Abstract
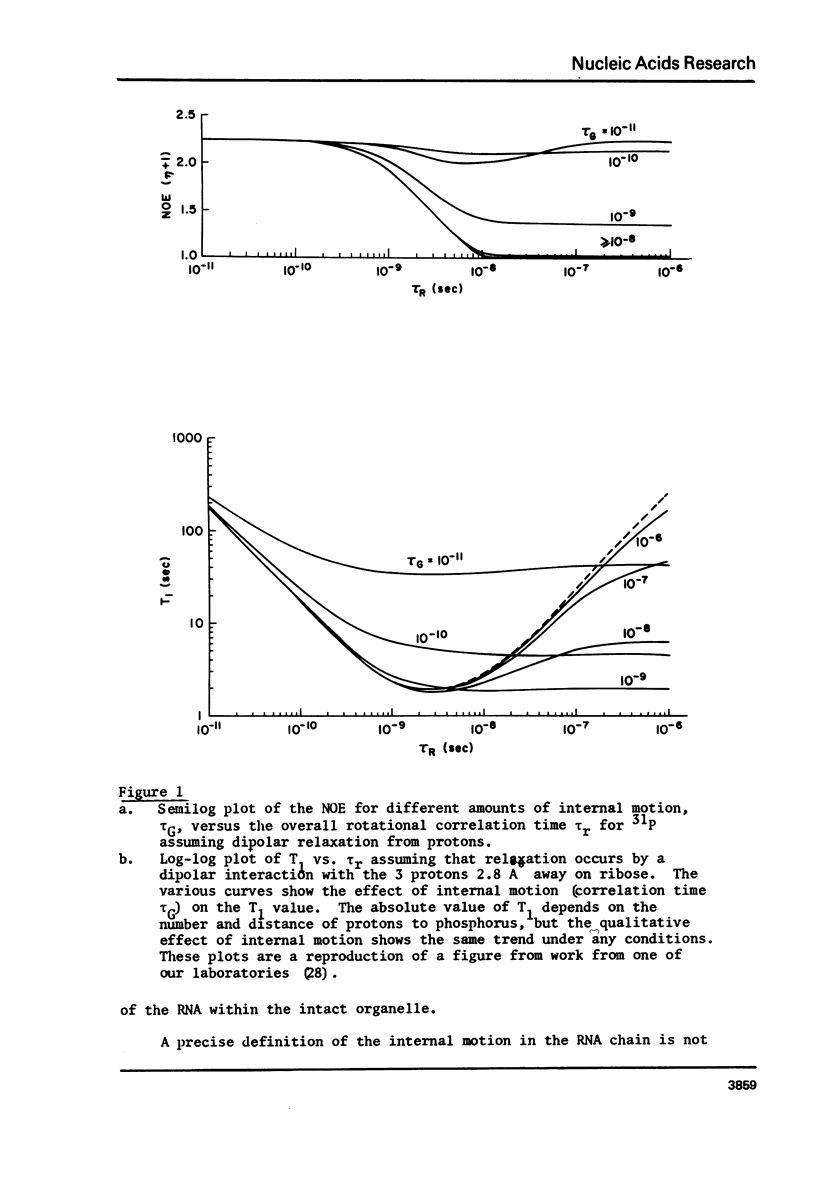
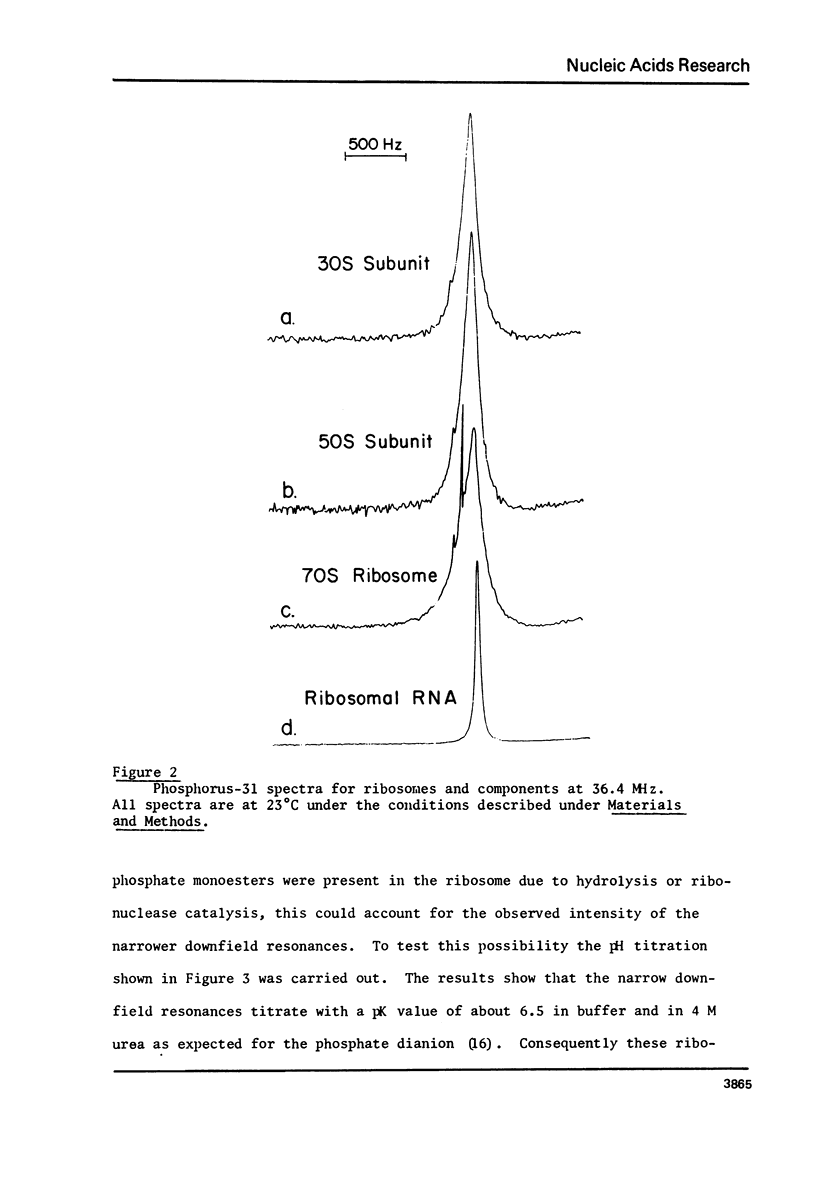
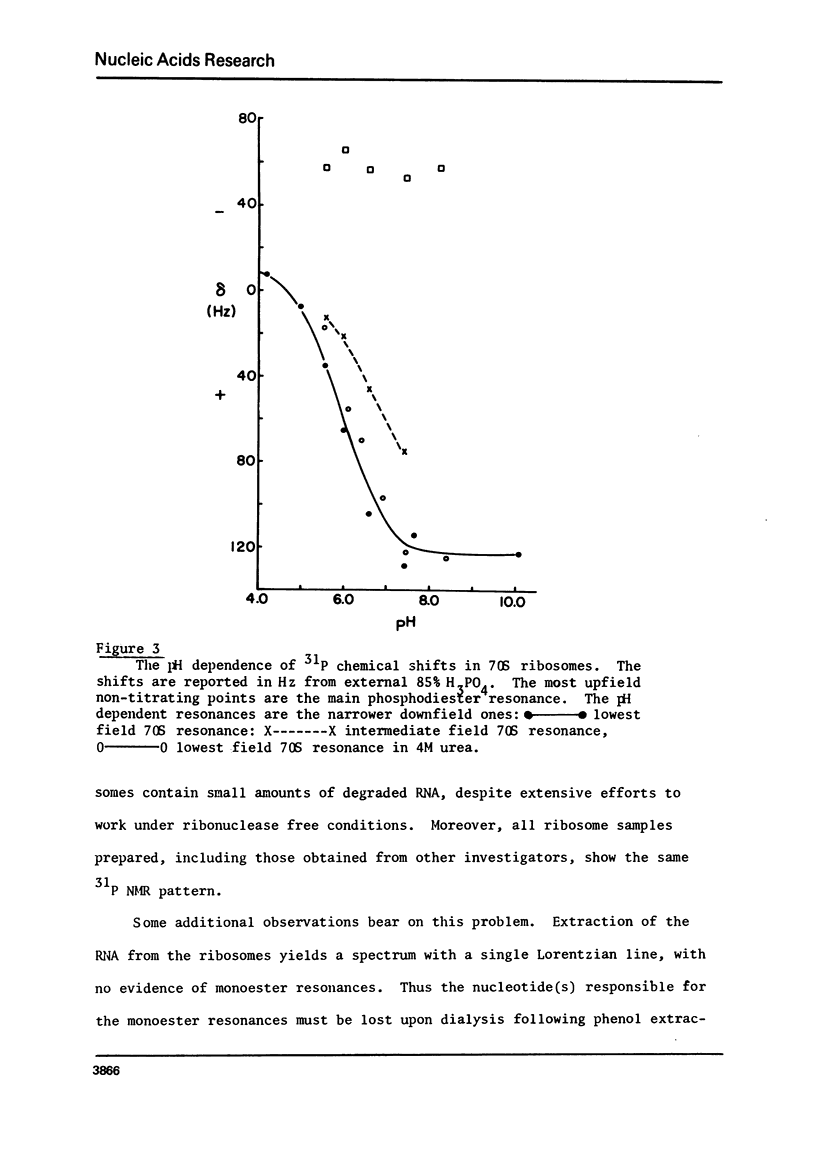
Phosphorus-31 nuclear magnetic resonance spectra, relaxation times and nuclear Overhauser (NOE) enhancement have been measured for E. coli ribosomes, subunits and rRNA. NOE and T1 experiments reveal that the phosphorus relaxation in this organelle is largely dipolar in origin. Moreover these results imply the presence of internal motion within the RNA chain with a correlation time of about 3-5 x 10(-9) sec. In all cases the predominant resonance is centered at about -1.5 ppm (relative to 85% H3PO4) as expected for a phosphodiester linkage where there is a large degree of double helix. The linewidth narrows by about a factor of four when the ribosomal proteins are removed indicating a substantial immobilization of the RNA when it is assembled into the ribosome. In addition to the phosphodiester resonance, ribosomes also reveal one or two narrower resonances shifted to low field by 1-4 ppm. Based on the observation that these resonances show a pH dependent chemical shift, we assign them to phosphate monoesters i.e. terminal 3' or 5' phosphate groups. These terminal phosphates are due to short oligomers of RNA derived from the terminus of the chain.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amand B., Pochon F., Lavalette D. Rotational diffusion of Escherichia coli ribosomes. I. - Free 70 S, 50 S and 30 S particles. Biochimie. 1977;59(10):779–784. doi: 10.1016/s0300-9084(77)80207-1. [DOI] [PubMed] [Google Scholar]
- Araco A., Belli M., Giorgi C., Onori G. The secondary structure of E. coli ribosomes and ribosomal RNA's: a spectrophotometric approach. Nucleic Acids Res. 1975 Mar;2(3):373–381. doi: 10.1093/nar/2.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berden J. A., Cullis P. R., Hoult D. I., McLaughlin A. C., Radda G. K., Richards R. E. Frequency dependence of 31P NMR linewidths in sonicated phospholipid vesicles: effects of chemical shift anisotropy. FEBS Lett. 1974 Sep 15;46(1):55–58. doi: 10.1016/0014-5793(74)80333-9. [DOI] [PubMed] [Google Scholar]
- Cotter R. I., Gratzer W. B. An infrared study of the conformation of RNA and protein in the ribosome. Eur J Biochem. 1969 Apr;8(3):352–356. doi: 10.1111/j.1432-1033.1969.tb00535.x. [DOI] [PubMed] [Google Scholar]
- Cotter R. I., McPhie P., Gratzer W. B. Internal organization of the ribosome. Nature. 1967 Dec 2;216(5118):864–868. doi: 10.1038/216864a0. [DOI] [PubMed] [Google Scholar]
- Cox R. A. A possible method for characterizing the secondary structure of ribonucleic acids. Biochem J. 1966 Jul;100(1):146–168. doi: 10.1042/bj1000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzone P. J., Jardetzky O. Phosphorus-31 Fourier transform nuclear magnetic resonance study of mononucleotides and dinucleotides. 2. Coupling constants. Biochemistry. 1976 Nov 2;15(22):4860–4865. doi: 10.1021/bi00667a017. [DOI] [PubMed] [Google Scholar]
- Debey P., Hui Bon Hoa G., Douzou P., Godefroy-Colburn T., Graffe M., Grunberg-Manago M. Ribosomal subunit interaction as studied by light scattering. Evidence of different classes of ribosome preparations. Biochemistry. 1975 Apr 22;14(8):1553–1559. doi: 10.1021/bi00679a001. [DOI] [PubMed] [Google Scholar]
- Dohme F., Nierhaus K. H. Total reconstitution and assembly of 50 S subunits from Escherichia coli Ribosomes in vitro. J Mol Biol. 1976 Nov 15;107(4):585–599. doi: 10.1016/s0022-2836(76)80085-x. [DOI] [PubMed] [Google Scholar]
- Gordon J. Determination of an upper limit to the phosphorus content of polypeptide chain elongation factor and ribosomal proteins in Escherichia coli. Biochem Biophys Res Commun. 1971 Aug 6;44(3):579–586. doi: 10.1016/s0006-291x(71)80122-5. [DOI] [PubMed] [Google Scholar]
- Gorenstein D. G., Findlay J. B., Momii R. K., Luxon B. A., Kar D. Temperature dependence of the 31P chemical shifts of nucleic acids. A prode of phosphate ester torsional conformations. Biochemistry. 1976 Aug 24;15(17):3796–3803. doi: 10.1021/bi00662a023. [DOI] [PubMed] [Google Scholar]
- Guéron M., Shulman R. G. 31P magnetic resonance of tRNA. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3482–3485. doi: 10.1073/pnas.72.9.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon S., Glonek T., Chan A. Comparison of the phosphorus magnetic resonance and circular dichroism properties of calf thymus DNA and chromatin. Biochemistry. 1976 Aug 24;15(17):3869–3875. doi: 10.1021/bi00662a034. [DOI] [PubMed] [Google Scholar]
- Hayashi F., Akasaka K., Hatano H. 31P Magnetic relaxation studies of yeast transfer RNAPhe. Biopolymers. 1977 Mar;16(3):655–667. doi: 10.1002/bip.1977.360160314. [DOI] [PubMed] [Google Scholar]
- Hochkeppel H. K., Craven G. R. Significant changes in 16 S RNA conformation accompanying assembly of the 30 S ribosome in vitro. J Mol Biol. 1977 Jul 15;113(4):623–634. doi: 10.1016/0022-2836(77)90226-1. [DOI] [PubMed] [Google Scholar]
- Hull W. E., Sykes B. D. Fluorotyrosine alkaline phosphatase: internal mobility of individual tyrosines and the role of chemical shift anisotropy as a 19F nuclear spin relaxation mechanism in proteins. J Mol Biol. 1975 Oct 15;98(1):121–153. doi: 10.1016/s0022-2836(75)80105-7. [DOI] [PubMed] [Google Scholar]
- Kallenbach N. R., Appleby D. W., Bradley C. H. 31P magnetic resonance of DNA in nucleosome core particles of chromatin. Nature. 1978 Mar 9;272(5649):134–138. doi: 10.1038/272134a0. [DOI] [PubMed] [Google Scholar]
- Mackie G. A., Zimmermann R. A. Characterization of fragments of 16 S ribonucleic acid protected against pancreatic ribonuclease digestion by ribosomal protein S4. J Biol Chem. 1975 Jun 10;250(11):4100–4112. [PubMed] [Google Scholar]
- Patel D. J. Peptide antibiotic-dinucleotide interactions. Nuclear magnetic resonance investigations of complex formation between actinomycin D and d-pGpC in aqueous solution. Biochemistry. 1974 May 21;13(11):2388–2395. doi: 10.1021/bi00708a024. [DOI] [PubMed] [Google Scholar]
- RICH A., DAVIES D. R., CRICK F. H., WATSON J. D. The molecular structure of polyadenylic acid. J Mol Biol. 1961 Feb;3:71–86. doi: 10.1016/s0022-2836(61)80009-0. [DOI] [PubMed] [Google Scholar]
- Staehelin T., Maglott D. M., Monro R. E. On the catalytic center of peptidyl transfer: a part of the 50 S ribosome structure. Cold Spring Harb Symp Quant Biol. 1969;34:39–48. doi: 10.1101/sqb.1969.034.01.008. [DOI] [PubMed] [Google Scholar]
- Sypherd P. S., Wireman J. W. Preparation of ribosomal subunits by large-scale zonal centrifugation. Methods Enzymol. 1974;30:349–354. doi: 10.1016/0076-6879(74)30037-7. [DOI] [PubMed] [Google Scholar]


