Abstract
Vitamin D deficiency is associated with a variety of skeletal, cardiometabolic, and immunologic co-morbidities that are present in chronic kidney disease (CKD). We performed a systematic review to investigate the effects of vitamin D supplementation, in the form of ergocalciferol or cholecalciferol, on various health outcomes in early CKD. Seventeen clinical trials were identified, only two of which were randomized, placebo controlled trials. The majority of studies supplementing with > 2,000 IU/day of cholecalciferol achieved optimal vitamin D status, whereas studies supplementing with ergocalciferol were less consistent. Studies varied widely in their effects on lowering serum parathyroid hormone concentrations. Few studies investigated effects of vitamin D treatment on other clinical health indicators in early CKD. Rigorous studies are necessary to investigate optimal vitamin D dosing strategies in early CKD for the maintenance of adequate vitamin D status, management of secondary hyperparathyroidism and improvement of non-skeletal related clinical outcomes.
Keywords: vitamin D, cholecalciferol, ergocalciferol, renal disease, secondary hyperparathyroidism, chronic kidney disease
Introduction
With the increasing prevalence of obesity, hypertension, and type 2 diabetes, there has also been an increase in the prevalence of chronic kidney disease (CKD).1 Complications of CKD include progression to end stage renal disease and need for costly dialysis or renal transplantation, bone disease and premature cardiovascular disease (CVD) morbidity and mortality.2 Strategies to manage CKD are thus a high priority, not only from a clinical, but also a public health perspective.3 Vitamin D may play a key role in the disease management of CKD. Accumulating evidence from epidemiological and experimental research suggests that vitamin D is integral not only for its classical effects on the skeletal system, but also for its extra-skeletal benefits such as those on cardiovascular health and immune function.4,5 In this regard, a recent meta-analysis indicated that higher circulating 25-hydroxyvitamin D (25(OH)D or calcidiol) concentrations were associated with lower all-cause mortality risk in all stages of CKD.6
Altered vitamin D metabolism and resultant changes in bone and mineral metabolism are key features of CKD. The kidneys are the primary site of 25(OH)D conversion to the hormonal form of vitamin D, calcitriol (1,25(OH)2D), by the enzyme 1α-hydroxylase.7 As kidney disease progresses and renal mass decreases, there is a decline in the availability of 1α-hydroxylase enzyme resulting in a decrease in calcitriol, followed by compensatory increases in parathyroid hormone (secondary hyperparathyroidism) to offset impaired intestinal calcium absorption and resultant hypocalcemia.8,9 Other abnormalities of CKD, such as elevated fibroblast growth factor (FGF)-23,10,11 accumulation of parathyroid hormone (PTH) fragments (N-terminally truncated or C-terminal)12 and uremic toxins,13 and reductions in glomerular filtration rate (GFR) and megalin contribute to calcitriol defects in CKD.8 Because of defects in renal calcitriol production, studies in the past have primarily focused on the use of calcitriol replacement (or other active vitamin D analogs) to control hyperparathyroidism in CKD.2,14 However, there is evidence that patients with end stage renal disease (ESRD) retain some capacity to produce calcitriol from 25(OH)D, either through residual renal functioning or extra-skeletal production of calcitriol.15,16 Extra-renal cells, such as parathyroid cells, smooth muscle cells, endothelial cells, pancreatic cells and immunomodulatory cells, contain the machinery to locally produce calcitriol.17 This may explain the associations of adequate vitamin D status with lower chronic disease risk. Thus, the ability to maintain sufficient serum concentrations of 25(OH)D, the substrate for extra-renal 1α-hydroxylase and local production of active vitamin D, is particularly important in CKD.
Recent reports estimate up to 84% prevalence of vitamin D deficiency and insufficiency (defined in this review as 25(OH)D < 20 ng/ml and 25(OH)D < 30 ng/ml, respectively18) in CKD populations.19,20 Reasons for high prevalence of vitamin D deficiency in the CKD population compared with the general population are not clear. Elevated proteinuria and thus loss of urinary vitamin D binding protein (VDBP),20,21 reduced recycling of 25(OH)D by megalin in proximal tubular cells,22,23 and differences in diet, reduced sun exposure, and other lifestyle factors such as limited outdoor activity2,24 may provide explanations. Given the dysregulated vitamin D metabolism and the high prevalence of vitamin D deficiency in patients with CKD, the increasingly recognized pleiotropic benefits of the vitamin D system,17 and the elevated risk of premature morbidity and mortality associated with both CKD25,26 and vitamin D deficiency,27,28 optimizing vitamin D status through supplementation may be of particular relevance in CKD patients, specifically in pre-dialysis CKD wherein renal capabilities of 25(OH)D conversion to calcitriol have not been exhausted. To this end, the purpose of this review is to investigate the effects of vitamin D supplementation (cholecalciferol or ergocalciferol) on health outcomes and biomarkers related to mineral and bone metabolism, CKD progression, diabetes, and cardiovascular disease in pre-dialysis stages of chronic kidney disease (Stages 2 to 4).
Materials and Methods
We searched the terms “cholecalciferol OR ergocalciferol AND chronic kidney disease” in PUBMED through the present date (January 30, 2011). Limits were pre-set to manuscripts published in the English language. Titles and abstracts were reviewed. Review articles, cross-sectional studies, and non-human studies were excluded. Manuscripts were further excluded for review if (1) they did not use oral cholecalciferol or ergocalciferol; (2) they used analog compounds of vitamin D such as calcitriol, doxercalciferol or paricalcitol; (3) study participants received dialysis; or (4) studies involved renal transplant patients. Additional manuscripts were identified from the references of the selected manuscripts and selected recent reviews. Two independent reviewers (J.A., H.W.) reviewed the literature with these search criteria. When there was disagreement regarding inclusion of the manuscript for this systematic review, a third reviewer (V.T.) determined whether the manuscript was eligible.
Specific outcomes of interest included: (1) serum/plasma 25(OH)D, (2) serum/plasma PTH, (3) albuminuria/proteinuria, (4) glomerular filtration rate and progression across kidney stages and to dialysis, (5) measures and biomarkers of insulin resistance, glucose intolerance, and glycemic control, (6) blood pressure/hypertension, (7) measures and biomarkers of vascular function, (8) inflammation, oxidative stress, and measures of immunity, (9) mortality and (10) CVD events.
PubMed search results
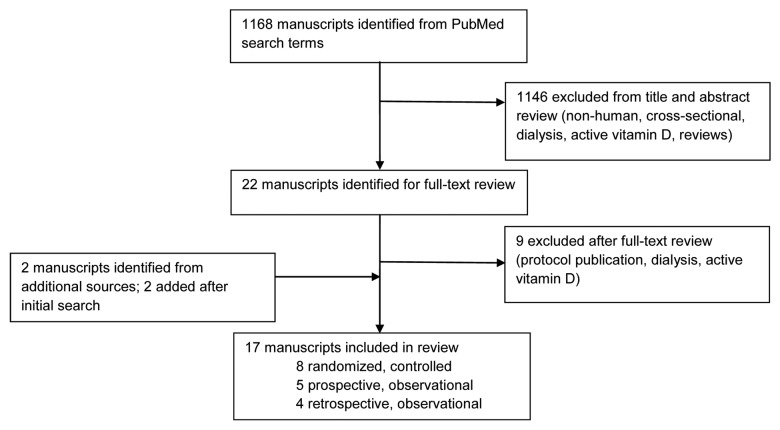
One thousand and sixty-eight manuscripts were identified from the specified search terms (Fig. 1). After applying additional exclusion criteria, 22 manuscripts were identified as potential eligible studies based on titles and abstracts. After review of full-text, 1 was excluded because it was a publication of a protocol, 3 were excluded because they were in dialysis or end stage renal disease patients and 5 were excluded because of use of active vitamin D or analog or calcidiol. Two studies were identified from additional sources and two were added following the initial search. A total of 17 studies were reviewed (Fig. 1).
Figure 1. Flow diagram of studies identified for review.
Results
Study design
Table 1 provides a summary of the reviewed studies. Of the 17 studies, two studies were double-blind, randomized placebo-controlled trials;29,30 6 were randomized controlled trials comparing cholecalciferol/ergocalciferol to active vitamin D analogs,31,32 no treatment,33-35 or different dose of cholecalciferol;36 4 were prospective, observation trials of cholecalciferol37 or ergocalciferol38-40 and 4 were retrospective, observational studies of ergocalciferol.41-44 One study45 appears to be a secondary analysis of a parent study,36 thus duplicate analyses are only reported for the parent study.
Table 1. Summary of cholecalciferol or ergocalciferol trials in early CKD.
| Author, yr |
Design, Intervention |
Duration/ Follow-up |
Participants |
CKD stage |
Baseline, Post 25(OH)D (ng/ml)e |
Baseline, Post PTH (pg/ml)e |
Other Outcomes |
|---|---|---|---|---|---|---|---|
| Trials with daily cholecalciferol | |||||||
| Rucker 2009 |
Prospective, randomized, controlled 1,000 IU/day vs. no treatment |
12 wks |
128 adults, age 69.0 ± 12.6 y |
3–5 |
16 ± 5.6, 26.8 ± 10.4* |
144.4 ± 81.7, 130 ± 72.2 |
↓GFR ↔ Serum Ca, P, albumin |
| Moe 2010 | Prospective, single-blind, randomized, active-controlled 4,000 IU/day × 1 mo then 2,000 IU/d vs Doxercalciferol (1 μg/d) |
12 wks | 47 adults, age 63.6 ± 10.2 y |
3/4 | 14.0 ± 6.1, 37.2 ± 10.1* |
109 ± 43, 97 ± 49 |
↔ Serum Ca, Serum P, urine ca/cr, urine alb/cr, blood pressure |
| Trials with bolus cholecalciferol | |||||||
|---|---|---|---|---|---|---|---|
| Hari 2010 |
Prospective, observational 600,000 IU total over 3 d (14,286 IU/day) |
6 weeks |
42 children, age 7 (2–15)a yrs |
2–4 |
17.9 (12.8–20.9)d, 48.2 (44–56.1)* |
55.3 (47.1–77.8), 41.4 (31.2–56.8)* |
↔ Serum Ca, P, alk phos, eGFR |
| Dogan 2008 | Prospective, randomized, controlled single dose 300,000 IU (10,714 IU/day) vs. no treatment |
4 wks | 40 adults, age 49 ± 14 y | 3/4 | 8.5 ± 3.6, 17.8 ± 21.4* |
368 ± 274, 279 ± 179* |
↔ Serum Ca, P, BUN, creatinine albumin |
| Trials with weekly or monthly cholecalciferol | |||||||
|---|---|---|---|---|---|---|---|
| Okša 2008 |
Prospective, randomized, open-label A: 5,000 IU/wk (714 IU/day) vs. B: 20,000 IU/wk (2857 IU/day) |
12 mo |
87 adults, age 66 (19–88) yra |
2–4 |
A: 15 (5–60)a, 28 (14–72)* B:16 (4–49), 37 (8–81)*b |
A: 63 (13–224), 48 (11–181)* B: 50 (10–184)b, 40 (11–203)* |
↑1,25(OH)2D; ↑Serum Cr (both groups), ↑Urine Ca at month 8, ↓GFR (group B) ↔ Serum Ca, Serum P, Urine P |
| Lajdova 2009 |
Prospective, observational 5,000 IU/wk (714 IU/day) |
12 mo |
44 adults, age 62 (19–77) yra |
2/3 |
Duplicate analysis of parent study |
Duplicate analysis of parent study |
↓ [Ca2+]i |
| Chandra 2008 |
Prospective, double-blind, randomized, placebo-controlled 50,000 IU/wk (7,143 IU/day) vs. placebo |
12 wks |
20 adults, age 60.9 ± 10.7 y |
3/4 |
17.3 (11.8–25.2)c, 49.4 (33.9–72.0)* |
288.9 (178.7–467.2), 200.5 (114.0–352.8) |
↔ Serum Ca, 1,25(OH)2D, BAP, CTX, TRAP5b |
| Taner 2011 | Prospective, randomized, controlled 300,000 IU/month (10,714 IU/day) vs. no treatment |
12 wks | 48 adults, age 57.5 ± 10.2 y |
3/4 | 13.4 ± 5.9, 82.9 ± 30.9* |
134.3 ± 92.5, 93.8 ± 67.7* |
↑serum Ca, P ↔ Serum albumin, eGFR |
| Trials with daily ergocalciferol | |||||||
|---|---|---|---|---|---|---|---|
| Deville 2006 |
Prospective, observational 800 IU/day to 100,000/wk (14,286 IU/day) |
90 d |
85 adults, age 67.2 ± 11.8 y |
3–5 |
17.0 ± 8.0, 42.1* |
176.2 ± 112.2, 148.9* |
———- |
| Menon 2008 |
Retrospective chart review 2,000–4,000 IU daily (KDOQI) |
12 wks |
22 children, age 10.7 ± 5.4 y |
2–4 |
Not reported |
122.1 ± 82.9, 80.1 ± 59.2* |
——– |
| Shroff 2012 | Prospective, double-blind, randomized, placebo-controlled 2,000–8,000 IU daily (KDOQI) vs. placebo |
6 mo-2 yrs | 40 children, age 9.3 ± 3.5 | 2–4 | 20.0 ± 7.8, 33.3* |
-8% change in PTH for vit D group (p = 0.30) vs +22.4% change in placebo (p = 0.046) | ↔ eGFR ↑1,25(OH)2D |
| Trials with weekly or monthly ergocalciferol | |||||||
|---|---|---|---|---|---|---|---|
| Trakarnvanich 2010 |
Prospective, observational 40,000 IU weekly or monthly (1,429–5,714 IU/day) (modified KDOQI) |
8 wks |
37 adults, age 63.6 ± 12.4 y |
3–5 |
22 ± 4.8, 34.5 ± 10.8* |
84.9 (18.3–379.9)a, 74.5 (18.3–751.6) |
↔ Serum Ca, PO4, albumin |
| Kovesdy 2011 |
Prospective, randomized, open-label, active-controlled 50,000 IU weekly or monthly (1,786–7,143 IU/day) (modified KDOQI) vs. Paracalcitol 1 µg daily |
16 wks |
76 adults, age 68.5 ± 10.0 y |
3/4 |
17.2 ± 5.9, 29 ± 9* |
175 ± 88, 166 ± 85 |
↔ Serum Ca, Serum P, urine ca/cr, BAP, total cholesterol, LDL, HDL, homocysteine, BNP, CRP, PWV, body fat |
| Zisman 2007 |
Prospective, observational Ergocalciferol, 50,000 IU weekly (1,786 IU/day) × 4 wks then 1,200–2,000 IU/d (modified KDOQI) |
7.1 ± 2.9 mo |
50 adults, age 72.7 ± 13.1 y |
3 4 |
20.3 ± 6.4, 31.6 ± 10.8* 18.8 ± 6.9, 35.4 ± 10.1* |
154.1 ± 90.6, 130.5 ± 93.6* 164.8 ± 155.0, 139.7 ± 98.4 |
↑1,25(OH)2D ↔ Serum Ca, P, GFR |
| Al-Aly 2007 |
Retrospective, observational Ergocalciferol 50,000 IU/wk for 12 wks then monthly for 12 wks (1,786–7,143 IU/day) (modified KDOQI) |
6 mo |
66 adults, age 70.4 ± 10.6 y |
3 4 |
17.5 ± 5.3, 27.6 ± 11.3* 14.7 ± 6.1, 26.4 ± 21.1* |
174 ± 139, 136 ± 80* 345 ± 272, 306 ± 305 |
↔ Serum Ca, Serum P |
| Qunibi 2010 |
Retrospective, observational Ergocalciferol, 50,000 IU/wk for 4–12 wks then monthly for 12 wks (1,786–7,143 IU/day) (KDOQI) |
6 mo |
88 adults, age 56.8 ± 9.5 y |
1–5 |
15.1 ± 5.8, 23.2 ± 11.8* |
157.9 ± 125.9, 150.7 ± 127.5 |
↑ Serum P ↔ Serum Ca |
| Lishmanov 2011 | Retrospective chart review Ergocalciferol, 50,000 IU weekly or monthly (1,786–7,143 IU/day) (modified KDOQI) |
6 mo | 126 men, age 71 ± 10 y |
3–4 | 17.9 ± 6.7, 34.3 ± 9.5* |
Not reported | ↓CVD events, ↑CVD survival, ↑overall survival |
p < 0.05 for change from baseline, ↑, increase; ↔, no change; amedian (range); bp < 0.05 for difference from other group; cmean (95% CI); dmedian (95% CI); emean ± SD (unless otherwise noted) in those receiving cholecalciferol/ergocalciferol only. Abbreviations: 25D, 25-hydoxyvitamin D [25(OH)D]; BAP, bone-specific alkaline phosphatase; CTX, C-telopeptide; TRAP5b, tartarate-resistant acid phosphates isoform 5b; Ca, calcium; P, phosphorus.
All but three studies30,37,43 were in adults. The dosing strategies widely varied. Eight studies based their vitamin D dosing strategies on the 2003 Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines.2 KDOQI recommends initiating ergocalciferol supplementation if PTH concentrations are above the target range (> 70 pg/ml in Stage 3 CKD or > 110 pg/ml in Stage 4 CKD) at a dose dependant on 25(OH)D concentrations [25(OH)D < 15 ng/ml: 50,000 IU weekly for 1 mo followed by 50,000 IU monthly for 4 mo; 25(OH)D 20–30 ng/ml: 50,000 IU monthly for 6 mo].
Only four studies reported sample size and power calculations,29-32 one of which was powered to detect treatment differences in 25(OH)D,29 two were powered to detect decreases in PTH,31,32 and the other to detect a risk reduction in secondary hyperparathyroidism.30
Vitamin D therapy on serum/plasma 25(OH)D
All studies reported significant improvements in 25(OH)D concentrations, although several studies did not reach a mean 25(OH)D concentration into the optimal range (≥ 30 ng/ml) after treatment, including a 12-wk study of cholecalciferol at 1,000 IU/day,34 a single-dose cholecalciferol study of 300,000 IU followed up to 4 weeks (~10,714 IU/day),33 two 5,000 IU/wk (~714 IU/day) cholecalciferol studies for 1 y36,45 and three studies based on the 2003 KDOQI guidelines2 for 16–24 wks (~1786–7143 IU/day ergocalciferol).31,42,44 Baseline serum 25(OH)D ranged from 13.4 to 22.0 ng/ml in those that achieved mean optimal concentrations29,32,35-41 vs 8.5 to 17.2 ng/ml in those that did not achieve mean optimal serum 25(OH)D concentrations.31,33,34,36,42,44,45 Lishmanov et al.41 retrospectively considered their treatment group as patients with an increase in serum 25(OH)D of at least 25%, thus group allocation was decided regardless of actual vitamin D supplementation. Shroff et al.30 reported normalization of serum 25(OH)D concentrations in children with Stage 2 CKD but not Stage 3 or 4 CKD.
Only half of the studies which followed protocols based on KDOQI guidelines achieved a mean serum 25(OH)D concentration in the optimal range,30,39-41 although the subjects in these studies generally had higher mean baseline serum 25(OH)D concentrations than the other studies. Qunibi et al.44 published the only study to use the exact adult KDOQI protocol; mean serum 25(OH)D concentrations in the optimal range were not achieved.
Vitamin D therapy on serum/plasma PTH
PTH significantly decreased in eight studies,33,35-38,40,42,43 with a variety of dosing protocols including both ergocalciferol and cholecalciferol. Okša et al.36 reported similar decreases in PTH using either 5,000 IU or 20,000 IU cholecalciferol per week (~714 or 2857 IU/day). Hari et al.37 reported greater median reductions in PTH in Stage 2 (38.5%) compared with Stages 3 (25.2%) and 4 (15.2%). Both Al-Aly42 and Zisman et al.40 reported a significant decrease in PTH in Stage 3 (21.8 and 13.1% decrease) but not Stage 4 (11.3 and 2% decrease) CKD patients. Deville et al.38 reported a significant decrease in PTH in stage 4 (17.9%) but not Stage 3 (12.7%) or five patients (7.0%), although the differences were attributed to sample size. Shroff et al.30 reported a significant effect of ergocalciferol treatment in preventing hyperparathyroidism compared with placebo in children.
Two studies compared the effectiveness of ergocalciferol vs an active vitamin D analog in reducing PTH.31,32 Moe et al.32 reported a within-group trend toward a decrease in PTH using ergocalciferol (p = 0.15) and a significant decrease in PTH using doxercalciferol (p = 0.002), yet no significant between-group difference (p = 0.11). Kovesdy et al.31 reported a significant decrease in PTH in patients treated with paracalcitriol (p < 0.001) but not ergocalciferol (p = 0.60), and a significant between group difference (p = 0.009).
Vitamin D therapy on other markers related to bone metabolism
Taner et al.35 reported an increase in serum calcium and phosphorus after 300,000 IU cholecalciferol per month for 12 wks, although the change was not significantly different than untreated controls. Qunibi et al.44 reported a statistically significant increase in serum phosphorus with no change in serum calcium in a retrospective study following KDOQI guidelines. Other studies reported no change in serum phosphorus or calcium.
Chandra et al.29 reported no changes in bone-specific alkaline phosphatase (BAP), C-telopeptide, or TRAP5b using 50,000 IU cholecalciferol per wk for 12 wks. Kovesdy et al.31 also did not find a significant change in BAP after ergocalciferol treatment for 16 wks, although there was a significant reduction in BAP using paricalcitol.
Vitamin D therapy on other markers related to CKD progression
Rucker et al.34 reported a decrease in estimated GFR (eGFR; 21 ± 8 to 20.5 ± 7.8 ml/min per 1.73m2, p = 0.01) after cholecalciferol supplementation compared with controls; serum creatinine concentrations were not provided. Okša et al.36 reported a decrease in eGFR in patients receiving 20,000 IU cholecalciferol/wk [mean eGFR (range): 51(15–89) to 50 (15–82) ml/min/1.73m2, p < 0.01) but not with 5,000 IU/wk after 1 y [mean eGFR (range): 43 (15–89) to 42 (7–80), p > 0.05]; serum creatinine increased in both groups. Four studies reported no change in eGFR.30,35,37,40
Moe et al.32 reported a non-significant decrease in urine albumin/creatinine ratio over 12 wks that did not differ between treatment with cholecalciferol or doxercalciferol (pooled mean baseline and follow-up urine albumin/creatinine ratio: 853 ± 1153 to 471 ± 770 μg/mg). No studies reported progression to dialysis as an outcome.
Vitamin D therapy on CVD events and mortality
Lishmanov et al.41 reported fewer CVD events leading up to a mean follow-up of 27.2 mo in patients whose serum 25(OH)D concentrations increased by at least 25% [from baseline 25(OH)D < 30 ng/ml] after 6 mo of ergocalciferol compared with those who were not treated or did not respond to treatment [OR (95% CI): 0.37 (0.14–1.0); p = 0.05], after adjusting for age, baseline PTH, statin use, CVD history, diabetes status, and eGFR]. All-cause survival and CVD-specific survival were higher in the treated group (p = 0.008 and 0.02, respectively). It should be noted that the treatment group had a lower history of diabetes compared with controls (53% vs. 75%, p = 0.02).
Vitamin D therapy on measures and biomarkers of cardiovascular risk
Only two studies reported outcomes specifically related to CVD risk,31,32 although they were not primary outcomes of the studies. Moe et al.32 reported a non-significant decrease in home-measured systolic and diastolic blood pressure (average of 1 week measurements; p = 0.17 and 0.11, respectively), yet no change in clinic-measured blood pressure (p > 0.40) after 2,000–4,000 IU cholecalciferol per day for 12 wks. Kovesdy et al.31 reported no change in cholesterol, B-type natriuretic peptide, pulse wave velocity, homocysteine, or body fat after ergocalciferol treatment, although there was a trend toward an increase in HDL cholesterol (p = 0.05).
Lajdova et al.45 reported a decrease in peripheral blood mononuclear cell intracellular calcium concentration ([Ca2+]i) after 5,000 IU/wk cholecalciferol treatment for 1 y, although [Ca2+]i was not the primary endpoint of the study. No studies reported outcomes related to measures and biomarkers of insulin resistance, glucose intolerance, or glycemic control.
Vitamin D therapy on inflammation, oxidative stress and measures of immunity
Kovesdy et al.31 measured C-reactive protein as a marker of inflammation, but did not find a significant change after ergocalciferol treatment. No studies have reported outcomes related to oxidative stress or immunity.
Adverse events
Hypercalcemia and hyperphosphatemia were reported in only one study,40 among stage 4 CKD patients receiving ergocalciferol based on KDOQI guidelines.2 This was an observational trial, and incidences were resolved spontaneously.
Discussion
Several health outcomes and co-morbidities that present in the early stages of CKD, including those related to bone and mineral disturbances, as well as cardiometabolic risk factors, have been linked to vitamin D deficiency,46 which is highly prevalent in patients with CKD.19 Moreover, vitamin D deficiency has been shown to be a predictor of CKD progression and death in this population in clinical and epidemiologic studies.6,27,47-49 We conducted a systematic review of the published literature to investigate the effects of vitamin D supplementation (in the form of cholecalciferol or ergocalciferol) in patients with pre-dialysis stage CKD. The studies reviewed varied widely in the form of vitamin D, dosage and protocol. The reported outcomes primarily reflected bone and mineral outcomes, such as changes in 25(OH)D and PTH. There was limited data available with respect to extra-skeletal outcomes.
The National Kidney Foundation recommends an optimal serum/plasma 25(OH)D of greater than or equal to 30 ng/mL to define optimal vitamin D status in patients with CKD.2 Although all studies reviewed noted a significant improvement in 25(OH)D concentrations, a mean optimal vitamin D concentration was not achieved by all and may be due to several reasons including an insufficient dose of vitamin D and/or use of a less effective form of vitamin D (ergocalciferol, as opposed to cholecalciferol). In general the studies supplementing with the equivalent of 700 to 1,000 IU vitamin D per day34,36 did not achieve mean optimal vitamin D status. In a dosage comparison study, Okša et al.36 found that 20,000 IU cholecalciferol/wk (~2,857 IU/day), but not 5,000 IU/wk (~714 IU/day) was sufficient to achieve optimal status in CKD patients. Collectively, studies in this review suggest daily doses of vitamin D > 2,000 IU/day (or weekly/monthly equivalents) are required to achieve optimal vitamin D status.
The KDOQI guidelines recommend the use of ergocalciferol with a specific dosing strategy to treat vitamin D deficiency/insufficiency2; however, several studies in healthy participants have shown cholecalciferol to be more effective in raising and maintaining 25(OH)D concentrations than ergocalciferol.50-52 No study has compared the effectiveness of cholecalciferol vs. ergocalciferol in a head-to-head trial in raising 25(OH)D concentrations in patients with CKD. However, a comparison of the placebo-controlled cholecalciferol trial in CKD by Chandra et al.29 to ergocalciferol trials with similar baseline serum 25(OH)D values and dosing strategies31,42,44 supports the hypothesis that cholecalciferol is more effective than ergocalciferol in CKD. Taken together, these data suggest KDOQI guidelines may need to be updated to recommend the use of cholecalciferol as opposed to ergocalciferol. Further study is needed to establish effective vitamin D dosing protocols specific to patients with CKD to achieve and maintain optimal serum 25(OH)D concentrations.
Alleviation of secondary hyperparathyroidism is a major target for CKD management2 and the most commonly investigated outcome for vitamin D supplementation in early CKD, as indicated by this review. Vitamin D is a known regulator of PTH secretion2; however the effective amount and form needed to manage or prevent secondary hyperparathyroidism in early CKD are not known. The 2003 KDOQI guidelines made opinion-based recommendations to assess serum 25(OH)D concentrations and treat vitamin D insufficiency with ergocalciferol in patients with CKD Stages 3 and 4 only if PTH concentrations are elevated.2 Several studies identified in this review used modified vitamin D dosing strategies modeled after KDOQI and found varying effects on PTH. The sole study published following the exact 2003 KDOQI protocol did not find a PTH-lowering effect of ergocalciferol treatment.44 Kandula et al.53 performed a meta-analysis on the PTH lowering effects of any form of vitamin D (including calcitriol) or vitamin D analog and reported a significant decrease in PTH in early CKD patients. A 30% decrease in PTH has been cited as being clinically effective.42 In the only randomized, double-blind, placebo-controlled trial of vitamin D adults with early CKD, serum PTH decreased ~30% after 12 weeks of cholecalciferol treatment, although the sample size may have not been large enough to detect a statistically significant change in PTH.29 Given the wide variability in the design of published vitamin D supplementation trials and the corresponding variability in PTH lowering effects, it is clear that further randomized, placebo-controlled trials are necessary to establish an optimal treatment strategy with vitamin D to manage secondary hyperparathyroidism.
CKD severity plays a role in the effectiveness of vitamin D in reducing serum PTH. Two studies reported a reduction in serum PTH in patients with Stage 3 but not Stage 4 CKD.40,42 These data suggest that vitamin D is most beneficial in lowering serum PTH when 1α-hydroxylase is more active.8,9 In this regard, it may be prudent to test and correct 25(OH)D concentrations prior to elevations in serum PTH among early stage CKD patients, despite current KDOQI guidelines.2 Indeed, ergocalciferol treatment in children with CKD stage 2–4 delayed the onset of secondary hyperparathyroidism compared with placebo,30 suggesting that optimization of vitamin D status may be especially important during early CKD. This remains to be examined in adult CKD patients.
Only two studies have investigated the use of active vitamin D analogs vs. supplemental vitamin D in CKD. One clinical trial showed paricalcitol more effectively suppressed PTH.31 The other suggested doxercalciferol may be better at reducing PTH but did not significantly differ from cholecalciferol.32 It is hypothesized that supplemental vitamin D may be a better choice given its lower cost, fewer side effects and potential extra-renal benefits that require adequate serum 25(OH)D as a substrate for conversion to 1,25(OH)2D to act as a paracrine/autocrine hormone in local tissue.17 Studies have not investigated differences between vitamin D forms on other outcomes in this regard. Vitamin D analogs have been hypothesized to interfere with local paracrine/autocrine VDR activation and effects by inhibiting local 1-α hydroxylase or promoting 24-hydroxylase expression thereby suppressing local calcitriol production.8,54 Furthermore, adequate serum 25(OH)D is able to suppress PTH synthesis and secretion in parathyroid cells.55 These reasons underscore the importance of maintaining adequate serum 25(OH)D levels in CKD.
The presence of proteinuria in CKD is an independent predictor for disease progression to ESRD and mortality.56,57 Cross-sectional studies indicate inverse relationships between circulating 25(OH)D and albuminuria in patients with CKD.58,59 Experimental evidence suggests that vitamin D may protect against or reduce proteinuria via its inhibitory effects on renin gene transcription and subsequent angiotensin II production;60 via inhibition of renal TNFα converting enzyme expression;8,61,62 via direct upregulation of nephrin;60,63 and/or via upregulation of renal megalin expression.22,64 Paricalcitol has been shown to significantly decrease proteinuria in clinical trials of patients with CKD,65-68 and may have anabolic effects, as indicated by increases in serum creatinine and blood urea nitrogen.65,69 eGFR is highly influenced by serum creatinine which may explain the reported decreases in eGFR identified in the review after cholecalciferol supplementation.34,36 Only one clinical trial of vitamin D supplementation identified by this review reported albuminuria as an outcome, albeit secondary, and did not observe a significant reduction in albuminuria.32 Given the possibility that proteinuria may promote vitamin D deficiency through urinary loss of VDBP,21 future clinical trials aimed at raising serum 25(OH)D concentrations with cholecalciferol and using proteinuria as a primary outcome are warranted. As accelerated protein energy wasting occurs with progressive CKD,70 the implications for a vitamin D protective effect on muscle integrity in this population should also be further explored.
Low circulating 25(OH)D concentrations predict increased all-cause mortality risk in pre-dialysis CKD patients in large cohort studies.6,27,47,49 Mortality was reduced by 26% in early CKD patients who received calcitriol, and this was independent of PTH and other risk factors.71 The mechanisms mediating a reduced mortality risk with higher vitamin D status are yet unknown; however vitamin D may play a role in infection-associated morbidity and mortality, which is significantly greater among patients with CKD.72 Calcitriol promotes upregulation of antimicrobial defenses by the immune system,5,73-75 and a higher rate of infection-associated mortality has been linked to severe vitamin D deficiency in ESRD.76 No studies have investigated the relationship between vitamin D status and infection in early chronic kidney disease.
The cardioprotective effects of vitamin D may also mediate the associations between better vitamin D status and reduced mortality in CKD. Vitamin D deficiency in ESRD patients is associated with an increased risk of CVD events.77 In contrast, a meta-analysis did not find a benefit of vitamin D analogs on cardiovascular outcomes.78 We identified only one study to-date examining the effect of vitamin D supplementation on CVD events in CKD. In a retrospective data review, Lishmanov et al.41 reported a reduced incidence of CVD events (albeit not statistically significant), as well as lower all-cause and CVD-related mortality, in early CKD patients successfully treated with ergocalciferol. Findings were independent of changes in PTH, suggesting other mechanisms mediated the reduced cardiovascular risk. Long-term, prospective, randomized-controlled trials of vitamin D supplementation are required to confirm effects in patients with early CKD.
Adequate vitamin D status may be important for regulation of several predictors of CVD risk that are relevant in patients with CKD, such as hypertension and left ventricular hypertrophy,79-81 insulin resistance/glucose intolerance,82 systemic inflammation83,84 and oxidative stress.85 A reduction in these CVD risk indicators with cholecalciferol and/or active vitamin D analog treatment has been shown in ESRD patients.79,85-93 Only two trials, as indicated in this review, have investigated effects of vitamin D supplementation on such measures of CVD risk with suggestive, but not significant, results, and not as primary outcomes.31,32 No study has specifically investigated if vitamin D supplementation improves glucose homeostasis in early CKD. Given the scarcity of data available, conclusions cannot be made regarding specific effects of vitamin D supplementation on CVD risk in early stage CKD. Prospective clinical trials are required to identify the best measure of cardioprotective effects of vitamin D supplementation in early stage CKD, as well as the optimal vitamin D formulation.
Conclusion
The current systematic review suggests that achievement of optimal vitamin D status (25(OH)D ≥ 30 ng/ml) in patients with early CKD may require greater than 2,000 IU vitamin D per day. Furthermore, cholecalciferol may be more effective than ergocalciferol in achieving and maintain optimal 25(OH)D concentrations, thus highlighting the need for a revision of the current KDOQI guidelines. Current recommendations suggest intervening with vitamin D after the recognition of hyperparathyroidism, however it is possible that treatment of vitamin D deficiency earlier in the progression of CKD could prevent the rise in secondary hyperparathyroidism. Although various studies reviewed found an improvement in serum/plasma 25(OH)D and a reduction in PTH, the clinical utility of such improvements in early CKD is not yet confirmed. Few studies examine tangible, relevant clinical outcomes such as CVD risk factors, progression to ESRD, and mortality. Future vitamin D supplementation trials in early CKD should be designed with these clinical indicators as the primary outcomes. Furthermore, future studies should investigate effective treatment strategies for maintaining optimal vitamin D status, as well as improving clinical outcomes.
Acknowledgments
J.A.A. is supported by the National Institutes of Health/National Institute of Diabetes And Digestive And Kidney Diseases (3T32DK007298-32S1).
Glossary
Abbreviations:
- 25(OH)D
25-hydroxyvitamin D or calcidiol
- 1,25(OH)2D
1,25-dihydroxyvitamin D or calctriol
- [Ca2+]i
intracellular calcium concentration
- BAP
bone-specific alkaline phosphatase
- CKD
chronic kidney disease
- CVD
cardiovascular disease
- eGFR
estimated glomerular filtration rate
- ESRD
end stage renal disease
- FGF-23
fibroblast growth factor-23
- GFR
glomerular filtration rate
- KDOQI
Kidney Disease Outcomes Quality Initiative
- PTH
parathyroid hormone
- VDBP
vitamin D binding protein
Footnotes
Previously published online: www.landesbioscience.com/journals/dermatoendocrinology/article/20014
References
- 1.Castro AF, Coresh J. CKD surveillance using laboratory data from the population-based National Health and Nutrition Examination Survey (NHANES) Am J Kidney Dis. 2009;53(Suppl 3):S46–55. doi: 10.1053/j.ajkd.2008.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Kidney Foundation K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42(Suppl 3):S1–201. doi: 10.1016/S0272-6386(03)00553-5. [DOI] [PubMed] [Google Scholar]
- 3.Couser WG, Remuzzi G, Mendis S, Tonelli M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011;80:1258–70. doi: 10.1038/ki.2011.368. [DOI] [PubMed] [Google Scholar]
- 4.Judd SE, Tangpricha V. Vitamin d therapy and cardiovascular health. Curr Hypertens Rep. 2011;13:187–91. doi: 10.1007/s11906-011-0190-2. [DOI] [PubMed] [Google Scholar]
- 5.Kamen DL, Tangpricha V. Vitamin D and molecular actions on the immune system: modulation of innate and autoimmunity. J Mol Med (Berl) 2010;88:441–50. doi: 10.1007/s00109-010-0590-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pilz S, Iodice S, Zittermann A, Grant WB, Gandini S. Vitamin D status and mortality risk in CKD: a meta-analysis of prospective studies. Am J Kidney Dis. 2011;58:374–82. doi: 10.1053/j.ajkd.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 7.Tebben PK, Vitamin R. D and the Kidney. In: Feldman DP, J.W.; Adams, J.S., ed. Vitamin D. Amsterdam: Elsevier Inc, 2011:471-91. [Google Scholar]
- 8.Dusso ASS. E. Vitamin D and Renal Disease. In: Feldman DP, J.W.; Adams, J.S., ed. Vitamin D. Amsterdam: Elsevier Inc, 2011:1325-57. [Google Scholar]
- 9.Jones G. Expanding role for vitamin D in chronic kidney disease: importance of blood 25-OH-D levels and extra-renal 1alpha-hydroxylase in the classical and nonclassical actions of 1alpha,25-dihydroxyvitamin D(3) Semin Dial. 2007;20:316–24. doi: 10.1111/j.1525-139X.2007.00302.x. [DOI] [PubMed] [Google Scholar]
- 10.Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16:2205–15. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 11.Perwad F, Zhang MY, Tenenhouse HS, Portale AA. Fibroblast growth factor 23 impairs phosphorus and vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin D-1alpha-hydroxylase expression in vitro. Am J Physiol Renal Physiol. 2007;293:F1577–83. doi: 10.1152/ajprenal.00463.2006. [DOI] [PubMed] [Google Scholar]
- 12.Usatii M, Rousseau L, Demers C, Petit JL, Brossard JH, Gascon-Barré M, et al. Parathyroid hormone fragments inhibit active hormone and hypocalcemia-induced 1,25(OH)2D synthesis. Kidney Int. 2007;72:1330–5. doi: 10.1038/sj.ki.5002532. [DOI] [PubMed] [Google Scholar]
- 13.Hsu CH, Patel S. Uremic plasma contains factors inhibiting 1 alpha-hydroxylase activity. J Am Soc Nephrol. 1992;3:947–52. doi: 10.1681/ASN.V34947. [DOI] [PubMed] [Google Scholar]
- 14.Kalantar-Zadeh K, Kovesdy CP. Clinical outcomes with active versus nutritional vitamin D compounds in chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:1529–39. doi: 10.2215/CJN.02140309. [DOI] [PubMed] [Google Scholar]
- 15.Jean G, Terrat JC, Vanel T, Hurot JM, Lorriaux C, Mayor B, et al. Evidence for persistent vitamin D 1-alpha-hydroxylation in hemodialysis patients: evolution of serum 1,25-dihydroxycholecalciferol after 6 months of 25-hydroxycholecalciferol treatment. Nephron Clin Pract. 2008;110:c58–65. doi: 10.1159/000151534. [DOI] [PubMed] [Google Scholar]
- 16.Wasse H, Huang R, Long Q, Singapuri S, Raggi P, Tangpricha V. Efficacy and safety of a short course of very-high-dose cholecalciferol in hemodialysis. Am J Clin Nutr. 2012;95:522–8. doi: 10.3945/ajcn.111.025502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 18.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Endocrine Society Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 19.LaClair RE, Hellman RN, Karp SL, Kraus M, Ofner S, Li Q, et al. Prevalence of calcidiol deficiency in CKD: a cross-sectional study across latitudes in the United States. Am J Kidney Dis. 2005;45:1026–33. doi: 10.1053/j.ajkd.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 20.Mehrotra R, Kermah D, Budoff M, Salusky IB, Mao SS, Gao YL, et al. Hypovitaminosis D in chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1144–51. doi: 10.2215/CJN.05781207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson AB, Thierry-Palmer M, Gibson KL, Rabinovich CE. Disease activity, proteinuria, and vitamin D status in children with systemic lupus erythematosus and juvenile dermatomyositis. J Pediatr. 2012;160:297–302. doi: 10.1016/j.jpeds.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dusso AS, Tokumoto M. Defective renal maintenance of the vitamin D endocrine system impairs vitamin D renoprotection: a downward spiral in kidney disease. Kidney Int. 2011;79:715–29. doi: 10.1038/ki.2010.543. [DOI] [PubMed] [Google Scholar]
- 23.Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, et al. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell. 1999;96:507–15. doi: 10.1016/S0092-8674(00)80655-8. [DOI] [PubMed] [Google Scholar]
- 24.Chonchol M, Kendrick J, Targher G. Extra-skeletal effects of vitamin D deficiency in chronic kidney disease. Ann Med. 2011;43:273–82. doi: 10.3109/07853890.2010.543923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 26.Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol. 2004;15:1307–15. doi: 10.1097/01.ASN.0000123691.46138.E2. [DOI] [PubMed] [Google Scholar]
- 27.Navaneethan SD, Schold JD, Arrigain S, Jolly SE, Jain A, Schreiber MJ, Jr., et al. Low 25-hydroxyvitamin D levels and mortality in non-dialysis-dependent CKD. Am J Kidney Dis. 2011;58:536–43. doi: 10.1053/j.ajkd.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zittermann A, Iodice S, Pilz S, Grant WB, Bagnardi V, Gandini S. Vitamin D deficiency and mortality risk in the general population: a meta-analysis of prospective cohort studies. Am J Clin Nutr. 2012;95:91–100. doi: 10.3945/ajcn.111.014779. [DOI] [PubMed] [Google Scholar]
- 29.Chandra P, Binongo JN, Ziegler TR, Schlanger LE, Wang W, Someren JT, et al. Cholecalciferol (vitamin D3) therapy and vitamin D insufficiency in patients with chronic kidney disease: a randomized controlled pilot study. Endocr Pract. 2008;14:10–7. doi: 10.4158/EP.14.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shroff R, Wan M, Gullett A, Ledermann S, Shute R, Knott C, et al. Ergocalciferol supplementation in children with CKD delays the onset of secondary hyperparathyroidism: a randomized trial. Clin J Am Soc Nephrol. 2012;7:216–23. doi: 10.2215/CJN.04760511. [DOI] [PubMed] [Google Scholar]
- 31.Kovesdy CP, Lu JL, Malakauskas SM, Andress DL, Kalantar-Zadeh K, Ahmadzadeh S. Paricalcitol versus ergocalciferol for secondary hyperparathyroidism in CKD stages 3 and 4: a randomized controlled trial. Am J Kidney Dis. 2012;59:58–66. doi: 10.1053/j.ajkd.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 32.Moe SM, Saifullah A, LaClair RE, Usman SA, Yu Z. A randomized trial of cholecalciferol versus doxercalciferol for lowering parathyroid hormone in chronic kidney disease. Clin J Am Soc Nephrol. 2010;5:299–306. doi: 10.2215/CJN.07131009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dogan E, Erkoc R, Sayarlioglu H, Soyoral Y, Dulger H. Effect of depot oral cholecalciferol treatment on secondary hyperparathyroidism in stage 3 and stage 4 chronic kidney diseases patients. Ren Fail. 2008;30:407–10. doi: 10.1080/08860220801964210. [DOI] [PubMed] [Google Scholar]
- 34.Rucker D, Tonelli M, Coles MG, Yoo S, Young K, McMahon AW. Vitamin D insufficiency and treatment with oral vitamin D3 in northern-dwelling patients with chronic kidney disease. J Nephrol. 2009;22:75–82. [PubMed] [Google Scholar]
- 35.Taner B, Abdulkadir U. Effect of cholecalciferol on parathyroid hormone and vitamin D levels in chronic kidney disease. Minerva Urol Nefrol. 2011;63:287–92. [PubMed] [Google Scholar]
- 36.Oksa A, Spustová V, Krivosíková Z, Gazdíková K, Fedelesová V, Lajdová I, et al. Effects of long-term cholecalciferol supplementation on mineral metabolism and calciotropic hormones in chronic kidney disease. Kidney Blood Press Res. 2008;31:322–9. doi: 10.1159/000157177. [DOI] [PubMed] [Google Scholar]
- 37.Hari P, Gupta N, Hari S, Gulati A, Mahajan P, Bagga A. Vitamin D insufficiency and effect of cholecalciferol in children with chronic kidney disease. Pediatr Nephrol. 2010;25:2483–8. doi: 10.1007/s00467-010-1639-2. [DOI] [PubMed] [Google Scholar]
- 38.DeVille J, Thorp ML, Tobin L, Gray E, Johnson ES, Smith DH. Effect of ergocalciferol supplementation on serum parathyroid hormone and serum 25-hydroxyvitamin D in chronic kidney disease. Nephrology (Carlton) 2006;11:555–9. doi: 10.1111/j.1440-1797.2006.00698.x. [DOI] [PubMed] [Google Scholar]
- 39.Trakarnvanich T, Chalapipat O, Disthabanchong S, Kurathong S, Praditpornsilpa K, Stitchantrakul W, et al. Effect of high dose ergocalciferol in chronic kidney disease patients with 25-hydroxyvitamin D deficiency. J Med Assoc Thai. 2010;93:885–91. [PubMed] [Google Scholar]
- 40.Zisman AL, Hristova M, Ho LT, Sprague SM. Impact of ergocalciferol treatment of vitamin D deficiency on serum parathyroid hormone concentrations in chronic kidney disease. Am J Nephrol. 2007;27:36–43. doi: 10.1159/000098561. [DOI] [PubMed] [Google Scholar]
- 41.Lishmanov A, Dorairajan S, Pak Y, Chaudhary K, Chockalingam A. Treatment of 25-OH Vitamin D Deficiency in Older Men With Chronic Kidney Disease Stages 3 and 4 Is Associated With Reduction in Cardiovascular Events. Am J Ther. 2011 doi: 10.1097/MJT.0b013e3182211b3b. [DOI] [PubMed] [Google Scholar]
- 42.Al-Aly Z, Qazi RA, González EA, Zeringue A, Martin KJ. Changes in serum 25-hydroxyvitamin D and plasma intact PTH levels following treatment with ergocalciferol in patients with CKD. Am J Kidney Dis. 2007;50:59–68. doi: 10.1053/j.ajkd.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 43.Menon S, Valentini RP, Hidalgo G, Peschansky L, Mattoo TK. Vitamin D insufficiency and hyperparathyroidism in children with chronic kidney disease. Pediatr Nephrol. 2008;23:1831–6. doi: 10.1007/s00467-008-0842-x. [DOI] [PubMed] [Google Scholar]
- 44.Qunibi WY, Abdellatif A, Sankar S, Hamdan Z, Lin FY, Ingle J, et al. Treatment of vitamin D deficiency in CKD patients with ergocalciferol: are current K/DOQI treatment guidelines adequate? Clin Nephrol. 2010;73:276–85. doi: 10.5414/cnp73276. [DOI] [PubMed] [Google Scholar]
- 45.Lajdova I, Spustova V, Oksa A, Chorvatova A, Chorvat D, Jr., Dzurik R. Intracellular calcium homeostasis in patients with early stages of chronic kidney disease: effects of vitamin D3 supplementation. Nephrol Dial Transplant. 2009;24:3376–81. doi: 10.1093/ndt/gfp292. [DOI] [PubMed] [Google Scholar]
- 46.Shroff R, Wan M, Rees L. Can vitamin D slow down the progression of chronic kidney disease? Pediatr Nephrol. 2011 doi: 10.1007/s00467-011-2071-y. [DOI] [PubMed] [Google Scholar]
- 47.Mehrotra R, Kermah DA, Salusky IB, Wolf MS, Thadhani RI, Chiu YW, et al. Chronic kidney disease, hypovitaminosis D, and mortality in the United States. Kidney Int. 2009;76:977–83. doi: 10.1038/ki.2009.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Melamed ML, Astor B, Michos ED, Hostetter TH, Powe NR, Muntner P. 25-hydroxyvitamin D levels, race, and the progression of kidney disease. J Am Soc Nephrol. 2009;20:2631–9. doi: 10.1681/ASN.2009030283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ravani P, Malberti F, Tripepi G, Pecchini P, Cutrupi S, Pizzini P, et al. Vitamin D levels and patient outcome in chronic kidney disease. Kidney Int. 2009;75:88–95. doi: 10.1038/ki.2008.501. [DOI] [PubMed] [Google Scholar]
- 50.Armas LA, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab. 2004;89:5387–91. doi: 10.1210/jc.2004-0360. [DOI] [PubMed] [Google Scholar]
- 51.Heaney RP, Recker RR, Grote J, Horst RL, Armas LA. Vitamin D(3) is more potent than vitamin D(2) in humans. J Clin Endocrinol Metab. 2011;96:E447–52. doi: 10.1210/jc.2010-2230. [DOI] [PubMed] [Google Scholar]
- 52.Trang HM, Cole DE, Rubin LA, Pierratos A, Siu S, Vieth R. Evidence that vitamin D3 increases serum 25-hydroxyvitamin D more efficiently than does vitamin D2. Am J Clin Nutr. 1998;68:854–8. doi: 10.1093/ajcn/68.4.854. [DOI] [PubMed] [Google Scholar]
- 53.Kandula P, Dobre M, Schold JD, Schreiber MJ, Jr., Mehrotra R, Navaneethan SD. Vitamin D supplementation in chronic kidney disease: a systematic review and meta-analysis of observational studies and randomized controlled trials. Clin J Am Soc Nephrol. 2011;6:50–62. doi: 10.2215/CJN.03940510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Repo JM, Rantala IS, Honkanen TT, Mustonen JT, Kööbi P, Tahvanainen AM, et al. Paricalcitol aggravates perivascular fibrosis in rats with renal insufficiency and low calcitriol. Kidney Int. 2007;72:977–84. doi: 10.1038/sj.ki.5002458. [DOI] [PubMed] [Google Scholar]
- 55.Ritter CS, Armbrecht HJ, Slatopolsky E, Brown AJ. 25-Hydroxyvitamin D(3) suppresses PTH synthesis and secretion by bovine parathyroid cells. Kidney Int. 2006;70:654–9. doi: 10.1038/sj.ki.5000394. [DOI] [PubMed] [Google Scholar]
- 56.Astor BC, Matsushita K, Gansevoort RT, van der Velde M, Woodward M, Levey AS, et al. Chronic Kidney Disease Prognosis Consortium Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int. 2011;79:1331–40. doi: 10.1038/ki.2010.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Menon V, Greene T, Wang X, Pereira AA, Marcovina SM, Beck GJ, et al. C-reactive protein and albumin as predictors of all-cause and cardiovascular mortality in chronic kidney disease. Kidney Int. 2005;68:766–72. doi: 10.1111/j.1523-1755.2005.00455.x. [DOI] [PubMed] [Google Scholar]
- 58.de Boer IH, Ioannou GN, Kestenbaum B, Brunzell JD, Weiss NS. 25-Hydroxyvitamin D levels and albuminuria in the Third National Health and Nutrition Examination Survey (NHANES III) Am J Kidney Dis. 2007;50:69–77. doi: 10.1053/j.ajkd.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 59.Isakova T, Gutiérrez OM, Patel NM, Andress DL, Wolf M, Levin A. Vitamin D deficiency, inflammation, and albuminuria in chronic kidney disease: complex interactions. J Ren Nutr. 2011;21:295–302. doi: 10.1053/j.jrn.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 60.Li YC. Vitamin D: roles in renal and cardiovascular protection. Curr Opin Nephrol Hypertens. 2012;21:72–9. doi: 10.1097/MNH.0b013e32834de4ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dusso A, Arcidiacono MV, Yang J, Tokumoto M. Vitamin D inhibition of TACE and prevention of renal osteodystrophy and cardiovascular mortality. J Steroid Biochem Mol Biol. 2010;121:193–8. doi: 10.1016/j.jsbmb.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Melenhorst WB, Visser L, Timmer A, van den Heuvel MC, Stegeman CA, van Goor H. ADAM17 upregulation in human renal disease: a role in modulating TGF-alpha availability? Am J Physiol Renal Physiol. 2009;297:F781–90. doi: 10.1152/ajprenal.90610.2008. [DOI] [PubMed] [Google Scholar]
- 63.Deb DK, Wang Y, Zhang Z, Nie H, Huang X, Yuan Z, et al. Molecular mechanism underlying 1,25-dihydroxyvitamin D regulation of nephrin gene expression. J Biol Chem. 2011;286:32011–7. doi: 10.1074/jbc.M111.269118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu W, Yu WR, Carling T, Juhlin C, Rastad J, Ridefelt P, et al. Regulation of gp330/megalin expression by vitamins A and D. Eur J Clin Invest. 1998;28:100–7. doi: 10.1046/j.1365-2362.1998.00253.x. [DOI] [PubMed] [Google Scholar]
- 65.Agarwal R, Acharya M, Tian J, Hippensteel RL, Melnick JZ, Qiu P, et al. Antiproteinuric effect of oral paricalcitol in chronic kidney disease. Kidney Int. 2005;68:2823–8. doi: 10.1111/j.1523-1755.2005.00755.x. [DOI] [PubMed] [Google Scholar]
- 66.Alborzi P, Patel NA, Peterson C, Bills JE, Bekele DM, Bunaye Z, et al. Paricalcitol reduces albuminuria and inflammation in chronic kidney disease: a randomized double-blind pilot trial. Hypertension. 2008;52:249–55. doi: 10.1161/HYPERTENSIONAHA.108.113159. [DOI] [PubMed] [Google Scholar]
- 67.de Zeeuw D, Agarwal R, Amdahl M, Audhya P, Coyne D, Garimella T, et al. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet. 2010;376:1543–51. doi: 10.1016/S0140-6736(10)61032-X. [DOI] [PubMed] [Google Scholar]
- 68.Fishbane S, Chittineni H, Packman M, Dutka P, Ali N, Durie N. Oral paricalcitol in the treatment of patients with CKD and proteinuria: a randomized trial. Am J Kidney Dis. 2009;54:647–52. doi: 10.1053/j.ajkd.2009.04.036. [DOI] [PubMed] [Google Scholar]
- 69.Weir MR. Short-term effects of vitamin D receptor activation on serum creatinine, creatinine generation, and glomerular filtration. Kidney Int. 2011;80:1016–7. doi: 10.1038/ki.2011.265. [DOI] [PubMed] [Google Scholar]
- 70.Kalantar-Zadeh K, Cano NJ, Budde K, Chazot C, Kovesdy CP, Mak RH, et al. Diets and enteral supplements for improving outcomes in chronic kidney disease. Nat Rev Nephrol. 2011;7:369–84. doi: 10.1038/nrneph.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shoben AB, Rudser KD, de Boer IH, Young B, Kestenbaum B. Association of oral calcitriol with improved survival in nondialyzed CKD. J Am Soc Nephrol. 2008;19:1613–9. doi: 10.1681/ASN.2007111164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang HE, Gamboa C, Warnock DG, Muntner P. Chronic kidney disease and risk of death from infection. Am J Nephrol. 2011;34:330–6. doi: 10.1159/000330673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fabri M, Stenger S, Shin DM, Yuk JM, Liu PT, Realegeno S, et al. Vitamin D is required for IFN-gamma-mediated antimicrobial activity of human macrophages. Sci Transl Med. 2011;3:ra102. doi: 10.1126/scitranslmed.3003045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hübel E, Kiefer T, Weber J, Mettang T, Kuhlmann U. In vivo effect of 1,25-dihydroxyvitamin D3 on phagocyte function in hemodialysis patients. Kidney Int. 1991;40:927–33. doi: 10.1038/ki.1991.296. [DOI] [PubMed] [Google Scholar]
- 75.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 76.Drechsler C, Pilz S, Obermayer-Pietsch B, Verduijn M, Tomaschitz A, Krane V, et al. Vitamin D deficiency is associated with sudden cardiac death, combined cardiovascular events, and mortality in haemodialysis patients. Eur Heart J. 2010;31:2253–61. doi: 10.1093/eurheartj/ehq246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang AY, Lam CW, Sanderson JE, Wang M, Chan IH, Lui SF, et al. Serum 25-hydroxyvitamin D status and cardiovascular outcomes in chronic peritoneal dialysis patients: a 3-y prospective cohort study. Am J Clin Nutr. 2008;87:1631–8. doi: 10.1093/ajcn/87.6.1631. [DOI] [PubMed] [Google Scholar]
- 78.Palmer SC, McGregor DO, Macaskill P, Craig JC, Elder GJ, Strippoli GF. Meta-analysis: vitamin D compounds in chronic kidney disease. Ann Intern Med. 2007;147:840–53. doi: 10.7326/0003-4819-147-12-200712180-00004. [DOI] [PubMed] [Google Scholar]
- 79.Kim HW, Park CW, Shin YS, Kim YS, Shin SJ, Kim YS, et al. Calcitriol regresses cardiac hypertrophy and QT dispersion in secondary hyperparathyroidism on hemodialysis. Nephron Clin Pract. 2006;102:c21–9. doi: 10.1159/000088295. [DOI] [PubMed] [Google Scholar]
- 80.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229–38. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Petchey WG, Johnson DW, Isbel NM. Shining D’ light on chronic kidney disease: mechanisms that may underpin the cardiovascular benefit of vitamin D. Nephrology (Carlton) 2011;16:351–67. doi: 10.1111/j.1440-1797.2011.01450.x. [DOI] [PubMed] [Google Scholar]
- 82.Alvarez JA, Ashraf A. Role of vitamin d in insulin secretion and insulin sensitivity for glucose homeostasis. Int J Endocrinol. 2010;2010:351385. doi: 10.1155/2010/351385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cohen-Lahav M, Shany S, Tobvin D, Chaimovitz C, Douvdevani A. Vitamin D decreases NFkappaB activity by increasing IkappaBalpha levels. Nephrol Dial Transplant. 2006;21:889–97. doi: 10.1093/ndt/gfi254. [DOI] [PubMed] [Google Scholar]
- 84.Talmor-Barkan Y, Bernheim J, Green J, Benchetrit S, Rashid G. Calcitriol counteracts endothelial cell pro-inflammatory processes in a chronic kidney disease-like environment. J Steroid Biochem Mol Biol. 2011;124:19–24. doi: 10.1016/j.jsbmb.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 85.Tanaka M, Tokunaga K, Komaba H, Itoh K, Matsushita K, Watanabe H, et al. Vitamin D receptor activator reduces oxidative stress in hemodialysis patients with secondary hyperparathyroidism. Ther Apher Dial. 2011;15:161–8. doi: 10.1111/j.1744-9987.2010.00890.x. [DOI] [PubMed] [Google Scholar]
- 86.Günal AI, Celiker H, Celebi H, Ustündağ B, Günal SY. Intravenous alfacalcidol improves insulin resistance in hemodialysis patients. Clin Nephrol. 1997;48:109–13. [PubMed] [Google Scholar]
- 87.Kautzky-Willer A, Pacini G, Barnas U, Ludvik B, Streli C, Graf H, et al. Intravenous calcitriol normalizes insulin sensitivity in uremic patients. Kidney Int. 1995;47:200–6. doi: 10.1038/ki.1995.24. [DOI] [PubMed] [Google Scholar]
- 88.Mak RH. Amelioration of hypertension and insulin resistance by 1,25-dihydroxycholecalciferol in hemodialysis patients. Pediatr Nephrol. 1992;6:345–8. doi: 10.1007/BF00869730. [DOI] [PubMed] [Google Scholar]
- 89.Mak RH. Intravenous 1,25 dihydroxycholecalciferol corrects glucose intolerance in hemodialysis patients. Kidney Int. 1992;41:1049–54. doi: 10.1038/ki.1992.159. [DOI] [PubMed] [Google Scholar]
- 90.Mak RH. 1,25-Dihydroxyvitamin D3 corrects insulin and lipid abnormalities in uremia. Kidney Int. 1998;53:1353–7. doi: 10.1046/j.1523-1755.1998.00865.x. [DOI] [PubMed] [Google Scholar]
- 91.Matias PJ, Jorge C, Ferreira C, Borges M, Aires I, Amaral T, et al. Cholecalciferol supplementation in hemodialysis patients: effects on mineral metabolism, inflammation, and cardiac dimension parameters. Clin J Am Soc Nephrol. 2010;5:905–11. doi: 10.2215/CJN.06510909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Park CW, Oh YS, Shin YS, Kim CM, Kim YS, Kim SY, et al. Intravenous calcitriol regresses myocardial hypertrophy in hemodialysis patients with secondary hyperparathyroidism. Am J Kidney Dis. 1999;33:73–81. doi: 10.1016/S0272-6386(99)70260-X. [DOI] [PubMed] [Google Scholar]
- 93.Stubbs JR, Idiculla A, Slusser J, Menard R, Quarles LD. Cholecalciferol supplementation alters calcitriol-responsive monocyte proteins and decreases inflammatory cytokines in ESRD. J Am Soc Nephrol. 2010;21:353–61. doi: 10.1681/ASN.2009040451. [DOI] [PMC free article] [PubMed] [Google Scholar]