Abstract
Vitamin D has received worldwide attention not only for its importance for bone health in children and adults but also for reducing risk for many chronic diseases including autoimmune diseases, type 2 diabetes, heart disease, many cancers and infectious diseases. Vitamin D deficiency is pandemic due to the fact that most humans have depended on sun for their vitamin D requirement which they now either avoid or wear sun protection for fear of skin cancer. There are few foods that naturally contain vitamin D. Some countries permit vitamin D fortification especially dairy products, some cereals and juice products. The Institute of Medicine made its recommendations based on a population-based model; the Endocrine Society's Practice Guidelines on Vitamin D was for the prevention and treatment of vitamin D deficiency, which helps explain the differences in the recommendations. The Guidelines defined vitamin D deficiency as a 25-hydroxyvitamin D < 20 ng/mL, insufficiency as 21–29 ng/mL and sufficiency as 30–100 ng/mL. To prevent vitamin D deficiency The Guidelines recommended vitamin D intake should be: children < 1 y 400–1,000 IU/d, children 1–18 y 600–1,000 IU/d and adults 1,500–2,000 IU/d.
Keywords: vitamin D, 25-hydroxyvitamin D, sunlight, Institute of Medicine, Endocrine Society Practice Guidelines
The IOM concluded most Americans can obtain an adequate amount of vitamin D from the diet and vitamin D deficiency is not as common a problem as has been suggested. To obtain 600 IU of vitamin D/d from dietary sources a child or adult would need to ingest wild caught salmon at least 5 d a week or drink or eat six servings of dairy fortified with vitamin D. The CDC reported that more than 30% of children and adults in the US are vitamin D deficient. Unless you have a granulomatous disorder there is no downside to increasing your vitamin D.
Who could have guessed that the sun-derived hormone vitamin D that has been produced in life forms for more than 500 million years would gain such attention and such controversy today? In 1997 the Institute of Medicine (IOM) recommended that all children and adults up to the age of 50 only required 200 IU of vitamin D/d to satisfy their bodies’ requirement for bone health.1 The 200 IU comes from studies in the 1940s demonstrating that 100 IU of vitamin D/d was all that was required to prevent overt skeletal signs of rickets in children. This amount was increased by factor of 2 for safety reasons and for more than 50 y it was believed that 200 IU of vitamin D/d was all that was needed to satisfy children and adults requirement. In 2010 the IOM reported that the RDA for vitamin D for most children and adults should be increased to 600 IU/d.2 The Committee’s recommendations were based on a population model, i.e., to satisfy 97.5% of the population (but didn’t even satisfy that criterion), and not a medical model and the recommendations were not intended to direct physicians on care of patients and suggested it was up to professional associations to established guidelines for care.
The IOM tripled the amount of vitamin D from an Adequate Intake (AI) of 200 IU to a Recommended Daily Allowance (RDA) of 600 IU for all children over the age of one year and adults up to the age of 50 and they doubled the safe upper limit (UL) from 2,000 IU/d to 4000 IU/d. This suggests that for the past 50 y previous recommendations based on “evidence-based medicine” were totally inadequate.
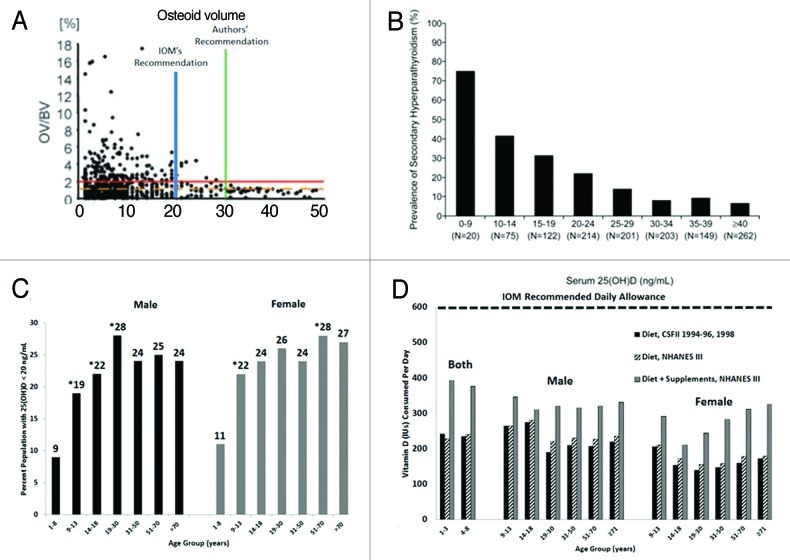
The IOM concluded that vitamin D deficiency should be defined as a 25-hydroxyvitamin D [25(OH)D] < 20 ng/ml. They made this recommendation based in part on a study of 675 German adult motor vehicle accident victims who had blood and bone recovered to evaluate serum 25(OH)D and related the concentrations to the presence of increased osteoid in the bone biopsy which was considered to be the gold standard for vitamin D deficiency bone disease.3 They concluded based on their population-based model that if 97.5% of the victims had no evidence of increased osteoid that should be the blood level that would define vitamin D deficiency. Based on the analysis of the data they concluded that 99% of the victims had no evidence of vitamin D deficiency osteomalacia when the 25(OH)D > 20 ng/ml. The authors of the study however concluded that to prevent vitamin D deficiency osteomalacia in 100% of adults the blood level of 25(OH)D should be > 30 ng/ml. A recent analysis of the data suggested that the IOM misinterpreted the data and that 8.5% (7 of 82) of the victims had evidence of vitamin D deficiency osteomalacia not 1% if the blood level of 25(OH)D > 20 ng/ml.4 (Fig. 1A)
Figure 1. (A) 25-hydroxyvitamin D [25(OH)D] concentrations in German motor vehicle accident victims and osteoid volume. Pathologic accumulations of osteoid are absent in all individuals with a 25(OH)D > 30 ng/ml (authors' recommendation). The Institute of Medicine concluded that 99% of subjects had no evidence of pathologic accumulations of osteoid when the blood level of 25(OH)D > 20 ng/ml (IOM recommendation). The horizontal line indicates a threshold of 2% osteoid volume used in this study as a conservative histopathologic border to osteomalacia. Reproduced with permission.3 (B) Percent of subjects with secondary hyperparathyroidism by 25(OH)D level. The percent of subjects with secondary hyperparathyroidism (PTH > 40 pg/ml) sorted by subgroups with serum 25(OH)D concentrations delineated by predefined cutoffs for analyses of 25(OH)D inadequacy. Adapted from reference 17 and reproduced with permission. (C) Prevalence at risk of vitamin D deficiency defined as a 25-hydroxyvitamin D < 20 ng/ml by age and sex: United States, 2001–2006. Adapted from and reproduced with permission.8 (D) Mean intake of vitamin D (IU) from food and food plus dietary supplements from Continuing Survey of Food Intakes by Individuals (CSFII) 1994–1996, 1998 and the Third National Health and Nutrition Examination Survey (NHANES III) 1988–1994. Adapted from and reproduced with permission.9
There have been several studies relating serum 25(OH)D with PTH concentrations. Most but not all of the studies that were evidence-based suggested that PTH concentrations begin to plateau when 25(OH)D were above 30 ng/ml and secondary hyperparathyroidism is minimized.(Fig. 1B and C)5
These findings are consistent with the threshold for hip and nonvertebral fracture prevention from a recent meta-analysis of double-blind randomized controlled trials (RCT) with oral vitamin D as noted in the Endocrine Society’s Clinical Practice Guideline on Vitamin D.5 The IOM also disregarded the observation that when postmenopausal women increased their blood level of 25(OH)D from 20 to 32 ng/ml, they increased the efficiency of intestinal calcium absorption by 45–65%. They argued that this was a small study that only indirectly measured intestinal calcium absorption but ignored the fact that the study was strengthened because the change in intestinal calcium absorption was the same women who had a blood level of 25(OH)D of ~20 ng/ml that was raised to an average of 32 ng/ml.5,6 Therefore the Endocrine Society’s Clinical Guidelines for vitamin D concluded, based on all of the evidence, that vitamin D deficiency be defined as a 25(OH)D < 20 ng/ml, insufficiency as a 25(OH)D of 21–29 ng/ml and sufficiency as a 25(OH)D of 30–100 ng/ml.5
Based on their definition of vitamin D deficiency, i.e., 25(OH)D < 20 ng/ml, the IOM concluded that concerns about widespread vitamin D deficiency in North American population is not well founded. However many studies have suggested that vitamin D deficiency is a significant health problem in North America. Fifty-four percent of community elders in Baltimore had a blood level of 25(OH)D < 10 ng/ml.7 Newborns, young children, teenagers and young adults are at high risk for being vitamin D deficient whether they are from Boston, Pittsburgh, Augusta or Sacramento.5-7 A study in Boston reported of 40 mother infant pairs 76% of moms and 81% of newborns were vitamin D deficient even though the mothers were documented to be ingesting on average 600 IU/d of vitamin D during their pregnancy. More than 40% of Hispanic and African American adolescents in Boston were found to be vitamin D deficient and 48% of white preadolescent girls from Maine had a 25(OH)D < 20 ng/ml.5,7 Data from the National Health and Nutrition Examination Surveys 2001–2006 ~33% of the US population was found to have a 25(OH)D < 20 ng/ml. (Fig. 1C)8 These results are similar to the observations made in Canada where 30–50% of children and adults have been reported to be vitamin D deficient.5,7
The IOM concluded that dietary and supplemental vitamin D intake is adequate to satisfy both children and adults. However Moore et al.9 estimated the vitamin D intake in US men, non-pregnant and non-lactating women, and non-breastfeeding children aged one year and older and found that in more than 9,000 female teenagers and adults the estimated vitamin D intake from food was 156–208 IU/d and with supplements 244–324 IU/d. For male teenagers and adults the estimated vitamin D intake from food was 208–320 IU/d and with supplements 308–392 IU/d. An estimate of the vitamin D intake from food for children of both sexes between the age of 1–8 y the intake was 228–240 IU/d and with supplements increased to 376–392 IU/d.(Fig. 1E) Based on the recommendation by the IOM that all children and adults (1–50 y) require 600 IU/d the Moore et al.9 study suggests that neither children nor adults in the US are obtaining the new RDA for vitamin D. The IOM suggests you can obtain your vitamin D requirement from the diet. However, the major source is from dairy, and since there is only 100 IU/8 oz. of milk this would require children and adults to drink 6 glasses of milk/d, which is unrealistic.
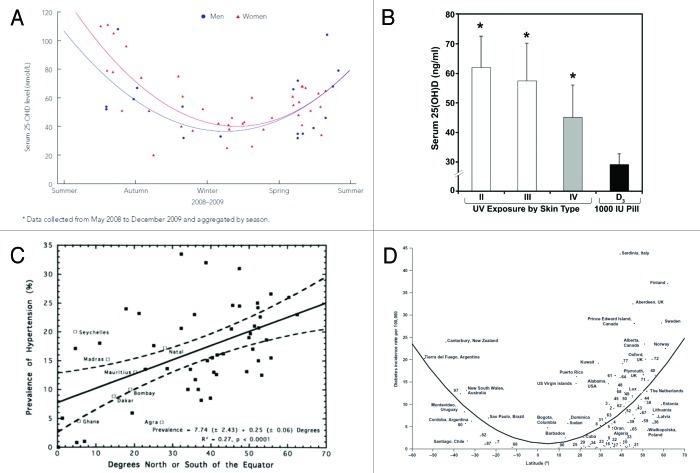
The IOM did appreciate the seasonal association with a person’s vitamin D status, i.e., the highest concentrations of 25(OH)D are at the end of the summer and at a nadir at the end of the winter. However they considered this to be of little consequence. Brot et al.10 reported that blood concentrations of 25(OH)D were ~18 ng/ml in the winter and rose to ~34 ng/ml at the end of the summer in Denmark. Australian Aborigines had blood concentrations of 45 ng/ml at the end of the summer and only 16 ng/ml in the winter.11
The body has a large capacity to make vitamin D and it has been estimated that an adult in a bathing suit exposed to one minimal erythema low-dose (MED) is equivalent to ingesting approximately 20,000 IU of vitamin D.7 Exposure to 0.5 MED UVB radiation once a week was more effective in raising 25(OH)D concentrations than taking vitamin D 1000 IU/d.(Fig. 1F) Although aging decreases the capacity of the skin to produce vitamin D because of the skin’s large capacity to make vitamin D even elders are able to raise their blood concentrations when exposed to 15 or 30 min of sunlight 3 times a week.7 The proper use of a sunscreen will markedly reduce the production of vitamin D in the skin contrary to what the IOM stated. Most farmers in a study in Pennsylvania and Illinois with a history of skin cancer and used a sunscreen daily were vitamin D deficient at the end of the summer.12 When used properly a sunscreen with a sun protection factor (SPF) 30 reduced vitamin D production by > 95%.7
The IOM ranked study designs with randomized controlled trials the highest level of evidence and cross-sectional and ecological studies were ignored. They heavily depended on two Agency for Health Care Research and Quality studies known as AHRQ-Ottawa and AHRQ-Tufts as well as the Women's Health Initiative Study (WHI) when they evaluated potential non-skeletal health benefits of vitamin D.2,13 They essentially ignored a multitude of association studies linking vitamin D deficiency with a wide variety of chronic illnesses including heart disease, infectious diseases, autoimmune diseases, type 2 diabetes, and some cancers.5-7,14-17 They also dismissed the evidence that vitamin D deficiency causes muscle weakness and increased risk for falling.
Although a well-designed and well-conducted RCT should be considered as the highest level of evidence to support a claim, often this is not the case.18 Many of the RCTs that were evaluated for non-skeletal benefits of vitamin D had problems with a high incidence of noncompliance, misinterpretation of the original data and used doses of vitamin D below the 2010 IOM recommendations.6,17 A good example is the Women’s Health Initiative Trial (WHI) which examined the effect of combined supplementation of vitamin D and calcium (400 IU of vitamin D3 in 1,000 mg of elemental calcium) for an average of 7 y.13 More than 50% of the participants admitted not taking the calcium and vitamin D daily and blood concentrations of 25(OH)D were often not measured at baseline and/or at study end. Furthermore the authors acknowledged that the 400 IU of vitamin D was inadequate to raise the blood level of 25(OH)D above 30 ng/ml which most studies have suggested is required to reduce cancer risk and other non-skeletal acute and chronic diseases. Typically baseline 25(OH)D in healthy white adults is 18–22 ng/ml and 15–18 ng/ml in healthy black adults.5-7 It has been estimated for every 100 IU/d of vitamin D ingested the blood level of 25(OH)D increases ~1 ng/ml.5,6 Virtually all of the subjects in the WHI were vitamin D deficient or insufficient; women in the lowest quartile of 25(OH)D < 12 ng/ml had an incidence of colorectal cancer that was 253% higher than the incidence in women who had a baseline 25(OH)D > 24 ng/ml.17 A reanalysis of the WHI concluded that 15,646 women (43%) who were not taking personal calcium or vitamin D supplements at randomization the calcium and vitamin D intervention significantly decreased the risk of total, breast and invasive breast cancers by 14–20% and risk of colorectal cancer by 17%.19 Lappe et al.20 conducted an RCT and reported a 60% reduction in all cancers in postmenopausal women who ingested 1,100 IU of vitamin D/d and 1500 mg of elemental calcium/d for 4 y.
The IOM in their synopsis concluded that the evidence of vitamin D on fall prevention is inconsistent.2 Vitamin D deficiency is associated with myopathy including proximal muscle weakness, diffuse muscle pain, gait impairments such as waddling way of walking.5 Double-blind RCTs demonstrated that 800 IU/d of vitamin D resulted in a 4–11% gain in lower extremity strength or function and a 72% reduction in rate of falling in older adults.5,21,22 This was substantiated by the recent meta-analysis of Murad et al.23 who reported that such interventions were associated with a statistically significant reduction in risk of falls [odds ratio (OR) = 0.84; 95% confidence interval, 0.76–0.93; inconsistency (I2 = 61%; 23 studies). This effect was more prominent in patients who were vitamin D deficient at baseline. The importance of dose of supplemental vitamin D in minimizing the risk of falls was confirmed by a multidose double-blind RCT among the 124 nursing home residents receiving 200, 400, 600 or 800 IU/d vitamin D or placebo for 5 mo and by a 2009 meta-analysis.22,24 Only participants receiving 800 IU/d of vitamin D had a substantially lower rate of falls than those taking placebo or doses of vitamin D that were 800 IU/d (rate ratio = 0.28; 95% Cl, 0.11–0.75). These observations are consistent with the 2010 assessment by International Osteoporosis Foundation and the 2011 assessment of the Agency for Healthcare Research and Quality for the US Preventative Services Task Force both of which identified vitamin D as an effective intervention to prevent falling in older adults.5
There has been a lot of debate as to whether vitamin D deficiency increases risk for cardiovascular disease. Several studies have found associations between 25(OH)D concentrations and hypertension, coronary artery calcification as well as prevalent and incident heart disease.5 Prevalent myocardial infarction was found to be inversely associated with 25(OH)D concentrations.25 Individuals with concentrations below 15 ng/ml had a multi-variable-adjusted hazard ratio of 1.62 (95% Cl, 1.11–2.36) for incident cardiovascular events compared with those with concentrations above 15 ng/ml. The IOM concluded there was insufficient evidence to support cardiovascular benefits of vitamin D and this was supported by a recent meta-analysis.26 However as with many of the other systematic reviews most of the intervention studies used an amount of vitamin D that was less than 600 IU/d. A recent RCT of 49 normotensive black boys and girls aged 16.3 ± 1.5 y who received either 400 IU or 2,000 IU/d of vitamin D for 4 mo27 supports the fact that most of the studies in systematic reviews were using an inadequate amount of vitamin D for cardiovascular benefit. The teenagers who ingested 2,000 IU/d of vitamin D raised their blood level of 25(OH)D from 11 ng/ml to 34 ng/ml and had a significant reduction in arterial stiffness. Teenagers who received 400 IU/d of vitamin D increased their blood level of 25(OH)D from 11 ng/ml to 24 ng/ml showed no reduction in arterial stiffness. This is supported by the observation that serum 25(OH)D < 30 ng/ml was strongly associated with hypertension, elevated blood sugar and metabolic syndrome in adolescents.28
At the turn of the last century, children with rickets were at higher risk for developing upper respiratory tract infections and dying from them.29 In the mid-1800s cod liver oil was used as a therapy to treat TB. In the early 1900s solariums were routinely used for TB patients and it was observed that people who live at higher altitudes in Switzerland were immune from TB. Macrophages have a vitamin D receptor (VDR) and when they ingest an infectious agent such as TB the toll-like receptors are activated resulting in signal transduction to increase the expression of both VDR and the 25-hydroxyvitamin D-1-hydroxylase (cyp27B1; 1-OHase).29 25(OH)D is in turn converted to 1,25-dihydroxyvitamin D [1,25(OH)2D] which signals the nucleus to increase the expression for cathelicidin, a defensen protein that kills infective agents like TB. The IOM recognized this important vitamin D enhanced immune killing function but had various reasons for not accepting that vitamin D reduces risk for upper respiratory tract infections. Infectious diseases have enormous health implications globally not only for increasing risk for morbidity but also mortality.17 A multicenter double-blind, placebo controlled trial assessed the effect of supplementing Japanese schoolchildren aged 6–15 y with 1,200 IU of vitamin D3/d from December to March and found with influenza antigen testing and nasopharyngeal swab specimen analysis a 42% relative risk reduction in children who received the vitamin D supplementation compared with the children who did not.30 They also observed that children who took the vitamin D daily had a relative risk reduction of 93% for having an asthma attack compared with the children who did not take a vitamin D supplement. The IOM disregarded this study for several reasons. They argued that although there was a significant reduced risk of influenza A infection in a Japanese children between days 1 and 30 this did not persist between days 61 and the end of the study and an analysis of other related secondary outcomes showed no significant difference in influenza B, influenza-like illness among others. But the Japanese study is consistent with a study in 198 healthy adults with blood concentrations of 25(OH)D > 37 ng/ml reduced risk of developing acute viral respiratory tract infections and numbers of days ill 2-fold.31
The IOM disregarded association studies as being too low evidence to support any non-skeletal beneficial claims for vitamin D. Many association studies have provided great insight into cause and effect relationships; the classic one being the cholera epidemic in Great Britain and the association with contaminated water. Semmelweis in 1840 introduced hand washing with chlorinated soda water as an effective method for reducing maternal and infant mortality in a Vienna Hospital. He reported the association of poor sanitation and lack of hand washing with increased mortality but the thought leaders were convinced the cause was bad humors and so derided his recommendations that he admitted himself to an insane asylum and he died of an infectious disease. In 1822, Sniadecki associated high incidence of rickets in Warsaw compared with the absence of rickets in areas outside of Warsaw with the lack of sun exposure.32 This insightful observation was disregarded by the thought leaders as being trivial. As a result tens of thousands of children would suffer the devastating consequences of the bone deforming disease rickets for another 100 years.
During that time rickets was so prevalent in the industrialized cities in the United States and Europe that numerous intervention studies were initiated to find a causal relationship. Findlay, a prominent Scottish physician, in 1908 was convinced rickets was caused by lack of activity and not lack of sun exposure and to prove the point he did an intervention study whereby he put rodents in a glass enclosure so that they could not move and exposed them to sunlight and demonstrated they developed rickets. He didn't realize that the lead containing glass absorbed all vitamin D producing solar radiation and therefore his conclusion was incorrect. Koch infected puppies with a bacillus bacterium and concluded that rickets was caused by an infectious disease.33 At the same time children exposed to radiation from a mercury arc lamp or sunlight were reported to be cured of their rickets.32 Therefore 100 years after the first association study suggesting sunlight deprivation was the cause of rickets was it finally accepted that this was a “definite and dependable cure of rickets.”32
The association of the beneficial effect of sun exposure for health can be dated back to the early Egyptians who worshiped the sun, as did many other cultures for its life giving benefits. Hoffman reported that living in cities and at higher latitudes between 1908 and 1912 was associated with increased cancer mortality. Peller and Stephenson in the 1930s reported the rate of skin cancer in the US. Navy was 8 times higher than in the civilian population but that the total number of deaths resulting from other cancers was 60% less.17 Apperly reported reduced cancer mortality in adults who lived in the Southern United States compared with living in the Northeast.17 In the 1980s and 1990s several investigators independently reported epidemiologic studies that evaluated correlation between cancer, type 1 diabetes, multiple sclerosis, schizophrenia and hypertension and higher risk when living at higher latitudes.14-17 Living at higher latitudes results in increased zenith angle for the sun’s radiation to penetrate to the earth thereby reducing the amount of UVB radiation that is responsible for producing vitamin D. A study in California suggested the overall increase in occurrence of colon cancer was 7.5–10.5% per degree latitude independent of race.17 If you were born near the equator you had a 10–15-fold reduced risk for developing type 1 diabetes (Fig. 2A) and 100% reduced risk for developing MS.17,34 Blood pressure was directly related to the latitude at which you lived35 (Fig. 2B) and children born at high latitudes and at the end of the winter are at higher risk for developing schizophrenia.36 Grant conducted a systematic review and reported an inverse relationship with cancer mortality in both men and women and exposure to solar UVB radiation.14 He calculated over a span of 24 y (between 1970 and 1994) a total of 566,400 Americans die prematurely of one of 13 cancers because of inadequate exposure to solar UVB radiation.14
Figure 2. (A) Seasonal variation of 25-hydroxyvitamin D in 58 Aboriginal Australian men (solid circle) and women (solid triangle). Reproduced with permission from (11). (B) Comparison of serum 25(OH)D concentrations in healthy adults who were either in a bathing suit and exposed to suberythemal doses (0.5 MED) of UVB radiation once a week for three months compared with healthy adults who received 1,000 units of vitamin D3 daily during the winter and early spring for a period of 11 weeks. Skin type is based on the Fitzpatrick scale. The data represents mean ± SEM. Reproduced with permission, copyright Michael F. Holick, 2008. (C) Relationship of prevalence of hypertension to distance North or South of the equator. Broken lines represent 95% confidence limits. Regression line and confidence limits are derived from INTERNSTAT centers only. Reproduced with permission.34
The IOM appropriately noted that the fat-soluble vitamin D is not as toxic as once thought and increased the upper limit for most children and adults to 4,000 IU/d. The IOM recognized that adults taking up to 10,000 IU/d of vitamin D for 5 mo did not cause toxicity but did not raise the UL for adults, unlike what the Endocrine Society's Clinical Practice Guideline recommended.5 This is of some consequence because even though the IOM stated that obesity was associated with vitamin D deficiency and that obese adults required more vitamin D to satisfy their requirement they did not consider this either in their recommendations or for the UL. The Endocrine Society’s Clinical Practice Guidelines recommended to treat vitamin D deficiency in adults with 50,000 IU of vitamin D once a week for 8 weeks; this is equivalent to ingesting ~7,000 IU/d.5 They further recommended that obese adults may require 2–3 times which would far exceed the UL. Recent studies in lactating women who receive 4,000 or 6,400 IU/d did not result in any toxicity but was effective in increasing the vitamin D content in their milk to satisfy their infants’ requirement.5,37,38
The IOM also cautioned about widespread increase in vitamin D supplementation because of a few reports suggesting that enhanced vitamin D supplementation and increasing 25(OH)D > 30 ng/ml increased mortality. They did acknowledge increased mortality when the 25(OH)D < 15 ng/ml. Their plotted data demonstrated decreased mortality until 30 ng/ml and then showed that the line reversed and there was increased risk for mortality above 30 ng/ml. One of the references that was used to support this conclusion was by Melamed et al.39 These authors however concluded there was a lower risk of mortality for 25(OH)D of 30–49 ng/ml and that there may be a higher risk for mortality in women but not in men when the blood level of 25(OH)D > 50 ng/ml. Similar analyses have been made by Zitterman et al.40 and Schottker et al.41
The IOM concluded that widespread vitamin D deficiency in the North American population is not well-founded. This statement is not supported by the evidence. Even the CDC reports vitamin D deficiency is common in all age groups in United States and that vitamin D deficiency is on the increase because of decreased milk consumption, increased sun protection and obesity.8,42 The IOM’s concern about skin cancer precluded them from making recommendations about sun exposure even though they acknowledged that casual sun exposure played an important role in a person’s vitamin D status. Humans have and continue to obtain a significant amount of their vitamin D requirement from sun exposure.7,10,11,17,42 Although excessive exposure to sunlight increases risk for nonmelanoma skin cancer which is easy to detect and easy to treat there is no evidence that sensible sun exposure, as our hunter gatherer forefathers likely experienced, increases risk. More importantly the most deadly form of skin cancer melanoma which occurs on the least sun exposed areas is less likely to occur in adults who have outdoor occupations.39,43,44 Therefore it is not unreasonable to consider sensible sun exposure as a good source of vitamin D.7,17
Essentially every organ and cell in the body has a vitamin D receptor. More than 200 genes, and some have estimated up to 2,000 genes, are directly or indirectly regulated by 1,25(OH)2D.14-17 This hormone is one of the most effective biochemicals capable of keeping cell growth in check by modulating proliferation and differentiation and when a cell has a propensity to become malignant to induce apoptosis and inhibit angiogenesis. This has been clinically demonstrated by the effective use of 1,25(OH)2D3 in treating the hyperproliferative skin disorder psoriasis.7 1,25(OH)2D3 has been reported in animal models and in cultured cells to improve insulin production, modulate T and B cell activity, enhance phagocytic killing activity, improve vascular smooth muscle resistance, reduce risk of developing autoimmune diseases and inhibit cancer cell growth.5,14-17
What can we conclude from the recent IOM report and the Endocrine Society’s Clinical Practice Guidelines relating vitamin D to overall health and well-being? The good news is that hopefully by the IOM increasing the vitamin D requirement 3-fold for most children and adults that this will be the impetus to have food processors increase the amount of vitamin D in fortified foods and increase the number of foods fortified with vitamin D. Supplement manufacturers have already begun to increase the amount of vitamin D in their calcium and multivitamin supplements. Many countries still do not either permit or promote vitamin D fortification of dairy products. Vitamin D fortification was common in Europe before 1950. An outbreak of hypercalcemia in infants that was thought to be caused by overfortification of milk with vitamin D that caused vitamin D intoxication and led to the banning of vitamin D fortification in most European countries.7,32,45 However this was never proven and some believe that these infants had William syndrome which made these infants hypersensitive to vitamin D. It is even difficult today to obtain a vitamin D supplement in some countries other than the US, Canada and some European countries without a doctor's prescription.
The IOM doubled the UL for vitamin D and the Endocrine Society’s Clinical Practice Guidelines recommended for adults a UL of 10,000 IU. These recommendations hopefully will also provide health care professionals and health care regulators with a level of comfort about making available more vitamin D fortified foods and supplements containing vitamin D.
There are now several thousand publications that support the non-skeletal health benefits of vitamin D that should not be ignored either because they are association studies or small randomized controlled trials. There is no evidence that there is a downside to increasing vitamin D intake in children and adults with the exception of those with chronic granuloma forming disorder or lymphoma.5,7 It will take several more years to hear from several ongoing large RCTs evaluating non-skeletal benefits of vitamin D. If you believed and followed the IOM recommendation of 200 IU/d in 1997 then for the past decade you were likely vitamin D deficient. 600 IU/d that the IOM now recommends will raise and maintain blood concentrations of 25(OH)D > 20 ng/ml but < 30 ng/ml. Based on the overwhelming cumulative reports this is not satisfactory to obtain all of the health benefits of vitamin D. The evidence-based recommendations by the Endocrine Society’s Clinical Practice Guidelines are more realistic (400–1,000 IU for children, 1,500–2,000 IU for adults to maintain 25(OH)D concentrations of 40–60 ng/ml for preventing and treating vitamin D deficiency.5,46
Acknowledgments
This work was supported in part by the UV Foundation.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/dermatoendocrinology/article/20015
References
- 1.Standing Committee on the Scientific Evaluation of Dietary Reference Intakes Food and Nutrition Board Institute of Medicine 1997 Vitamin D. In: Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. National Academy Press, Washington, DC, USA; 1999. p. 250-287. [Google Scholar]
- 2.IOM (Institute of Medicine) 2011. Dietary Reference Intakes for Calcium and Vitamin D. Committee to Review Dietary Reference Intakes for Calcium and Vitamin D. Washington DC: The National Academies Press. Institute of Medicine. 2011.
- 3.Priemel M, von Domarus C, Klatte TO, Kessler S, Schlie J, Meier S, et al. Bone mineralization defects and vitamin D deficiency: histomorphometric analysis of iliac crest bone biopsies and circulating 25-hydroxyvitamin D in 675 patients. J Bone Miner Res. 2010;25:305–12. doi: 10.1359/jbmr.090728. [DOI] [PubMed] [Google Scholar]
- 4.Maxmen A. Nutrition advice: the vitamin D-lemma. Nature. 2011;475:23–5. doi: 10.1038/475023a. [DOI] [PubMed] [Google Scholar]
- 5.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Endocrine Society Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 6.Heaney RP, Holick MF. Why the IOM recommendations for vitamin D are deficient. J Bone Miner Res. 2011;26:455–7. doi: 10.1002/jbmr.328. [DOI] [PubMed] [Google Scholar]
- 7.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 8.Looker AC, Johnson CL, Lacher DA, Pfeiffer CM, Schleicher RL, Sempos CT. Vitamin D status: United States, 2001-2006. NCHS Data Brief. 2011:1–8. [PubMed] [Google Scholar]
- 9.Moore C, Murphy MM, Keast DR, Holick MF. Vitamin D intake in the United States. J Am Diet Assoc. 2004;104:980–3. doi: 10.1016/j.jada.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 10.Brot C, Vestergaard P, Kolthoff N, Gram J, Hermann AP, Sørensen OH. Vitamin D status and its adequacy in healthy Danish perimenopausal women: relationships to dietary intake, sun exposure and serum parathyroid hormone. Br J Nutr. 2001;86(Suppl 1):S97–103. doi: 10.1079/BJN2001345. [DOI] [PubMed] [Google Scholar]
- 11.Vanlint SJ, Morris HA, Newbury JW, Crockett AJ. Vitamin D insufficiency in Aboriginal Australians. Med J Aust. 2011;194:131–4. doi: 10.5694/j.1326-5377.2011.tb04195.x. [DOI] [PubMed] [Google Scholar]
- 12.Matsuoka LY, Wortsman J, Hanifan N, Holick MF. Chronic sunscreen use decreases circulating concentrations of 25-hydroxyvitamin D. A preliminary study. Arch Dermatol. 1988;124:1802–4. doi: 10.1001/archderm.1988.01670120018003. [DOI] [PubMed] [Google Scholar]
- 13.Wactawski-Wende J, Kotchen JM, Anderson GL, Assaf AR, Brunner RL, O’Sullivan MJ, et al. Women’s Health Initiative Investigators Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006;354:684–96. doi: 10.1056/NEJMoa055222. [DOI] [PubMed] [Google Scholar]
- 14.Grant WB. An estimate of premature cancer mortality in the U.S. due to inadequate doses of solar ultraviolet-B radiation. Cancer. 2002;94:1867–75. doi: 10.1002/cncr.10427. [DOI] [PubMed] [Google Scholar]
- 15.Moan J, Porojnicu AC, Dahlback A, Setlow RB. Addressing the health benefits and risks, involving vitamin D or skin cancer, of increased sun exposure. Proc Natl Acad Sci U S A. 2008;105:668–73. doi: 10.1073/pnas.0710615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 17.Holick MF. The D-batable Institute of Medicine report: a D-lightful perspective. Endocr Pract. 2011;17:143–9. doi: 10.4158/ep.17.1.143. [DOI] [PubMed] [Google Scholar]
- 18.Kristal AR. Are clinical trials the “gold standard” for cancer prevention research? Cancer Epidemiol Biomarkers Prev. 2008;17:3289–91. doi: 10.1158/1055-9965.EPI-08-1066. [DOI] [PubMed] [Google Scholar]
- 19.Bolland MJ, Grey A, Gamble GD, Reid IR. Calcium and vitamin D supplements and health outcomes: a reanalysis of the Women’s Health Initiative (WHI) limited-access data set. Am J Clin Nutr. 2011;94:1144–9. doi: 10.3945/ajcn.111.015032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85:1586–91. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- 21.Pfeifer M, Begerow B, Minne HW, Suppan K, Fahrleitner-Pammer A, Dobnig H. Effects of a long-term vitamin D and calcium supplementation on falls and parameters of muscle function in community-dwelling older individuals. Osteoporos Int. 2009;20:315–22. doi: 10.1007/s00198-008-0662-7. [DOI] [PubMed] [Google Scholar]
- 22.Broe KE, Chen TC, Weinberg J, Bischoff-Ferrari HA, Holick MF, Kiel DP. A higher dose of vitamin d reduces the risk of falls in nursing home residents: a randomized, multiple-dose study. J Am Geriatr Soc. 2007;55:234–9. doi: 10.1111/j.1532-5415.2007.01048.x. [DOI] [PubMed] [Google Scholar]
- 23.Elamin KB, AbuElnour NO, Elamin KB, Fatourechi MM, Alkatib AA, Almandoz JP, et al. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:1931–42. doi: 10.1210/jc.2011-0398. [DOI] [PubMed] [Google Scholar]
- 24.Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, Orav JE, Stuck AE, Theiler R, et al. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. BMJ. 2009;339:b3692. doi: 10.1136/bmj.b3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–11. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elamin MB, Abu Elnour NO, Elamin KB, Fatourechi MM, Alkatib AA, Almandoz JP, et al. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:1931–42. doi: 10.1210/jc.2011-0398. [DOI] [PubMed] [Google Scholar]
- 27.Dong Y, Pollock N, Stallmann-Jorgensen IS, Gutin B, Lan L, Chen TC, et al. Low 25-hydroxyvitamin D levels in adolescents: race, season, adiposity, physical activity, and fitness. Pediatrics. 2010;125:1104–11. doi: 10.1542/peds.2009-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar J, Muntner P, Kaskel FJ, Hailpern SM, Melamed ML. Prevalence and associations of 25-hydroxyvitamin D deficiency in US children: NHANES 2001-2004. Pediatrics. 2009;124:e362–70. doi: 10.1542/peds.2009-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adams JS, Hewison M. Update in vitamin D. J Clin Endocrinol Metab. 2010;95:471–8. doi: 10.1210/jc.2009-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr. 2010;91:1255–60. doi: 10.3945/ajcn.2009.29094. [DOI] [PubMed] [Google Scholar]
- 31.Sabetta JR, DePetrillo P, Cipriani RJ, Smardin J, Burns LA, Landry ML. Serum 25-hydroxyvitamin d and the incidence of acute viral respiratory tract infections in healthy adults. PLoS One. 2010;5:e11088. doi: 10.1371/journal.pone.0011088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 2006;116:2062–72. doi: 10.1172/JCI29449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eliot MM, Park EA. 1938. Rickets. In Brennemann’s Practice of Pediatrics. Volume 1. W.F. Prior Company, Inc., 1-110. [Google Scholar]
- 34.Mohr SB, Garland CF, Gorham ED, Garland FC. The association between ultraviolet B irradiance, vitamin D status and incidence rates of type 1 diabetes in 51 regions worldwide. Diabetologia. 2008;51:1391–8. doi: 10.1007/s00125-008-1061-5. [DOI] [PubMed] [Google Scholar]
- 35.Rostand SG. Ultraviolet light may contribute to geographic and racial blood pressure differences. Hypertension. 1997;30:150–6. doi: 10.1161/01.hyp.30.2.150. [DOI] [PubMed] [Google Scholar]
- 36.McGrath J, Eyles D, Mowry B, Yolken R, Buka S. Low maternal vitamin D as a risk factor for schizophrenia: a pilot study using banked sera. Schizophr Res. 2003;63:73–8. doi: 10.1016/S0920-9964(02)00435-8. [DOI] [PubMed] [Google Scholar]
- 37.Hollis BW. Vitamin D requirement during pregnancy and lactation. J Bone Miner Res. 2007;22(Suppl 2):V39–44. doi: 10.1359/jbmr.07s215. [DOI] [PubMed] [Google Scholar]
- 38.Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res. 2011;26:2341–57. doi: 10.1002/jbmr.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Melamed ML, Michos ED, Post W, Astor B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168:1629–37. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zittermann A, Iodice S, Pilz S, Grant WB, Bagnardi V, Gandini S. Vitamin D deficiency and mortality risk in the general population: a meta-analysis of prospective cohort studies. Am J Clin Nutr. 2012;95:91–100. doi: 10.3945/ajcn.111.014779. [DOI] [PubMed] [Google Scholar]
- 41.Schöttker B, Ball D, Gellert C, Brenner H. Serum 25-hydroxyvitamin D levels and overall mortality. A systematic review and meta-analysis of prospective cohort studies. Ageing Res Rev. 2012 doi: 10.1016/j.arr.2012.02.004. [Epub] [DOI] [PubMed] [Google Scholar]
- 42.Nesby-O’Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988-1994. Am J Clin Nutr. 2002;76:187–92. doi: 10.1093/ajcn/76.1.187. [DOI] [PubMed] [Google Scholar]
- 43.Kennedy C, Bajdik CD, Willemze R, De Gruijl FR, Bouwes Bavinck JN, Leiden Skin Cancer Study The influence of painful sunburns and lifetime sun exposure on the risk of actinic keratoses, seborrheic warts, melanocytic nevi, atypical nevi, and skin cancer. J Invest Dermatol. 2003;120:1087–93. doi: 10.1046/j.1523-1747.2003.12246.x. [DOI] [PubMed] [Google Scholar]
- 44.Garland FC, White MR, Garland CF, Shaw E, Gorham ED. Occupational sunlight exposure and melanoma in the U.S. Navy. Arch Environ Health. 1990;45:261–7. doi: 10.1080/00039896.1990.10118743. [DOI] [PubMed] [Google Scholar]
- 45.Gillie O. The Scots’ Paradox: can sun exposure, or lack of it, explain major paradoxes in epidemiology? Anticancer Res. 2012;32:237–48. [PubMed] [Google Scholar]
- 46.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Guidelines for Preventing and Treating Vitamin D Deficiency and Insufficiency Revisited. J Clin Endocrinol Metab. 2012 doi: 10.1210/jc.2011-2601. In press. [DOI] [PubMed] [Google Scholar]