Abstract
<u>Background:</u> Vitamin D insufficiency is common in cystic fibrosis (CF) and vitamin D repletion may have an important role in improving clinical outcomes in CF. This randomized, placebo-controlled, pilot study examined the feasibility and impact of a single, large dose of cholecalciferol on vitamin D status and clinical outcomes in subjects with CF.
<u>Methods:</u> Thirty adults with were randomized in a double-blinded, pilot study to receive 250,000 IU cholecalciferol or placebo within 48 h of hospital admission for a pulmonary exacerbation. Concentrations of 25-hydroxyvitamin D (25(OH)D), clinical outcomes and potential adverse events were assessed up to one year after randomization. Mixed effects linear regression models were used to evaluate the difference in mean serum concentrations and log-rank analyses were used to evaluate survival.
<u>Results:</u> Data from all subjects was analyzed. Serum 25(OH)D concentrations increased from a mean of 30.6 ± 3.2 ng/mL to 58.1 ± 3.5 ng/mL (p < 0.001) at one week and 36.7 ± 2.6 ng/mL by 12 weeks (p = 0.06) in the vitamin D group; in contrast, serum 25(OH)D concentrations remained unchanged in the placebo group. Unadjusted, one-year survival and hospital-free days were increased in the vitamin D group (p = 0.029, p = 0.036; respectively). There was also a trend toward increased IV antibiotic therapy-free days in the vitamin D group (p = 0.073). There were no signs of hypervitaminosis D or adverse events. Serum PTH and calcium concentrations were similar across both groups.
<u>Conclusions:</u> In this pilot study, a single, oral bolus of cholecalciferol increased serum 25(OH)D concentrations and was associated with a trend toward improved clinical outcomes in CF subjects hospitalized for a pulmonary exacerbation. Further investigation is needed into the clinical impact of improved vitamin D status in patients with CF.
Keywords: vitamin D, cystic fibrosis, parathyroid hormone, anti-microbial peptide, pulmonary exacerbation
Introduction
Cystic fibrosis (CF) is the most common life-shortening, inherited disease among Caucasians in the United States.1 Morbidity and mortality in individuals with CF is primarily due to progressive lung disease and recurrent pulmonary infections. Adults and children with CF have a high prevalence of vitamin D deficiency despite increased awareness and guidelines for treatment of vitamin D deficiency.2-5
Vitamin D insufficiency [25(OH)D < 30 ng/mL] may be particularly detrimental in the CF population.6 Individuals with CF are at greater risk of several conditions reported to have strong epidemiologic associations with vitamin D insufficiency including: low bone mineral density, diabetes, decreased lung function, respiratory infections and dysregulation of the adaptive and innate immune response.3,7-10 Vitamin D insufficiency has been associated with increased systemic inflammation, which has been identified as a contributor to respiratory failure in patients with CF.11-14 Therefore, increasing vitamin D status may translate into improved clinical outcomes in CF.
The primary objective of this pilot study was to evaluate the feasibility and clinical impact of a high-dose vitamin D supplementation in patients with CF hospitalized for treatment of a pulmonary exacerbation. The hypothesis of this study was that high dose vitamin D would rapidly increase vitamin D status in CF patients, which would translate into improved clinical outcomes and markers of health. The findings reported in this manuscript help advance the science of vitamin D in improving health outcomes in individuals with CF. We report the impact of a single 250,000 IU dose of cholecalciferol vs. placebo on serum concentrations of 25(OH)D, parathyroid hormone (PTH), and calcium. We also evaluated key clinical outcomes: survival, hospitalizations, IV antibiotic therapy and lung function up to 12 mo after randomization.
Results
Subject demographics
Thirty subjects, 15 per group, were enrolled. Baseline characteristics of the treatment and placebo groups were similar (Table 1). Three patients in the placebo group died due to CF-related causes before completing the 12-week visit, and one patient in the placebo group was unable to be studied at the 1-week visit. There were no significant differences between the groups in microbiology at the time of hospitalization or the types of antibiotic therapy during hospitalization. The length of IV therapy was similar between the groups. In the vitamin D group, the median length of IV therapy was 5 d and in the placebo group, 6 d (p = 0.2).
Table 1. Baseline characteristics of the study sample.
| Vitamin D3† | Placebo† | p value‡ | |
|---|---|---|---|
|
Age, years |
24.9 (16.01) |
28.2 (30.89) |
0.06 |
|
Gender, % male |
60.0 (9) |
53.3 (8) |
0.71 |
|
BMI, kg/m2 |
18.5 (12.04) |
21.0 (15.70) |
0.30 |
|
Race, % Caucasian |
100.0 (15) |
80.0 (12) |
0.07 |
|
CF mutation: % homozygous ΔF508/ΔF508 |
53.3 (8) |
53.3 (8) |
0.51 |
|
% Heterozygous ΔF508/____ |
33.3 (5) |
13.3 (2) |
|
|
% Unknown |
13.3 (2) |
33.3 (5) |
|
|
Reported vitamin D supplementation, % |
73.3 (11) |
80.0 (12) |
0.67 |
|
Vitamin D intake from supplements, IU/day |
400.0 (2600) |
400.0 (2800) |
0.80 |
|
Season of admission, % |
|
|
|
|
Spring/summer |
60.0 (9) |
46.7 (7) |
0.46 |
|
Pancreatic insufficiency, % |
86.7 (13) |
100.0 (15) |
0.14 |
|
CF-related diabetes mellitus, % |
40.0 (6) |
60.0 (9) |
0.27 |
|
Serum creatinine, mg/dL |
0.8 (0.79) |
0.8 (0.61) |
0.85 |
|
Serum albumin, mg/Dl |
3.4 (1.03) |
3.1 (1.50) |
0.06 |
|
Number of hospital-free days in previous year |
355.0 (51.0) |
357.0 (96.0) |
1.00 |
|
Best FEV1% of predicted since most recent pulmonary exacerbation |
51.0 (100.0) |
45.5 (71.0) |
0.48 |
|
Baseline FEV1% of predicted§ |
67.0 (103.0) |
50.0 (71.0) |
0.45 |
|
Admission FEV1% of predicted |
33.5 (98.0) |
35.0 (71.0) |
0.95 |
|
Mean decrease in FEV1 and of predicted from baseline to admission |
7.0 (20.0) |
6.0 (31.0) |
0.33 |
|
% of subjects per group with > 10% decrease in FEV1 from baseline to admission (n, total = 18) |
71.4 (10) | 57.1 (8) | 0.43 |
† Median (range) or percentage (n); ‡Wilcoxan-Mann-Whitney test to compare means, Fisher’s exact test to compare categorical variables; §Best FEV1% of predicted > 30 d and < 6 mo before randomization.
Impact of 250,000 IU of vitamin D3 on serum 25(OH)D, calcium and PTH concentrations
The mean serum 25(OH)D concentrations at baseline did not differ between the groups (p = 0.71). At baseline, 60% of subjects in the vitamin D group and 47% in the placebo group were vitamin D sufficient (serum 25(OH)D concentration ≥ 30 ng/mL). At 7 d, all subjects in the vitamin D group were sufficient; there was no change in the proportion of vitamin D sufficiency in the placebo group. The mean serum 25(OH)D concentrations for both groups at all three time points, with p-values for time point-specific mean comparisons, are shown in Table 2. At 1 week, mean serum 25(OH)D increased in the vitamin D group by 27.5 ± 3.8 ng/mL (p < 0.001) and in the placebo group it decreased by 0.2 ± 1.4 ng/mL (p = 0.64). Correspondingly, the week 1 difference in mean concentrations between groups was highly significant (p < 0.001). Figure 1 provides a visual representation of these relationships over the three time points. Adjusting for baseline age and FEV1 (forced expired volume in 1 second), did not change this relationship (Table 2). The maximum 25(OH)D concentration in an individual subject at 1-week was 83 ng/mL and 47 ng/mL in the vitamin D and placebo groups, respectively.
Table 2. Measures of vitamin D and calcium status of adult CF patients receiving 250,000 IU cholecalciferol vs. placebo at baseline (prior to treatment), 1 week and 12 weeks.
| Vitamin D3† | Placebo† | p value‡ | p value§ | ||
|---|---|---|---|---|---|
|
25(OH)D, ng/ml |
Baseline |
30.6 (3.2) |
28.7 (3.5) |
0.69 |
0.71 |
| |
1 week |
58.1 (3.5) |
28.9 (3.6) |
< 0.001 |
< 0.001 |
| |
12 weeks |
36.7 (2.6) |
28.0 (4.1) |
0.09 |
0.13 |
|
PTH,‖pg/ml |
Baseline |
44.6 (9.2) |
75.8 (15.9) |
0.11 |
0.19 |
| |
1 week |
39.8 (12.8) |
56.6 (23.2) |
0.81 |
0.89 |
| |
12 weeks |
32.4 (6.0) |
32.5 (6.7) |
0.85 |
0.79 |
|
Calcium, mg/dl |
Baseline |
9.0 (0.12) |
8.7 (0.17) |
0.12 |
0.15 |
| |
1 week |
8.9 (0.08) |
8.9 (0.11) |
0.90 |
0.99 |
| 12 weeks | 9.0 (0.09) | 8.9 (0.09) | 0.90 | 0.96 |
† Unadjusted mean values (standard error), mixed effects linear regression model;
† Mixed linear regression model;
§ Mixed linear model adjusted for age and FEV1;
‖ Data was log transformed prior to modeling.
Figure 1. Unadjusted, mean change in 25-hydroxyvitamin D in response to a bolus dose of 250,000 IU of vitamin D3 or placebo. The dashed line (vitamin D group) and solid line (placebo group) represent the unadjusted mean change in serum 25-hydroxyvitamin D concentrations [25(OH)D] at baseline, 1 week and 12 weeks in adult CF subjects randomized to either a single 250,000 IU dose of cholecalciferol or placebo. The change in mean serum 25(OH)D from baseline in the vitamin D group was +27.5 (± 13) ng/mL and +6.2 (± 11) ng/mL at week 1 and 12, respectively; contrasted to the change from baseline in the placebo group of -0.2 (± 13) ng/mL and -0.6 (± 14) ng/mL at week 1 and 12, respectively. (Mean with standard error bars, comparison between groups of the change from baseline at week 1, p < 0.001; at week 12, p = 0.06, Student’s t-test).
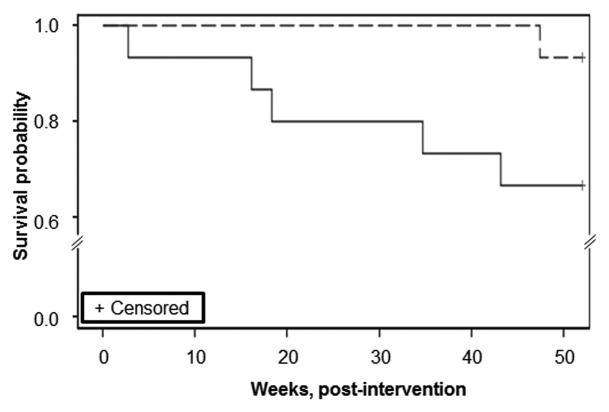
Figure 2. Survival analysis by treatment group. The dashed line (vitamin D group) and solid line (placebo group) represent the unadjusted survival curves of adult CF patients (n = 30) randomized to either a single, oral 250,000 IU dose of cholecalciferol or identically matched placebo. Mortality was determined by CF clinic records over the following 12 mo. Subjects receiving cholecalciferol had a significantly increased survival over placebo (Log-rank analysis, p = 0.029). When adjusted for age and FEV1% of predicted, there continued to be a trend for increased survival in the vitamin D group (Log-rank analysis, p = 0.09).
Subjects exhibited good tolerance of the 250,000 IU dose of cholecalciferol; there were no reported symptoms of vitamin D toxicity as assessed by patient questionnaire at any study visit. There were also no clinical signs of hypercalcemia. As seen in Table 2, there were no significant changes in mean serum calcium or PTH concentrations in either group and no significant differences in these means across groups at any time point.
Clinical outcomes
Subjects were followed up to 12 mo post-randomization or until death for clinical outcomes. Subjects in the vitamin D group exhibited an increase in the number of hospital-free and IV antibiotic therapy-free days as reported in Table 3. Prior to admission, the groups had an equal number of hospital-free days (Table 1). The total number of hospital-free days in 6 mo post-intervention in the vitamin D group was 168.6 ± 3.8 compared with 133.3 ± 14.8 in the placebo group (p = 0.036), a difference of 26.4%. There was also a positive trend in the number of IV antibiotic therapy-free days over 6 mo post-intervention in the vitamin D vs. the placebo group (153.6 ± 7.4 and 121.1 ± 15.4, respectively; p = 0.08).
Table 3. Change in clinical outcomes post-intervention of adult CF patients receiving 250,000 IU cholecalciferol vs. placebo.
| Vitamin D3 | Placebo | p value | |
|---|---|---|---|
| Number of hospitalization-free days, 6 mo post-intervention |
169 (4) |
133 (15) |
0.04† |
| Number of IV antibiotic-free days, 6 mo post-intervention |
154 (7) |
121 (15) |
0.07† |
| FEV1% of predicted (n = 18‖) Proportion returned to > 95% of baseline before each subject’s next recorded pulmonary exacerbation, 3 mo post-intervention, % |
90 (9) |
50 (4) |
0.12‡ |
| Mortality 12 weeks post-intervention (n) 12 mo post-intervention (n) |
0 1 |
3 5 |
0.03§ |
Values are the mean (± SEM); †Student’s t-test; ‡Fisher’s exact test; §Log-rank analysis, when adjusted for age and FEV1% of predicted p = 0.09; ‖Only subjects whose FEV1% of predicted decreased greater than 10% from baseline to admission were included in this analysis.
There was also a trend toward improved lung function in subjects receiving vitamin D compared with placebo assessed by the proportion of subjects who returned to ≥ 95% of baseline lung function. At admission, 18 of the 30 subjects had a ≥ 10% decrease in FEV1 from baseline. Of the subjects who had a decrease in FEV1 at admission, 9 out of 10 (90%) subjects receiving vitamin D returned to ≥ 95% FEV1, compared with 4 out of 8 (50%) subjects in the placebo group (p = 0.12).
Subjects who received vitamin D had a lower, unadjusted one-year mortality rate compared with subjects who received placebo, with 5 deaths in the placebo vs. 1 death in the treatment group at 12 mo (Table 3). Baseline FEV1, BMI, age, and CF-related diabetes status were assessed as potential confounders, but none were individually significant. Survival analysis via the log-rank test suggested a statistically significantly higher risk for death in the placebo group over the 12 mo follow-up period (p = 0.029; Fig. 2). When adjusted for age and baseline FEV1, this p-value increased to 0.09. There was no difference between the vitamin D and placebo groups in changes in BMI.
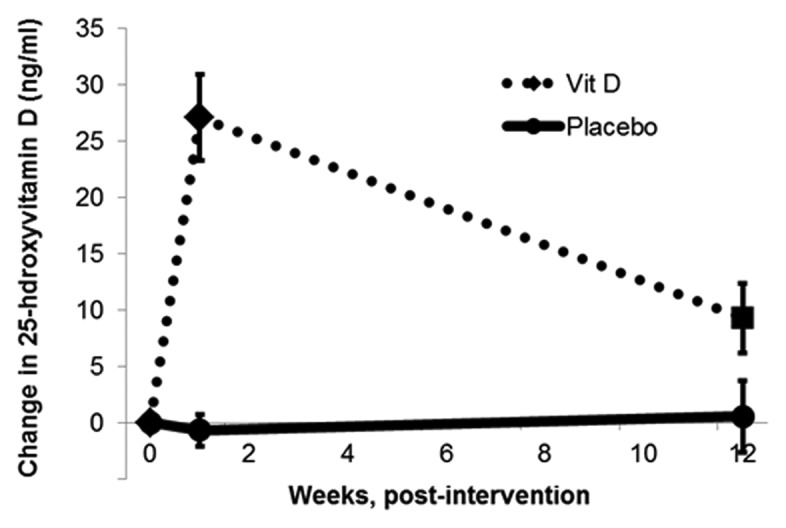
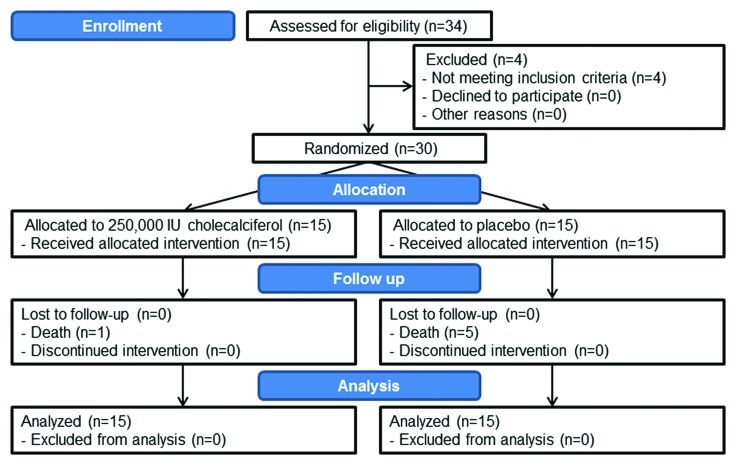
Figure 3. Recruitment and allocation of subjects. Subjects were recruited from the Emory Adult CF Center within 48 h of admission to the Emory University Hospital for the treatment of an acute pulmonary exacerbation. A total of 34 subjects were assessed for eligibility, 4 were ineligible and 30 subjects were block randomized to either 250,000 IU vitamin D3 or placebo. All subject deaths were related to complications of cystic fibrosis. All patients were followed for 1-y or until death and data from all patients was analyzed for this study.
Discussion
The goal of this double-blinded, randomized, placebo-controlled pilot study was to assess the feasibility and clinical impact of administering a single 250,000 IU dose of cholecalciferol to CF subjects hospitalized for a pulmonary exacerbation. We demonstrate that this novel dosing strategy safely improved or maintained vitamin D status at one week and did not produce evidence of vitamin D toxicity in any subject at any subject visit. Vitamin D supplementation may also have improved clinical outcomes by increasing the number of hospital-free days over 6 mo post-intervention. There was also a trend toward increased recovery of lung function, increased 1-y survival and decreased home IV antibiotic therapy. Considered together, the results of this pilot study strongly support further analyses of the impact of vitamin D supplementation in CF.
Optimal vitamin D status continues to be debated both within the CF and general populations as demonstrated by the discrepancy in recommendations for vitamin D status and supplementation by the IOM and the Endocrine Society.15,16 We found a striking improvement in vitamin D status at 1 week with a trend for improved status over 12 weeks. As has been observed in other studies of high dose vitamin D, we found no adverse effects relating to hypervitaminosis D or hypercalcemia in the vitamin D group.2,17,18
A bolus dosing strategy is attractive in the CF population given the long circulating half-life of 25(OH)D, improved adherence, and the potential role of vitamin D in acute infection, especially during pulmonary exacerbation. Additionally, bolus dosing of cholecalciferol has improved vitamin D status in several studies in both healthy subjects and subjects with chronic disease.19,20 Vitamin D supplementation using daily or weekly dosing has not consistently proven effective in normalizing vitamin D status in patients with CF.21 In adults, weekly oral dosing of ergocalciferol, 50,000 IU or 100,000 IU, did not produce sufficiency in 92% of adult CF patients.2 In pediatric CF patients, doses up to 150,000 IU of ergocalciferol weekly produced sufficiency in a maximum of 42% of patients.17,22 However, in another pediatric trial over a shorter time span, 700,000 IU of ergocalciferol over two weeks did produce sufficiency in 17 of 18 subjects.18 There have been few studies that have compared ergocalciferol to cholecalciferol; we found a single study comparing ergocalciferol to cholecalciferol in a randomized, controlled trial of 600,000 IU over 12 weeks. In this study, the cholecalciferol group exhibited a more than 3 times greater increase in vitamin D status compared with the ergocalciferol group.4 These studies demonstrate the ineffectiveness of ergocalciferol and suggest that larger doses of vitamin D or the use of specifically cholecalciferol given over a shorter time period may be necessary to correct vitamin D status in patients with CF. Our pilot study demonstrated that a large, single dose of cholecalciferol was successful in correcting or maintaining vitamin D status and should be evaluated as a treatment for vitamin D insufficiency in CF, especially in the inpatient setting.
This is the first randomized, placebo-controlled pilot study of vitamin D repletion in patients with CF during acute pulmonary exacerbation. We found that unadjusted 1-y survival, hospitalization rates and use of IV antibiotics were improved in those receiving vitamin D compared with placebo. The subjects in this study had a high risk of mortality, at the baseline visit, 38% of subjects had an FEV1% of predicted < 30% and more than 75% had been hospitalized in the previous year. Patients with FEV1% of predicted < 30% have a 2-y mortality of 50% and those with increased frequency of pulmonary exacerbation also have a greater risk for mortality.23,24 Therefore, the high rate of mortality in this study may be related to the overall health of the subjects. Vitamin D deficiency has been associated with increased risk for all-cause, cardiovascular and infection-related mortality in the general population.25-28 Increased vitamin D status is associated with decreased markers of inflammation as well as better lung function, which are important factors in CF prognosis.7,29 Vitamin D sufficiency may increase the production of antimicrobial peptides which may decrease requirements for antibiotic therapy and hospitalization.12,13,30 As a modulator of inflammation, vitamin D status may decrease inflammatory damage to lung tissue thereby increasing lung function and survival in CF subjects. These factors may contribute to the favorable one-year survival and increase in hospital-free days we found in our high dose vitamin D group compared with placebo
Vitamin D status has also been positively associated with pulmonary function as measured by FEV1 in CF subjects.3,7,31 A positive association between vitamin D status and lung function has also been observed in other chronic lung diseases such as asthma and chronic obstructive pulmonary disease.32 The potential mechanisms of vitamin D that may improve lung function involve its effect on increasing muscle strength, decreasing the frequency of respiratory infection and/ or decreasing the severity of inflammation.9,13,33 Our study demonstrated a trend toward improved recovery of lung function following pulmonary exacerbation; however, this suggestive finding was not statistically significant, possibly due to our small sample size.
This pilot study has several limitations; primarily, as a pilot study, it is limited by a small sample size that may have contributed to our inability to draw statistically significant conclusions regarding outcomes such as FEV1 and IV therapy-free days. However, these pilot data suggest that a study appropriately powered to evaluate these outcomes (and the sustainability of vitamin D changes at 12 weeks or beyond) could be very useful. This study is also limited by lack of information regarding the initiation of new medications and oral antibiotic therapy following the intervention. Future studies should examine how vitamin D supplementation may interact with other therapies and impact the frequency of pulmonary exacerbations. The placebo group was slightly, but not significantly, older than the vitamin D group, this may have been due to the small sample size. Both groups did have similar rates of hospitalization and lung function in the previous year. The current study is not an evaluation of the efficacy of this dose to treat vitamin D insufficiency in CF, since our study included patients who were vitamin D insufficient and sufficient at baseline. However, the effects of vitamin D may be more pronounced in subjects with lower baseline vitamin D status. Further, administration of this large dose of vitamin D to sufficient subjects without toxicity is a further indication of the safety of the supplementation strategy used in this study and the possible benefits of vitamin D supplementation in hospitalized CF patients.
In a population where correction of vitamin D status has been difficult, this pilot study describes a novel, single-dose strategy. When administered during acute pulmonary exacerbation of CF, it provides preliminary evidence that vitamin D supplementation may improve clinical outcomes. This method did not produce any evidence of vitamin D toxicity, even when administered to subjects with blood 25(OH)D concentrations > 30 ng/mL. Larger and longer term studies should be conducted to evaluate the impact of vitamin D on clinical outcomes and other markers of health in CF patients.
Materials and Methods
Subjects
This study was approved by the Emory University Institutional Review Board (project approval number: IRB00007572) before subject recruitment began in August 2008; recruitment was completed in May 2009. The study was registered with clinicaltrials.gov (NCT00788138). Subject recruitment and allocation are summarized in Figure 3, the CONSORT flow diagram.34,35 All subjects gave written informed consent before the initiation of study protocols.
All adult patients with CF followed at the Emory University CF Center and admitted to Emory University Hospital for treatment of a pulmonary exacerbation were eligible for this study. Pulmonary exacerbations were diagnosed by a pulmonologist (VS or AS) and were characterized by a constellation of symptoms and signs including increased cough, increased sputum production, increased crackles on auscultation, weight loss, and/or a decrease in lung function. Eligibility criteria included: age ≥ 18 y, serum 25(OH)D concentrations 5–75 ng/mL in the past year and current supplemental vitamin D intake < 2,000 IU/day. Subjects were excluded if they had a history of disorders that affect vitamin D, calcium or phosphorous metabolism; a history of organ transplant; currently pregnant or planning to become pregnant.
Design
Within 48 h of admission, subjects were screened for inclusion and exclusion criteria. Subjects were randomized to receive either a single oral dose of 250,000 IU cholecalciferol or an identically matched placebo according to a computer-generated, balanced randomization scheme in blocks of six. All vitamin D capsules were administered by the Emory Investigational Drug Service at Children’s Healthcare of Atlanta. Study personnel, caregivers, subjects and those assessing outcomes were blinded to the allocation of the study drug.
The cholecalciferol dose consisted of five capsules of 50,000 IU of cholecalciferol (total dose 250,000 IU, in a powder vehicle composed of lactose, microcrystalline cellulose and magnesium stearate contained in a capsule of hard gelatin from Tishcon, Inc.) or a matched, identical placebo (five matched capsules containing only the vehicle used in the cholecalciferol capsule). Vitamin D content of the capsules was certified by an independent laboratory (Analytical Research Laboratories) at the end of the study period to contain 96.7% of the expected amount of cholecalciferol. The study drug was ingested without regard to the timing of nutrient or pancreatic enzyme supplementation.
Blood was collected immediately prior to the administration of the study capsules, at 1 week and at 12 weeks post-intervention. Subjects were also followed at 6 mo and 12 mo through their scheduled quarterly CF clinic visits. The primary outcomes included: the change in serum 25(OH)D, calcium, and PTH concentrations. Secondary endpoints included lung function measured by percent of predicted FEV1; hospitalizations, IV antibiotic therapy and mortality. These outcomes were verified by a review of medical records and local data submitted to the national CF patient registry and accessed via Port CF. Symptoms of vitamin D toxicity (nausea, appetite, thirst, frequent urination, constipation, abdominal pain, muscle weakness, muscle and joint pain, confusion, lethargy and fatigue) were assessed by patient questionnaire at all study visits.
Hospital-free days were calculated as: number of days survival – days hospitalized = hospital-free days. Home IV antibiotic therapy-free days were also evaluated. IV antibiotic therapy-free days were calculated as: number of days survival – days treated with IV antibiotic therapy in the outpatient setting = IV antibiotic therapy-free days. Baseline FEV1 was determined as the subject’s highest value since their most recent pulmonary exacerbation recorded in the CF clinic records or in the previous 6 mo. FEV1 recovery to baseline was determined only in those subjects who had a > 10% decrease in FEV1 from baseline to admission. Recovery FEV1 was defined as the subject’s highest FEV1 in the 3 mo following randomization.
Analytical methods
Serum 25(OH)D and PTH were analyzed by ELISA (IDS, Ltd. and Immunotopics International, L.L.C., respectively). To ensure accuracy of the serum 25(OH)D measurements, our laboratory participates in the vitamin D external quality assessment scheme (DEQAS, site 606) and the NIST/NIH Vitamin D Metabolites Quality Assurance Program (VitDQAP). Serum calcium, albumin and creatinine were analyzed by standard methods in the Emory University Hospital clinical laboratories. Antibiotic therapy during hospitalization was determined from hospital patient records. Pathogens present at admission were determined by sputum analysis. Vitamin D insufficiency was defined as serum 25(OH)D < 30 ng/mL in accordance with the CF Foundation guidelines.36
Statistics
All statistical analyses were performed using SAS 9.2 (SAS Institute, Cary, NC). Previous analysis demonstrated that the mean 25(OH)D status of patients from the Emory CF center was 22.6 (± 10) ng/ml.4 A 10 ng/ml increase in 25(OH)D would produce mean 25(OH)D > 30 ng/ml; therefore, the enrollment of 30 subjects provided > 90% power to detect a 10 ng/mL difference in serum 25(OH)D between the two groups at a significance of level of α = 0.05. Fisher's exact chi-square tests and Wilcoxon-Mann-Whitney's tests were used to compare frequencies for categorical variables and means of continuous variables at baseline between the treatment groups. Variable assessments at each time point confirmed approximate normality of serum 25(OH)D and calcium concentrations; PTH values were log transformed due to their right-skewed distributions before inclusion in analysis. Paired t-tests were applied to assess within-group changes in mean serum concentrations from baseline.
Mixed effects linear regression models with a random intercept to account for within-subject correlations were used to evaluate the difference in mean serum concentrations of the vitamin D vs. the placebo group at each time point, based on repeated measurements of serum 25(OH)D, calcium, and PTH. Specifically, the following model was fit separately via the SAS MIXED procedure for four outcome variables.
| Y [serum 25(OH)D, calcium, and log(PTH):Yij = (β0 + b0i)] + β1GROUP + β2WEEK1 + β3WEEK12 + β4GROUP * WEEK1 + β5GROUP * WEEK12 + εij |
where i indexes subject, j indexes time point, GROUP, WEEK1 and WEEK12 are binary indicator variables, b0i is a random intercept and εij is a random within-subject error term. Contrasts were constructed and tested in order to compare mean levels between groups at each time point (e.g., the null hypothesis for the week 1 comparison is equivalent to the condition β1 + β4 = 0). These tests were assessed for qualitative agreement with simple two-sample t tests at each time point. The same time point-specific contrasts were then assessed after refitting the mixed models while adjusting for potentially influential baseline patient characteristics. The following baseline variables were evaluated as potential confounders: age, race, BMI, FEV1 and CF-related diabetes status.
The log-rank test was used to compare overall mortality across the two groups over the 12 mo of follow-up. Cox proportional hazards regression was applied univariately to assess the importance of baseline FEV1, BMI, age and CF-related diabetes status. A multivariable Cox model was then fit to assess the treatment effect after controlling for other variables deemed clinically or univariately statistically significant.
Acknowledgments
This project was supported by grants from the CF Foundation (R.G.), and NIH (T32DK007734 to R.G.; T32DK007298 to M.K.; K24 RR023356 to T.R.Z.; UL1 RR025008 to V.T. T.R.Z., R.L. and S.L.; and K23AR054334 to V.T.); and the Center for Cystic Fibrosis Research, Children’s Healthcare of Atlanta (V.T., S.Z., M.S., A.S.). Supported in part by PHS Grant (UL1 RR025008) from the Clinical and Translational Science Award program, National Institutes of Health, National Center for Research Resources.
The authors would also like to acknowledge the support of JoAnne and James Grote (BTR, Group) for their financial support of the study.
The study sponsors did not have any involvement in the study design; collection, analysis and interpretation of data; or in the writing of the manuscript and the decision to submit the manuscript for publication.
Glossary
Abbreviations:
- 25(OH)D
25-hydroxyvitamin D
- BMI
body mass index
- CF
cystic fibrosis
- DEQAS
vitamin D external quality assessment scheme
- FEV1
forced expired volume in 1 second
- IV
intravenous
- NIH
National Institutes of Health
- PTH
parathyroid hormone
Disclosure of Potential Conflicts of Interest
V.T. received an unrestricted research grant from BTR, Group (a vitamin D supplement company). The remaining authors have no financial conflicts of interest to report.
Author Contributions
V.T., R.E.G., T.R.Z., A.A.S., M.S.S. and S.M.Z. designed the research; V.T., R.E.G., V.S., S.S. and M.K. conducted the research; V.T., R.E.G., R.H.L., S.L., M.S.S., A.S.S. and T.R.Z. analyzed data; and V.T. and R.E.G. wrote the paper. All authors have participated in editing and have approved of the manuscript
Clinical Trial Registration Number
clinicaltrials.gov (NCT00788138)
Footnotes
Previously published online: www.landesbioscience.com/journals/dermatoendocrinology/article/20332
References
- 1.Foundation, Fibrosis C. Patient Registry 2009 Annual Report ed. Bethesda, MD, USA: Cystic Fibrosis Foundation 2010. [Google Scholar]
- 2.Boyle MP, Noschese ML, Watts SL, Davis ME, Stenner SE, Lechtzin N. Failure of high-dose ergocalciferol to correct vitamin D deficiency in adults with cystic fibrosis. Am J Respir Crit Care Med. 2005;172:212–7. doi: 10.1164/rccm.200403-387OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolfenden LL, Judd SE, Shah R, Sanyal R, Ziegler TR, Tangpricha V. Vitamin D and bone health in adults with cystic fibrosis. Clin Endocrinol (Oxf) 2008;69:374–81. doi: 10.1111/j.1365-2265.2008.03216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khazai NB, Judd SE, Jeng L, Wolfenden LL, Stecenko A, Ziegler TR, et al. Treatment and prevention of vitamin D insufficiency in cystic fibrosis patients: comparative efficacy of ergocalciferol, cholecalciferol, and UV light. J Clin Endocrinol Metab. 2009;94:2037–43. doi: 10.1210/jc.2008-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall WB, Sparks AA, Aris RM. Vitamin d deficiency in cystic fibrosis. Int J Endocrinol. 2010;2010:218691. doi: 10.1155/2010/218691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.West NE, Lechtzin N, Merlo CA, Turowski JB, Davis ME, Ramsay MZ, et al. Appropriate goal level for 25-hydroxyvitamin D in cystic fibrosis. Chest. 2011;140:469–74. doi: 10.1378/chest.10-2114. [DOI] [PubMed] [Google Scholar]
- 7.Pincikova T, Nilsson K, Moen IE, Karpati F, Fluge G, Hollsing A, et al. Scandinavian Cystic Fibrosis Study Consortium Inverse relation between vitamin D and serum total immunoglobulin G in the Scandinavian Cystic Fibrosis Nutritional Study. Eur J Clin Nutr. 2011;65:102–9. doi: 10.1038/ejcn.2010.194. [DOI] [PubMed] [Google Scholar]
- 8.Kamen DL, Tangpricha V. Vitamin D and molecular actions on the immune system: modulation of innate and autoimmunity. J Mol Med (Berl) 2010;88:441–50. doi: 10.1007/s00109-010-0590-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr. 2010;91:1255–60. doi: 10.3945/ajcn.2009.29094. [DOI] [PubMed] [Google Scholar]
- 10.Jeffery LE, Burke F, Mura M, Zheng Y, Qureshi OS, Hewison M, et al. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J Immunol. 2009;183:5458–67. doi: 10.4049/jimmunol.0803217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stenbit A, Flume PA. Pulmonary complications in adult patients with cystic fibrosis. Am J Med Sci. 2008;335:55–9. doi: 10.1097/MAJ.0b013e31815d2611. [DOI] [PubMed] [Google Scholar]
- 12.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 13.Adams JS, Ren SY, Liu PT, Chun RF, Lagishetty V, Gombart AF, et al. Vitamin d-directed rheostatic regulation of monocyte antibacterial responses. J Immunol. 2009;182:4289–95. doi: 10.4049/jimmunol.0803736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belderbos ME, Houben ML, Wilbrink B, Lentjes E, Bloemen EM, Kimpen JL, et al. Cord blood vitamin D deficiency is associated with respiratory syncytial virus bronchiolitis. Pediatrics. 2011;127:e1513–20. doi: 10.1542/peds.2010-3054. [DOI] [PubMed] [Google Scholar]
- 15.Boucher BJ. The 2010 recommendations of the American Institute of Medicine for daily intakes of vitamin D. Public Health Nutr. 2011;14:740–50. doi: 10.1017/S136898001100022X. [DOI] [PubMed] [Google Scholar]
- 16.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Endocrine Society Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 17.Green D, Carson K, Leonard A, Davis JE, Rosenstein B, Zeitlin P, et al. Current treatment recommendations for correcting vitamin D deficiency in pediatric patients with cystic fibrosis are inadequate. J Pediatr. 2008;153:554–9. doi: 10.1016/j.jpeds.2008.04.058. [DOI] [PubMed] [Google Scholar]
- 18.Boas SR, Hageman JR, Ho LT, Liveris M. Very high-dose ergocalciferol is effective for correcting vitamin D deficiency in children and young adults with cystic fibrosis. J Cyst Fibros. 2009;8:270–2. doi: 10.1016/j.jcf.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Wasse H, Huang R, Long Q, Singapuri S, Raggi P, Tangpricha V. Efficacy and safety of a short course of very-high-dose cholecalciferol in hemodialysis. Am J Clin Nutr. 2012;95:522–8. doi: 10.3945/ajcn.111.025502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leventis P, Kiely PDW. The tolerability and biochemical effects of high-dose bolus vitamin D2 and D3 supplementation in patients with vitamin D insufficiency. Scand J Rheumatol. 2009;38:149–53. doi: 10.1080/03009740802419081. [DOI] [PubMed] [Google Scholar]
- 21.Ferguson JH, Chang AB. Vitamin D supplementation for cystic fibrosis. Cochrane Database Syst Rev. 2009:CD007298. doi: 10.1002/14651858.CD007298.pub2. [DOI] [PubMed] [Google Scholar]
- 22.Green DM, Leonard AR, Paranjape SM, Rosenstein BJ, Zeitlin PL, Mogayzel PJ., Jr. Transient effectiveness of vitamin D2 therapy in pediatric cystic fibrosis patients. J Cyst Fibros. 2010;9:143–9. doi: 10.1016/j.jcf.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Kerem E, Reisman J, Corey M, Canny GJ, Levison H. Prediction of mortality in patients with cystic fibrosis. N Engl J Med. 1992;326:1187–91. doi: 10.1056/NEJM199204303261804. [DOI] [PubMed] [Google Scholar]
- 24.de Boer K, Vandemheen KL, Tullis E, Doucette S, Fergusson D, Freitag A, et al. Exacerbation frequency and clinical outcomes in adult patients with cystic fibrosis. Thorax. 2011;66:680–5. doi: 10.1136/thx.2011.161117. [DOI] [PubMed] [Google Scholar]
- 25.Ginde AA, Scragg R, Schwartz RS, Camargo CA., Jr Prospective study of serum 25-hydroxyvitamin D level, cardiovascular disease mortality, and all-cause mortality in older U.S. adults. J Am Geriatr Soc. 2009;57:1595–603. doi: 10.1111/j.1532-5415.2009.02359.x. [DOI] [PubMed] [Google Scholar]
- 26.Braun A, Chang D, Mahadevappa K, Gibbons FK, Liu Y, Giovannucci E, et al. Association of low serum 25-hydroxyvitamin D levels and mortality in the critically ill. Crit Care Med. 2011;39:671–7. doi: 10.1097/CCM.0b013e318206ccdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melamed ML, Michos ED, Post W, Astor B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168:1629–37. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zittermann A, Iodice S, Pilz S, Grant WB, Bagnardi V, Gandini S. Vitamin D deficiency and mortality risk in the general population: a meta-analysis of prospective cohort studies. Am J Clin Nutr. 2012;95:91–100. doi: 10.3945/ajcn.111.014779. [DOI] [PubMed] [Google Scholar]
- 29.Proesmans M, Els C, Vermeulen F, De Boeck K. Change in IgG and evolution of lung function in children with cystic fibrosis. J Cyst Fibros. 2011;10:128–31. doi: 10.1016/j.jcf.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 30.McNally P, Coughlan C, Bergsson G, Doyle M, Taggart C, Adorini L, et al. Vitamin D receptor agonists inhibit pro-inflammatory cytokine production from the respiratory epithelium in cystic fibrosis. J Cyst Fibros. 2011;10:428–34. doi: 10.1016/j.jcf.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 31.Stephenson A, Brotherwood M, Robert R, Atenafu E, Corey M, Tullis E. Cholecalciferol significantly increases 25-hydroxyvitamin D concentrations in adults with cystic fibrosis. Am J Clin Nutr. 2007;85:1307–11. doi: 10.1093/ajcn/85.5.1307. [DOI] [PubMed] [Google Scholar]
- 32.Finklea JD, Grossmann RE, Tangpricha V. Vitamin D and chronic lung disease: a review of molecular mechanisms and clinical studies. Adv Nutr. 2011;2:244–53. doi: 10.3945/an.111.000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Førli L, Bjortuft O, Boe J. Vitamin D status in relation to nutritional depletion and muscle function in patients with advanced pulmonary disease. Exp Lung Res. 2009;35:524–38. doi: 10.1080/01902140902763193. [DOI] [PubMed] [Google Scholar]
- 34.Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869. doi: 10.1136/bmj.c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bian ZX, Shang HC. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2011;154:author reply291–2. doi: 10.7326/0003-4819-154-4-201102150-00016. [DOI] [PubMed] [Google Scholar]
- 36.Aris RM, Merkel PA, Bachrach LK, Borowitz DS, Boyle MP, Elkin SL, et al. Guide to bone health and disease in cystic fibrosis. J Clin Endocrinol Metab. 2005;90:1888–96. doi: 10.1210/jc.2004-1629. [DOI] [PubMed] [Google Scholar]