Abstract
Obesity develops from a prolonged imbalance of energy intake and energy expenditure. However, the relatively recent discovery that the composition and function of the gut microbiota impacts on obesity has lead to an explosion of interest in what is now a distinct research field. Here, research relating to the links between the gut microbiota, diet and obesity will be reviewed under five major headings: (1) the gut microbiota of lean and obese animals, (2) the composition of the gut microbiota of lean and obese humans, (3) the impact of diet on the gut microbiota, (4) manipulating the gut microbiota and (5) the mechanisms by which the gut microbiota can impact on weight gain.
Keywords: gut microbiota, intervention, prebiotic, probiotic, diet and obesity
Introduction
Obesity has become one of the most prevalent health issues of our time. In 2008, the World Health Organization (WHO) estimated that there were over 1.5 billion overweight adults in the world and, of these, approximately 500 million are clinically obese. Indeed, more deaths are caused worldwide by excessive weight than those caused by being underweight.1 Obesity is a multifactorial condition but it can be most simply described as being the result of a long-term imbalance between energy intake and energy expenditure. While modern eating habits and ever increasingly sedentary lifestyles are major contributory factors, researchers are gaining an ever greater appreciation of other important risk factors. One such issue that has emerged in recent years is the link between obesity and the composition and functionality of the microorganisms in the gut. Here we review the literature related to this topic under five major headings i.e., (1) the gut microbiota of lean and obese animals, (2) the composition of the gut microbiota of lean and obese humans, (3) the impact of diet on the gut microbiota, (4) manipulating the gut microbiota and (5) the mechanisms by which the gut microbiota can impact on weight gain (Fig. 1).
Figure 1.
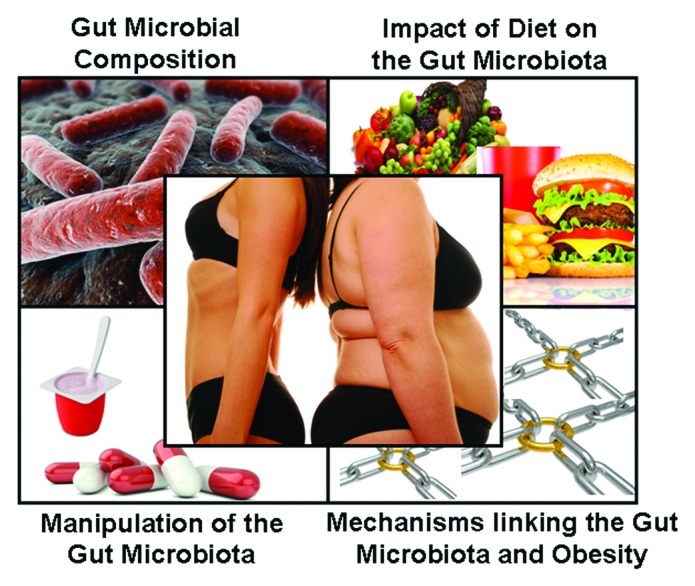
The study of the role of the gut microbiota in obesity can be subdivided into four broad areas.
Gut Microbiota of Lean and Obese Animals
Mouse models are frequently employed by researchers investigating obesity and the role of the gut microbiota in obesity. The following sub-sections will focus on the various mouse models that have been employed and the outcome from studies performed to date.
Microbiota of genetically obese mice.
Many studies have examined the difference in the composition of the gut microbiota of lean and obese mice using a wide range of molecular methods (listed in Table 1). The possible existence of a link between obesity and the gut microbiota only became apparent upon the application of DNA sequencing on a large scale to facilitate an unbiased analysis of the entire gut microbiota (i.e., including culturable and unculturable microbes). This DNA sequencing approach initially focused on the 16S rRNA gene which, because of the presence of highly conserved and variable regions, can be employed to classify bacteria (Fig. 2). In 2005, Ley et al.2 first employed this approach in an obesity context to analyze 5,088 bacterial 16S rRNA sequences corresponding to the cecal microbiota of ob/ob, ob/+ and +/+ mice. ob/ob mice are leptin deficient, eat excessively and are obese as a consequence of this genotype. Traditional “Sanger” sequencing was employed in this instance.3 The investigation revealed that the two most abundant bacterial divisions in mice were the phylum Firmicutes (60–80% of sequences) and the phylum Bacteroidetes (20–40% of sequences) and it was established that the proportions of Bacteroidetes and Firmicutes were reduced and increased, respectively, in the obese animals relative to their lean counterparts. These shifts were division wide (i.e., no particular subgroup of Firmicutes and/or Bacteroidetes were lost or gained).2 Turnbaugh et al.4 added to our knowledge in this area in 2006 in a study which differed by virtue of being performed on a larger scale and the use of an alternative approach, i.e., random or shotgun metagenomic sequencing of the murine (ob/ob, ob/+ and +/+) cecal microbial DNA (Fig. 2). In this case both traditional Sanger and high throughput 454 pyrosequencing technologies were employed.5 This study again highlighted an increased ratio of Firmicutes to Bacteroidetes in obese mice relative to their lean counterparts. It was also noted that the gut microbiota of ob/ob mice contained a higher proportion of Archaea than were present in the cecum of their lean counterparts.4 Though these results are interesting, the possibility that the animal’s genotype could also influence the gut microbial composition cannot be excluded. Notably, since this study, high throughput DNA sequencing approaches have essentially replaced Sanger sequencing and other strategies when the objective is to assess alternations in the gut microbiota composition. The methods used to analyze the gut microbiota in the studies referred to in this review are summarized in Table 2.
Table 1. Molecular methods used in studies discussed in this review.
| Approach | Description |
|---|---|
|
Sequencing strategies employed to study eubacterial (bacterial) populations |
|
| Shotgun sequencing |
Metagenomic DNA is randomly sheared into smaller fragments and sequenced at random to reveal information regarding the functional potential and, to a lesser extent, composition of a microbial population. |
| 16S rRNA amplicon sequencing |
Amplification of the 16S rRNA gene from eubacteria using degenerate PCR primers and sequencing thereof to reveal information regarding the bacterial population present in the environment. |
|
Sequencing platforms currently employed |
|
| Sanger sequencing |
Dideoxy chain termination method in which fluorescent labeled ddNTPs or dNTPs are incorporated into the newly synthesized DNA thereby preventing further synthesis and leaving products of different lengths. Products are separated by size by gel or capillary electrophoresis. |
| Pyrosequencing |
Sequence by synthesis method involving the detection of pyrophosphate release upon incorporation of a known nucleotide. A number of sequencing platforms, including the Roche-454 sequencers, employ this technology. |
| Illumina sequencing |
Sequence by synthesis method in which small DNA fragments are bound to a slide and amplified in clusters. Double stranded DNA in the clusters is separated and sequenced. Lasers detect nucleotide addition and from this the sequence of the DNA fragment is generated. |
|
Other technologies employed to investigate microbial ecosystems |
|
| Fluorescent in situ hybridization |
Fluorescent labeled oligonucleotide probes used to identify taxa in situ through hybridization. |
| Denaturing gradient gel electrophoresis (DGGE) |
Separation of DNA (usually 16S amplicons) based on GC content using gel electrophoresis and a denaturing agent to differentiate between microbial populations. |
| Terminal restriction fragment length polymorphism (T-RFLP) |
Separation of terminally labeled PCR amplicons that have been enzymatically digested with restriction enzymes. |
| Quantitative real time polymerase chain reaction (qPCR) | Modified form of PCR which allows for detection and quantification of fluorescent PCR amplicons or amplicons to which fluorescent probes have attached, the fluorescence of which increases in line with increasing DNA concentrations. |
Figure 2.
Next generation sequencing (NGS)-high throughput. Left-16S rRNA gene amplification using specific PCR primers followed by sequencing to reveal eubacterial composition. Right-random shearing of metagenomic DNA into small fragments followed by sequencing to reveal functional potential of bacterial population.
Table 2. Culture independent methods used by studies in this review*.
| Cohort/model | Method | Reference |
|---|---|---|
|
ob/ob, ob/+ and +/+ mice |
16S by Sanger |
2 |
| Obese human on low calorie diets |
16S by Sanger |
12 |
|
ob/ob, ob/+ and +/+ mice |
16S by Sanger and Pyrosequencing |
4 |
| Humans on weight loss diets |
FISH |
15 |
| CD14-/- and Wt mice |
FISH |
43 |
|
ob/ob, +/+, CD14-/- and ob/ob CD14-/- mice |
DGGE and qPCR |
75 |
| Children |
FISH and qPCR |
31 |
| DIO mice and WT mice |
16S by Sanger |
6 |
| Overweight and normal weight pregnant women |
qPCR and FISH |
27 |
| Adolescents on diets and exercise |
FISH |
42 |
| Monozygotic and dizygotic human twins and mothers |
16S by Sanger and Pyrosequencing |
13 |
|
ob/ob mice |
DGGE and qPCR |
67 |
| Lean, obese and post gastric bypass humans |
16S by Sanger and Pyrosequencing |
24 |
| NMRI-KI mice on LF/HS, HF/HS or LF/PP diets |
qPCR |
45 |
| Adolescents on diets and exercise |
qPCR |
41 |
| Lean, overweight and obese humans |
qPCR |
14 |
| Hamsters |
16S by Pyrosequencing, DGGE and qPCR |
56 |
| Children |
qPCR |
32 |
| RELMβ knockout and wild-type mice |
16S by Pyrosequencing |
7 |
| Syrian hamsters |
DGGE, 16S Sanger and qPCR |
44 |
| Obese, lean and anorexic humans |
qPCR |
18 |
| Apoa-I−/− and Wt C57BL/6J mice |
16S on DGGE, T-RFLP and Pyrosequencing |
46 |
| African Americans and Caucasian Americans |
DGGE, FISH and qPCR |
16 |
| Humanized gnotobiotic mice |
16S by Pyrosequencing |
11 |
| Overweight and normal weight pregnant women |
qPCR |
29 |
| C57bI6/J mice |
DGGE and qPCR |
68 |
| Lean and obese humans |
qPCR |
26 |
| HF-fed, ob/ob and Wt mice |
Pyrosequencing |
10 |
| Overweight and normal weight children |
FISH |
33 |
| Overweight humans |
qPCR, DGGE and Sanger |
49 |
| Humans of different nationalities |
Sanger and Pyrosequencing |
19 |
| Gnotobiotic mice |
Illumina shotgun sequencing |
48 |
| Wistar rats |
16S Pyrosequencing |
25 |
| Mammals |
Shotgun Pyrosequencing |
53 |
| JCR:LA-cp rats |
qPCR |
69 |
| Healthy humans |
16S by Pyrosequencing and shotgun metagenomics |
54 |
| In vitro three stage colonic model | FISH and DGGE | 52 |
Table done in chronological order.
Microbiota of diet-induced obese mice.
Another murine model has been developed which focuses on obesity that arises due to consumption of a high-fat, “western” diet (i.e., diet induced obesity or DIO), rather than genetics. In 2008, Turnbaugh et al.6 showed that the western diet associated cecal microbial community had a significantly lower proportion of Bacteroidetes and a specific increase in the Mollicutes subpopulation of the Firmicutes. In 2009, Hildebrandt et al.7 investigated the microbial communities from both wild-type and resistin-like molecule (RELM) β knockout (KO) mice fed a standard chow diet and a high fat diet. The RELMβ gene is expressed by colonic goblet cells and its expression has been shown to be dependant on the gut microbiome8 and can be induced by a high fat diet.9 Wild-type and RELMβ KO mice were compared in order to further investigate the relationships between diet, obesity and microbiota composition. A sequence based analysis of murine fecal samples revealed that the gut microbiota communities of 13 week old wild-type and RELMβ KO mice fed a standard chow diet were very similar, with Bacteroidetes, followed by Firmicutes, being the dominant groups. The phyla Proteobacteria, Tenericutes and TM7 were also detected. After three months consumption of a high fat diet, the gut microbiota of both groups of animals differed from those fed the standard chow diet. More specifically, the phylum Firmicutes class Clostridiales, Actinobacteria and Deltaproteobacteria increased their respective proportions in the gut of both groups of animals fed a high fat diet which was accompanied by a reduction in the abundance of class Bacteroidales. An increase in the Mollicutes population was also noted in these animals, although this bloom was not as dramatic as had been observed by Turnbaugh et al.6 Despite these similarities with respect to gut microbial composition, the RELMβ KO mice consuming a high fat diet remained lean, whereas the corresponding wild-type mice became obese. From this study, the authors concluded that, because the general changes in the composition of the gut microbiota were similar in the wild-type and KO mice, the effect of diet was dominant i.e., the high fat diet, and not the obese state, accounted for the alteration in the gut microbial communities. We will return to the implications of this study later. A recent 16S rRNA-based study by Murphy et al. has provided further insight. Here the fecal microbiota of lean (+/+), ob/ob as well as +/+ mice fed a high fat (HF) diet, was investigated at 7 weeks (the time points at which the low and high fat groups were separated), 11 weeks and 15 weeks of age. It was established that, in addition to the Firmicutes and Bacteroidetes, a high proportion of Actinobacteria was present in the gut. While no significant changes in the proportions of the different microbial populations was observed in lean mice over the 8 week period, there was a progressive increase in the proportions of Firmicutes in the fecal microbiota of HF-fed and ob/ob mice. Bacteroidetes levels decreased overtime in all groups but this reduction reached statistical significance in ob/ob mice only. The levels of Actinobacteria fluctuated in all three groups with significant increases in their proportions being apparent in the ob/ob and HF-fed mice when samples from weeks 7 and 11 and from weeks 7 and 15 were compared. It was also noted that Proteobacteria decreased in HF-fed mice from weeks 11 to 15 while in ob/ob mice Deferribacteria and Lactococcus decreased overtime.10
Humanized mice.
Studies have revealed that although the distal gut of both mice and humans contains microbes from the same dominant range of bacterial phyla (i.e., Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria and, to a lesser extent, Verrucomicrobia, Cyanobacteria, TM7, Fusobacteria and Spirochaeates), many of the bacterial genera and species present in mice are not detected in humans and vice versa.2 With a view to addressing this, and to thus improve the murine model, Turnbaugh et al. 2009,11 created an animal model of the human gut ecosystem by transplanting human fecal microbial communities into germ-free mice. Then this demonstrated how these humanized animals can be utilized to conduct controlled proof-of-principle “clinical” metagenomic studies of host-microbiome interrelations. It was established that all bacterial phyla, 11 of 12 bacterial classes, and 88% (58 of 66) of genus-level taxa detected in the sample from the human donor were present in the recipient mice. Furthermore, the genera that were absent from the humanized mice were those present at low abundance (0.008% on average) in the donor sample. It was also established that the human gut microbiota could be successfully transferred from the humanised mice to germ-free recipient mice without a significant drop in diversity. Use of these model animals, and examination of the gut microbial community thereof, revealed that the gut microbiota in those consuming a “western” diet contained a higher proportion of the Firmicutes classes Erysipelotrichi and a lower proportion of Bacteroidetes and Bacillus than did the microbiota of those in receipt of a to low fat/plant polysaccharide diet.
The Composition of the Gut Microbiota of Lean and Obese Humans
Mice are useful models in that they can be housed in controlled environments and fed specific diets. For obvious reasons, human studies lack these levels of control, and thus shifts in the associated microbiota can be considerably more variable. However, any shifts in populations deemed to be of importance with respect to weight gain ultimately need to be validated through human studies.
Gut microbiota of lean and obese adult humans.
The gut microbiota of lean and obese individuals was compared in 2006 by Ley et al.12 through 16S rRNA sequencing of DNA extracted from fecal samples. It was revealed that the Bacteroidetes and Firmicutes divisions again dominated the microbiota (92.6%) but with obese individuals possessing a lower proportion of Bacteroidetes and higher levels of Firmicutes than their lean counterparts, thus resembling the patterns established in previous murine studies.2 In 2009, Turnbaugh et al.13 characterized the gut microbiota of 154 individuals, consisting of monozygotic or dizygotic twins and their mothers. The study revealed that the composition of the gut microbiota is more similar between family members than unrelated individuals. However, it was also evident that each individual’s gut microbiota was distinct and that a similar degree of co-variation existed between adult monozygotic and dizygotic twin pairs. Notably with respect to the topic of this review, it was also apparent that there was a lower proportion of Bacteroidetes in the gut of obese individuals compared with their lean counterparts, that the proportions of Actinobacteria was elevated in obese individuals and that the microbial population was in general less diverse in obese individuals. Notably, unlike the previous study, no significant difference in proportions of Firmicutes was apparent when the gut microbiota of lean and obese individuals was compared.
Indeed, considerable debate continues regarding the significance of the Firmicutes and Bacteroidetes proportions with respect to obesity in humans. In 2009 Schwiertz et al. studied lean and obese volunteers of both sexes and assessed the associated fecal microbiota. Firmicutes belonging to the Clostridium leptum and the Clostridium coccoides groups as well as Bacteroides spp (phylum Bacteroidetes) were the most abundant bacterial groups in general. In contrast to the studies cited above, it was noted that proportions of the genus Bacteroides were greater in overweight volunteers than lean and obese volunteers (p = 0.002 and p = 0.145, respectively) whereas the Ruminococcus flavefaciens subgroup, C. leptum group, Methanobrevibacter and the genus Bifidobacterium was less abundant in overweight and obese subjects. The significance of the proportion of Bacteroidetes and Firmicutes present was also questioned by Duncan et al.15 when they found that weight loss did not change the relative proportions of the Bacteroides spp, or the percentage of Firmicutes present, in the human gut. The composition of the gut microbiota of both African Americans and Caucasian Americans was also investigated by Mai et al. in 2009.16 Of greatest relevance to this review is the observation by Mai et al. that while a number of diet related trends were observed i.e., individuals that consumed high levels of fat had fewer clostridia, lactic acid bacteria levels were higher in subjects that consumed fiber, and levels of Clostridium cluster XIVa were elevated in subjects with a higher intake of heterocyclic amines (HCAs), none of these changes were statistically significant. Similarly, no association between the body mass index (BMI) and the proportions of Bacteroidetes and Clostridium cluster XIVa was detected. Finally, in 2009 Armougom et al.18 assessed the gut microbiota of obese, lean and patients suffering from anorexia nervosa. The study revealed that the proportion of Firmicutes present in the gut of all three groups was similar, the proportion of Bacteroidetes was reduced in obese individuals and that Methanobrevibacter smithii was present in higher proportions in the gut of the anorexic group. A recent development in the area of human gut microbial composition is the potential discovery of distinct clusters or enterotypes in the human microbiome using sequencing data from 30 three gut microbiomes across different nationalities (French, Spanish, Italian, Danish, Japanese and American).19 The three enterotypes can be identified on the basis of variations in the relative levels of Bacteroides, Prevotella and Ruminococcus. Enterotype 1 is enriched in Bacteroides and the co-occurring Parabacteroides. These both derive energy mainly from carbohydrates and proteins by fermentation.20 Enterotype 2 is enriched in Prevotella which co-occurs with Desulfovibrio. These are known to operate in synergy to break down mucin glycoproteins.21 Enterotype 3 is the most common enterotype and is distinguished on the basis of enrichment in the levels of Ruminococcus and the co-occurring Akkermansia, both of which contain species capable of degrading mucins.22 The relationship between diet and enterotype is addressed below.
Impact of bariatric surgery on the gut microbiota.
Bariatric surgery is increasingly employed as an anti-obesity treatment, and for a morbidly obese patient it is the only option available that can deliver substantial and sustained weight loss.23 The surgery can be performed in a number of different ways in that, in some cases, it involves a reduction in the size of the stomach using a gastric band, in others a portion of the stomach is removed, while another option involves the creation a small stomach pouch and resecting/re-routing it to the small intestine. The impact of such surgery on the composition of the gut microbiota of patients has been investigated through a comparison of the gut microbiota of 3 obese, 3 lean and 3 post gastric bypass individuals.24 The investigation established that the gut microbiota of individuals who had undergone gastric bypass differed from that of both the obese and lean individuals by virtue of an increase in proportions of Gammaproteobacteria (including the Enterobacteriaceae) and Fusobacteriaceae and a proportional decrease in Clostridia. In addition, the gut microbiota of lean individuals contained elevated proportions of sequences corresponding to the Lachnospira (order Clostridiales) compared with that of obese and gastric bypass individuals. It was also noted that the gut microbiota of obese individuals contained a lower proportion Verrucomicrobia and a higher proportion of Archaea relative to the other two groups. Finally, Methanobacteriales was found in all obese individuals but in only one gastric bypass patient.24
Another recent paper also focused on this topic but incorporated a larger number of individuals, i.e., 30 obese individuals who had undergone bariatric surgery and 13 lean volunteers, and instead relied on a qPCR-based analysis.25 The investigation established that after surgery the levels of Bacteroides/Prevotella were higher than they had been prior to surgery. This increase brought the Bacteroides/Prevotella more closely in line what that observed in the lean controls. An increase in Escherichia coli and Faecalibacterium prausnitzii was also noted when post-surgical samples from individuals were compared with their pre-surgery equivalents. The relatively low levels of F. prausnitzii in pre-surgical samples were more apparent in the obese diabetic cohort than in their obese non diabetic counterparts. In contrast, decreases in levels of the combined Lactobacillus/Leuconostoc/Pediococcus group and in Bifidobacterium sp were apparent in post-, relative to pre-, surgical samples. Body weight, BMI, body fat mass and leptin concentration all negatively correlated with Bacteroides/Prevotella and E. coli but positively correlated with Bifidobacterium populations. The F. prausnitzzi population strongly negatively correlated with changes in inflammatory markers and orosomucoid serum levels. While these results are interesting and suggest that this area requires further attention, the possibility exists that the roux-en-Y gastric bypass procedure may contribute to changes in gut microbial composition as a consequence of the associated change in pH and the downstream delivery of bile acids.25
Finally, in one case the impact of bariatric surgery on the microbial composition of wistar rats was assessed. Although differing from the previous studies by virtue of its reliance on an animal model, an increase in Gammaproteobacteria was again apparent. However, in this instance a reduction in Firmicutes and Bacteroidetes was observed,26 the significance or cause of which is not known.
Gut microbiota of normal and overweight pregnant women.
The gut microbiota of normal weight and overweight pregnant women was investigated by Collado et al.27 In addition to being of interest with respect to the health and weight of the mother, such investigations are also of importance given that, in cases of natural delivery, the child is first colonized by microbiota from the mother.28 While an overall increase in the number of bacteria was observed between the first and third trimester in both groups, significant differences between the gut microbiota composition of pregnant women of different weight were noted. More specifically, higher numbers of representatives of the Bacteroides group and Staphylococcus aureus were recorded in overweight individuals. Indeed a one kilogram gain in weight correlated with a corresponding increase in Bacteroides numbers by 0.006 log units. The levels of the Clostridium group increased in overweight women from the first trimester to the third (p = 0.054). Bifidobacterium proportions were higher in women who exhibited a relatively lower weight gain during pregnancy. Finally, it was also noted that overweight women tended to give birth to heavier infants. More recently, Santacruz et al.29 investigated the fecal microbiota of 50 pregnant women who were assigned into one of two groups, i.e., overweight or normal weight, based on their BMI. As with the Collado study, higher numbers of Staphylococcus and lower numbers of Bifidobacterium were noted in overweight women. However, in contrast with the previous study, Bacteroides numbers were found to be lower in overweight women. Increased numbers of Enterobacteriaceae in general and of E. coli in particular, were also associated with overweight women. The gut microbiota of women who gained excessive weight during pregnancy underwent similar increases and decreases in microbial numbers as were associated with overweight women. Santacruz et al.29 also investigated the relationship between gut microbiota composition and metabolomic parameters. It was found that increased Staphylococcus numbers corresponded with increased serum levels of cholesterol, a rise in numbers of Enterobacteriaceae and E. coli was linked with increased levels of serum ferritin, saturation transferrin index and decreased levels of transferrin, while greater numbers of Bifidobacterium correlated with reduced levels of ferritin, saturation transferring index and increased levels of transferrin and folic acid. Finally, increased Bacteroides numbers were associated with increased levels of high density lipoprotein (HDL)-cholesterol, folic acid and lower levels of triacylglycerol (TAG).
Gut microbiota of lean and obese children.
The WHO estimated that in 2010 there were over 42 million overweight children under the age of five worldwide.30 The alarming increase in obesity rates in children has lead to a particular interest in investigating the gut microbiota of lean and obese children. A seven year study to investigate the composition of the fecal microbiota of children, published by Kalliomäki et al.31 in 2008, found that normal weight development was linked to a lower number of fecal S. aureus number and a higher number of bifidobacteria relative to those present in the feces of overweight children. The S. aureus finding is doubly interesting in the context of the increase in number of Staphylococcus in the gut of overweight pregnant women referred to above. Balamurugan et al.32 noted that obese and non-obese Indian children had similar dietary intakes of energy and, thus, it was apparent that other factors were at play. Thus the nature of the dominant fecal microbiota within each group was investigated. Although this revealed that there were no significant differences with respect to the levels of the Bacteroides-Prevotella, Bifidobacterium, Eubacterium rectale or Lactobacillus acidophilus groups in the gut, it did reveal that, unlike the aforementioned study by Furet et al.25 the obese subjects had significantly higher levels of F. prausnitzii, a representative of the Firmicutes which can ferment unabsorbed carbohydrate. It was thus postulated that the presence of this bacterium in greater numbers in obese children could lead to increased energy extraction from carbohydrate that would not otherwise contribute to dietary energy intake. Luoto et al. analyzed the fecal microbiota of a group of children over 10 y.33 At 3 mo of age there was no statistically significant difference in the fecal bacterial counts of the children. However children who were overweight by the time they reached age 10 y tended to have lower bifidobacterial numbers in their feces when it were assessed at 3 mo of age. Interestingly 10 y old normal weight children had significantly higher mean concentrations of serum-soluble innate microbial receptor (sCD14) than overweight children. sCD14 is involved in innate immunity and its expression is increased by the presence of LPS and fatty acids that resemble the lipid portion of LPS.34 It was also noted that mothers of children who were normal weight at age 10 y had statistically significantly higher mean concentrations of adiponectin in maternal colostrum than mothers of overweight children. Adiponectin is a protein hormone secreted from adipose tissue35 and the placenta36 into the blood stream. It has an important role in glucose regulation and fatty acid metabolism37,38 and provides protection against metabolic syndrome39 as well as having antiatherogenic and anti-inflammatory properties.40 Diabetics and obese individuals have low levels of adiponectin.35
Impact of Diet on the Gut Microbiota
Until recently, the relationship between diet, microbes and, in turn, optimal health has remained obscure. However, a number of recent studies have investigated the primacy of diet among lifestyle factors that influence the composition of the gut microbiota. That which is known with respect to the impact of diet on the gut microbiota is summarized in Table 3.
Table 3. Dietary related influences on gut microbiota.
| Human | ||
|---|---|---|
| Diet | Populations increasing | Populations decreasing |
| Fat restricted |
Bacteroidetes12 |
Firmicutes12 |
| Carbohydrate restriction |
Bacteroidetes12 |
Firmicutes12 |
| Low carbohydrate/high protein |
Oscillibacter valerigens
41
|
Roseburia, E. rectale
41
and Bifidobacterium
15
|
| Calorie restriction and exercise |
Bacteroides fragilis, Lactobacillus42 and Bacteroides43 |
C. coccoides, B. longum, B. adolescentis,42C. histolyticum and E. rectale-C. coccoides43 |
| Resistant starch |
Ruminococcus bromii, Oscillibacter valerigens, Roseburia and E. rectale41 |
|
| High dietary fiber |
Bifidobacterium, Ruminococcus, Lactobacillus-Enterococcus, Faecalibacterium prausnitzii and E. rectale-C. coccoides44 |
|
|
Animal | ||
|
Diet |
Populations increasing |
Populations decreasing |
| High fat |
Mollicutes M2 cluster45 |
Mollicutes M1 and M3 cluster, Bifidobacteriaceae,45 Bacteroides, E. rectale-C. coccoides and Bifidobacterium46 |
| Western diet (high fat/high sugar) |
C. innocuum, E. dolichum, C. mitsuokai and Bacilli11 |
Bacteroidetes11 and E. rectale47 |
| Western diet reduced fat |
Bacteroidetes6 |
Mollicutes6 and E. rectale47 |
| Western diet reduced carbohydrate |
Bacteroidetes6 |
Mollicutes6 |
| Fasting |
Desulfovibrionaceae48 |
Clostridium48 |
| Increased casein | E. rectale, D. piger and M. formatexigens49 | |
Low carbohydrate/calorie diets.
The effects of a fat restricted or carbohydrate restricted low calorie diet randomly assigned to 12 obese people has been investigated by Ley et al.12 While it was established that over time the relative abundance of Bacteroidetes increased and the abundance of Firmicutes decreased, these changes appeared to be irrespective of the low calorie diet consumed. Duncan et al.15 also studied the effect of an altered carbohydrate intake on the gut microbiota. They recruited 19 obese, but otherwise healthy individuals and allocated them to three different diets i.e., a maintenance diet, a high protein/medium carbohydrate diet (HPMC) and a high protein/low carbohydrate diet (HPLC). The investigation established that bacterial numbers were greatest in individuals on the maintenance diet and that the gram negative Bacteroides and the gram positive C. coccoides were the most abundant bacterial groups (approximately 29% and 22% of total bacteria respectively) in all cases. It was apparent that the bifidobacteria, Roseburia spp and E. rectale from the Clostridium group were all negatively impacted upon by decreased carbohydrate intake. The consumption of the HPMC and HPLC diets also resulted in the lowering of the short chain fatty acid (SCFA) concentrations, with butyrate concentrations being most dramatically reduced.
The influence of an obesity treatment program on the gut microbiota and body weight of overweight adolescents has been examined by Santacruz et al.42 The participants in this study were subjected to a calorie restricted diet and increased physical activity program over 10 weeks. After the treatment, a group of subjects, experiencing a > 4 kg weight loss and showing significant BMI reductions, was identified. The remaining individuals lost < 2.0 kg in weight despite the fact that there were no significant differences in the dietary intake of the two groups. In general, the treatment led to an increase in Bacteroides fragilis and Lactobacillus groups and a decrease in the C. coccoides, Bifidobacterium longum and Bifidobacterium adolescentis numbers. The post intervention microbiota shifts were most significant in individuals that responded more successfully to treatment. It was also noted that Bifidobacterium bifidum, the C. coccoides group, the Lactobacillus group, Bifidobacterium and Bifidobacterium breve were significantly lower in the high weight loss group compared with the low weight loss group before and after the treatment. Conversely total bacteria, the B. fragilis, the C. leptum, and the Bifidobacterium catenulatum groups were significantly higher in the more abundant weight loss group before and after treatment.42 In another such study by Nadal et al.43 the adolescent obesity treatment programmes incorporated nutritional and individual diet counselling, calorie restriction and increased physical activity over a ten week period. The maximum energy intake permitted was 1,800 kcal/d for females and 2,200 kcal/d for males. Most of the participants experienced significant weight loss ranging from 4.1 to 16.6 kg after the 10 week period. Overall, the intervention program led to reductions in the proportions of Clostridium histolyticum and, in line with previous investigations, E. rectale-C. coccoides. A correlation between C. histolyticum and E. rectale-C. coccoides proportions and BMI was also evident. Bacteroides proportions increased as a consequence of the intervention and almost achieved significant levels of correlation with weight loss. Although the Lactobacillus-Enterococcus populations also increased as weight and BMI correlations decreased, these correlations were not significant. In the group which did not experience a significant loss in weight (< 2.5 kg), the bacterial groups analyzed did not differ significantly as a consequence of the intervention program. No correlations were detected between bacterial proportions and either body weight or BMI reductions in this low weight loss group. Unsurprisingly, a number of animal studies have also taken place. The cecal microbial composition of high fat fed and control mice was quantified by Cani et al.46 They found that, in the high fat diet mice, Bacteroides-like microbes were significantly reduced compared with controls. Furthermore, although the E. rectale-C. coccoides group were found to be the dominant microbiota, a reduction in this group, and of Bifidobacterium, relative to controls was also noted.
In a study discussed briefly above Turnbaugh et al.6 took an alternative approach to investigate the relationship between diet and the gut microbiota. More specifically, conventionally raised (CONV-R; i.e., mice which have been allowed to acquire their microbiota naturally from birth) mice were weaned onto a “western” or a low-fat chow diet rich in structurally complex plant polysaccharides (CHO diet) for 8–9 weeks. Mice on the “western” diet unsurprisingly gained more weight and had a significantly larger adiposity. The researchers then performed investigations to determine if the gut microbiota of these DIO animals possessed attributes that can more successfully increase host adiposity than the microbiota of CHO fed animals. This involved the transplantation of the cecal microbiota of the lean and obese animals to GF, CHO-fed recipients. Recipients of the DIO associated microbiota recipients brought about a significantly greater proportional increase in body fat compared with recipients than the CHO-associated microbiota was capable of Turnbaugh et al.6 also tested the impact of defined shifts in diet on the body weight, adiposity and distal gut microbial ecology of obese mice. CONV-R mice were fed either a “western” diet, a “western” diet with reduced carbohydrates (CARB-R) or a “western” diet with reduced fat (FAT-R). Mice on CARB-R or FAT-R diets had reduced Mollicutes levels and an increased abundance of Bacteroidetes. It would thus seem that both FAT-R and CARB-R diets repress the multiple effects associated with “western” diet induced obesity.
The impact of fasting on the gut microbiota of hibernating animals has also been the subject of investigation. Sonoyama et al.48 compared the gut microbiota of male Syrian hamsters which were separated into a (1) fed, active, non-hibernating group, (2) a fasted, active, non-hibernating group and a (3) hibernating group, with the latter being housed in constant darkness at 4°C in order to bring on hibernation. It was established that the total bacterial populations were significantly reduced in fasted active hamsters when compared with fed active and hibernating hamsters, whereas there was no significant difference between the latter two groups. HPLC analysis of the cecal contents also showed that fasted active hamsters had significantly lower concentrations of total SCFA and acetic acid than fed active or hibernating hamsters. A 16S rRNA-based investigation of cecal bacteria showed that the class Clostridia was the most abundant taxonomic group in all treatment groups but that the proportion of the Clostridia in fasted active hamsters tended to be lower than that in fed active and hibernating hamsters. Verrucomicrobia and Proteobacteria were the second and third most abundant groups. Notably, all Verrucomicrobia-associated sequences in fasted active hamsters were classified to the genus Akkermansia. The family Desulfovibrionaceae was the most common family in the phylum Proteobacteria and the proportion of this family was higher in fasted active hamsters than in the other two groups. It has been suggested that fasting stimulates the growth of sulfate-reducing bacteria such as Desulfovibrio spp through increased degradation of mucins by A. muciniphila in the cecum of fasted active hamsters. Overall the results suggest that gut microbiota respond differently to fasting and hibernation in Syrian hamsters.
An examination of humanized mice fed a “western” diet revealed an increased representation of the Erysipelotrichi class of bacteria or, more specifcially, Clostridium innocuum, Eubacterium dolichum and Catenibacterium mitsuokai, in the fecal samples of these animals compared with those of mice fed a low fat/plant polysaccharide (LF/PP) diet.11 A significant increase in the relative abundance of another class of Firmicutes, the Bacilli (corresponding primarily to Enterococcus sp), was also associated with the “western” diet. These increases were apparent along the entire length of the gut. A significant decrease in the representation of members of the Bacteroidetes in “western” diet fed mice was also apparent. A parallel assessment of the gene composition of the gut microbiome revealed an obvious shift within 1 d of the switch to the “western” diet in the form of the enrichment of ATP-binding cassette transporters and phosphotransferase systems. The microbiome associated with the LF/PP diet was enriched for pathways including N-glycan degradation, sphingolipid metabolism and glycosaminoglycan degradation, all of which are pathways which are also enriched in Bacteroidetes.
The effects of a LF/PP and “western” diet on two dominant phyla has been investigated by Mahowald et al.47 In this study the authors specifically selected E. rectale and Bacteroides thetaiotaomicron as representatives of the two dominant bacterial phyla, Firmicutes and Bacteroidetes, and investigated how these microbes were affected by changes in the host diet. Co-colonized mice were fed one of three diets, i.e., a standard LF/PP, a high fat, high sugar “western-type” diet (HF/HS) or a low fat, high sugar, control diet (LF/HS). B. thetaiotaomicron was not affected by diet but colonisation by E. rectale was significantly reduced in mice fed either the LF/HS or HF/HS diets. The authors propose a number of explanations for this occurrence, i.e., (1) E. rectale does not possess the glycoside hydrolase and polysaccharide lyases that can process host glycans, (2) it cannot use the sugars that are derived from mucosal polysaccharides and/or (3) the glycobiome of the host includes enzymes that can directly process the simple sugars in these two diets. Transcriptional profiling of B. thetaiotaomicron revealed that it significantly upregulated polysaccharide utilization loci (PULs) involved in breakdown of host polysaccharides and downregulated PULs involved in breakdown of plant polysaccharides when mice were subjected to a HF/HS or LF/HS diet. E. rectale responded to the HF/HS and LF/HS diets by downregulating several glycoside hydrolases and sugar transporters.47
The impact of a high fat diet on the gut microbiota was again examined by Zhang et al. in 2009.45 In contrast to animals fed a normal chow diet (NC), it was noted that apolipoprotein a-I (Apoa-I) knockout and wild-type mice fed a high fat diet (HFD) lacked Bifidobacteriaceae in their faeces. Apoa-I knockout mice were included in the study as they have been shown to have impaired glucose tolerance (IGT) and increased body fat.50 After 25 weeks on a HFD the WT mice also exhibited IGT. The family Desulfovibrionaceae was more prevalent in Apoa-I-/- on NC or HFD and WT fed a HFD than WT NC control mice. An examination of the microbiota of the mice failed to identify phylum-wide changes associated with IGT/obesity. It was apparent, however, that diet and host health can have different effects on lineages within the family more specifically, levels of Erysipelotrichaceae from the class Mollicutes. The family Erysipelotrichaceae of the class Mollicutes can be subdivided into four phylogenetic clusters i.e., M1, M2, M3 and M4. The M1 cluster were reduced in Apoa-I-/- and HFD mice. Levels of the M2 cluster increased in HFD mice, the M3 cluster was severely diminished in the HFD mice and the M4 cluster was only present in the HFD mice. WT mice fed a HFD were the most obese group after the 25 week trial.
Finally, it is notable that the impact of diet on a combination of ten human gut bacteria has been the subject of a recent investigation.49 Strains of Blautia hydrogenotrophica, Bacteroides ovatus, Bacteroides caccae, B. thetaiotaomicron, Clostridium symbiosum, Collinsella aerofaciens, Desulfovibrio piger, E. rectale, E. coli and Marvinbryantia formatexigens were introduced into the gut of germ free mice which were then provided with refined diets which changed every two weeks. Each diet systematically varied the concentrations of four ingredients, i.e., casein, corn oil, cornstarch and sucrose. It was revealed that changes in diet impacted on the relative abundance of the the various species. In particular, it was noted that the abundance of all ten species was significantly associated with casein, i.e., seven species showed positive correlation to increasing casein while the abundance of the others (E. rectale, D. piger and M. formatexigens) decreased with increased casein levels. A parallel increase in the expression of pathways associated with amino acid metabolism was apparent in the seven species with which a positive correlation with casein existed.49
Other dietary-related influences.
In addition to digestible carbohydrate, the impact of other dietary components on the gut microbiota has also been investigated. In one case the impact of controlled changes in the main type of non-digestible carbohydrate components upon the microbial community of 14 overweight humans was examined.41 A significant increase in the percent of Ruminococcus bromii-like bacteria was apparent in individuals consuming a resistant starch (RS) diet relative to those in receipt of a non-starch polysaccharide (NSP) diet. A significant increase in Oscillibacter valericigenes-like bacteria was also noted in individuals on RS and a reduced carbohydrate/high protein (WL) diet compared with those fed a maintenance or NSP diets.41 Finally, Roseburia and E. rectale were also significantly increased among individuals consuming RS diets but were decreased in individuals consuming a WL diet.41
New evidence has also demonstrated a link between dietary fat and the metabolism of the intestinal microbiota with atherosclerosis.51 It has been reported that the atherosclerosis-associated upregulation of two macrophage scavenger receptors, CD36 and SR-A1, occurs in mice that have choline, trimethylamine N-oxide (TMAO) or betaine [three metabolites of the lipid phosphatidylcholine (PC)] added to their diet52 and that supplementation with choline and TMAO promotes the formation of atherosclerosis in mice.51 The link between these phenomena and the gut microbiota was made by comparing the microbiota of mice in receipt of choline or PC with or without antibiotics. Mice fed a diet supplemented with 1% choline displayed augmented atherosclerosis but this impact was lessened in mice on the same diet but which were treated with broad spectrum antibiotics. Similarly the presence of the gut microbiota is required for the formation of TMAO. More specifically, the formation of TMAO is observed in mice in receipt of PC or choline but is suppressed in their antibiotic-treated mice equivalents.51
Using a three stage colonic model the impact of a high dietary fiber intake was examined with relation to the effect on microbial composition.44 An increase in dietary fiber resulted in a significant increase in the numbers of Bifidobacterium, Ruminococcus and Lactobacillus-Enterococcus group in an in vitro three stage colonic model. High fiber intake in the vessel representing the proximal colon significantly increased the numbers of F. prausnitzii and E. rectale-C. coccoides groups.
The impact of diet on the microbiota is also very much evident when the microbiota of omnivores, herbivores and carnivores (30 three mammals and 18 humans) is compared.53 Principal coordinate analysis (PCoA) plots of both bacterial 16S rRNA and whole community gene data sets separated carnivores and omnivores from herbivores. Twelve amino acids biosynthetic enzymes were enriched in herbivores whereas as no such enrichment was apparent among carnivores. In contrast, the enrichment of nine amino acid degradation pathways was observed in carnivores. PCoA plots of diet and the human microbiome revealed total protein intake was significantly associated with KEGG orthology (KO) data whereas, insoluble dietary fiber was significantly associated with bacterial operational taxonomic units (OTU) content.53
Finally, in a recent study of 98 individuals, the effects of diet on the gut microbial enterotypes were examined.54 It was noted that only long-term diet correlated with enterotypes. Short-term controlled identical feeding was shown not to affect intersubject variation. A food frequency questionnaire found that the Bacteroides enterotype was highly associated with animal protein. In contrast Prevotella enterotype was linked to high carbohydrate and simple sugars. Vegans (n = 1) and vegetarians (n = 11) were enriched in the Prevotella enterotype.
Manipulation of the Gut Microbiota
In addition to the aforementioned studies, which have highlighted the impact of the overall levels of carbohydrate and/or fat on the composition of the gut microbiota, the impact of other specific components of diet, i.e., food additives, probiotics and prebiotics, have also been investigated.
Grain sorghum is an abundant source of phytochemicals that are of possible benefit to health.55 Martinez et al.56 used the hamster model of hypercholesterolemia to investigate if changes in the gut microbiota are linked with the positive effects of grain sorghum lipid extract (GSL) on cholesterol metabolism. The hamsters’ diet was supplemented with 0%, 1% or 5% GSL. Although high animal-to-animal variability at both the family and genus level was apparent, it was established that one family, the Coriobacteriaceae, and two genera, both of which were unclassified members of the family Erysipelotrichaceae, were significantly reduced in abundance as a consequence of the addition of GSL to the hamsters’ diet. Furthermore, 5% GSL was shown to reduce the overall diversity of the gut microbiota. In contrast, levels of the genus Pseudoramibacter and Allobaculum increased with increased GSL levels. A significant increase in Bifidobacterium in hamsters fed GSL was also observed and this increase was positively linked with high-density lipoprotein (HDL) plasma cholesterol levels (r = 0.75, p = 0.001). GLS feeding caused a decrease in the proportion of Coriobacteriaceae. This decrease in Coriobacteriaceae showed a strong correlation with non-HDL plasma cholesterol (r = 36 0.84, p = 0.0002). These findings suggest that GLS feeding influences the HDL/non-HDL equilibrium via a mechanism that appears to be through the alteration of the intestinal microbiota. Overall this analysis would imply that bifidobacteria are beneficial and Coriobacteriaceae are detrimental with respect to plasma cholesterol levels in hamsters.
The impact of probiotics on the composition of the gut microbiota has been the focus of particularly great attention in recent years. Probiotics are defined by the food and agriculture organization of the united nations57 and the WHO as “live microorganisms which when administered in adequate amounts confer a health benefit on the host.”57 Notably, Schiffrin et al.58 performed an investigation to determine if administration of a probiotic containing yoghurt can ameliorate or treat small intestinal bacterial overgrowth (SIBO) by improving gut barrier function or as a consequence of their antibacterial, immunomodulatory and/or anti-inflammatory effects. SIBO most frequently occurs in older people and can lead to chronic diarrhea, anorexia and nausea as well as malabsorption and malnutrition.59,60 In the study, 23 elderly subjects with a positive glucose/H2 breath test (SPH) and 13 subjects with a negative test (SNH) were administered the probiotic yoghurt Lactobacillus johnsonii La1 for 4 weeks. The glucose/H2 breath test measures the excretion of hydrogen in the breath which is reflective of glucose metabolism by microbes in the intestine. Patients with a positive result are suspected of having SIBO.61,62 After 4 weeks of yoghurt consumption, the authors noticed a trend toward a reduction of endotoxin concentration in the SPH group and a significant decrease in plasma endotoxin was also noted in the SNH after probiotic consumption. In conclusion, Schiffrin and colleagues suggest that an altered intestinal ecology underlies the low grade inflammatory status that favors catabolism and loss of lean body mass in the elderly and thus, redressing such ecological imbalances could provide health benefits.
In 2009, Hamad et al.63 investigated the ability of milk fermented by Lactobacillus gasseri SBT2055 (LGSP) to impact on adipocyte size by inhibition of dietary fat absorption in Zucker rats. Obese Zucker rats have a spontaneous mutation in the leptin receptor gene causing early onset severe obesity due to over-eating.64,65 Rats fed a LGSP diet had reduced total, mesenteric and subcutaneous adipose tissue masses compared with those fed a control skimmed milk diet (SM). No significant effect was observed on other white adipose tissue. The mesenteric fat mass in lean rats was reduced even more dramatically than that of obese rats fed the LGSP diet (p < 0.05). The LGSP diet also had a significant effect on serum leptin concentrations which were decreased by 36% in lean rats but were not significantly altered in obese rats. However, the LGSP diet had no effect on the serum levels of both glucose and adiponectin in lean or obese rats. The LGSP diet caused a significant reduction in the levels of total and HDL-cholesterol in serum and a significant increase in fecal cholesterol in both groups. Overall, the results of this study revealed that the milk fermented by L. gasseri reduced visceral adipose tissue mass and adipocyte hypertrophy in lean Zucker rat through a decrease in fatty acid absorption.63
The benefits of consuming prebiotics have also been the focus of ever greater attention in recent years. Prebiotics are defined as “non-digestible food ingredients that beneficially affect the host by selectively stimulating the growth and/or activity of one or a limited number of bacterial species already resident in the colon, and thus attempt to improve host health.”66 Cani and colleagues67 hypothesized that the control of gut permeability through the selective modulation of gut microbiota by prebiotics helps to protect ob/ob mice from metabolic diseases and performed investigations which revealed that mice fed the prebiotic (oligofructose) had lower levels of the cytokines TNFα, IL1b, IL1α, IL6 and INFγ, all of which are known to promote tight-junction disruption. This study also showed that altering the gut microbiota through the increase in abundance of Bifidobacterium spp through the use of prebiotics is linked with a notable reduction of gut permeability. The improved gut barrier of ob/ob prebiotic (Ob-Pre) fed mice also correlated with lower plasma LPS levels and inflammatory tone. A decrease in markers of oxidative and inflammatory stress in liver tissue significantly correlated with the lowering of systemic inflammation by prebiotics. Overall these data suggest that prebiotics could act favorably on the gut barrier, hence improving metabolic disorders.67 The authors also noted that when the gut microbiota is changed using prebiotics, an increase in endogenous production of the glucagon-like peptide-2 (GLP-2) occurs which may explain the associated improvement in intestinal barrier function. Evidence for this was provided when it was established that a GLP-2 antagonist completely blocked the positive effects of prebiotic treatment on both intestinal tight-junction proteins and proglucagon mRNA.67 The effect of inulin-type fructans (ITFs) with prebiotic properties on the gut microbiota of animals fed a high fat diet was investigated by Dewulf et al. in 2010. While it was established that cecal content was significantly reduced in mice fed a high fat (HF) diet relative to that of mice fed a control (CT) diet, this effect was reversed in mice co-administered ITFs with the HF diet. Total bacterial numbers were reduced in HF fed mice relative to CT fed animals while qPCR revealed a 100-fold increase in bifidobacteria numbers and a reduction in Roseburia spp and Clostridium cluster XIVa in ITF-HF mice compared with CT and HF fed mice. Notably, weight gain and subcutaneous adipose tissue accumulation was reduced in ITF treated HF mice even though they ingested fat to a level similar to that for non-ITF treated HF mice.68
The consequences of consuming different concentrations of prebiotic fiber (inulin and oligofructose), over a ten week period, on the gut microbiota of lean and obese rats was the subject of another recent investigation.69 Obese and lean rats were broken into three diet groups: controls (C), 10% prebiotic fiber (LF) and 20% prebiotic fiber (HF). In control rats the levels of Bacteroides/Prevotella, C. leptum and Enterobacteriaceae as well as the overall bacterial population were greater in lean than obese rats. Obese rats on the HF diet had significantly increased levels of total bacteria, Bacteroides/Prevotella, C. leptum, Lactobacillus, Bifidobacterium and Enterobacteriaceae when compared with obese controls. Meanwhile lean rats fed a HF diet had significantly increased Bacteroides/Prevotella and Bifidobacterium populations while a significant decrease in the C. leptum and C. coccoides populations was apparent. No statistically significant difference was seen between LF diet rats and controls. Percentage body fat, body weight, fasting insulin, insulin, incremental area under the curve (iAUC) and energy intake negatively correlated with Bacteroides and total bacteria. Total energy intake, glucose iAuc, body weight and fat positively correlate with Lactobacillus spp. The population of Enterobacteriaceae increases with increases in glucose iAuc and GLP-1 total area under the curve (tAUC). A positive correlation was noted between Bacteroides and total bacteria and ghrelin tAUC.
Despite these promising results, there has been some debate recently, prompted by a proposal by Prof Didier Raoult that a link may exist between probiotics and obesity.70 Prof Raoult has suggested that there are dangers associated with promoting the consumption of products containing bacteria that have been associated with weight gain in the animal food industry. Raoult’s opinion is based on four points: (1) Ley et al.12 found that the gut of obese individuals contained more Firmicutes than that of lean individuals, (2) The farming industry uses probiotics which can contain Firmicutes, including Lactobacillus spp, Bifidobacterium spp and Enterococcus spp, as growth promoters. (3) L. acidophilus, at levels equivalent to those found in functional foods, causes weight gain in piglets and, finally, (4) Lactobacillus species have shown to cause weight gain in children.17 This view has been rejected in responses by other scientists71,72 and, indeed, the arguments made by Dr Raoult do not accurately reflect the data present in the papers he has cited, or consider the data presented by other papers, some of which have already been discussed in this review. Most notably, lactobacilli represent just a small fraction of Firmicutes in the gut. Furthermore, Raoult’s editorial refers to Bifidobacterium as a Firmicute, when in fact it belongs to the phylum Actinobacteria. In addition, in the studies cited by Raoult,17,73 probiotics did not significantly impact on weight gain in children. Finally, in a recent review in reference 74, it was revealed that only 3 out of 22 studies showed a significant weight gain in piglets.
Many antibiotics used to threat humans have a broad spectrum of activity which facilitates the treatment of infections of unknown etiology. As these antibiotics are not selective in their killing, there are associated impacts on the natural biota of the human gut. In this instance the importance of the gut microbiota with respect to obesity and metabolism was investigated through disruption of the gut microbiota through treatment with ampicillin or neomycin.75 WT mice were fed a control, control with antibiotics, high fat or high fat with antibiotics (carbohydrate free) diet for 4 weeks. ob/ob mice were also fed a control diet or a control diet with antibiotics. The 4 week antibiotic therapy had considerable impacts. The endotoxin content per gram of cecal content was considerably decreased after antibiotic treatment in both the control and high fat diet groups. Adiposity was also reduced in high fat, antibiotic-treated mice compared with high fat, untreated controls and, furthermore, antibiotic treatment significantly lowered plasma LPS levels, gut permeability. The occurrence of visceral (mesentric) adipose tissue inflammation, oxidative stress, macrophage infiltration and metabolic disorders was also lowered. Microbiological analysis revealed that the high fat diet mice reduced Lactobacillus spp and Bifidobacterium spp numbers, but increased Bacteroides-Prevotella spp numbers, compared with control mice. High-fat fed, antibiotic-treated mice had reduced numbers of all three of these groups compared with the high fat diet mice. Indeed the microbiota of the high-fat fed, antibiotic-treated mice had numbers of all three groups which were very similar to those present in control antibiotic mice. ob/ob mice had higher Lactobacillus spp and Bifidobacterium spp, but lower Bacteroides-Prevotella numbers, than ob/ob mice. Unsurprisingly, antibiotic treatment dramatically changed the ob/ob mice gut microbiota, reducing Lactobacillus spp Bifidobacterium spp and Bacteroides-Prevotella spp.75
Mechanisms by which the Gut Microbiota may Impact Obesity
Thus far this review has focused on studies in which differences in the microbiota of lean and obese animals and humans and the impact of diet on these microbial populations were investigated. However, a key question is how these microbial populations are impacting on weight gain. In this section mechanisms via which these gut microbes can impact on obesity is discussed (see also Fig. 3).
Figure 3.

Comparison of germ free and conventionally raised mice with respect to weight gain and associated biomarkers. Fasting induced adipose factor (Fiaf), phosphorylated AMP activated protein kinase (AMPK), lipoprotein lipase (LPL) and western diet (WD).
Energy extraction, leptin, Fiaf and AMPK.
As noted briefly above, the gut microbiota is capable of the breakdown of otherwise indigestible components of the mammalian diet, thus affecting the energy balance. In 2004 Bäckhed et al.76 analyzed germ free (GF), CONV-R and conventionalized (CONV-D) mice to examine the hypothesis that the microbiota acts through host signaling pathways to regulate energy storage in the host. GF mice are raised in the absence of any microbiota, while CONV-D mice are initially germ free but are then colonized with the microbiota from CONV-R donors. The study found that CONV-R animals had 42% more total body fat and 47% more epididymal fat pad weight than GF mice and that their epididymal fat pad weights were significantly greater. This was despite the fact that their consumption of chow was 29% less than their GF counterparts. CONV-D mice had 57% greater total body fat content and 61% greater epididymal fat pad weight than GF mice. Like CONV-R mice, CONV-D animals also consumed less food, in this case 27% less than GF mice. Increased energy expenditure by GF mice was excluded as an explanation for the decreased body fat content of GF mice as these animals were shown to have a metabolic rate that was 27% lower than either CONV-R or CONV-D mice. Further investigation revealed that microbial colonization caused an increase in leptin levels which was proportional to the increase in body fat. Leptin is mainly an adipocyte-derived hormone which reduces food intake and increases energy expenditure in mice and thus the impact of the microbiota on leptin levels may be of importance.77,78 An increased in lipoprotein lipase (LPL) has also been observed in the epididymal fat pads of CONV-D mice compared with GF mice. This is significant as LPL is a vital regulator in the release of fatty acids from lipoproteins in muscle, heart and fat.79 Notably, the Bäckhed study also showed that the presence of a microbial population in the gut promotes increased monosaccharide uptake.76 Analysis of the gut microbiome by Turnbaugh et al.4 in 2006 also revealed that the gut microbiome of ob/ob mice had an increased capacity to ferment polysaccharides compared with the lean-associated equivalent. It was thus postulated that this could lead to more energy being extracted from complex carbohydrates, leading to increased energy in the host. Bomb calorimetry supported this theory, in that it was found that stool samples from obese mice contained less energy than their lean counterparts. To test this idea further, GF mice were colonised with the gut microbiota from ob/ob or +/+ donors. Noticeably the mice colonized with the ob/ob microbiota had a significantly greater percentage increase in body fat over 2 weeks than mice colonized with a +/+ microbiota. This prompted further investigation and in 2007 Bäckhed and colleagues80 again studied GF and CONV-R mice to establish if GF mice are resistant to DIO. After being fed a “western” diet for 8 weeks it was again found that CONV-R mice had gained significantly more weight than their germ free counterparts and, as had been found previously in reference 76, the epididymal fat pad weights were also significantly greater in conventionalised mice than GF mice. GF mice fed a “western” diet for 8 weeks showed no significant weight gain when compared with GF mice on a low fat diet. However, unlike the previous cited investigation, it was found that the amounts of chow consumed by CONV-R and GF mice were similar. Notably, it was established that GF mice had higher levels of Fiaf (fasting induced adipose factor) expression in the intestine than CONV-R mice. Fiaf is a circulating lipoprotein lipase inhibitor whose expression is normally selectively suppressed in the gut epithelium by the microbiota.76 The relevance of Fiaf expression was highlighted when it was established that when GF wild-type and Fiaf-/- mice were fed a “western diet,” Fiaf deficient animals gained significantly more weight and had significantly greater epididymal fat pads than their wild-type littermates.80 Higher levels of LPL activity (67%) in the epididymal fat pads of Fiaf-/- mice compared with GF wild-type mice was also noted.76 GF mice were also found to have increased skeletal muscle and liver levels of phosphorylated AMP activated protein kinase (AMPK). AMPK is a heterotrimeric enzyme that functions as a “fuel gauge” that monitors cellular energy status. Increased intracellular ratios of AMP to ATP results in its activation.81 40% and 50% higher levels of AMPK and AMP, respectively, were found in the gastrocnemius muscle harvested from GF mice than CONV-D mice fed a “western diet.” This indicates that GF mice are sheltered from diet induced obesity by two mechanisms that result in increased fatty acid metabolism i.e., elevated levels of Fiaf and AMPK.80
As discussed briefly above, Hilderbrandt et al.7 in 2009 investigated the effects of a high fat diet on WT and RELMβ KO mice. Higher levels of RELMβ expression were observed in high fat diet mice when compared with mice fed a standard chow diet. It was also noted that oral treatment of mice with antibiotics reduced the expression of colonic RELMβ in both mice fed a standard chow or a high fat diet. Thus it was concluded that the induction of RELMβ expression by a high fat diet is dependent upon the commensal gut microbiota. It was noted that although KO and wild-type mice weighed the same at 13 weeks of age when fed a standard chow diet, following 21 weeks on a high fat diet RELMβ KO mice displayed diminished weight gain due to a decreased build-up of fat mass compared with the wild-type controls. This difference was not due to an alteration in food intake, fat absorption or core body temperature and it was also established that RELMβ KO mice did not exhibit any differences with respect to physical activity when compared with wild-type controls during the period. However, indirect calorimetry revealed that the relative reduction in diet induced obesity in KO mice was caused by an increase in energy expenditure. Further analysis also revealed that the expression of a collection of genes encoding ABC transporters was increased in wild-type mice fed the high fat diet when compared with expression of the same genes in wild-type mice on a standard chow diet. The corresponding proteins are responsible for the transport of lipids, sugars and peptides as well as metals. Expression of genes for amino acid metabolism and carbohydrate metabolism were relatively decreased. In summary, the results from this study demonstrate the importance of diet as a determinant of gut microbiome composition and suggest the need to control for dietary variation when evaluating the composition of the human gut microbiome.
The possibility that manipulation of the gut microbiota with B. breve might influence the fatty acid composition of host tissues has been investigated by Wall et al.82 In this study different animal models were fed with B. breve NCIMB 702258, which was selected because of its ability to synthesize bioactive isomers of conjugated linoleic acid (CLA) from free linoleic acid. The authors hypothesized that the administration of NCIMB 702258 could have an anti-inflammatory impact by virtue of this metabolite. An 8 week dietary intervention study involving BALB/c mice showed that the cis-9, trans-11 CLA (c9,t11 CLA) content of the livers of mice fed the B. breve and a linoleic acid substrate was 2.4-fold higher than in the control mice. This CLA isomer has been previously shown to exhibit a number of beneficial activities in experimental animal models and human cell culture studies including the ability to reduce body fat and to bring about anti-diabetic effects.83-85 These studies should be distinguished from those involving the cis-12, trans-10 CLA isomer, which can have negative effects.86,87 The c9,t11 CLA content of the large intestine, small intestine, cecal contents and faeces of these test mice was also higher than that of controls. In the same study, the supplementation of the diet of severe combined immunodeficient (SCID; most frequently employed as animal models of inflammatory bowel disease) mice with B. breve and linoleic acid resulted in 4-fold higher levels of c9,t11 CLA in the liver than the group receiving linoleic acid alone. Examination of the former group also revealed 3.0- and 2.0-fold higher levels of c9,t11 CLA in the large intestines and ceca, respectively. Indeed, all tissues from SCID mice fed pure c9,t11 CLA had higher levels of this compound when compared with those fed linoleic acid alone. It was also noted that levels of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in adipose tissue in mice supplemented with B. breve alone were 3-fold higher than the corresponding tissue from control mice. EPA and DHA are also known to exert anti-inflammatory properties. From corresponding porcine studies it was established that the livers of pigs fed the B. breve strain and linoleic acid had 1.5-fold greater c9,t11 CLA levels than those from unsupplemented controls. Overall this study showed that the fatty acid composition of host tissue can be positively influenced by the oral administration of a metabolically active commensal acting on a dietary substrate.
The occurrence of sexual dimorphism in the mouse model in relation to total body fat content (TBFC) was observed in a recent study.88 It was demonstrated that male mice had a significantly higher TBFC than female mice. Interestingly GF male and female mice did not display this sexual dimorphism. GF animals showed lower brown adipose tissue lactate levels and circulating levels of very low density lipoprotein while greater levels of (D)-3-hydroxybutyrate in liver, plasma and brown adipose tissue. These results imply that the gut microbiota adjust the lipid metabolism in brown adipose tissue as the loss of gut microbiota inhibits lipogenesis while also promoting hepatic and brown adipose tissue lipolysis.
Short chain fatty acids.
Fecal SCFAs are predominantly produced from the fermentation of fiber in the large intestine by bacteria. In the absence of these intestinal microbes, the host would not be able to completely hydrolyse this fiber. These SCFAs, consisting mainly of acetate, propionate and butyrate, represent an additional source of energy and, indeed, it is estimated that microbially generated SCFAs provide 10% of the total dietary energy supply in humans.81 SCFA levels were investigated in the previously referred to study by Schwiertz et al. 2009.14 In total, 98 volunteers (34 males and 64 females) were analyzed and their stool samples revealed the presence of acetate, propionate, butyrate and valerate as well as iso-valerate and iso-butyrate. It was noted that the samples from obese volunteers had 20% higher mean total SCFA concentrations than those from lean volunteers. Of the SCFA, propionate levels were most dramatically increased in this group (41%), followed by levels of butyrate (28%), valerate (21%) and acetate (18%). In contrast, the iso-SCFAs concentrations did not differ considerably. In 2007 Duncan et al.15 measured the changes in fecal SCFA in response to changes in dietary intake of carbohydrates. Volunteers were given a maintenance (M) (13% protein, 52% carbohydrate and 35% fat) diet for 3 d. After this they were given a high protein (30%), low carbohydrate (4%) (HPLC) diet or a high protein (30%), moderate carbohydrate (35%) (HPMC) diet for 4 weeks. The analysis of fecal samples revealed that concentrations of SCFA were lower when the volunteers were in receipt of the HPLC and HPMC diets than when they consumed the M diet. While the concentrations of the predominant SCFAs, i.e., acetate, propionate and valerate, decreased due to the shift from the maintenance to the low carbohydrate diets (50%), butyrate levels decreased even more dramatically (75%). Notably, a linear relationship existed between carbohydrate intake and butyrate concentration. In contrast, although Murphy et al. 2010,10 did find that the fecal energy content of ob/ob mice was decreased and cecal SCFA concentrations increased at 7 weeks of age relative to lean controls, these patterns did not continue with time and were not observed in DIO mice. The Murphy et al.10 study also indicated that SCFA concentrations were unrelated to changes in proportions of Firmicutes, Bacteroidetes or Actinobacteria. These findings suggest that the connection between the microbial composition and energy harvest capacity is more complex than previously thought.
LPS-mediated impacts on obesity.
Obesity and metabolic syndrome are associated with low grade inflammation, and data from several studies provides evidence that the lipopolysaccharide (LPS) endotoxin derived from certain components of the gut microbiota contributes to the increased development of adipose tissue and impaired glucose tolerance in obesity. LPS is a component of the Gram-negative bacterial cell wall and is composed of lipid and a polysaccharide. The impact of LPS on the host is mediated through the Toll-like receptor 4 (TLR4)/MyD88/NFκB signaling pathway. Endogenous LPS is continuously produced in the gut as a consequence of the death of Gram-negative bacteria and is absorbed into capillaries of the intestine through a TLR4-dependant mechanism. In 2007, Cani et al.46 showed that LPS could be an early factor in the triggering of high-fat diet induced metabolic diseases. More specifically, their data showed that high fat feeding caused plasma LPS concentrations to remain high throughout the whole day compared with controls which showed dinural variations in plasma LPS concentrations. Due to the fact that high fat feeding induced plasma LPS concentrations were lower than values associated with septicemia and infections, the authors defined this phenomenon as metabolic endotoxemia. To causally link high fat diet increased LPS concentrations to metabolic disease, the authors mimicked LPS concentrations of high fat feeding by implanting a subcutaneous osmotic minipump in mice and continuously infused LPS or saline for a month. It was then revealed that fasted glycemia, blood glucose, fasted insulinemia, liver triglyceride content and body weight levels were greater in mice infused with LPS than those infused with saline. Furthermore, the magnitude of weight gain and visceral and subcutaneous adipose depots in LPS infused mice was similar to that observed in mice fed a high fat diet. It was also apparent that mRNA concentrations corresponding to the genes for the main inflammatory factors involved in metabolic disease (i.e., tumor necrosis factor (TNF)α, interleukin (IL)-1, IL-6 and plasminogen activator inhibitor (PAI)-1) were increased in both high fat diet and LPS infused mice. In a further study, WT and CD14 mutant mice were intravenously infused with LPS for 3 h. It was noted that, as a consequence, levels of IL-6, PAI-1, IL-1, phosphorylated nuclear factorκB and IkappaB kinase89 forms increased in WT mice whereas levels of the same factors decreased or were unchanged in CD14 mutant mice. In addition, the body weight, visceral and subcutaneous adipose depot weight and liver weight of WT mice were increased but were unchanged in CD14 mutant mice.
The possibility that metabolic endotoxemia could be controlled by changes of the gut microbiota has been examined by Cani et al.75 This involved a 4 week study during which mice were fed a control, or a high fat, carbohydrate-free diet. While mice fed a high fat diet had increased plasma LPS levels relative to controls, plasma LPS levels were not increased in high fat diet mice treated with the antibiotics ampicillin and neomycin. This study also revealed that the high fat diet significantly increased intestinal permeability through a mechanism that resulted in reduced expression of ZO-1 and occludin, i.e., tight junction proteins. Antibiotic treatment reversed this effect, suggesting that gut bacteria affected by antibiotic administration are involved in the control of intestinal permeability and hence the occurrence of metabolic endotoxemia.
A link may also exist between serum amyloid A (SAA) proteins and LPS. SAA proteins are suspected mediators of inflammation and atherosclerosis.90-92 Increased serum levels of SAA proteins have been linked with obesity, chronic hyperglycemia, insulin resistance and cardiovascular disease.91,93-97 Four functional SAA isoforms have been identified in mice i.e., SAA1–4. SAA3 is the most abundant SAA isoform and, significantly with respect to the link between LPS and obesity, its expression is induced in adipose tissue after intraperitoneal administration of LPS.98 The human isoform, SAA1, is most similar to the mouse SAA3 when their amino acid sequences are compared. The expression of human SAA1 has been shown to augument lipolysis in adipocytes91 and increased expression is observed in hypertrophic adipocytes of obese humans.99 Scheja et al.97 showed that SAA3 is also upregulated in the adipose tissue of mice fed a high-fat diet and proposed that SAA3 could be a mediator of the chronic inflammation associated with insulin resistance in obesity. Reigstad et al.98 took these investigations a step further by comparing levels of SAA3 mRNA in adipose and intestinal tissue from GF and CONV-R mice to determine if components of the gut microbiota impacted on SAA3 levels. It was established that SAA3 mRNA levels in adipose tissue were significantly higher (9.9-fold) in CONV-R mice than in GF mice, whereas SAA1 and SAA2 levels did not increase significantly in these tissues. With respect to the gut, an analysis of cDNAs from the duodenum, jejunum, ileum and proximal colon revealed that SAA3 mRNA levels were highest in the colon of both GF and CONV-R mice and that, here too, SAA3 mRNA levels were significantly higher (7.0 fold) in CONV-R mice than GF controls. Expression of TNFα, a commonly expressed cytokine with a likely role in chronic inflammatory disease and a known regulator of SAA3 expression, was also found to be significantly higher in the colon of CONV-R mice. Co-staining of colonic sections from CONV-R mice revealed that SAA3 is expressed by the intestinal epithelial cells and intraepithelial macrophages which may contribute to the elevated SAA3 levels identified in the colon of these animals. Indeed, the treatment of CMT-93 colonic epithelial cell lines and RAW 264.7 mouse macrophages with increasing concentrations of purified E. coli LPS caused a concentration-dependent increase in SAA3 mRNA levels. More specifically, a 27-fold increase was observed in the CMT-93 cells upon the addition of 1 μg/ml LPS. Furthermore, SAA3 mRNA levels also increased in RAW 264.7 macrophages treated with 1 μg/ml LPS, but only to a maximum of 1.6-fold. The authors speculated that mouse SAA3 might be functionally similar to human SAA1 and could represent a link between low grade microbiota-induced inflammation and obesity.
Conclusion
The debate regarding the significance of the Firmicutes:Bacteroidetes ratio with respect to obesity is still ongoing, and the basis for differences between the various studies investigating this phenomenon has not yet been established. While differences in host genetics represent an obvious variable, other factors such as the degree of weight change, the severity of calorie restriction and/or the duration of the study are just some of a number of other important factors. Possibly the most important of these factors relates to diet associated differences in the composition of the gut microbiota. The question as to whether specific populations are responsible for weight gain or are simply flourishing as a consequence of the diet being consumed (i.e., are these populations a “cause” or an “effect”) remains a key one. The use of GF mice and mice conventionalized with known microbial species should help to reveal the influence of specific populations on host health and in turn reveal therapeutic targets in the fight against obesity. Further investigations, combined with the ever increasing capacity of next generation sequencing technologies, and the standardization of methods to facilitate comparisons between studies will overcome the inconsistencies that have plagued investigations into the link between human gut microbiota and obesity. Such developments are crucial as the obesity epidemic is showing no sign of abating and all possible treatments, including potentially the targeting of specific components of the gut microbiota of overweight and obese individuals, need to be considered.
Acknowledgments
S.F.C. is the recipient of a Teagasc Walsh Fellowship.
Footnotes
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/20168
References
- 1.WHO. Fact sheet: obesity and overweight. Available at: https://wwwwhoint/mediacentre/factsheets/fs311/en/indexhtml Accessed 13 April 2011.
- 2.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102:11070–5. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977;74:5463–7. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 5.Margulies M, Egholm M, Altman WE, Attiya S, Bader JS, Bemben LA, et al. Genome sequencing in microfabricated high-density picolitre reactors. Nature. 2005;437:376–80. doi: 10.1038/nature03959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turnbaugh PJ, Bäckhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3:213–23. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, Keilbaugh SA, Hamady M, Chen YY, et al. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology. 2009;137:1716–24, e1-2. doi: 10.1053/j.gastro.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He WM, Wang ML, Jiang HQ, Steppan CM, Shin ME, Thurnheer MC, et al. Bacterial colonization leads to the colonic secretion of RELMbeta/FIZZ2, a novel goblet cell-specific protein. Gastroenterology. 2003;125:1388–97. doi: 10.1016/j.gastro.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 9.Shojima N, Ogihara T, Inukai K, Fujishiro M, Sakoda H, Kushiyama A, et al. Serum concentrations of resistin-like molecules beta and gamma are elevated in high-fat-fed and obese db/db mice, with increased production in the intestinal tract and bone marrow. Diabetologia. 2005;48:984–92. doi: 10.1007/s00125-005-1735-1. [DOI] [PubMed] [Google Scholar]
- 10.Murphy EF, Cotter PD, Healy S, Marques TM, O’Sullivan O, Fouhy F, et al. Composition and energy harvesting capacity of the gut microbiota: relationship to diet, obesity and time in mouse models. Gut. 2010;59:1635–42. doi: 10.1136/gut.2010.215665. [DOI] [PubMed] [Google Scholar]
- 11.Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1:ra14. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–3. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 13.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–4. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwiertz A, Taras D. Schäfer Kl, Beijer S, Bos NA, Donus C, et al. Microbiota and SCFA in Lean and Overweight Healthy Subjects. Obesity 2009. [DOI] [PubMed] [Google Scholar]
- 15.Duncan SH, Belenguer A, Holtrop G, Johnstone AM, Flint HJ, Lobley GE. Reduced dietary intake of carbohydrates by obese subjects results in decreased concentrations of butyrate and butyrate-producing bacteria in feces. Appl Environ Microbiol. 2007;73:1073–8. doi: 10.1128/AEM.02340-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mai V, McCrary QM, Sinha R, Glei M. Associations between dietary habits and body mass index with gut microbiota composition and fecal water genotoxicity: an observational study in African American and Caucasian American volunteers. Nutr J. 2009;8:49. doi: 10.1186/1475-2891-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chouraqui JP, Grathwohl D, Labaune JM, Hascoet JM, de Montgolfier I, Leclaire M, et al. Assessment of the safety, tolerance, and protective effect against diarrhea of infant formulas containing mixtures of probiotics or probiotics and prebiotics in a randomized controlled trial. Am J Clin Nutr. 2008;87:1365–73. doi: 10.1093/ajcn/87.5.1365. [DOI] [PubMed] [Google Scholar]
- 18.Armougom F, Henry M, Vialettes B, Raccah D, Raoult D. Monitoring bacterial community of human gut microbiota reveals an increase in Lactobacillus in obese patients and Methanogens in anorexic patients. PLoS One. 2009;4:e7125. doi: 10.1371/journal.pone.0007125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, et al. MetaHIT Consortium Enterotypes of the human gut microbiome. Nature. 2011;473:174–80. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martens EC, Koropatkin NM, Smith TJ, Gordon JI. Complex glycan catabolism by the human gut microbiota: the Bacteroidetes Sus-like paradigm. J Biol Chem. 2009;284:24673–7. doi: 10.1074/jbc.R109.022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wright DP, Rosendale DI, Robertson AM. Prevotella enzymes involved in mucin oligosaccharide degradation and evidence for a small operon of genes expressed during growth on mucin. FEMS Microbiol Lett. 2000;190:73–9. doi: 10.1111/j.1574-6968.2000.tb09265.x. [DOI] [PubMed] [Google Scholar]
- 22.Derrien M, Vaughan EE, Plugge CM, de Vos WM. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol. 2004;54:1469–76. doi: 10.1099/ijs.0.02873-0. [DOI] [PubMed] [Google Scholar]
- 23.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 24.Zhang HS, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu YS, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci U S A. 2009;106:2365–70. doi: 10.1073/pnas.0812600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furet JP, Kong LC, Tap J, Poitou C, Basdevant A, Bouillot JL, et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59:3049–57. doi: 10.2337/db10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li JV, Ashrafian H, Bueter M, Kinross J, Sands C, le Roux CW, et al. Metabolic surgery profoundly influences gut microbial-host metabolic cross-talk. Gut. 2011;60:1214–23. doi: 10.1136/gut.2010.234708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collado MC, Isolauri E, Laitinen K, Salminen S. Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. Am J Clin Nutr. 2008;88:894–9. doi: 10.1093/ajcn/88.4.894. [DOI] [PubMed] [Google Scholar]
- 28.Langhendries JP. [Early bacterial colonisation of the intestine: why it matters?] Arch Pediatr. 2006;13:1526–34. doi: 10.1016/j.arcped.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 29.Santacruz A, Collado MC, García-Valdés L, Segura MT, Martín-Lagos JA, Anjos T, et al. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br J Nutr. 2010;104:83–92. doi: 10.1017/S0007114510000176. [DOI] [PubMed] [Google Scholar]
- 30.WHO. Set of recommendations on the marketing of foods and non-alcoholic beverages to children. World Health Organization: Geneva 2010. [Google Scholar]
- 31.Kalliomäki M, Collado MC, Salminen S, Isolauri E. Early differences in fecal microbiota composition in children may predict overweight. Am J Clin Nutr. 2008;87:534–8. doi: 10.1093/ajcn/87.3.534. [DOI] [PubMed] [Google Scholar]
- 32.Balamurugan R, George G, Kabeerdoss J, Hepsiba J, Chandragunasekaran AMS, Ramakrishna BS. Quantitative differences in intestinal Faecalibacterium prausnitzii in obese Indian children. Br J Nutr. 2010;103:335–8. doi: 10.1017/S0007114509992182. [DOI] [PubMed] [Google Scholar]
- 33.Luoto R, Kalliomäki M, Laitinen K, Delzenne NM, Cani PD, Salminen S, et al. Initial dietary and microbiological environments deviate in normal-weight compared to overweight children at 10 years of age. J Pediatr Gastroenterol Nutr. 2011;52:90–5. doi: 10.1097/MPG.0b013e3181f3457f. [DOI] [PubMed] [Google Scholar]
- 34.Manco M, Fernandez-Real JM, Vecchio FM, Vellone V, Moreno JM, Tondolo V, et al. The decrease of serum levels of human neutrophil alpha-defensins parallels with the surgery-induced amelioration of NASH in obesity. Obes Surg. 2010;20:1682–9. doi: 10.1007/s11695-010-0129-8. [DOI] [PubMed] [Google Scholar]
- 35.Gavrila A, Chan JL, Yiannakouris N, Kontogianni M, Miller LC, Orlova C, et al. Serum adiponectin levels are inversely associated with overall and central fat distribution but are not directly regulated by acute fasting or leptin administration in humans: cross-sectional and interventional studies. J Clin Endocrinol Metab. 2003;88:4823–31. doi: 10.1210/jc.2003-030214. [DOI] [PubMed] [Google Scholar]
- 36.Chen J, Tan B, Karteris E, Zervou S, Digby J, Hillhouse EW, et al. Secretion of adiponectin by human placenta: differential modulation of adiponectin and its receptors by cytokines. Diabetologia. 2006;49:1292–302. doi: 10.1007/s00125-006-0194-7. [DOI] [PubMed] [Google Scholar]
- 37.Okamoto Y, Kihara S, Funahashi T, Matsuzawa Y, Libby P. Adiponectin: a key adipocytokine in metabolic syndrome. Clin Sci (Lond) 2006;110:267–78. doi: 10.1042/CS20050182. [DOI] [PubMed] [Google Scholar]
- 38.Tsatsanis C, Zacharioudaki V, Androulidaki A, Dermitzaki E, Charalampopoulos I, Minas V, et al. Peripheral factors in the metabolic syndrome: the pivotal role of adiponectin. Ann N Y Acad Sci. 2006;1083:185–95. doi: 10.1196/annals.1367.013. [DOI] [PubMed] [Google Scholar]
- 39.Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2009;302:179–88. doi: 10.1001/jama.2009.976. [DOI] [PubMed] [Google Scholar]
- 40.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–9, quiz 920. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 41.Walker AW, Ince J, Duncan SH, Webster LM, Holtrop G, Ze X, et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011;5:220–30. doi: 10.1038/ismej.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Santacruz A, Marcos A, Wärnberg J, Martí A, Martin-Matillas M, Campoy C, et al. EVASYON Study Group Interplay between weight loss and gut microbiota composition in overweight adolescents. Obesity (Silver Spring) 2009;17:1906–15. doi: 10.1038/oby.2009.112. [DOI] [PubMed] [Google Scholar]
- 43.Nadal I, Santacruz A, Marcos A, Warnberg J, Garagorri M, Moreno LA, et al. Shifts in clostridia, bacteroides and immunoglobulin-coating fecal bacteria associated with weight loss in obese adolescents. Int J Obes (Lond) 2009;33:758–67. doi: 10.1038/ijo.2008.260. [DOI] [PubMed] [Google Scholar]
- 44.Shen Q, Zhao L, Tuohy KM. High-level dietary fibre upregulates colonic fermentation and relative abundance of saccharolytic bacteria within the human faecal microbiota in vitro. Eur J Nutr. 2011;51:693–705. doi: 10.1007/s00394-011-0248-6. [DOI] [PubMed] [Google Scholar]
- 45.Zhang C, Zhang M, Wang S, Han R, Cao Y, Hua W, et al. Interactions between gut microbiota, host genetics and diet relevant to development of metabolic syndromes in mice. ISME J. 2010;4:232–41. doi: 10.1038/ismej.2009.112. [DOI] [PubMed] [Google Scholar]
- 46.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–72. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 47.Mahowald MA, Rey FE, Seedorf H, Turnbaugh PJ, Fulton RS, Wollam A, et al. Characterizing a model human gut microbiota composed of members of its two dominant bacterial phyla. Proc Natl Acad Sci U S A. 2009;106:5859–64. doi: 10.1073/pnas.0901529106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sonoyama K, Fujiwara R, Takemura N, Ogasawara T, Watanabe J, Ito H, et al. Response of gut microbiota to fasting and hibernation in Syrian hamsters. Appl Environ Microbiol. 2009;75:6451–6. doi: 10.1128/AEM.00692-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Faith JJ, McNulty NP, Rey FE, Gordon JI. Predicting a human gut microbiota’s response to diet in gnotobiotic mice. Science. 2011;333:101–4. doi: 10.1126/science.1206025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Han R, Lai R, Ding Q, Wang Z, Luo X, Zhang Y, et al. Apolipoprotein A-I stimulates AMP-activated protein kinase and improves glucose metabolism. Diabetologia. 2007;50:1960–8. doi: 10.1007/s00125-007-0752-7. [DOI] [PubMed] [Google Scholar]
- 51.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuchibhotla S, Vanegas D, Kennedy DJ, Guy E, Nimako G, Morton RE, et al. Absence of CD36 protects against atherosclerosis in ApoE knock-out mice with no additional protection provided by absence of scavenger receptor A I/II. Cardiovasc Res. 2008;78:185–96. doi: 10.1093/cvr/cvm093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muegge BD, Kuczynski J, Knights D, Clemente JC, González A, Fontana L, et al. Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science. 2011;332:970–4. doi: 10.1126/science.1198719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–8. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carr TP, Weller CL, Schlegel VL, Cuppett SL, Guderian DM, Jr., Johnson KR. Grain sorghum lipid extract reduces cholesterol absorption and plasma non-HDL cholesterol concentration in hamsters. J Nutr. 2005;135:2236–40. doi: 10.1093/jn/135.9.2236. [DOI] [PubMed] [Google Scholar]
- 56.Martínez I, Wallace G, Zhang CM, Legge R, Benson AK, Carr TP, et al. Diet-induced metabolic improvements in a hamster model of hypercholesterolemia are strongly linked to alterations of the gut microbiota. Appl Environ Microbiol. 2009;75:4175–84. doi: 10.1128/AEM.00380-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.WHO/FAO. Health and Nutritional Properties of Probiotics in Food including Powder Milk with Live Lactic Acid Bacteria 2001.
- 58.Schiffrin EJ, Parlesak A, Bode C, Bode JC, van’t Hof MA, Grathwohl D, et al. Probiotic yogurt in the elderly with intestinal bacterial overgrowth: endotoxaemia and innate immune functions. Br J Nutr. 2009;101:961–6. doi: 10.1017/S0007114508055591. [DOI] [PubMed] [Google Scholar]
- 59.Donald IP, Kitchingmam G, Donald F, Kupfer RM. The diagnosis of small bowel bacterial overgrowth in elderly patients. J Am Geriatr Soc. 1992;40:692–6. doi: 10.1111/j.1532-5415.1992.tb01961.x. [DOI] [PubMed] [Google Scholar]
- 60.Riordan SM, McIver CJ, Wakefield D, Bolin TD, Duncombe VM, Thomas MC. Small intestinal bacterial overgrowth in the symptomatic elderly. Am J Gastroenterol. 1997;92:47–51. [PubMed] [Google Scholar]
- 61.Parlesak A, Klein B, Schecher K, Bode JC, Bode C. Prevalence of small bowel bacterial overgrowth and its association with nutrition intake in nonhospitalized older adults. J Am Geriatr Soc. 2003;51:768–73. doi: 10.1046/j.1365-2389.2003.51259.x. [DOI] [PubMed] [Google Scholar]
- 62.Romagnuolo J, Schiller D, Bailey RJ. Using breath tests wisely in a gastroenterology practice: an evidence-based review of indications and pitfalls in interpretation. Am J Gastroenterol. 2002;97:1113–26. doi: 10.1111/j.1572-0241.2002.05664.x. [DOI] [PubMed] [Google Scholar]
- 63.Hamad EM, Sato M, Uzu K, Yoshida T, Higashi S, Kawakami H, et al. Milk fermented by Lactobacillus gasseri SBT2055 influences adipocyte size via inhibition of dietary fat absorption in Zucker rats. Br J Nutr. 2009;101:716–24. doi: 10.1017/S0007114508043808. [DOI] [PubMed] [Google Scholar]
- 64.Chua SC, Jr., White DW, Wu-Peng XS, Liu SM, Okada N, Kershaw EE, et al. Phenotype of fatty due to Gln269Pro mutation in the leptin receptor (Lepr) Diabetes. 1996;45:1141–3. doi: 10.2337/diabetes.45.8.1141. [DOI] [PubMed] [Google Scholar]
- 65.Zucker LM, Zucker TF. Fatty, A new mutation in the rat. J Hered. 1961;52:275–8. [Google Scholar]
- 66.Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401–12. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 67.Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091–103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dewulf EM, Cani PD, Neyrinck AM, Possemiers S, Van Holle A, Muccioli GG, et al. Inulin-type fructans with prebiotic properties counteract GPR43 overexpression and PPARγ-related adipogenesis in the white adipose tissue of high-fat diet-fed mice. J Nutr Biochem. 2011;22:712–22. doi: 10.1016/j.jnutbio.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 69.Parnell JA, Reimer RA. Prebiotic fibres dose-dependently increase satiety hormones and alter Bacteroidetes and Firmicutes in lean and obese JCR:LA-cp rats. Br J Nutr. 2012;107:601–13. doi: 10.1017/S0007114511003163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Raoult D. Probiotics and obesity: a link? Nat Rev Microbiol. 2009;7:616. doi: 10.1038/nrmicro2209. [DOI] [PubMed] [Google Scholar]
- 71.Delzenne N, Reid G. No causal link between obesity and probiotics. Nat Rev Microbiol. 2009;7:901–, author reply 901. doi: 10.1038/nrmicro2209-c2. [DOI] [PubMed] [Google Scholar]
- 72.Ehrlich SD. Probiotics - little evidence for a link to obesity. Nat Rev Microbiol. 2009;7:901–, author reply 901. doi: 10.1038/nrmicro2209-c1. [DOI] [PubMed] [Google Scholar]
- 73.Guandalini S, Pensabene L, Zikri MA, Dias JA, Casali LG, Hoekstra H, et al. Lactobacillus GG administered in oral rehydration solution to children with acute diarrhea: a multicenter European trial. J Pediatr Gastroenterol Nutr. 2000;30:54–60. doi: 10.1097/00005176-200001000-00018. [DOI] [PubMed] [Google Scholar]
- 74.Simon O. Micro-organisms as feed additives—probiotcs. Advances in Pork Production. 2005;16:161–7. [Google Scholar]
- 75.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–81. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 76.Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101:15718–23. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maffei M, Fei H, Lee GH, Dani C, Leroy P, Zhang Y, et al. Increased expression in adipocytes of ob RNA in mice with lesions of the hypothalamus and with mutations at the db locus. Proc Natl Acad Sci U S A. 1995;92:6957–60. doi: 10.1073/pnas.92.15.6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–3. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 79.Preiss-Landl K, Zimmermann R, Hämmerle G, Zechner R. Lipoprotein lipase: the regulation of tissue specific expression and its role in lipid and energy metabolism. Curr Opin Lipidol. 2002;13:471–81. doi: 10.1097/00041433-200210000-00002. [DOI] [PubMed] [Google Scholar]
- 80.Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci U S A. 2007;104:979–84. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McNeil NI. The contribution of the large intestine to energy supplies in man. Am J Clin Nutr. 1984;39:338–42. doi: 10.1093/ajcn/39.2.338. [DOI] [PubMed] [Google Scholar]
- 82.Wall R, Ross RP, Shanahan F, O’Mahony L, O’Mahony C, Coakley M, et al. Metabolic activity of the enteric microbiota influences the fatty acid composition of murine and porcine liver and adipose tissues. Am J Clin Nutr. 2009;89:1393–401. doi: 10.3945/ajcn.2008.27023. [DOI] [PubMed] [Google Scholar]
- 83.Terpstra AH. Effect of conjugated linoleic acid on body composition and plasma lipids in humans: an overview of the literature. Am J Clin Nutr. 2004;79:352–61. doi: 10.1093/ajcn/79.3.352. [DOI] [PubMed] [Google Scholar]
- 84.de Roos B, Rucklidge G, Reid M, Ross K, Duncan G, Navarro MA, et al. Divergent mechanisms of cis9, trans11-and trans10, cis12-conjugated linoleic acid affecting insulin resistance and inflammation in apolipoprotein E knockout mice: a proteomics approach. FASEB J. 2005;19:1746–8. doi: 10.1096/fj.05-3953fje. [DOI] [PubMed] [Google Scholar]
- 85.Moloney F, Toomey S, Noone E, Nugent A, Allan B, Loscher CE, et al. Antidiabetic effects of cis-9, trans-11-conjugated linoleic acid may be mediated via anti-inflammatory effects in white adipose tissue. Diabetes. 2007;56:574–82. doi: 10.2337/db06-0384. [DOI] [PubMed] [Google Scholar]
- 86.Poirier H, Niot I, Clément L, Guerre-Millo M, Besnard P. Development of conjugated linoleic acid (CLA)-mediated lipoatrophic syndrome in the mouse. Biochimie. 2005;87:73–9. doi: 10.1016/j.biochi.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 87.Poirier H, Shapiro JS, Kim RJ, Lazar MA. Nutritional supplementation with trans-10, cis-12-conjugated linoleic acid induces inflammation of white adipose tissue. Diabetes. 2006;55:1634–41. doi: 10.2337/db06-0036. [DOI] [PubMed] [Google Scholar]
- 88.Mestdagh R, Dumas ME, Rezzi S, Kochhar S, Holmes E, Claus SP, et al. Gut microbiota modulate the metabolism of brown adipose tissue in mice. J Proteome Res. 2012;11:620–30. doi: 10.1021/pr200938v. [DOI] [PubMed] [Google Scholar]
- 89.Claesson MJ, O’Sullivan O, Wang Q, Nikkilä J, Marchesi JR, Smidt H, et al. Comparative analysis of pyrosequencing and a phylogenetic microarray for exploring microbial community structures in the human distal intestine. PLoS One. 2009;4:e6669. doi: 10.1371/journal.pone.0006669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lewis KE, Kirk EA, McDonald TO, Wang S, Wight TN, O’Brien KD, et al. Increase in serum amyloid a evoked by dietary cholesterol is associated with increased atherosclerosis in mice. Circulation. 2004;110:540–5. doi: 10.1161/01.CIR.0000136819.93989.E1. [DOI] [PubMed] [Google Scholar]
- 91.Yang RZ, Lee MJ, Hu H, Pollin TI, Ryan AS, Nicklas BJ, et al. Acute-phase serum amyloid A: an inflammatory adipokine and potential link between obesity and its metabolic complications. PLoS Med. 2006;3:e287. doi: 10.1371/journal.pmed.0030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Urieli-Shoval S, Linke RP, Matzner Y. Expression and function of serum amyloid A, a major acute-phase protein, in normal and disease states. Curr Opin Hematol. 2000;7:64–9. doi: 10.1097/00062752-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 93.Andersson CX, Gustafson B, Hammarstedt A, Hedjazifar S, Smith U. Inflamed adipose tissue, insulin resistance and vascular injury. Diabetes Metab Res Rev. 2008;24:595–603. doi: 10.1002/dmrr.889. [DOI] [PubMed] [Google Scholar]
- 94.Johnson BD, Kip KE, Marroquin OC, Ridker PM, Kelsey SF, Shaw LJ, et al. National Heart, Lung, and Blood Institute Serum amyloid A as a predictor of coronary artery disease and cardiovascular outcome in women: the National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Circulation. 2004;109:726–32. doi: 10.1161/01.CIR.0000115516.54550.B1. [DOI] [PubMed] [Google Scholar]
- 95.Lin Y, Rajala MW, Berger JP, Moller DE, Barzilai N, Scherer PE. Hyperglycemia-induced production of acute phase reactants in adipose tissue. J Biol Chem. 2001;276:42077–83. doi: 10.1074/jbc.M107101200. [DOI] [PubMed] [Google Scholar]
- 96.Ogasawara K, Mashiba S, Wada Y, Sahara M, Uchida K, Aizawa T, et al. A serum amyloid A and LDL complex as a new prognostic marker in stable coronary artery disease. Atherosclerosis. 2004;174:349–56. doi: 10.1016/j.atherosclerosis.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 97.Scheja L, Heese B, Zitzer H, Michael MD, Siesky AM, Pospisil H, et al. Acute-phase serum amyloid A as a marker of insulin resistance in mice. Exp Diabetes Res 2008; 2008:230837; PMID:18584041; 10.1155/2008/230837. [DOI] [PMC free article] [PubMed]
- 98.Reigstad CS, Lundén GO, Felin J, Bäckhed F. Regulation of serum amyloid A3 (SAA3) in mouse colonic epithelium and adipose tissue by the intestinal microbiota. PLoS One. 2009;4:e5842. doi: 10.1371/journal.pone.0005842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sjöholm K, Palming J, Olofsson LE, Gummesson A, Svensson PA, Lystig TC, et al. A microarray search for genes predominantly expressed in human omental adipocytes: adipose tissue as a major production site of serum amyloid A. J Clin Endocrinol Metab. 2005;90:2233–9. doi: 10.1210/jc.2004-1830. [DOI] [PubMed] [Google Scholar]



