Abstract
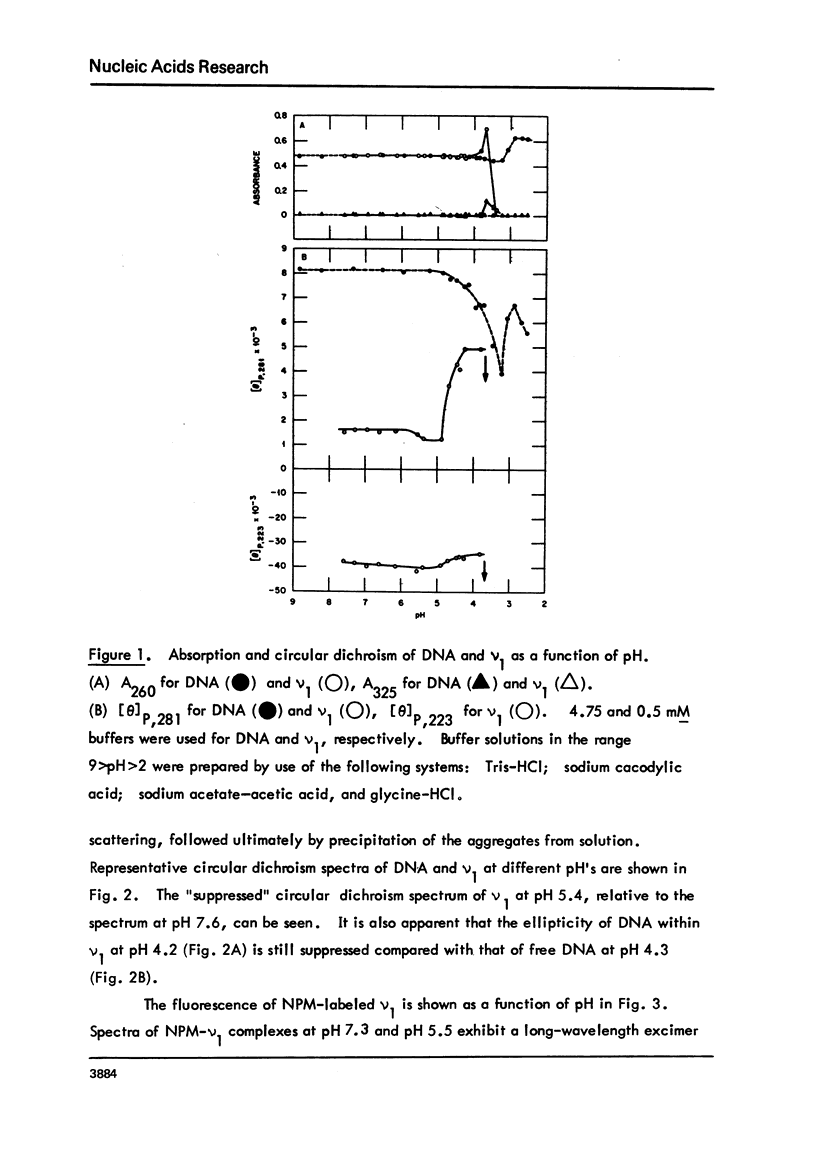
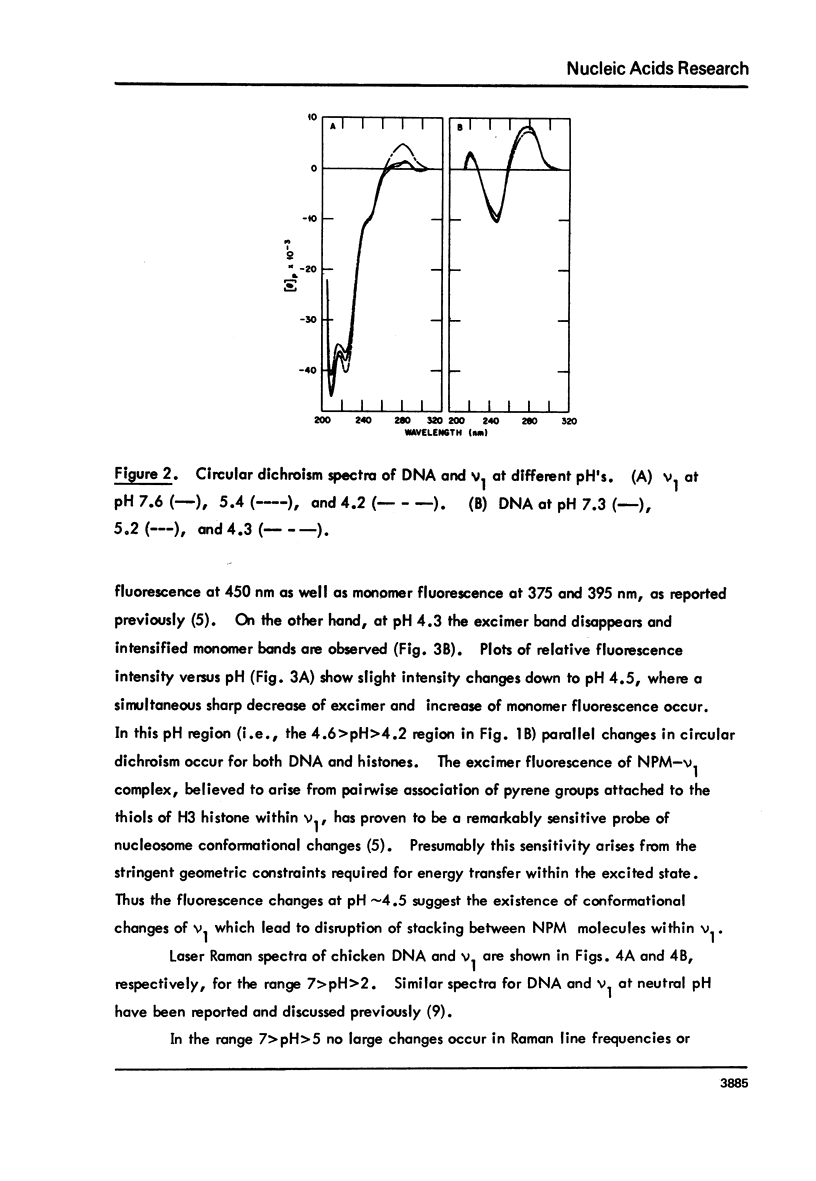
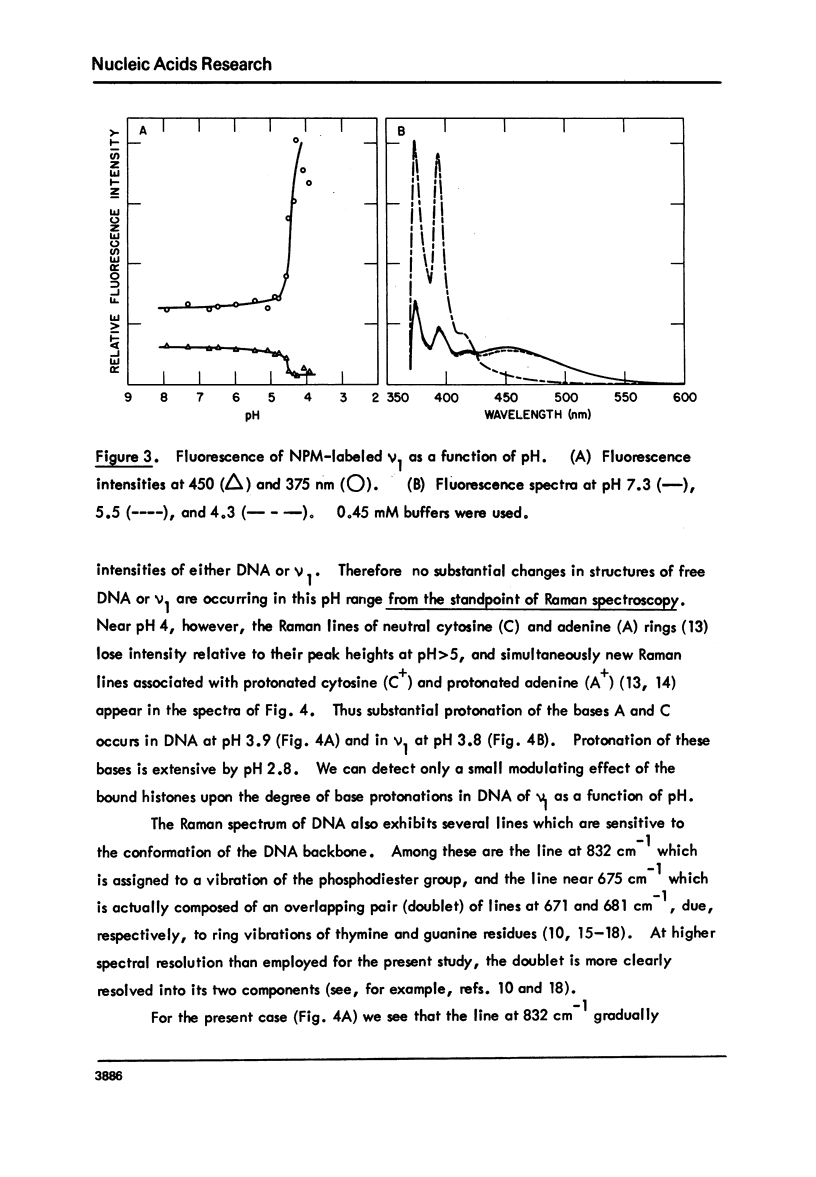
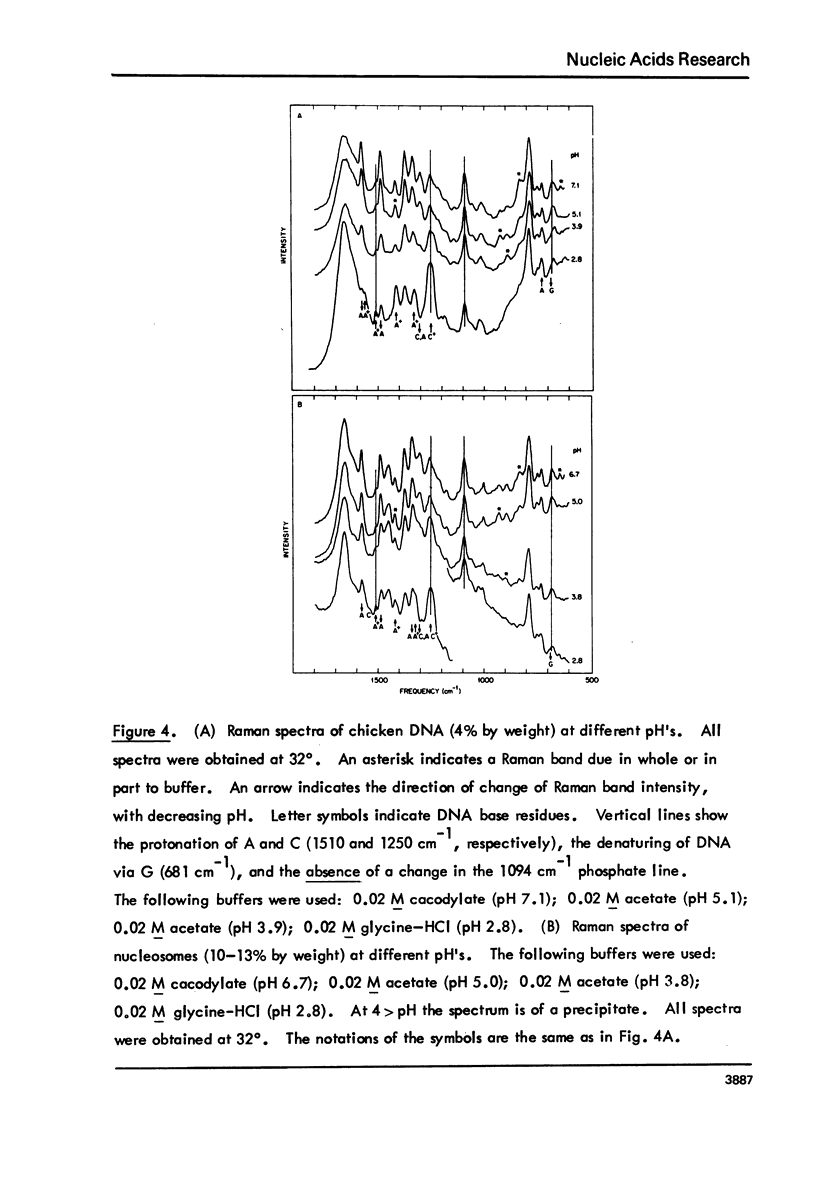
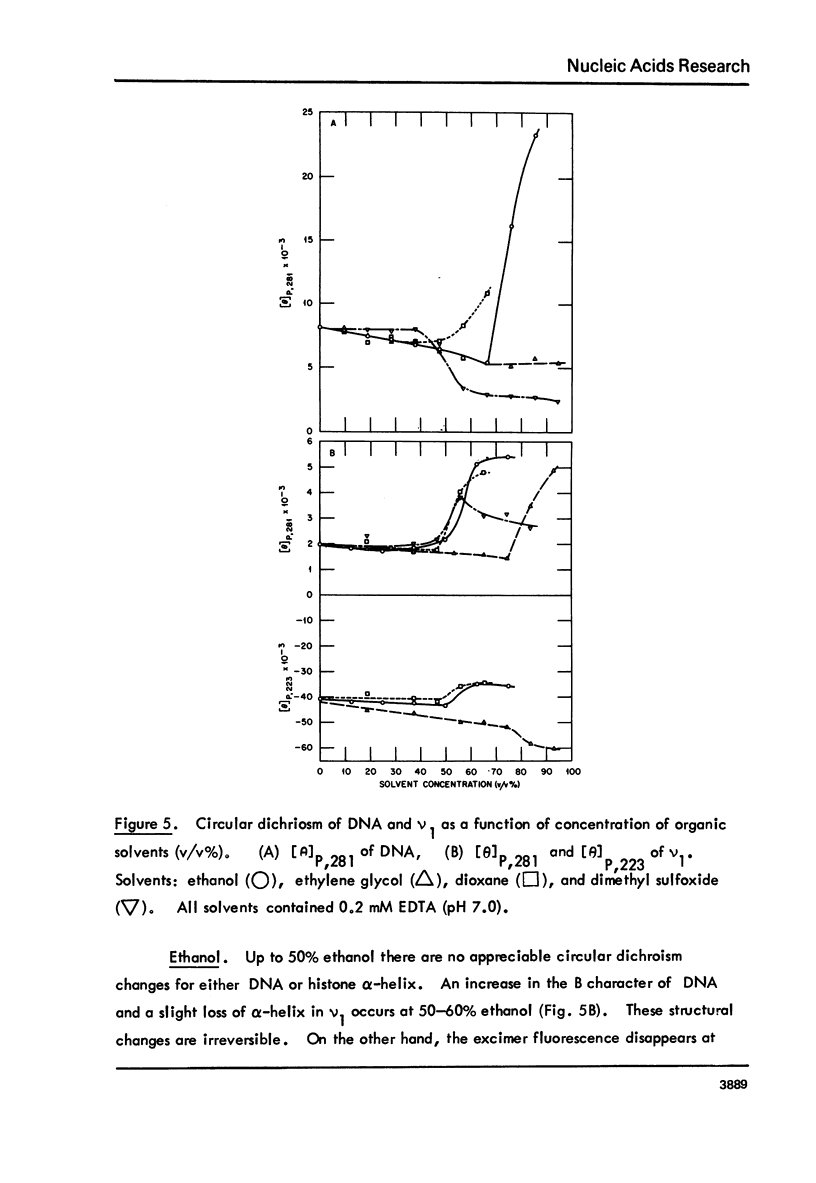
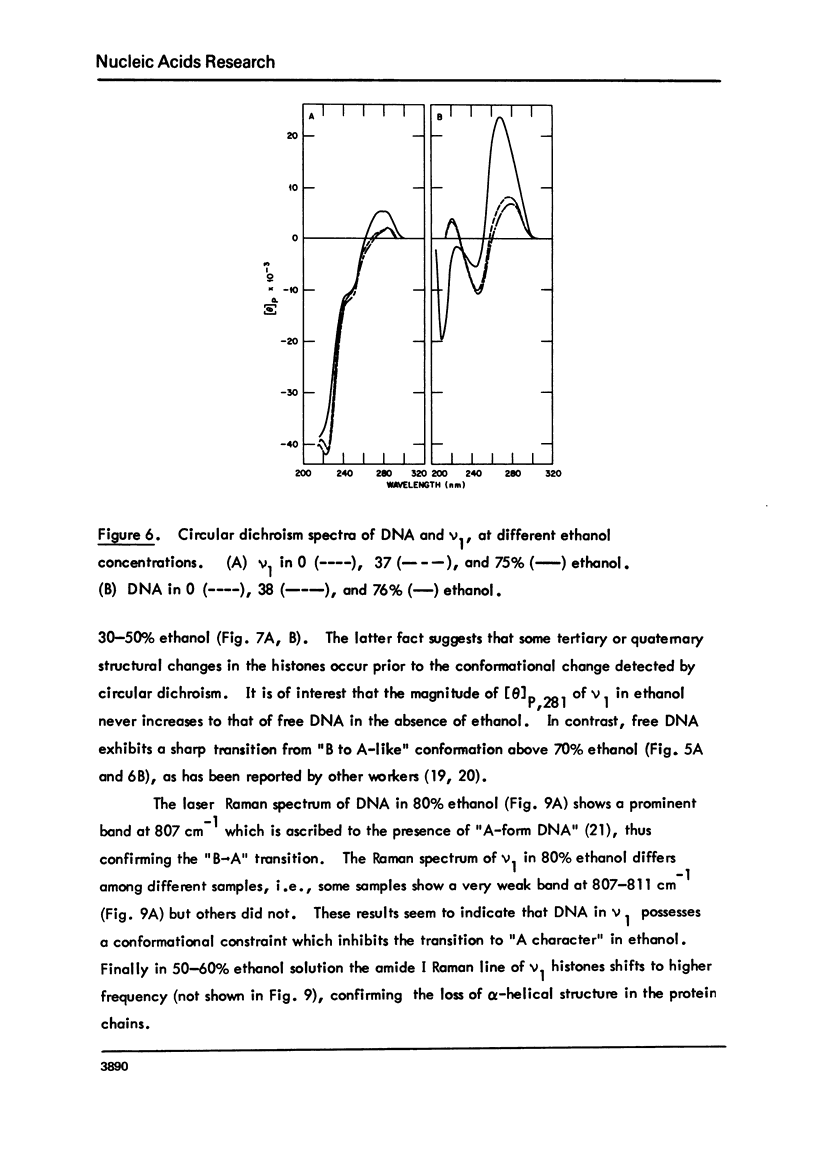
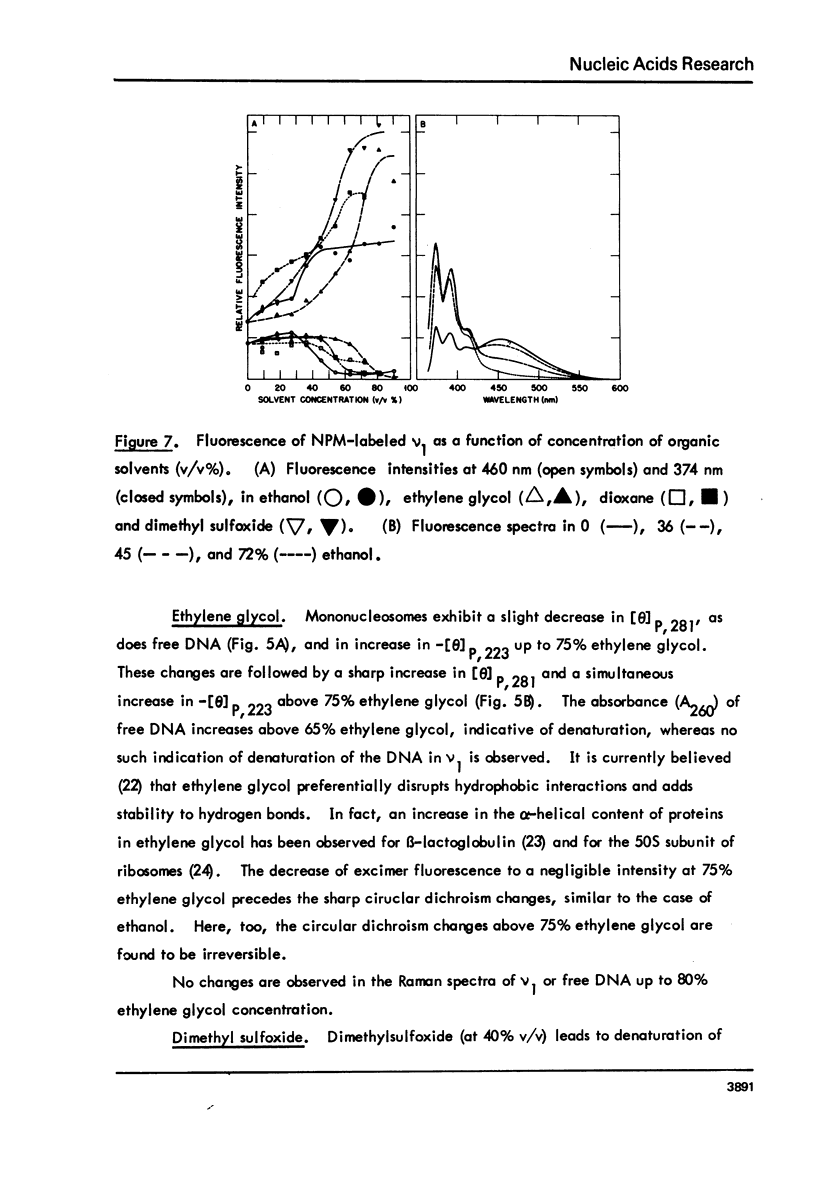
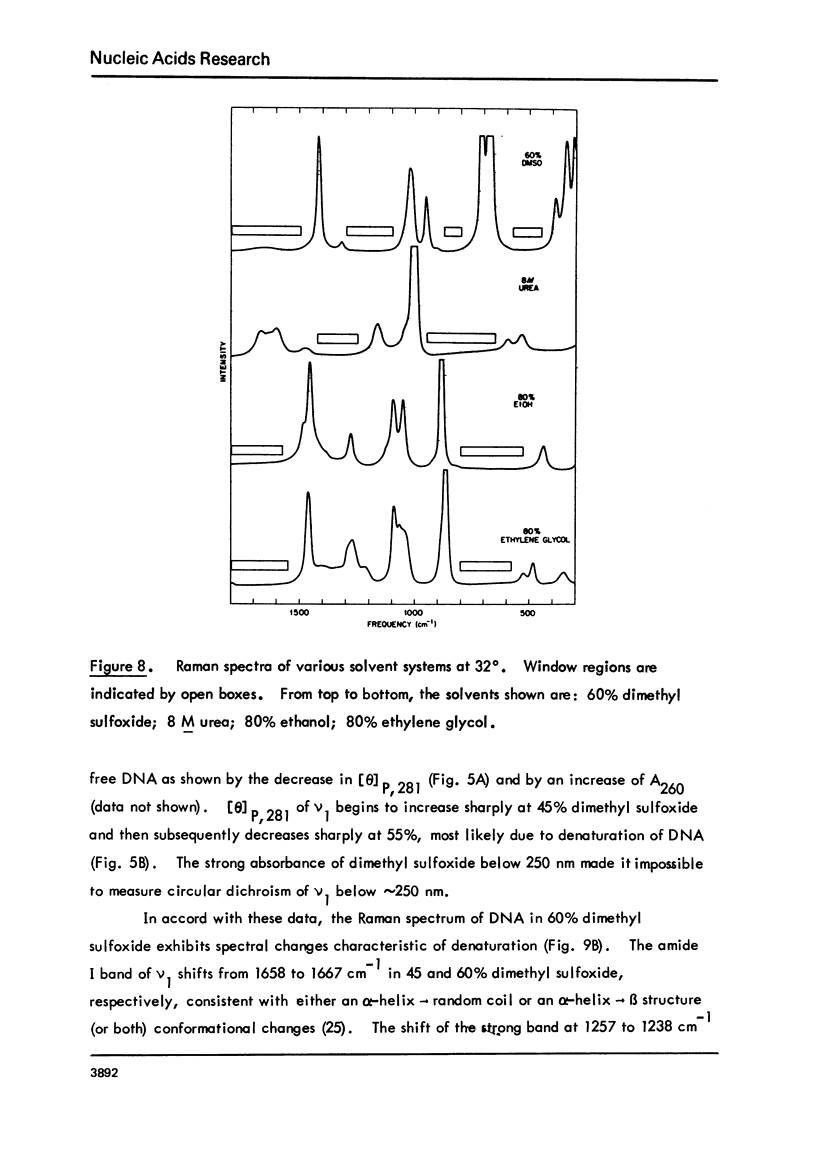
Monomer nucleosomes (nu 1) from chicken erythrocyte nuclei were examined in aqueous buffers (8 greater than pH greater than 3) and in solvent mixtures (i.e., water and ethanol, ethylene glycol, dioxane, dimethyl sulfoxide, 2-methyl-2,4-pentanediol, polyethylene glycol, sucrose, or urea). Circular dichroism, laser Raman spectroscopy of nu 1, and the fluorescence of nu 1 labeled with N-(3-pyrene) maleimide on thiol groups of H3 histone were employed to detect conformational transitions in nu 1. The results of pH studies were as follows: 5.5 greater than pH greater than 4.8, suppression of DNA ellipticity and no change of histone alpha-helix; 4.6 greater than pH greater than 4.2 an irreversible increase in the B character of DNA, a slight loss of histone alpha-helix, and a parallel loss of pyrene excimer fluorescence; 4 greater than pH, aggregation of nu 1 and protonation of the DNA bases C and A. Results obtained in the studies of nu 1 in solvent mixtures included the following: sharp conformational transitions that variously involved an increase in the B character of DNA, a slight loss of histone alpha-helix, and a loss of pyrene excimer. Different solvents required different concentrations to effect these conformational changes.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chou C. H., Thomas G. J., Jr Raman spectral studies of nucleic acids. XVI. Structures of polyribocytidylic acid in aqueous solution. Biopolymers. 1977 Apr;16(4):765–789. doi: 10.1002/bip.1977.360160406. [DOI] [PubMed] [Google Scholar]
- Erfurth S. C., Kiser E. J., Peticolas W. L. Determination of the backbone structure of nucleic acids and nucleic acid oligomers by laser Raman scattering. Proc Natl Acad Sci U S A. 1972 Apr;69(4):938–941. doi: 10.1073/pnas.69.4.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erfurth S. C., Peticolas W. L. Melting and premelting phenomenon in DNA by laser Raman scattering. Biopolymers. 1975 Feb;14(2):247–264. doi: 10.1002/bip.1975.360140202. [DOI] [PubMed] [Google Scholar]
- Felsenfeld G. Chromatin. Nature. 1978 Jan 12;271(5641):115–122. doi: 10.1038/271115a0. [DOI] [PubMed] [Google Scholar]
- Fox J. W., Owens D. P., Wong K. P. Structural changes of ribosome by the action of ethylene glycol. Biochemistry. 1978 Apr 18;17(8):1357–1364. doi: 10.1021/bi00601a001. [DOI] [PubMed] [Google Scholar]
- Garel A., Axel R. Selective digestion of transcriptionally active ovalbumin genes from oviduct nuclei. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3966–3970. doi: 10.1073/pnas.73.11.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon V. C., Knobler C. M., Olins D. E., Schumaker V. N. Conformational changes of the chromatin subunit. Proc Natl Acad Sci U S A. 1978 Feb;75(2):660–663. doi: 10.1073/pnas.75.2.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray D. M., Taylor T. N., Lang D. Dehydrated circular DNA: circular dichroism of molecules in ethanolic solutions. Biopolymers. 1978 Jan;17(1):145–157. doi: 10.1002/bip.1978.360170111. [DOI] [PubMed] [Google Scholar]
- Herbeck R., Yu T. J., Peticolas W. L. Effect of cross-linking on the secondary structure of DNA I. Cross-linking by photodimerization. Biochemistry. 1976 Jun 15;15(12):2656–2660. doi: 10.1021/bi00657a027. [DOI] [PubMed] [Google Scholar]
- Ivanov V. I., Minchenkova L. E., Schyolkina A. K., Poletayev A. I. Different conformations of double-stranded nucleic acid in solution as revealed by circular dichroism. Biopolymers. 1973;12(1):89–110. doi: 10.1002/bip.1973.360120109. [DOI] [PubMed] [Google Scholar]
- Mansy S., Engstrom S. K., Peticolas W. L. Laser Raman identification of an interaction site on DNA for arginine containing histones in chromatin. Biochem Biophys Res Commun. 1976 Feb 23;68(4):1242–1247. doi: 10.1016/0006-291x(76)90330-2. [DOI] [PubMed] [Google Scholar]
- Nozaki Y., Tanford C. The solubility of amino acids and related compounds in aqueous thylene glycol solutions. J Biol Chem. 1965 Sep;240(9):3568–3575. [PubMed] [Google Scholar]
- Olins A. L., Carlson R. D., Wright E. B., Olins D. E. Chromatin nu bodies: isolation, subfractionation and physical characterization. Nucleic Acids Res. 1976 Dec;3(12):3271–3291. doi: 10.1093/nar/3.12.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olins D. E., Bryan P. N., Harrington R. E., Hill W. E., Olins A. L. Conformational states of chromatin nu bodies induced by urea. Nucleic Acids Res. 1977 Jun;4(6):1911–1931. doi: 10.1093/nar/4.6.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TANFORD C., BUCKLEY C. E., 3rd, DE P. K., LIVELY E. P. Effect of ethylene glycol on the conformation of gama-globulin and beta-lactoglobulin. J Biol Chem. 1962 Apr;237:1168–1171. [PubMed] [Google Scholar]
- Thomas G. J., Jr, Prescott B., Olins D. E. Secondary structure of histones and DNA in chromatin. Science. 1977 Jul 22;197(4301):385–388. doi: 10.1126/science.560060. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Palter K., Van Lente F. Histones H2a, H2b, H3, and H4 form a tetrameric complex in solutions of high salt. Cell. 1975 Sep;6(1):85–110. doi: 10.1016/0092-8674(75)90077-x. [DOI] [PubMed] [Google Scholar]
- Yu N. T. Raman spectroscopy: a conformational probe in biochemistry. CRC Crit Rev Biochem. 1977;4(3):229–280. doi: 10.3109/10409237709102559. [DOI] [PubMed] [Google Scholar]
- Zimmer C., Luck G., Venner H., Fric J. Studies on the conformation of protonated DNA. Biopolymers. 1968 Apr;6(4):563–574. doi: 10.1002/bip.1968.360060410. [DOI] [PubMed] [Google Scholar]
- Zimmer C., Venner H. Protonation of cytosine in DNA. Biopolymers. 1966 Dec;4(10):1073–1079. doi: 10.1002/bip.1966.360041004. [DOI] [PubMed] [Google Scholar]


