Abstract
Synbiotic supplements, which contain multiple functional ingredients, may enhance the immune system more than the use of individual ingredients alone. A double blind active controlled parallel trial over a 21 day exercise training period was conducted to evaluate the effect of Gut BalanceTM, which contains Lactobacillus paracasei subsp paracasei (L. casei 431®), Bifidobacterium animalis ssp lactis (BB-12®), Lactobacillus acidophilus (LA-5®), Lactobacillus rhamnosus (LGG®), two prebiotics (raftiline and raftilose) and bovine whey derived lactoferrin and immunoglobulins with acacia gum on fecal microbiota, short chain fatty acids (SCFA), gut permeability, salivary lactoferrin and serum cytokines. All subjects randomized were included in the analysis. There was a 9-fold (1.2-fold to 64-fold; 95% confidence intervals p = 0.03) greater increase in fecal L. paracasei numbers with Gut BalanceTM compared with acacia gum supplementation. Gut BalanceTM was associated with a 50% (-12% to 72%; p = 0.02) smaller increase in the concentration of serum IL-16 in comparison to acacia gum from pre- to post-study. No substantial effects of either supplement were evident in fecal SCFA concentrations, measures of mucosal immunity or GI permeability. Clinical studies are now required to determine whether Gut BalanceTM may exert beneficial GI health effects by increasing the recovery of fecal L. paracasei. Both supplements had little effect on immunity. Twenty-two healthy physically active male subjects (mean age = 33.9 ± 6.5 y) were randomly allocated to either daily prebiotic or synbiotic supplementation for 21 day. Saliva, blood, urine and fecal samples were collected pre-, mid- and post-intervention. Participants recorded patterns of physical activity on a self-reported questionnaire.
Keywords: Synbiotics, athletes, immune function, prebiotics, acacia gum, Gut Balance, microbiota
Introduction
Synbiotic supplements combine probiotics and prebiotics to yield greater efficacy than use of either functional ingredient alone. These supplements exert beneficial health effects by modifying GI microbiota and enhancing immunity. The two most common commercial strains of probiotics are strains from the lactobacilli and bifidobacteria families, while fructooligosaccharides (FOS or inulin) and galactooligosaccharides (GOS) are the prebiotics most often added to foods. A substantial body of animal, in vitro and ex vivo evidence now indicates that these probiotic strains and prebiotics modify GI microbiota and may have immuno-enhancing1 and health promoting effects2 although there are conflicting findings in vivo.3 FOS and GOS may also increase the fecal concentration of SCFA, which may alter immune cell activity by binding to G protein coupled receptors. The growing body of evidence for these functional ingredients supports their use as supplements to enhance health in the general community.
Combining prebiotics and probiotics with other ingredients may augment their effects. Recent technological advances have allowed the manufacture of ingredients derived from plant and animal sources, such as bovine whey derived lactoferrin and immunoglobulins. Lactoferrin and immunoglobulins are present in humoral components of the body and act to prevent and limit infection. Furthermore, they are known to contribute to the biological activity of milk and initial research indicates that bovine whey derived lactoferrin is safe for consumption by humans.4
Research with athletes indicates that those more prone to respiratory tract illness have a dysregulated cytokine response to exhaustive exercise.5 We chose to investigate the pro-inflammatory cytokines IL-16, IL-18, IFNγ and IL-12 given their role in regulating CD4+ T cell status, innate immune cell trafficking and the activation of inflammatory mediators.6,7 Furthermore, altered gut permeability from heavy exercise is proposed to increase mucosal and systemic inflammation via the translocation of bacterial products.8 Initial studies of probiotics in active individuals have provided insight into the immunomodulatory and health promoting effects of these supplements. Use of this cohort is consistent with the proposition that research in immuno-nutrition studies in healthy people utilize models that challenge homeostasis to determine the ability of the body to respond and adapt to stress.9
This study examined the effects of Gut BalanceTM, containing four probiotics, two prebiotics and bovine whey derived lactoferrin and immunoglobulins with a potential prebiotic (acacia gum) on fecal microbiology, SCFA concentration, cytokines, salivary lactoferrin and gut permeability. Gut BalanceTM was chosen as it is available commercially and its constituents have a substantial body of pre-clinical and clinical research completed.10-12 As the effects of individual probiotic strains and prebiotic additives and their interaction are strain and dose dependent13 it is necessary to conduct research specific to this formulation. Acacia gum was chosen as a positive control given evidence of its bifidogenic effect and that it is considered a surrogate prebiotic.14 Employing a positive/active control is consistent with ethical approaches in the use of placebo intervention.15 New treatments must show greater efficacy than current practices, and a traditional placebo treatment, to justify production and manufacture of a new and novel nutrition supplement.
Results
Subjects.
All 22 subjects completed the study. The groups were well-matched on all characteristics (Table 2). There were five episodes of mild GI symptoms that included flatulence and stomach rumbles in both groups during supplementation. Both supplements were otherwise well tolerated.
Table 2. Differences in key measures between the groups at baseline.
| Measure | Prebiotic | Synbiotic | Qualitative difference |
|---|---|---|---|
| n |
11 |
11 |
|
| Age (y) |
31.4 ± 4.9 |
34.4 ± 3.5 |
Trivial |
| Mass (kg) |
73.1 ± 4.9 |
79.1 ± 10.4 |
Trivial |
| VO2 max (mlkg-1min-1) |
56.4 ± 4.9 |
57.9 ± 7.3 |
Trivial |
| Training load p/week (duration × intensity) | 21.3 ± 18.5 | 21.4 ± 16.8 | Trivial |
Fecal microbiology and biochemistry.
Analysis of similarity of the DGGE patterns indicated that synbiotic supplementation significantly altered the composition of the gut microflora compared with prebiotic supplementation (R = 0.27, p < 0.001). SIMPER analysis of the DGGE patterns revealed that no one band contributed more than 5% to the dissimilarity between treatments (data not shown). QPCR revealed that there was a relative 9-fold (2-fold to 43-fold; p = 0.03) difference in fecal L. paracasei between the groups. There were no substantial changes with total Lactobacilli, L. acidophilus, L. rhamnosus, B. lactis and E. coli in either group or in the concentrations of the individual short chain fatty acid concentrations (Table 3).
Table 3. The effect of supplementation on the concentration on fecal variables.
| Measure |
Prebiotic Mean ×/÷ SD |
Synbiotic Mean ×/÷ SD |
Difference in change |
|||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Mean (95% CI) | Inference | |
| Total bacteria* |
4 × 108 ×/÷ 4.0 |
6 × 108 ×/÷ 3.0 |
6 × 108 ×/÷ 3.0 |
6 × 108 ×/÷ 3.0 |
-2% (-65 to 173%) |
Unclear |
| Total Lactobacillus* |
5 × 103 ×/÷ 12 |
2 × 104 ×/÷ 21 |
10 × 103 ×/÷ 26 |
1 × 104 ×/÷ 6.0 |
-37% (-91 to 364%) |
Unclear |
|
L. paracasei* |
4 × 102 ×/÷ 4.0 |
2 × 102 ×/÷ 5.0 |
2 × 102 ×/÷ 5.0 |
1 × 103 ×/÷ 5.0 |
9-fold (25 to 6264%) |
Large |
|
L. rhamnosus# |
2 |
8 |
5 |
9 |
-0.1 (-0.5 to 0.3) |
Unclear |
|
B. lactis* |
2 × 102 ×/÷ 8.0 |
5 × 103 ×/÷ 12 |
3 × 102 ×/÷ 23 |
3 × 104 ×/÷ 12 |
4-fold (0.02- to 754-fold) |
Unclear |
| Acetate (μmol/g) |
58 ×/÷ 1.3 |
52 ×/÷ 1.3 |
54 ×/÷ 1.5 |
52 ×/÷ 1.4 |
0.8 (-25 to 35) |
Unclear |
| Propionate (μmol/g) |
17.5 ×/÷ 1.4 |
16 ×/÷ 1.4 |
16 ×/÷ 1.6 |
14 ×/÷ 1.4 |
-9.4 (-32 to 22) |
Unclear |
| Butyrate μmol/g) | 20 ×/÷ 1.3 | 17 ×/÷ 1.4 | 18 ×/÷ 1.7 | 16 ×/÷ 1.7 | 1.2 (-31 to 49) | Unclear |
SD, factor standard deviation; CI, confidence interval; L. Paracasei, Lactobacillus paracasei; L. rhamnosus, Lactobacillus rhamnosus; B. Lactis, Bifidobacterium lactis. *Copies of the 16S rRNA gene per g ww; #samples with recoverable bacteria.
Systemic immunity. The concentration of IL-16 over the course of the study is shown in Figure 1. Relative to the synbiotic group, there was a 50% (20 to 68%; 90% confidence interval; p = 0.02) greater increase in the concentration of IL-16 in the prebiotic group from pre- to post-supplementation. There was no substantial difference between the groups in the resting concentration of IL-18. Covariate analysis did not find any association between changes in microbiota and changes in resting cytokines. The concentration of both IL-16 and IL-18 was characterized by large between- and within-subject variability (~100–300%). No data are reported for IL-12 and IFNγ as the concentration of both cytokines in the samples was below the detection limit of the assay.
Figure 1.
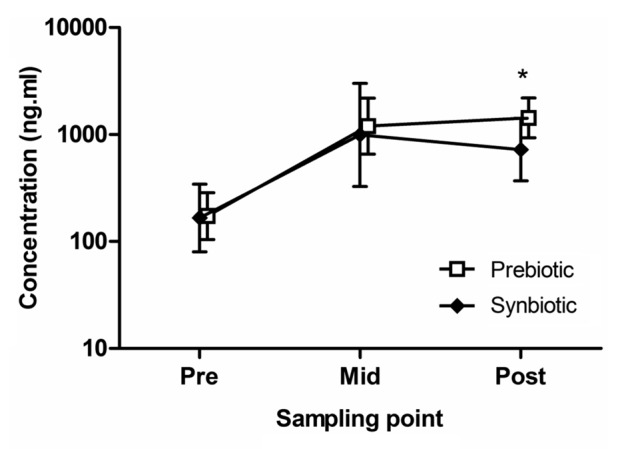
The effect of supplementation on the concentration of IL-16. The values presented are mean and standard deviation of the mean. *p < 0.02.
Mucosal immunity.
There was no substantial effect of supplementation on salivary lactoferrin (-39%; -74 to 41%; 90% confidence interval; p = 0.3) or gut permeability (lactulose/mannitol ratio; -75%; -96 to 53%; p = 0.19).
Discussion
We show for the first time in healthy physically active individuals that a synbiotic supplement elicits favorable changes in colonic microbiota in comparison to a prebiotic supplement. Supplementation with Gut BalanceTM increased the fecal recovery of L. paracasei while supplementation with acacia gum, in contrast, was associated with a reduction in fecal L. paracasei numbers. There were only trivial effects of supplementation on other species of fecal bacteria analyzed. Both supplements had relatively little effect on the immune system, with the only substantial effect associated with supplementation being a 4-fold increase in the synbiotic group and 8-fold increase in the acacia gum group in resting IL-16 concentration. No substantial effects of supplementation on other cytokines or on parameters of mucosal immunity were evident. An increase in the fecal recovery of L. paracasei from supplementation with Gut BalanceTM justifies undertaking further research to determine whether supplementation is associated with clinical benefit.
Pre- and pro-biotics purportedly exert their positive effects on the immune system by increasing beneficial species of bacteria colonizing the GI tract. In this study, only the synbiotic supplement fostered a substantial change in fecal microbiota, eliciting a 14-fold increase in the recovery of fecal L. paracasei. Given that there were four strains of bacteria in the synbiotic, however, it was expected that a greater number of bacteria would be recovered following supplementation. Our findings regarding L. casei 431® and B. lactis BB-12 are in contrast to previous research in which BB-12 was recoverable and L. casei 431® was not16,17 while our inability to recover L. acidophilus LA-5 is consistent with the findings of Shioya et al. The lack of recoverable BB-12 following supplementation with the synbiotic is also surprising given the bifidogenic effect reported for FOS and GOS.18 The results from the present research indicate that the dosage of probiotic bacteria and the dosage of the prebiotics in Gut BalanceTM (90 mg Raftiline and 10 mg Raftilose GR per capsule) were not sufficient to elicit further changes in microbiota as evident from the bacterial diversity analysis. That the dosage of prebiotics was too low was further confirmed by the lack of effect of supplementation on fecal SCFA. While this study shows for the first time that the concentration of SCFAs in healthy physically active individuals are similar to the general population, our findings confirm previous research that dosages of 5 to 10 g/day of FOS and GOS are needed to induce changes in fecal bacteria and short chain fatty acid concentrations.19 The synbiotic formulation may have greater effects on fecal microbiota by removal of FOS and GOS and an increase in the other probiotic species to counts over one billion CFU. Consumption of L. casei 431®, in conjunction with L. acidophilus LA-5, has been shown to prevent and/or reduce the severity of diarrhea in infants.20,21 Determining whether increased recovery of fecal L. paracasei from L. casei 431® supplementation is associated with enhanced intestinal and extra-intestinal health is warranted.
The only effect of supplementation in this study on immunology was an increase in resting IL-16 concentration, which occurred in both treatment groups. However, supplementation with Gut Balance appears to have reduced the magnitude of the increase in IL-16 by 50% relative to acacia gum. The limited effect of Gut BalanceTM supplementation on the immune system is similar to a recent study of another synbiotic containing only prebiotics and probiotics, which found that supplementation in healthy adults for six weeks increased the expression of L-selectin but not lymphocyte subsets, phagocytic activity, serum C-reactive protein, ceruloplasmin or other adhesion molecule concentrations.22 Examination of the training data from participants in the present study indicates that the increase in cytokine concentration was not the result of a change in physical activity patterns from pre- to post-supplementation. While L. paracasei can modulate various aspects of innate and adaptive immunity,23 covariate analysis did not identify any clear trends between supplement-induced changes in microbiota and the increase in IL-16 concentration. IL-16 is a pro-inflammatory cytokine that is chemotactic for immune cells, particularly T-cells.24 The health benefits of regular exercise are attributed to the promotion of anti-inflammatory effects, in particular by altering cytokine balance.25 The blunting of IL-16 in this study tentatively indicates that supplementation with Gut BalanceTM may augment the anti-inflammatory effects of regular exercise. Given the inherent redundancy within the immune system and the large variance in the range of healthy immune markers, it is necessary to include a wider panel of appropriate markers and utilize systems analysis approaches in future investigations to confirm such an effect.
There was no substantial effect with either Gut BalanceTM or acacia gum on gut permeability or the concentration of salivary lactoferrin. While probiotic administration enhances epithelial barrier integrity in vitro and in animal models26 and in some in vivo studies of critically ill patients,27 there are few studies reporting effects on healthy individuals. GI permeability was measured in the present study given evidence that individuals undertaking prolonged intense exercise may have higher barrier permeability.28 Baseline measures of the mean lactulose/mannitol ratio in each group were within normal values and it may be that improvements in GI permeability are more likely to occur only when permeability has been disturbed. Interest in examining the concentration of lactoferrin was based on previous research showing prolonged intense exercise reduces the concentration of lactoferrin by 60%.29 Future studies examining lactoferrin should focus on cellular activation to antigenic challenge given this may be where benefits are most likely to occur.
The limited effects of supplementation on markers of immunity in this study are consistent with studies of gut health supplements in healthy active populations. Four studies have examined the effects of probiotics on markers of immunity in active populations. While Gleeson and colleagues30 recently reported reduced respiratory illness and the maintenance of SIgA with four months of L. casei shirota supplementation in men and women engaged in endurance exercise, no substantial effects were noted for plasma immunoglobulins, leucocyte subsets or secretion of whole blood culture cytokines. In contrast, a study of L. fermentum (PCC) in elite male runners over four months of winter training found that plasma IFNγ was maintained during supplementation but there was no substantial effect on SIgA subclasses or whole blood culture IL-4 or IL-12 secretion.31 A follow up study of L. fermentum (PCC) in 99 well trained cyclists for 11 weeks confirmed the lack of an effect of supplementation on SIgA along with salivary lactoferrin and lysozyme and resting serum cytokines while reporting clinical benefits related to respiratory illness.32 There were, however, substantial reductions in the acute post-exercise cytokine perturbations in the well trained cyclists following an exercise test to exhaustion. This finding may indicate that probiotics enhance the resilience of the immune system to stress and complements the proposition that health be defined as the ability to adapt to stress.33 The difference in findings between the studies may relate to the population cohorts (e.g., elite vs. moderately active or sedentary), training mode (e.g., cycling, running) and training load and the strain or dosage of the relevant probiotics.
In conclusion, supplementation with Gut BalanceTM in healthy physically active individuals elicited a substantial increase in the recovery of fecal L. paracasei. While there were little effects of supplementation on immunity overall, Gut BalanceTM also attenuated the increase in resting IL-16 concentration to half that of the those supplementing with acacia gum. Further research focusing on a broader number of cytokines and cellular markers of activity and on conditions associated with aberrant immune responses, particularly inflammatory disorders, should provide further evidence on the usefulness of synbiotics in healthy active adults. In conjunction with evidence that L. casei 431® has clinical benefits with regard to infantile diarrhea, the findings from this study that Gut BalanceTM increases the fecal recovery of L. paracasei justify undertaking research to determine the clinical effects of this multi-formulation supplement in GI health.
Material and Methods
Study design.
The study was a randomized, double blind, parallel controlled trial to compare the effects of a synbiotic supplement with a prebiotic supplement on markers of gut health and immunity. The prebiotic was chosen as the control treatment to determine whether the combination of ingredients in the synbiotic supplement had greater efficacy than the use of a single ingredient. The study consisted of a 14 d pre-intervention period where participants stopped eating yoghurt and supplements that modulate enteric microbiota, a 21 d supplementation period in which subjects consumed with the prebiotic or synbiotic supplement daily and a 14 d post-intervention observation period. Fecal and urine samples were obtained at day 0 (after pre-intervention) and day 21 (end of intervention) for assessment of fecal microbiology and chemistry and GI permeability while serum and saliva samples were collected at days 0, 11 (mid-intervention) and 21 for assessment of immune status. Subjects provided samples for the assessment of fecal microbiology, fecal chemistry and immune status. Subjects were paired on age and maximal oxygen uptake (VO2 max) and randomly allocated to either prebiotic (acacia gum) or synbiotic supplementation by the study statistician who did not have contact with the subjects. VO2 max was used as a measure that ensured subjects were undertaking regular endurance activity and performed as previously described in reference 34. During supplementation subjects consumed three capsules of either the synbiotic or prebiotic daily. Subjects were able to consume the supplements with or without food and in the morning or evening. The number of capsules consumed was recorded and all subjects returned their supply bottle following supplementation to verify compliance. The study was conducted according to the guidelines prescribed in the Declaration of Helsinki, and all procedures involving human subjects/patients were approved by the Human Research Ethics Committees of the Australian Institute of Sport and Griffith University. All subjects provided written informed consent.
Subjects.
A total of 22 physically active healthy males aged 33.9 ± 6.5 y (mean ± SD) were recruited from the local cycling community and completed the study. Subjects were required to declare their use of dietary and/or ergogenic aids that may influence underlying immune function and/or exercise performance. All subjects on immunomodulatory medications were excluded. Inclusion to the study was dependent upon the subjects not taking antibiotics and supplements or foods with probiotics for 14 d prior to and during the study period. At the start of the study subjects undertook a VO2 max test as a measure the subject’s fitness for inclusion to the study as physically active individuals. A VO2 max of > 45 ml/kg-1/min-1 is indicative of individuals undertaking regular physical activity. The test was performed on an electromagnetic cycle ergometer (Excalibur Sport, Lode NV Groningen).
Product.
The synbiotic capsules (BiosourceTM Gut Balance, Probiotech Pharma) contained 200 mg Glycomax Immunoglobulin, 50 mg Glycomax Lactoferrin, 100 mg CBAR-Blend-100 (Chr. Hansen A/S, Horsholm, Denmark), which contained 4.6 × 108 Lactobacillus paracasei subs Paracasei (L. casei 431®), 6 × 108 Bifidobacterium animalis ssp lactis (BB-12®), 4.6 × 108 Lactobacillus acidophilus LA-5, 4.6 × 108 Lactobacillus rhamnosus GG, 90 mg Raftiline, 10 mg Raftilose GR and 10 mg magnesium stearate. The prebiotic supplement contained 116 mg acacia powder, 23 mg of microcrystalline cellulose, 8 mg of silica colloidal anhydrous British Pharmacopeia/United States Pharmacopeia (BP/USP), 31 mg chocolate flavor, 174 mg calcium hydrogen phosphate, 116.5 lecithin epikuron and 5 mg magnesium stearate BP.
Sample collection.
Saliva was collected using an eye spear (Defries Industries Pty. Ltd.). The eye spear was placed between the cheek and teeth for five min, centrifuged for 5 min at 778 g and frozen at -80°C until analysis. Albumin concentration was assessed to control for changes in salivary flow rate. Blood (5 ml) was drawn from an antecubital vein at rest and prior to the VO2 max test. Blood samples were collected directly into K3EDTA tubes (Greiner Bio-one; Frickenhausen, Germany). Plasma was separated by centrifugation at 4,974 g for 5 min and stored frozen at -80°C until analysis. Subjects were provided with a fecal sample collection kit and a portable -20°C freezer. A fecal sample was collected in a sealable plastic bag and frozen immediately at -20°C. Following collection of the freezers the samples were frozen at -80°C until analysis. Urine samples were collected using a commercial collection kit (ARL Pathology Pty. Ltd.). In brief, subjects were required to eliminate residual urine after an overnight fast and then consume an isomolar solution (120 ml) containing 6.0 g lactulose and 3.0 g mannitol. Participants then collected urine into a sealed flask over a period of 6 h. At the end of this period a 2.5 ml aliquot was taken from the flask and immediately frozen at -20°C. Saliva, serum and urine samples were taken at the same time of the day to control for diurnal variation.
Measures of mucosal immunity.
Salivary lactoferrin concentration was measured spectrophotometrically by an enzyme linked immunosorbant assay (ELISA) using a commercial kit (lactoferrin—EMD Chemicals). Albumin concentration was measured by immunoturbidimetric assay on a Hitachi 911 Chemistry Analyzer (Roche). The between-run coefficient of variation was 6.8%. Urinary lactulose and mannitol were analyzed by high-performance liquid chromatography35 and expressed as the mean of two separate runs.
Measures of systemic immunity.
The cytokines analyzed were IL 16, IL-18, IL-12 (p70) and IFNγ. The concentration of plasma cytokines were measured on a Bio-Plex Suspension Array System (Bio-Rad Laboratories Pty. Ltd.). The samples were analyzed on custom manufactured Multiplex Cytokine Kits (Bio-Rad Laboratories Pty. Ltd.) according to the manufacturer’s instructions and read using the Bio-Plex Suspension Array System (Bio-Rad Laboratories Pty. Ltd.
Physical activity log and adverse symptoms.
Subjects recorded details of their exercise training during the study. For each session, training mileage (km/wk-1), duration (h/wk-1) and intensity (scored on a 1–5 scale: 1, easy; 5, maximal) were recorded. Subjects recorded daily information of symptoms of gastrointestinal illness during the study as previously described to note any adverse or unusual effects or events during supplementation.36 Symptoms of GI illness included nausea, diarrhea, bloating and pain. A classification of GI illness was made when two or more symptoms were recorded on consecutive days. The severity of symptoms were self-rated as mild, moderate or severe based on the impact of the symptoms on activities for that day, with mild symptoms associated with no change to normal activities, moderate symptoms resulting in a reduction in or modification to activities and severe symptoms requiring the total cessation of normal activities.
Microbial analysis.
DNA was extracted according to Abell et al.37 and quantified using Quant-iTTM Picogreen (Invitrogen). Microbial diversity was examined using a universal bacteria 16S rRNA primer set (907f-1392rgc). The amplified product was analyzed by denaturing gradient gel electrophoresis (DGGE). The PCR and DGGE gel conditions follow the protocol of Abell et al.37 with the exception that a 35%–70% denaturing gradient was used. Dominant DGGE bands were extracted from the gels and sequenced for putative identification. DGGE banding patterns were analyzed to estimate bacterial diversity for each specimen using GelCompar II version 6.0 (Applied Maths, Inc.) software package and the normalized banding patterns were further analyzed with Primer6 version 6.1.12 and Permanova+ addition version 1.02 (PRIMER-E Ltd.).38 SIMPER analysis was conducted as previously described in reference 39. Total Lactobacilli, L. paracasei, L. acidophilus, L. rhamnosus, B. lactis and E. coli were quantified by real time PCR. The primer pairs, and their annealing temperature and concentration, used to detect groups and specific bacteria are outlined in Table 1.
Table 1. Quantitative real-time PCR primers.
| Target group | Primer name | Primer sequence (5'-3') | Annealing (°C) | Primer conc. (nM) | Reference |
|---|---|---|---|---|---|
| Total bacteria |
1114f 1275r |
CGG CAA CGA GCG CAA CCC CCA TTG TAG CAC GTG TGT AGC C |
60 |
150 |
45 |
| Lactobacillus group |
Lacto-F Lacto-R |
AGC AGT AGG GAA TCT TCC ACA CCG CTA CAC ATG GAG |
58 |
500 |
46 |
|
47 | |||||
|
Lactobacillus paracasei |
LcaseF LcaseR |
GCA CCG AGA TTC AAC ATG GGG TTC TTG GAT YTA TGC GGT ATT AG |
60 |
500 |
48 |
|
Lactobacillus rhamnosus |
LrhamF LcaseR |
TGC TTG CAT CTT GAT TTA ATT TTG GGT TCT TGG ATY TAT GCG GTA TTA G |
62 |
500 |
48 |
| Bifidobacterium lactis | Bflact2F Bflact5R |
GTG GAG ACA CGG TTT CCC CAC ACC ACA CAA TCC AAT AC |
60 | 600 | 49 |
Short chain fatty acids.
Fecal samples were thawed at 4°C, pooled, homogenized and then sub-sampled for analysis. Weighed portions for the determination of free (unesterified) SCFA were diluted 1:3 w/w with deionized water containing 1.68 mM heptanoic acid as an internal standard (Sigma Chemical Co.). Unesterified SCFA were analyzed as described previously in reference 40. A three point linear standard curve containing acetic, propionic, isobutyric, butyric, isovaleric, valeric and caproic acids was used for calibration at concentrations spanning the range of those measured in samples from this study.
Statistical analysis.
The statistical approach to determine the effect of supplementation was based on clinical and statistical significance.41 Smallest clinical values were derived by standardization; in this case 0.20 of the pooled between-subject standard deviation of the supplement groups. The differences between group means of outcome variables were assessed with a modification42 of Cohen’s scale as described previously in reference 43.
Descriptive statistics of all measures are presented as mean ± standard deviation or mean ×/÷ factor standard deviation. To calculate the factor standard deviation the mean is multiplied by the SD for the upper level while the lower level is calculated by dividing the mean by the SD. DGGE banding patterns were analyzed using the Bray-Curtis similarity matrix as previously described in reference 37. Relationships between diet and DGGE banding patterns were examined using the analysis of similarity (ANOSIM) test (one-way). The R statistic denotes the similarity between two groups with a value of 0 if similarities within diets and between diets are the same on average, and a value of 1 only if all replicates within diets are more similar to each other than any replicates from different diets. All measures were log-transformed before analysis to reduce non-uniformity of error, and permit the effect of the treatment to be analyzed as a percent difference. Differences in the change in mean saliva and serum protein concentrations, and fecal numbers, between the groups were analyzed with a Student’s t-test for independent samples (unequal variance).44 Baseline values of the dependent variable were included as a covariate in these analyses to account for regression to the mean. The extent to which bacterial counts accounted for changes in outcome measures was investigated through covariate analysis. In these analyses the baseline log-transformed bacterial count or the pre-post change in the log-transformed count was the covariate, and the dependent variable was rank-transformed. The effects of supplementation are shown with 95% confidence limits.
Sample size was determined based on variance analysis (standard deviations) from previous studies on the parameters of interest. To demonstrate a difference of 0.20 of the pooled between-subject standard deviation in the salivary immune parameters, which have previously shown the largest variance, a total of nine subjects per group were required to give 80% power at an α level of 0.05. To account for the potential of drop outs 11 subjects were recruited per group.
Acknowledgments
The study was funded by Griffith University, Probiotec Pharma Pty. Ltd., and the Australian Institute of Sport (AIS) and conducted at the AIS. The authors would like to thank the subjects and members of the Department of Physiology at the AIS for their assistance during the study, particularly Dr Amanda Cox. The authors would also like to thank Corinna Bennett from CSIRO for her assistance in fecal processing and SCFA analysis.
Glossary
Abbreviations:
- IFN-γ
interferon gamma
- IL
interleukin
- GI
gastrointestinal
- FOS
fructooligosaccharides
- GOS
galactooligosaccharides
- SCFA
short chain fatty acids
- ANOSIM
analysis of similarity
- CFU
colony forming units
- VO2 max
maximal oxygen uptake
Disclosure of Potential Conflicts of Interest
A.W.C. receives research support from and provides consultancy services to Probiotec Pharma Pty. Ltd.
Footnotes
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/19579
References
- 1.Vulevic J, Drakoularakou A, Yaqoob P, Tzortzis G, Gibson GR. Modulation of the fecal microflora profile and immune function by a novel trans-galactooligosaccharide mixture (B-GOS) in healthy elderly volunteers. Am J Clin Nutr. 2008;88:1438–46. doi: 10.3945/ajcn.2008.26242. [DOI] [PubMed] [Google Scholar]
- 2.Gerritsen J, Smidt H, Rijkers GT, de Vos WM. Intestinal microbiota in human health and disease: the impact of probiotics. Genes Nutr 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christensen HR, Larsen CN, Kaestel P, Rosholm LB, Sternberg C, Michaelsen KF, et al. Immunomodulating potential of supplementation with probiotics: a dose-response study in healthy young adults. FEMS Immunol Med Microbiol. 2006;47:380–90. doi: 10.1111/j.1574-695X.2006.00109.x. [DOI] [PubMed] [Google Scholar]
- 4.Yamauchi K, Wakabayashi H, Hashimoto S, Teraguchi S, Hayasawa H, Tomita M. Effects of orally administered bovine lactoferrin on the immune system of healthy volunteers. Adv Exp Med Biol. 1998;443:261–5. doi: 10.1007/978-1-4757-9068-9_32. [DOI] [PubMed] [Google Scholar]
- 5.Cox AJ, Pyne DB, Saunders PU, Callister R, Gleeson M. Cytokine responses to treadmill running in healthy and illness-prone athletes. Med Sci Sports Exerc. 2007;39:1918–26. doi: 10.1249/mss.0b013e318149f2aa. [DOI] [PubMed] [Google Scholar]
- 6.Trøseid M, Seljeflot I, Arnesen H. The role of interleukin-18 in the metabolic syndrome. Cardiovasc Diabetol. 2010;9:11. doi: 10.1186/1475-2840-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braunstahl GJ, Fokkens WJ, Overbeek SE, KleinJan A, Hoogsteden HC, Prins JB. Mucosal and systemic inflammatory changes in allergic rhinitis and asthma: a comparison between upper and lower airways. Clin Exp Allergy. 2003;33:579–87. doi: 10.1046/j.1365-2222.2003.01652.x. [DOI] [PubMed] [Google Scholar]
- 8.West NP, Pyne DB, Peake JM, Cripps AW. Probiotics, immunity and exercise: a review. Exerc Immunol Rev. 2009;15:107–26. [PubMed] [Google Scholar]
- 9.van Ommen B, Keijer J, Heil SG, Kaput J. Challenging homeostasis to define biomarkers for nutrition related health. Mol Nutr Food Res. 2009;53:795–804. doi: 10.1002/mnfr.200800390. [DOI] [PubMed] [Google Scholar]
- 10.Chr Hansen A/S. Study summaries L. Casei 431. date accessed 28 November 2011 from http://www.chr-hansen.com/products/product-areas/probiotics-for-human-health/strains.html
- 11.Zenhom M, Hyder A, de Vrese M, Heller KJ, Roeder T, Schrezenmeir J. Prebiotic oligosaccharides reduce proinflammatory cytokines in intestinal Caco-2 cells via activation of PPARγ and peptidoglycan recognition protein 3. J Nutr. 2011;141:971–7. doi: 10.3945/jn.110.136176. [DOI] [PubMed] [Google Scholar]
- 12.Borromei C, Careri M, Cavazza A, Corradini C, Elviri L, Mangia A, et al. Evaluation of Fructooligosaccharides and Inulins as Potentially Health Benefiting Food Ingredients by HPAEC-PED and MALDI-TOF MS. Int J Anal Chem. 2009;2009:530639. doi: 10.1155/2009/530639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagpal R, Kaur A. Synbiotic effect of various prebiotics on in vitro activities of probiotic lactobacilli. Ecol Food Nutr. 2011;50:63–8. doi: 10.1080/03670244.2011.539161. [DOI] [PubMed] [Google Scholar]
- 14.Calame W, Weseler AR, Viebke C, Flynn C, Siemensma AD. Gum arabic establishes prebiotic functionality in healthy human volunteers in a dose-dependent manner. Br J Nutr. 2008;100:1269–75. doi: 10.1017/S0007114508981447. [DOI] [PubMed] [Google Scholar]
- 15.Powell T, Bailey J. Against placebos. Am J Bioeth. 2009;9:23–5. doi: 10.1080/15265160903244234. [DOI] [PubMed] [Google Scholar]
- 16.Larsen CN, Nielsen S, Kaestel P, Brockmann E, Bennedsen M, Christensen HR, et al. Dose-response study of probiotic bacteria Bifidobacterium animalis subsp lactis BB-12 and Lactobacillus paracasei subsp paracasei CRL-341 in healthy young adults. Eur J Clin Nutr. 2006;60:1284–93. doi: 10.1038/sj.ejcn.1602450. [DOI] [PubMed] [Google Scholar]
- 17.Shioya M, Nakaoka K, Iizuka N, Sato M, Benno Y. Effect of fermented milk containing Bifidobacterium lactis FK 120 on the fecal flora, with special reference to Bifidum species and fecal properties in elderly volunteers. Food Health and Nutrition Research (Journal of Nutritional Food) 2000:33–44. [Google Scholar]
- 18.Kolida S, Meyer D, Gibson GR. A double-blind placebo-controlled study to establish the bifidogenic dose of inulin in healthy humans. Eur J Clin Nutr. 2007;61:1189–95. doi: 10.1038/sj.ejcn.1602636. [DOI] [PubMed] [Google Scholar]
- 19.Meyer D, Stasse-Wolthuis M. The bifidogenic effect of inulin and oligofructose and its consequences for gut health. Eur J Clin Nutr. 2009;63:1277–89. doi: 10.1038/ejcn.2009.64. [DOI] [PubMed] [Google Scholar]
- 20.Gaón D, García H, Winter L, Rodríguez N, Quintás R, González SN, et al. Effect of Lactobacillus strains and Saccharomyces boulardii on persistent diarrhea in children. Medicina (B Aires) 2003;63:293–8. [PubMed] [Google Scholar]
- 21.Gonzalez SN, Cardozo R, Apella MC, Oliver G. Biotherapeutic role of fermented milk. Biotherapy. 1994;8:129–34. doi: 10.1007/BF01878496. [DOI] [PubMed] [Google Scholar]
- 22.Nova E, Viadel B, Wärnberg J, Carreres JE, Marcos A. Beneficial effects of a synbiotic supplement on self-perceived gastrointestinal well-being and immunoinflammatory status of healthy adults. J Med Food. 2011;14:79–85. doi: 10.1089/jmf.2008.0328. [DOI] [PubMed] [Google Scholar]
- 23.Castellazzi AM, Valsecchi C, Montagna L, Malfa P, Ciprandi G, Avanzini MA, et al. In vitro activation of mononuclear cells by two probiotics: Lactobacillus paracasei I 1688, Lactobacillus salivarius I 1794, and their mixture (PSMIX) Immunol Invest. 2007;36:413–21. doi: 10.1080/08820130701361160. [DOI] [PubMed] [Google Scholar]
- 24.Glass WG, Sarisky RT, Vecchio AM. Not-so-sweet sixteen: the role of IL-16 in infectious and immune-mediated inflammatory diseases. J Interferon Cytokine Res. 2006;26:511–20. doi: 10.1089/jir.2006.26.511. [DOI] [PubMed] [Google Scholar]
- 25.Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11:607–15. doi: 10.1038/nri3041. [DOI] [PubMed] [Google Scholar]
- 26.Madsen K, Cornish A, Soper P, McKaigney C, Jijon H, Yachimec C, et al. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology. 2001;121:580–91. doi: 10.1053/gast.2001.27224. [DOI] [PubMed] [Google Scholar]
- 27.Alberda C, Gramlich L, Meddings J, Field C, McCargar L, Kutsogiannis D, et al. Effects of probiotic therapy in critically ill patients: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2007;85:816–23. doi: 10.1093/ajcn/85.3.816. [DOI] [PubMed] [Google Scholar]
- 28.Pals KL, Chang RT, Ryan AJ, Gisolfi CV. Effect of running intensity on intestinal permeability. J Appl Physiol. 1997;82:571–6. doi: 10.1152/jappl.1997.82.2.571. [DOI] [PubMed] [Google Scholar]
- 29.West NP, Pyne DB, Kyd JM, Renshaw GM, Fricker PA, Cripps AW. The effect of exercise on innate mucosal immunity. Br J Sports Med. 2010;44:227–31. doi: 10.1136/bjsm.2008.046532. [DOI] [PubMed] [Google Scholar]
- 30.Gleeson M, Bishop NC, Oliveira M, Tauler P. Daily probiotic’s (Lactobacillus casei Shirota) reduction of infection incidence in athletes. Int J Sport Nutr Exerc Metab. 2011;21:55–64. doi: 10.1123/ijsnem.21.1.55. [DOI] [PubMed] [Google Scholar]
- 31.Cox AJ, Pyne DB, Saunders PU, Fricker PA. Oral administration of the probiotic Lactobacillus fermentum VRI-003 and mucosal immunity in endurance athletes. Br J Sports Med. 2010;44:222–6. doi: 10.1136/bjsm.2007.044628. [DOI] [PubMed] [Google Scholar]
- 32.West NP, Pyne DB, Cripps AW, Hopkins WG, Eskesen DC, Jairath A, et al. Lactobacillus fermentum (PCC®) supplementation and gastrointestinal and respiratory-tract illness symptoms: a randomised control trial in athletes. Nutr J. 2011;10:30. doi: 10.1186/1475-2891-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rizzardini G, Eskesen D, Calder PC, Capetti A, Jespersen L, Clerici M. Evaluation of the immune benefits of two probiotic strains Bifidobacterium animalis ssp. lactis, BB-12® and Lactobacillus paracasei ssp. paracasei, L. casei 431® in an influenza vaccination model: a randomised, double-blind, placebo-controlled study. Br J Nutr. 2012;107:876–84. doi: 10.1017/S000711451100420X. [DOI] [PubMed] [Google Scholar]
- 34.Lee H, Martin DT, Anson JM, Grundy D, Hahn AG. Physiological characteristics of successful mountain bikers and professional road cyclists. J Sports Sci. 2002;20:1001–8. doi: 10.1080/026404102321011760. [DOI] [PubMed] [Google Scholar]
- 35.Camilleri M, Nadeau A, Lamsam J, Nord SL, Ryks M, Burton D, et al. Understanding measurements of intestinal permeability in healthy humans with urine lactulose and mannitol excretion. Neurogastroenterol Motil. 2010;22:e15–26. doi: 10.1111/j.1365-2982.2009.01361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fricker PA, Pyne DB, Saunders PU, Cox AJ, Gleeson M, Telford RD. Influence of training loads on patterns of illness in elite distance runners. Clin J Sport Med. 2005;15:246–52. doi: 10.1097/01.jsm.0000168075.66874.3e. [DOI] [PubMed] [Google Scholar]
- 37.Abell GC, Cooke CM, Bennett CN, Conlon MA, McOrist AL. Phylotypes related to Ruminococcus bromii are abundant in the large bowel of humans and increase in response to a diet high in resistant starch. FEMS Microbiol Ecol. 2008;66:505–15. doi: 10.1111/j.1574-6941.2008.00527.x. [DOI] [PubMed] [Google Scholar]
- 38.Clarke K, Warwick R. A further biodiversity index applicable to species lists: variation in taxonomic distinctness. Mar Ecol Prog Ser. 2001;216:265–78. doi: 10.3354/meps216265. [DOI] [Google Scholar]
- 39.J Abell GC, Christophersen CT, McOrist AL, Clarke JM. Dietary resistant and butyrylated starches have different effects on the faecal bacterial flora of azoxymethane-treated rats. Br J Nutr. 2011;105:1480–5. doi: 10.1017/S0007114510005349. [DOI] [PubMed] [Google Scholar]
- 40.Bajka BH, Topping DL, Cobiac L, Clarke JM. Butyrylated starch is less susceptible to enzymic hydrolysis and increases large-bowel butyrate more than high-amylose maize starch in the rat. Br J Nutr. 2006;96:276–82. doi: 10.1079/BJN20061807. [DOI] [PubMed] [Google Scholar]
- 41.Stang A, Poole C, Kuss O. The ongoing tyranny of statistical significance testing in biomedical research. Eur J Epidemiol. 2010;25:225–30. doi: 10.1007/s10654-010-9440-x. [DOI] [PubMed] [Google Scholar]
- 42.Hopkins WG, Marshall SW, Batterham AM, Hanin J. Progressive statistics for studies in sports medicine and exercise science. Med Sci Sports Exerc. 2009;41:3–13. doi: 10.1249/MSS.0b013e31818cb278. [DOI] [PubMed] [Google Scholar]
- 43.Cohen J. Statistical power analysis for the behavioral sciences, Hillsdale NJ. Lawrence Erlbaum 1988. [Google Scholar]
- 44.Hopkins WG. Estimating sample size for magnitude-based inferences. Sports Science. 2006;10:63–70. [Google Scholar]
- 45.Denman SE, McSweeney CS. Development of a real-time PCR assay for monitoring anaerobic fungal and cellulolytic bacterial populations within the rumen. FEMS Microbiol Ecol. 2006;58:572–82. doi: 10.1111/j.1574-6941.2006.00190.x. [DOI] [PubMed] [Google Scholar]
- 46.Walter J, Hertel C, Tannock GW, Lis CM, Munro K, Hammes WP. Detection of Lactobacillus, Pediococcus, Leuconostoc, and Weissella species in human feces by using group-specific PCR primers and denaturing gradient gel electrophoresis. Appl Environ Microbiol. 2001;67:2578–85. doi: 10.1128/AEM.67.6.2578-2585.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heilig HG, Zoetendal EG, Vaughan EE, Marteau P, Akkermans AD, de Vos WM. Molecular diversity of Lactobacillus spp. and other lactic acid bacteria in the human intestine as determined by specific amplification of 16S ribosomal DNA. Appl Environ Microbiol. 2002;68:114–23. doi: 10.1128/AEM.68.1.114-123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Byun R, Nadkarni MA, Chhour KL, Martin FE, Jacques NA, Hunter N. Quantitative analysis of diverse Lactobacillus species present in advanced dental caries. J Clin Microbiol. 2004;42:3128–36. doi: 10.1128/JCM.42.7.3128-3136.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ventura M, Reniero R, Zink R. Specific identification and targeted characterization of Bifidobacterium lactis from different environmental isolates by a combined multiplex-PCR approach. Appl Environ Microbiol. 2001;67:2760–5. doi: 10.1128/AEM.67.6.2760-2765.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]


