon of Escherichia coli. Mol Gen Genet. 1972;119(3):207–222. doi: 10.1007/BF00333859. [DOI] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Meselson M. S., Radding C. M. A general model for genetic recombination. Proc Natl Acad Sci U S A. 1975 Jan;72(1):358–361. doi: 10.1073/pnas.72.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosharrafa E., Pilacinski W., Zissler J., Fiandt M., Szybalski W. Insertion sequence IS2 near the gene for prophage lambda excision. Mol Gen Genet. 1976 Aug 10;147(1):103–109. doi: 10.1007/BF00337943. [DOI] [PubMed] [Google Scholar]
- Potter H., Dressler D. On the mechanism of genetic recombination: the maturation of recombination intermediates. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4168–4172. doi: 10.1073/pnas.74.10.4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulleyblank D. E., Morgan A. R. The sense of naturally occurring superhelices and the unwinding angle of intercalated ethidium. J Mol Biol. 1975 Jan 5;91(1):1–13. doi: 10.1016/0022-2836(75)90368-x. [DOI] [PubMed] [Google Scholar]
- Rosenberg B. H., Ungers G., Deutsch J. F. Variation in DNA swivel enzyme activity during the mammalian cell cycle. Nucleic Acids Res. 1976 Dec;3(12):3305–3311. doi: 10.1093/nar/3.12.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmir M., Révet B. M., Vinograd J. Dependence of the sedimentation coefficient of denatured closed circular DNA in alkali on the degree of strand interwinding. The absolute sense of supercoils. J Mol Biol. 1974 Feb 15;83(1):35–45. doi: 10.1016/0022-2836(74)90422-7. [DOI] [PubMed] [Google Scholar]
- Wang J. C. Interaction between DNA and an Escherichia coli protein omega. J Mol Biol. 1971 Feb 14;55(3):523–533. doi: 10.1016/0022-2836(71)90334-2. [DOI] [PubMed] [Google Scholar]
- Wang J. C. Interlocked DNA rings. II. Physicochemical studies. Biopolymers. 1970;9(4):489–502. doi: 10.1002/bip.1970.360090410. [DOI] [PubMed] [Google Scholar]
- Wang J. C. The degree of unwinding of the DNA helix by ethidium. I. Titration of twisted PM2 DNA molecules in alkaline cesium chloride density gradients. J Mol Biol. 1974 Nov 15;89(4):783–801. doi: 10.1016/0022-2836(74)90053-9. [DOI] [PubMed] [Google Scholar]
- Yoshida S., Ungers G., Rosenberg B. H. DNA swivel enzyme activity in a nuclear membrane fraction. Nucleic Acids Res. 1977 Jan;4(1):223–228. doi: 10.1093/nar/4.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]


