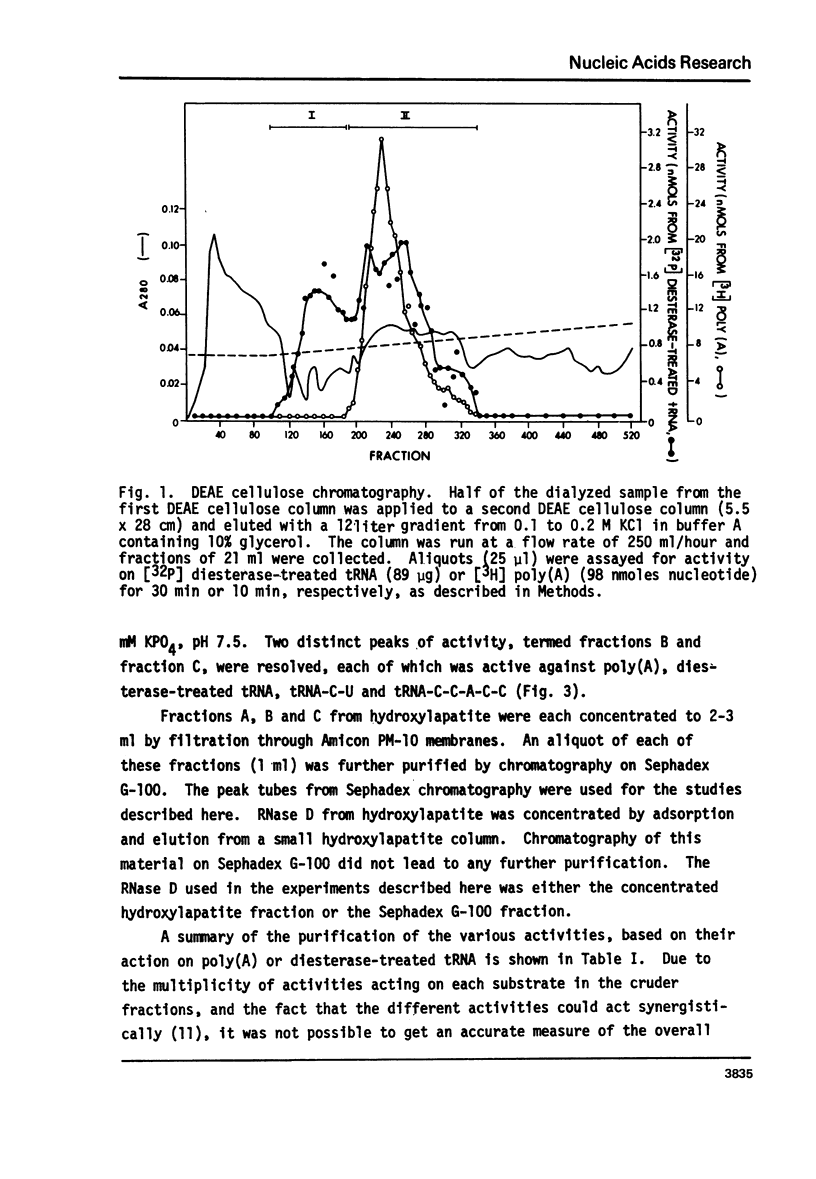
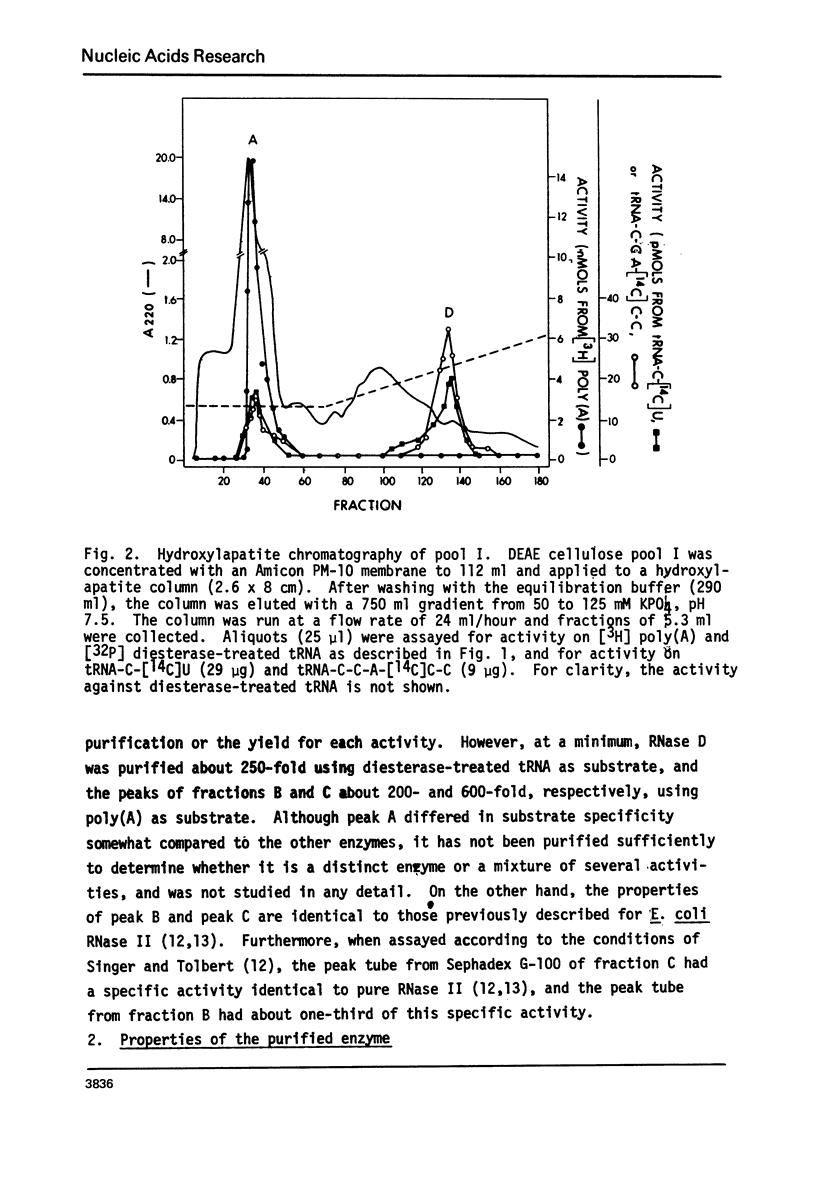
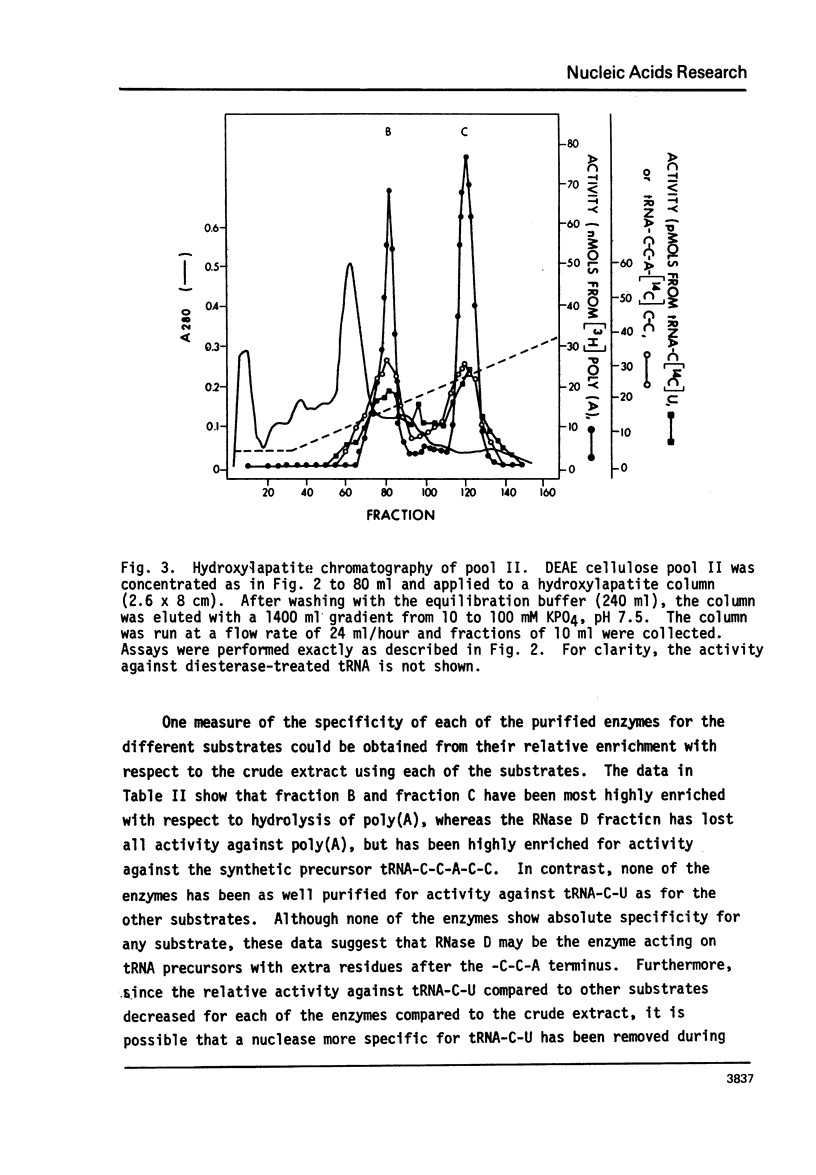
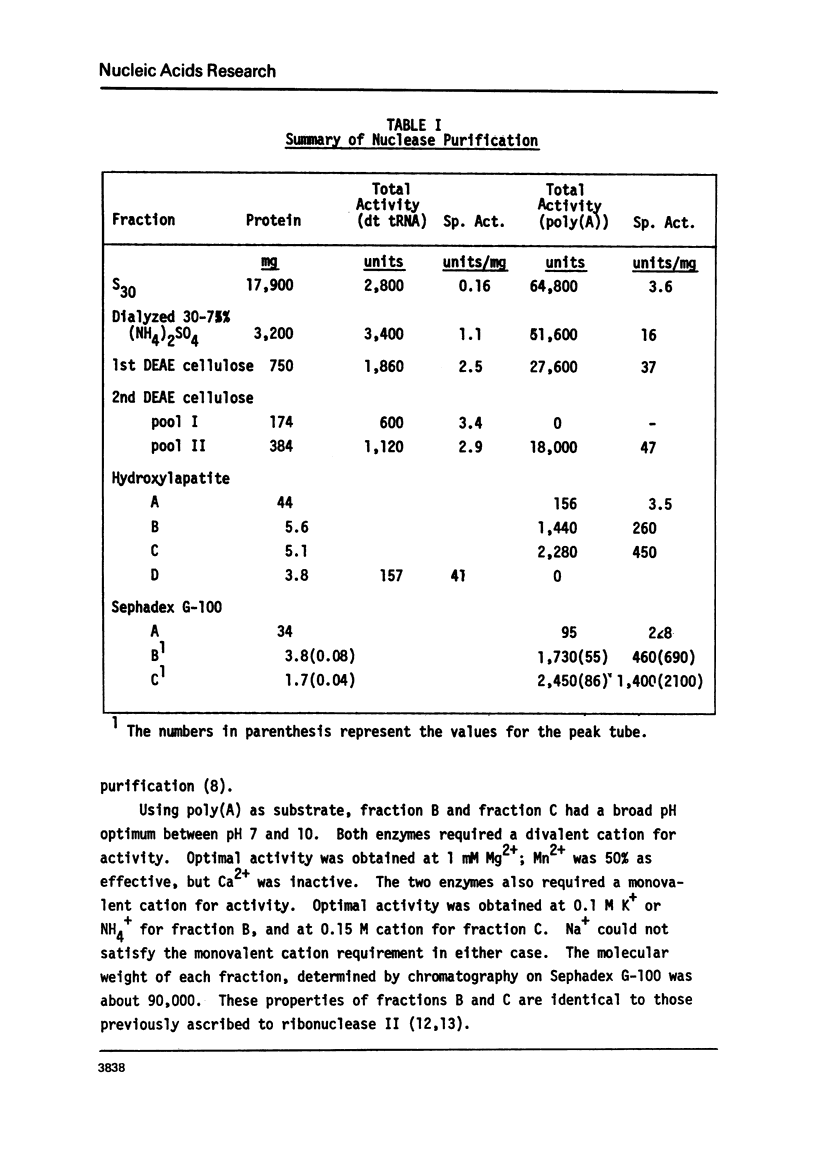
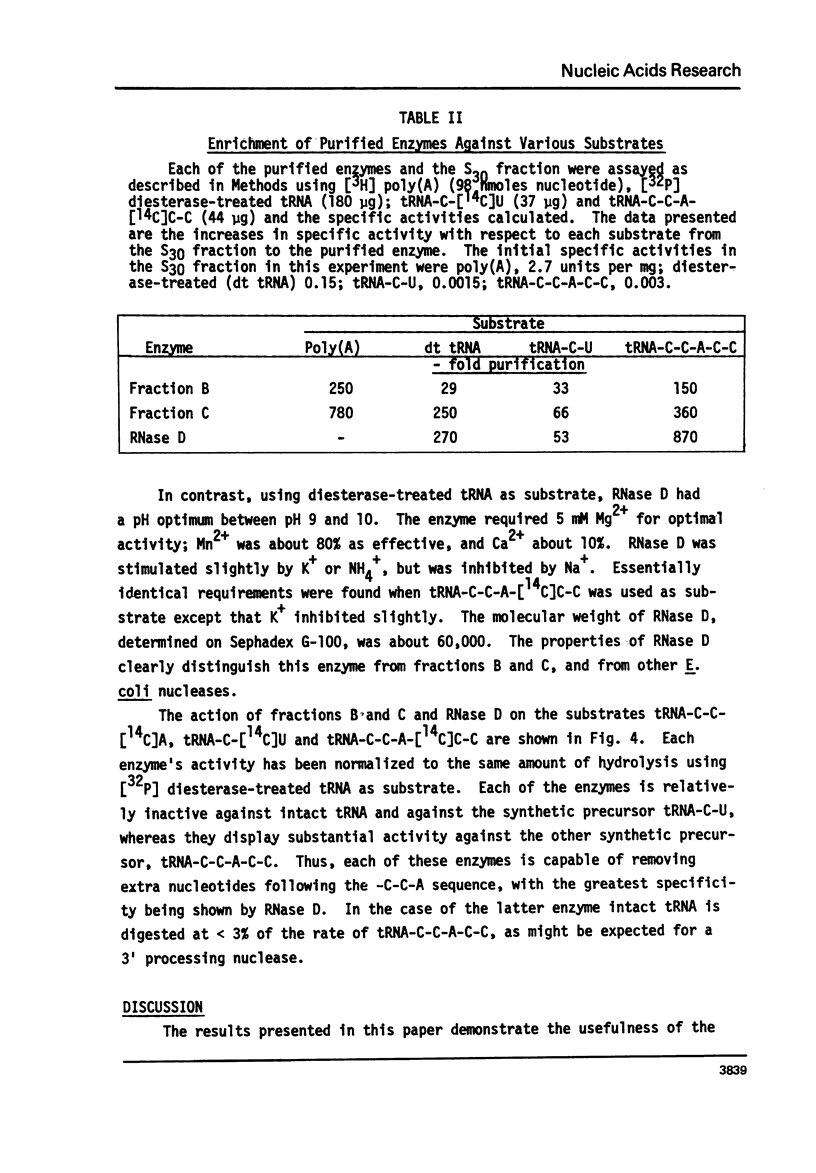
Abstract
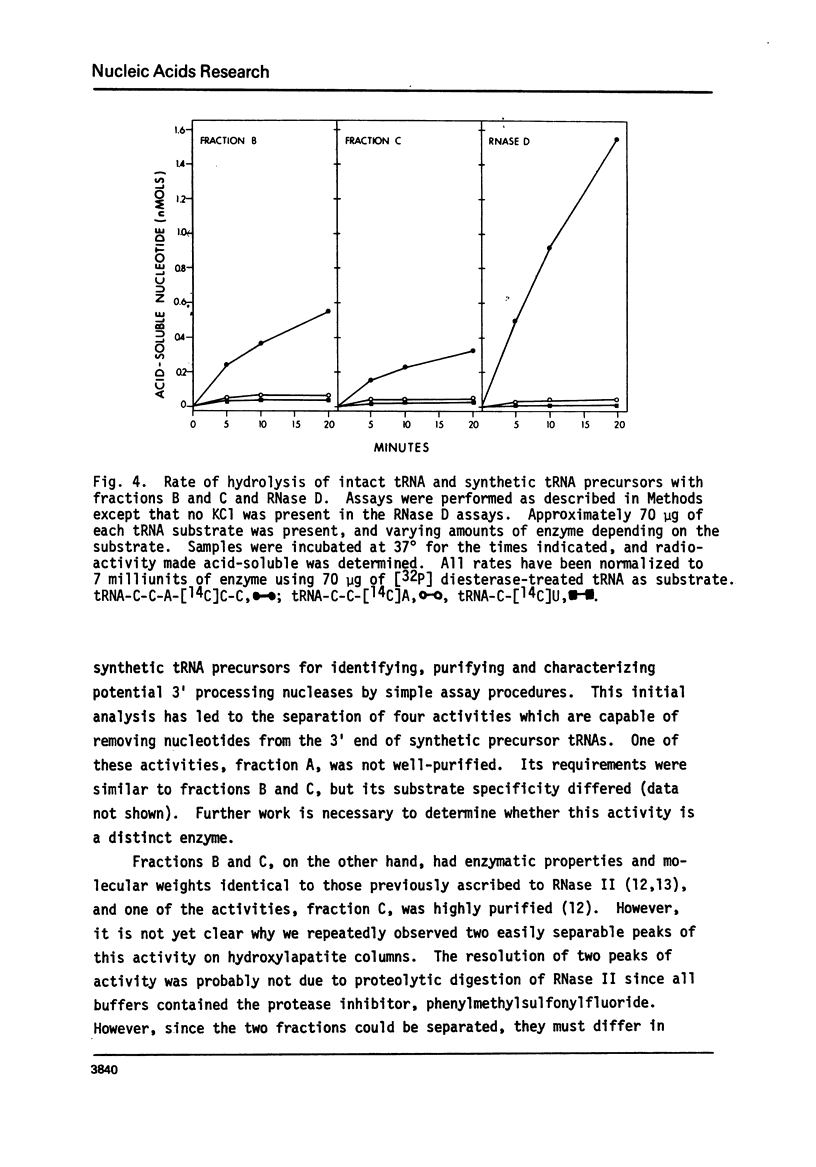
The synthetic tRNA precursors, tRNA-C-114C]U and tRNA-C-C-A-[14C]C-C, as well as poly (a) and diesterase-treated tRNA, have been used to identify and purify potential 3'processing nucleases. Four activities have been separated by this analysis; and three of them have been characterized. Two of the enzymes, which are well-separated on hydroxylapatite columns, act on poly(A), require K+ and Mg2+ for activity, and have molecular weights of about 90,000. These activities have properties previously ascribed to RNase II. The third enzyme does not act on poly(A), requires Mg2+ for activity, and has a molecular weight of about 60,000. It is identical to RNase D, previously characterized as an exonuclease acting on tRNAs with altered structure. Each of the enzymes can remove nucleotides from the tRNA precursor containing extra nucleotides beyond the 3'terminus, whereas they are relatively inactive with intact tRNA or tRNA-C-U. The greatest specificity was displayed by RNase D. The possibility that RNase D is a 3'processing nuclease is discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bikoff E. K., LaRue B. F., Gefter M. L. In vitro synthesis of transfer RNA. II. Identification of required enzymatic activities. J Biol Chem. 1975 Aug 25;250(16):6248–6255. [PubMed] [Google Scholar]
- Deutscher M. P., Hilderman R. H. Isolation and partial characterization of Escherichia coli mutants with low levels of transfer ribonucleic acid nucleotidyltransferase. J Bacteriol. 1974 May;118(2):621–627. doi: 10.1128/jb.118.2.621-627.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher M. P., Lin J. J., Evans J. A. Transfer RNA metabolism in Escherichia coli cells deficient in tRNA nucleotidyltransferase. J Mol Biol. 1977 Dec 25;117(4):1081–1094. doi: 10.1016/s0022-2836(77)80014-4. [DOI] [PubMed] [Google Scholar]
- Fournier M. J., Webb E., Tang S. In vitro biosynthesis of functional Escherichia coli su3+ tyrosine transfer RNA. Biochemistry. 1977 Aug 9;16(16):3608–3618. doi: 10.1021/bi00635a017. [DOI] [PubMed] [Google Scholar]
- Ghosh R. K., Deutscher M. P. Identification of an Escherichia coli nuclease acting on structurally altered transfer RNA molecules. J Biol Chem. 1978 Feb 25;253(4):997–1000. [PubMed] [Google Scholar]
- Maisurian A. N., Buianovskaia E. A. Effekt podavleniia deistviia supressora amber-mutatsii, soderzhashchegosia v genome bakteriofaga T4. Genetika. 1975;11(6):114–118. [PubMed] [Google Scholar]
- Morse J. W., Deutscher M. P. Letter: Apparent non-involvement of transfer RNA nucleotidyltransferase in the biosynthesis of Escherichia coli suppressor transfer RNAs. J Mol Biol. 1975 Jun 15;95(1):141–144. doi: 10.1016/0022-2836(75)90342-3. [DOI] [PubMed] [Google Scholar]
- Schedl P., Primakoff P. Mutants of Escherichia coli thermosensitive for the synthesis of transfer RNA. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2091–2095. doi: 10.1073/pnas.70.7.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedl P., Roberts J., Primakoff P. In vitro processing of E. coli tRNA precursors. Cell. 1976 Aug;8(4):581–594. doi: 10.1016/0092-8674(76)90226-9. [DOI] [PubMed] [Google Scholar]
- Seidman J. G., McClain W. H. Three steps in conversion of large precursor RNA into serine and proline transfer RNAs. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1491–1495. doi: 10.1073/pnas.72.4.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman J. G., Schmidt F. J., Foss K., McClain W. H. A mutant of escherichia coli defective in removing 3' terminal nucleotides from some transfer RNA precursor molecules. Cell. 1975 Aug;5(4):389–400. doi: 10.1016/0092-8674(75)90058-6. [DOI] [PubMed] [Google Scholar]
- Shimura Y., Sakano H., Nagawa F. Specific ribonucleases involved in processing of tRNA precursors of Escherichia coli. Partial purification and some properties. Eur J Biochem. 1978 May;86(1):267–281. doi: 10.1111/j.1432-1033.1978.tb12308.x. [DOI] [PubMed] [Google Scholar]
- Singer M. F., Tolbert G. Purification and properties of a potassium-activated phosphodiesterase (RNAase II) from Escherichia coli. Biochemistry. 1965 Jul;4(7):1319–1330. doi: 10.1021/bi00883a016. [DOI] [PubMed] [Google Scholar]


