Abstract
There is increasing interest in the administration of microbes or microbial metabolites for the prevention and treatment of aberrant inflammatory activity. The protective effects associated with these microbes are mediated by multiple mechanisms involving epithelial cells, DCs and T cells, but most data are derived from animal models. In this addendum, we summarize our recent data, showing that oral consumption of Bifidobacterium infantis 35624 is associated with enhanced IL-10 secretion and Foxp3 expression in human peripheral blood. In addition, we discuss the potential DC subset-specific mechanisms, which could contribute to DCREG and TREG programming by specific gut microbes.
Keywords: bifidobacteria, dendritic cells, immunoregulation, microbiota, pattern recognition receptors, retinoic acid
Introduction
It is accepted that the microbiota is required for optimal host development and ongoing immune homeostasis.1-3 The microbiota aids in the digestion of foods, competes with pathogens, degrades mucin and promotes the differentiation of epithelial cells and mucosa-associated lymphoid tissue. In addition, the composition and metabolic activity of the microbiota has profound effects on proinflammatory activity and the induction of immune tolerance. Accumulating evidence suggests that certain bacterial strains may provide protective signals, while others stimulate damaging immune responses. Thus, the activity of the mammalian immune system seems to be governed by the balance between symbiotic and pathogenic factors derived from our indigenous microbes.4-6 This raises the possibility that an imbalance or “dysbiosis” might lead to inappropriate inflammatory responses and contribute to the increase in several immune-mediated disorders, including allergy and inflammatory bowel disease, in recent decades. However, whether dysbiosis is a cause, epiphenomenon or consequence of disease is uncertain.
Bifidobacterium infantis 35624
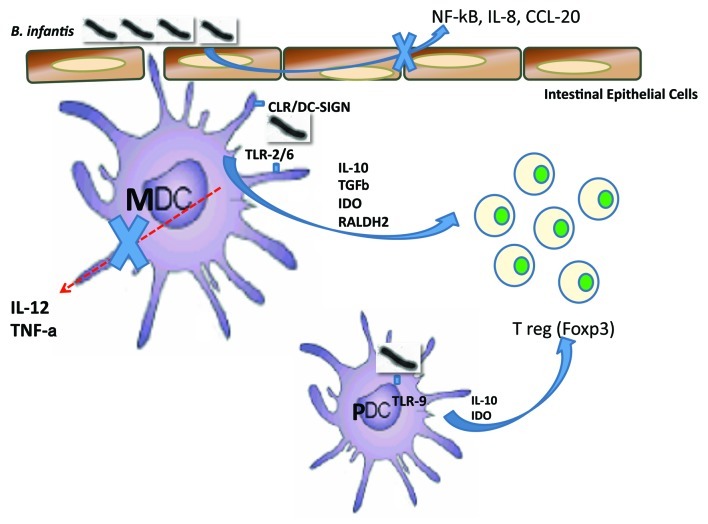
Bifidobacterium longum subsp infantis 35624 (B. infantis) was originally isolated from resected human healthy gastrointestinal tissue about 15 years ago. At the time, it was hypothesized that the isolation of probiotic candidates among those populations of lactic acid bacteria adhering to surgically-removed specimens of healthy human gastrointestinal mucosa might result in the identification of strains capable of functioning effectively within the anatomically and physiochemically complex human gastrointestinal environment. It was also hypothesized that bacterial strains, which existed in close proximity to human epithelial cells without inducing an inflammatory reaction, might possess potent immunoregulatory properties. B. infantis was one of a number of isolates that were obtained and this bacterial strain has been extensively studied for its potential immunoregulatory effects on epithelial cells, dendritic cells, lymphocytes, murine models and human studies (Fig. 1).
Figure 1.
B. infantis immunoregulatory effects on epithelial cells, dendritic cells and lymphocytes are illustrated.
Epithelial Cells
Within the gastrointestinal mucosa, epithelial cells are the first cells to encounter microbes. B. infantis was shown to adhere to gastrointestinal epithelial cell lines, without inducing NF|B activation or the secretion of chemokines.7 This is in contrast to other microbes, which induced proinflammatory transcription factor expression and chemokine secretion. In addition, exposure to B. infantis reduced epithelial cell secretion of IL-8 and CCL-20 in response to pathogens and TLR ligands such as flagellin.7,8 These studies suggest that, although the intestinal epithelium is immunologically quiescent when it encounters B. infantis, this microbe exerts immunomodulatory effects on intestinal cells that mediate host proinflammatory responses to enteric pathogens.
Dendritic Cells
Dendritic cells (DCs) are professional antigen presenting cells that are present as immature sentinel cells sampling their local environment for antigenic material particularly at sites vulnerable to pathogenic entry. Upon activation by signals released from microorganisms, sentinel DCs undergo maturation into potent effector DCs and migrate toward the T-cell areas of draining lymphoid organs. There, effector DCs activate naive TH cells with pathogen-specific [MHC peptide complexes, signal (1)] and costimulatory [B7 family molecules, signal (2)]molecules.9 In addition to signals 1 and 2, DCs are responsible for a third signal, which determines the polarization of naive TH cells into TH1, TH2, TH9, TH17 or TREG cells.10 Signal 3 is heterogeneous and can be mediated by various soluble or membrane-bound molecules, including interleukin (IL)-6, IL-10, IL-12, IL-18, TGFβ, IFNα, OX40 ligand (OX40L) and retinoic acid.11 Importantly, in vitro studies suggest that the expression levels of these TH cell-polarizing molecules by mature effector DCs depends on the conditions prevailing during their initial activation as sentinel DCs. This implies that different microbes may promote the development of distinct DC phenotypes by provoking tissues to release mediators involved in polarization. B. infantis has been shown to selectively induce DCs with regulatory activity. DCs isolated from human mesenteric lymph nodes (MLN), obtained following surgical resection of inflamed gut from patients with inflammatory bowel disease, secreted IL-10 and TGFβ in response to stimulation with B. infantis, while IL-12 and TNF〈 were not induced.12 In contrast Salmonella typhimurium induced both IL-12 and TNF〈 by the same MLN-derived DCs. Interestingly, peripheral blood-derived DCs secreted TNF〈 in response to both B. infantis and S. typhimurium suggesting that commensal-pathogen divergence in cytokine responses is more marked in cells isolated from the mucosal immune system than those derived from peripheral blood. However, IL-12 secretion in response to B. infantis was not observed for either mucosal or peripheral blood-derived DCs. This observation was confirmed in a more comprehensive analysis of monocyte-derived DCs (MDDCs), directly isolated myeloid (MDC) and plasmacytoid DCs (PDCs).13 All of these DC subsets secreted IL-10, but not IL-12, in response to B. infantis. However, other bacterial strains induced both IL-10 and IL-12 secretion by DCs. In addition, PDCs did not upregulate IFNα gene expression upon B. infantis stimulation, while control stimulation of these cells with CpG DNA resulted in a potent upregulation of IFNα. These data suggest that human DCs respond to B. infantis in a specific manner, resulting in selective secretion of IL-10, but not IL-12 or IFNα.
It was also demonstrated by Konieczna et al. that MDDCs, MDCs and PDCs directly bind and phagocytose B. infantis. However, only a subset of DCs bound B. infantis (approximately 20–30%), even when B. infantis cells were added at 100x saturating concentrations. Within MDDCs, there is a well-described subset that is CD1a+ or CD1a-. We repeated the binding experiments to determine if CD1a expression was associated with enhanced or diminished B. infantis binding (Fig. 2). Both CD1a+ and CD1a- MDDCs bound B. infantis, albeit more CD1a+ MDDCs bound B. infantis than CD1a- MDDCs. In conclusion, CD1a expression does not correlate definitively with the ability to bind B. infantis and other, currently unknown, receptors are also involved.
Figure 2.
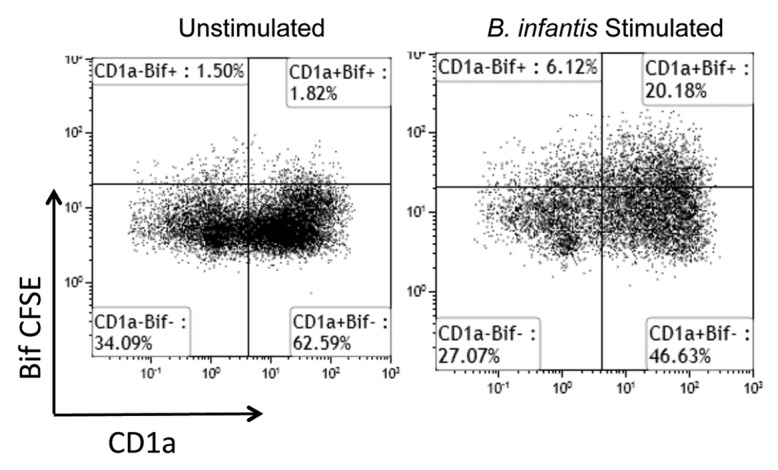
CD1a expression does not correlate with MDDC capture of B. infantis. Following gating of the B. infantis +ve and B. infantis -ve cells, CD1a is not exclusively associated with B. infantis binding to MDDCs. Unstimulated cells were not incubated with B. infantis.
Pattern Recognition Receptors and Mucosal Homeostasis
The selection of an appropriate cytokine secretion pattern by microbial stimulated DCs is dependent on a number of factors but is significantly influenced by the binding of microbial ligands, termed pathogen-associated molecular patterns (PAMPs), to pattern recognition receptors (PRRs) such as Toll-like receptors (TLR) and C-type lectin receptors (CLR). The differential binding of specific PRRs activates a number of intracellular signaling pathways, which ultimately result in cytokine secretion and/or cellular maturation. Certain intracellular pathways have been well described (e.g., the TLR-4 pathway) while others are still being explored. However, in vivo, multiple DC PRRs are simultaneously activated and the co-operation or competition between the resultant signaling cascades is not well understood. The specificity of these responses is achieved through the activation of a particular mosaic of PRRs, that is determined by the available PAMPs and the innate immune cells involved. For example, Toll-like receptor (TLR)-2 recognition of zymosan results in the secretion of retinoic acid and IL-10 leading to Foxp3 induction, while dectin-1 activation by zymosan leads to IL-23 secretion and TH17 induction.14 Recent findings on the role of PRR signaling in mucosal homeostasis have emphasized the delicate balance that exists between different PRR functions, and revealed that defective PRR signaling can result in inflammation and atopic sensitization.15,16 TLR-2 knockout animals are more sensitive to dextran sodium sulfate-induced colitis, while TLR-2 gene variants are associated with disease phenotype in inflammatory bowel disease patients. Indeed, TLR-2 has been demonstrated to promote Foxp3 expression in response to intestinal microbes in murine models.17,18 In addition, Konieczna et al. now describe that cross-talk between TLR-2/6, DC-specific intercellular adhesion molecule-grabbing non-integrin receptor (DC-SIGN) and TLR-9 activation on human DCs, in response to B. infantis, are partially responsible for inducing high levels of IL-10 secretion and lymphocyte Foxp3 expression.13 TLR-2 blockade also resulted in increased secretion of IL-12 and TNF〈 in response to B. infantis suggesting that signaling via TLR-2 provides a negative intracellular signal (yet to be elucidated), which blocks IL-12 gene expression. However, it is likely that many other Bifidobacterium strains also activate TLR-2/6, TLR-9 and DC-SIGN so these PRRs may not be entirely responsible for the observed selectivity in cytokine and metabolite induction by B. infantis stimulated DCs. Thus, additional PRRs are currently being examined.
T Regulatory Cells (TREG)
A feature of immunoregulatory microbes, which is being increasingly recognized, is their ability to induce TREG cells. TREG cells are required to maintain tolerance to self-antigens and their depletion results in autoimmune diseases. They are derived from the thymus but may also be induced in peripheral organs, including the gut mucosa, where TREG cells play an important role in maintaining tolerance to the gastrointestinal microbiota and food antigens.19,20 CD103+ DCs within the mucosa are largely responsible for the conversion of TREG cells via TGFβ and retinoic acid dependent processes.21,22 The conversion is probably driven by gastrointestinal-specific environmental factors associated with the presence of large numbers of commensal organisms. Indeed, we have recently reported that retinoic acid metabolizing enzymes can be upregulated in myeloid, but not plasmacytoid, DCs following in vitro incubation with B. infantis.13 In vitro, human DCs stimulated with B. infantis selectively promoted the upregulation of Foxp3 expression in naïve lymphocytes. Murine studies have demonstrated that B. infantis administration results in an increase in CD25+Foxp3+ lymphocytes, which are functional as they protect against LPS or pathogen-induced NF|B activation.23 Furthermore, administration to healthy human volunteers also results in increased frequency of Foxp3+ lymphocytes within peripheral blood.13 Thus, the induction of Foxp3+ TREG by B. infantis seems to be a robust and reproducible phenomenon, which is potentially mediated by the interaction with DCs. However, it is unlikely that all commensal microbes are equally effective at inducing TREG cells in vivo. A comparison of three different commensal organisms (Bifidobacterium longum AH1206, Bifidobacterium breve AH1205 and Lactobacillus salivarius AH102), has shown that Bifidobacterium longum AH1206 induced TREG cells and was also able to protect against respiratory allergic inflammation.24 The other bacterial strains did not effectively induce TREG cells and were unable to protect against allergic inflammation. Therefore, the induction of TREG cells may be a critical characteristic of a healthy microbiota, which is protective against the development of aberrant immunological reactivity.
Animal Models of Inflammatory Disease
B. infantis has been demonstrated to reduce the severity of colitis in the IL-10 knockout, the SCID lymphocyte adoptive transfer and the DSS models.25-27 The induction of regulatory cell populations was not examined in these studies; however, the potential role for a tonic cholinergic anti-inflammatory efferent vagal effect was shown not to be required for the B. infantis protective effect. Interestingly, B. infantis retains anti-inflammatory activity in the IL-10 knockout mouse, suggesting that additional mechanisms, such as retinoic acid metabolism, are also important.
In addition to colitis models, B. infantis has been examined for anti-inflammatory effects in murine models of infection, arthritis and respiratory inflammation. The host response to infection requires innate and acquired cellular and humoral immune reactions, designed to limit spread of the offending organism and to restore organ homeostasis.28 However, to limit the aggressiveness of collateral damage to host tissues, a range of regulatory constraints may be activated, such as induction of TREG cells.29 A successful immune response is characterized by the efficient elimination of the pathogenic organism with minimal inflammatory damage to the host and the associated inflammatory cascades which may promote inflammatory disease. Infection with Salmonella typhimurium results in a potent inflammatory response, characterized by NF|B activation, release of pro-inflammatory cytokines and tissue damage. The Salmonella associated inflammatory response was attenuated by B. infantis administration in a TREG-dependent manner.23 Administration of B. infantis to the collagen-induced arthritis model attenuated joint swelling and joint inflammation (unpublished data). Two unrelated Bifidobacterium strains were also examined and these strains had no impact on arthritis severity. Similarly, B. infantis reduced bronchoalveolar lavage eosinophil numbers and TNF〈 levels in the ovalbumin-induced respiratory inflammation model, which was not observed with other Bifidobacterium strains (unpublished results).
The ability of B. infantis to modulate inflammatory activity, at intestinal and extra-intestinal sites, across a wide range of different models, suggests that this bacterium exploits a central tolerogenic mechanism, which subsequently influences pro-inflammatory activity.
Human Studies
B. infantis has been demonstrated to reduce symptom severity in patients with irritable bowel syndrome (IBS) in two placebo controlled human clinical studies.30,31 IBS has been associated with altered immunoregulatory function both within the mucosa and systemically and it is tempting to speculate that the protective effects of B. infantis in IBS patients could be related to its immunoregulatory activity.32-34 The report by Konieczna et al. suggests that the immunoregulatory activity in murine studies is relevant to humans.13B. infantis administration to healthy human volunteers for 8 weeks resulted in significantly increased numbers of Foxp3+ TREG cells in peripheral blood. This increase in T REG cells was accompanied by increased secretion of IL-10 by in vitro anti-CD3/CD28 stimulated peripheral blood mononuclear cells (PBMCs). However, anti-CD3/CD28 stimulated PBMC secretion of TNFα, IFNγ, IL-2 or IL-12 was unaffected, suggesting that B. infantis selectively induces a regulatory immune response in humans.
Role for Metabolic Factors in Probiotic-Mediated Immunoregulation
Konieczna et al. demonstrate that induction of vitamin A and tryptophan metabolic pathways in DCs by B. infantis is important for adaptive immune cell responses.13 These metabolic pathways are recognized participants in immunoregulatory mechanisms. That food-grade microbes deploy these mechanisms raises the possibility of a new link between diet, microbiota, metabolism and immunoregulation. Simply put, B. infantis administration to an individual, with a diet deficient in vitamin A and/or tryptophan, may not be as effective as B. infantis administration to an individual with a diet incorporating sufficient quantities of these nutrients. At present this is a hypothesis, however recent findings regarding other dietary factors support that certain dietary factors may be important for microbiota and probiotic-associated immunoregulation.35 For example, microbial fermentation of dietary oligosaccharides results in short chain fatty acid secretion and immunoregulation via GPCR41 and GPCR43.36
Conclusions and Unanswered Questions
While it is clear that the microbiota influences host immune maturation and immune activity, the molecular basis of these immunomodulatory mechanisms are still emerging. The report by Konieczna et al. supports the concept that the presence of certain bacterial strains, such as B. infantis, influence host immunoregulatory mechanisms, either due to direct interactions with the host (e.g., via DC PRR activation) or by their metabolic activity in vivo. In addition, we suggest that studies focused on the impact of dietary factors and microbiota-associated molecular pathways should be a priority, so that preventive and therapeutic strategies may be formulated by matching essential microbes with dietary components. It remains to be shown whether the B. infantis immunoregulatory responses described in healthy human volunteers will also be observed in patients with inflammatory disorders. The data are encouraging and call for the further evaluation of this microbe in patients.
In addition to using live microbes for the treatment of inflammation, another exciting approach is to identify the microbial factor(s) responsible for the beneficial effect and to use these isolated factor(s) alone. Mining B. infantis and other microbiota for metabolites and ligands that modulate host immune function will likely lead to a new class of immunotherapeutic agents for inflammatory states. The continued identification of new microbes and microbial compounds, which induce tolerogenic DCs and TREG activity, promise to reveal novel therapeutic candidate molecules.
Footnotes
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/20358
References
- 1.O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7:688–93. doi: 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shanahan F. Gut microbes: from bugs to drugs. Am J Gastroenterol. 2010;105:275–9. doi: 10.1038/ajg.2009.729. [DOI] [PubMed] [Google Scholar]
- 3.Lee YK, Mazmanian SK. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science. 2010;330:1768–73. doi: 10.1126/science.1195568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grainger JR, Hall JA, Bouladoux N, Oldenhove G, Belkaid Y. Microbe-dendritic cell dialog controls regulatory T-cell fate. Immunol Rev. 2010;234:305–16. doi: 10.1111/j.0105-2896.2009.00880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–58. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 6.Feng T, Elson CO. Adaptive immunity in the host-microbiota dialog. Mucosal Immunol. 2011;4:15–21. doi: 10.1038/mi.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Hara AM, O’Regan P, Fanning A, O’Mahony C, Macsharry J, Lyons A, et al. Functional modulation of human intestinal epithelial cell responses by Bifidobacterium infantis and Lactobacillus salivarius. Immunology. 2006;118:202–15. doi: 10.1111/j.1365-2567.2006.02358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sibartie S, O’Hara AM, Ryan J, Fanning A, O’Mahony J, O’Neill S, et al. Modulation of pathogen-induced CCL20 secretion from HT-29 human intestinal epithelial cells by commensal bacteria. BMC Immunol. 2009;10:54. doi: 10.1186/1471-2172-10-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 10.Kaliński P, Hilkens CMU, Wierenga EA, Kapsenberg ML. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol Today. 1999;20:561–7. doi: 10.1016/S0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- 11.Akdis M, Burgler S, Crameri R, Eiwegger T, Fujita H, Gomez E, et al. Interleukins, from 1 to 37, and interferon-γ: receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2011;127:701–21, e1-70. doi: 10.1016/j.jaci.2010.11.050. [DOI] [PubMed] [Google Scholar]
- 12.O’Mahony L, O’Callaghan L, McCarthy J, Shilling D, Scully P, Sibartie S, et al. Differential cytokine response from dendritic cells to commensal and pathogenic bacteria in different lymphoid compartments in humans. Am J Physiol Gastrointest Liver Physiol. 2006;290:G839–45. doi: 10.1152/ajpgi.00112.2005. [DOI] [PubMed] [Google Scholar]
- 13.Konieczna P, Groeger D, Ziegler M, Frei R, Ferstl R, Shanahan F, et al. Bifidobacterium infantis 35624 administration induces Foxp3 T regulatory cells in human peripheral blood: potential role for myeloid and plasmacytoid dendritic cells. Gut. 2012;61:354–66. doi: 10.1136/gutjnl-2011-300936. [DOI] [PubMed] [Google Scholar]
- 14.Manicassamy S, Ravindran R, Deng J, Oluoch H, Denning TL, Kasturi SP, et al. Toll-like receptor 2-dependent induction of vitamin A-metabolizing enzymes in dendritic cells promotes T regulatory responses and inhibits autoimmunity. Nat Med. 2009;15:401–9. doi: 10.1038/nm.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukata M, Abreu MT. Pathogen recognition receptors, cancer and inflammation in the gut. Curr Opin Pharmacol. 2009;9:680–7. doi: 10.1016/j.coph.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tesse R, Pandey RC, Kabesch M. Genetic variations in toll-like receptor pathway genes influence asthma and atopy. Allergy. 2011;66:307–16. doi: 10.1111/j.1398-9995.2010.02489.x. [DOI] [PubMed] [Google Scholar]
- 17.Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, et al. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 2011;332:974–7. doi: 10.1126/science.1206095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A. 2010;107:12204–9. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karim M, Kingsley CI, Bushell AR, Sawitzki BS, Wood KJ. Alloantigen-induced CD25+CD4+ regulatory T cells can develop in vivo from CD25-CD4+ precursors in a thymus-independent process. J Immunol. 2004;172:923–8. doi: 10.4049/jimmunol.172.2.923. [DOI] [PubMed] [Google Scholar]
- 20.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coombes JL, Siddiqui KR, Arancibia-Cárcamo CV, Hall J, Sun CM, Belkaid Y, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–64. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–85. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Mahony C, Scully P, O’Mahony D, Murphy S, O’Brien F, Lyons A, et al. Commensal-induced regulatory T cells mediate protection against pathogen-stimulated NFkappaB activation. PLoS Pathog. 2008;4:1000112. doi: 10.1371/journal.ppat.1000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lyons A, O’Mahony D, O’Brien F, MacSharry J, Sheil B, Ceddia M, et al. Bacterial strain-specific induction of Foxp3+ T regulatory cells is protective in murine allergy models. Clin Exp Allergy. 2010;40:811–9. doi: 10.1111/j.1365-2222.2009.03437.x. [DOI] [PubMed] [Google Scholar]
- 25.van der Kleij H, O’Mahony C, Shanahan F, O’Mahony L, Bienenstock J. Protective effects of Lactobacillus rhamnosus [corrected] and Bifidobacterium infantis in murine models for colitis do not involve the vagus nerve. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1131–7. doi: 10.1152/ajpregu.90434.2008. [DOI] [PubMed] [Google Scholar]
- 26.McCarthy J, O’Mahony L, O’Callaghan L, Sheil B, Vaughan EE, Fitzsimons N, et al. Double blind, placebo controlled trial of two probiotic strains in interleukin 10 knockout mice and mechanistic link with cytokine balance. Gut. 2003;52:975–80. doi: 10.1136/gut.52.7.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunne C, Murphy L, Flynn S, O’Mahony L, O’Halloran S, Feeney M, et al. Probiotics: from myth to reality. Demonstration of functionality in animal models of disease and in human clinical trials. Antonie Van Leeuwenhoek. 1999;76:279–92. doi: 10.1023/A:1002065931997. [DOI] [PubMed] [Google Scholar]
- 28.Slack E, Hapfelmeier S, Stecher B, Velykoredko Y, Stoel M, Lawson MA, et al. Innate and adaptive immunity cooperate flexibly to maintain host-microbiota mutualism. Science. 2009;325:617–20. doi: 10.1126/science.1172747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat Immunol. 2005;6:353–60. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- 30.O’Mahony L, McCarthy J, Kelly P, Hurley G, Luo F, Chen K, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541–51. doi: 10.1053/j.gastro.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 31.Whorwell PJ, Altringer L, Morel J, Bond Y, Charbonneau D, O’Mahony L, et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101:1581–90. doi: 10.1111/j.1572-0241.2006.00734.x. [DOI] [PubMed] [Google Scholar]
- 32.Macsharry J, O’Mahony L, Fanning A, Bairead E, Sherlock G, Tiesman J, et al. Mucosal cytokine imbalance in irritable bowel syndrome. Scand J Gastroenterol. 2008;43:1467–76. doi: 10.1080/00365520802276127. [DOI] [PubMed] [Google Scholar]
- 33.Dinan TG, Quigley EM, Ahmed SM, Scully P, O’Brien S, O’Mahony L, et al. Hypothalamic-pituitary-gut axis dysregulation in irritable bowel syndrome: plasma cytokines as a potential biomarker? Gastroenterology. 2006;130:304–11. doi: 10.1053/j.gastro.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 34.Scully P, McKernan DP, Keohane J, Groeger D, Shanahan F, Dinan TG, et al. Plasma cytokine profiles in females with irritable bowel syndrome and extra-intestinal co-morbidity. Am J Gastroenterol. 2010;105:2235–43. doi: 10.1038/ajg.2010.159. [DOI] [PubMed] [Google Scholar]
- 35.Frei R, Lauener RP, Crameri R, O’Mahony L. Microbiota and dietary interactions: an update to the hygiene hypothesis? Allergy. 2012;67:451–61. doi: 10.1111/j.1398-9995.2011.02783.x. [DOI] [PubMed] [Google Scholar]
- 36.Maslowski KM, Mackay CR. Diet, gut microbiota and immune responses. Nat Immunol. 2011;12:5–9. doi: 10.1038/ni0111-5. [DOI] [PubMed] [Google Scholar]