Abstract
Type IV pili (Tfp) play a primary role in mediating the adherence of pathogenic bacteria to their hosts. The pilus filament can retract with an immense force. However, the role of this activity in microbial pathogenesis has not been rigorously explored. Experiments performed on volunteers suggested that the retraction capacity of enteropathogenic Escherichia coli (EPEC) Tfp is required for full virulence. Here we review our recent study1 in which we showed that the retraction capacity of the EPEC Tfp facilitates tight-junction disruption and actin-rich pedestal formation by promoting efficient bacterial protein effector translocation into epithelial host cells. We also present new data using live imaging confocal microscopy suggesting that EPEC adheres to monolayers in microcolonies and that Tfp retraction facilitates significant changes in the microcolony shape, which may be critical for efficient effector delivery. Our studies hence suggest novel insights into the role of pili retraction in EPEC pathogenesis.
Keywords: enteropathogenic E. coli, type IV pili, bundle forming pili, tight junctions, polarized epithelial cells
Type IV Pili
Type IV pili (Tfp) are long (several microns in length), thin (6–7 nm thick), but remarkably strong and flexible rod-like filamentous organelles that extend from the surface of many Gram negative and some Gram positive bacteria (reviewed in ref. 2). Tfp are expressed in pathogenic [e.g., Neisseria gonorrhea, N. meningitidis, Pseudomonas aeruginosa, Moraxella bovis, Vibrio cholera and enteropathogenic Escherichiae coli (EPEC)], as well as environmental species such as Synechocystis aquifex, Myxococcus xanthus and Shewanella oneidensis. All pili are comprised primarily of oligomeric pilin proteins, which are conserved in their structure and part of their amino acid sequences (reviewed in ref. 3). Many Tfp are adhesins that play a role in mediating inter-bacterial contacts, which are manifest as bacterial aggregates, microcolonies or biofilms, and in bacterial interactions with host cells. These interactions have been shown to play a role in the induction of pathogenesis, hence highlighting Tfp as critical virulence factors in several important human diseases.2,4,5 Studying the molecular basis underlying Tfp-mediated bacterial aggregation and bacterial-host cell interactions is crucial for obtaining deep insights into the mechanisms of microbial pathogenesis.
In addition to their adhesive properties, Tfp are involved in secretion and uptake of various macromolecules. A unique and fascinating function of Tfp concerns their ability to mediate flagellum-independent bacterial movements in solid or semi-solid surfaces. Social gliding motility of M. xanthus and twitching motility in P. aeruginosa and N. gonorrhoeae are perhaps the best studied examples of such activities.6 The recently described Tfp-dependent bacterial movement in a vertical (upright) orientation represents another very interesting mode of bacterial locomotion.7 However, despite considerable knowledge regarding Tfp-mediated movement, little is known about Tfp-dependent bacterial motility on host cell surfaces and its role in infection.
Tfp are dynamic organelles. Bacterial motion depends upon their ability to extend and retract their pili,8,9 utilizing an amazingly sophisticated and powerful ATP-driven machinery (reviewed in refs. 10 and 11). Intriguingly, the pilus retracts through the cell wall while remaining firmly adhered to the surface targeted by the pilus’ tip. The retraction generates an enormous force, exceeding 100 pN per single fiber, as measured for N. gonorrhoeae, distinguishing the ATPase retraction apparatus as one of the most powerful molecular motors in nature.12,13 A mysterious question concerning Tfp retraction is how external cues, typically contributed by environmental and host cell components, trigger the retraction machinery, which resides within the bacterium. The signal must somehow be transmitted along the extended pili to the retraction machine, possibly through force induced conformational changes in the pilin subunits.14
Bundle Forming Pili of Enteropathogenic E. coli are Important Virulence Factors
EPEC is a major cause of acute childhood diarrhea primarily in developing countries.15 Adhesive type IV bundle forming pili (BFP) are key components in promoting bacterial aggregation to form microcolonies,16 and in their binding to enterocytes lining the human small intestine in a process called “localized adherence” (LA).17 Following LA, EPEC employs a type III secretion system (T3SS), a molecular syringe that translocates protein effectors from the bacterium to the host cell to induce cytoskeletal remodeling, the formation of attaching and effacing (A/E) lesions,18 and numerous other effects on host cell functions.19 One such effector is the translocated intimin receptor (Tir), which is inserted into the host cell plasma membrane where it is phosphorylated by host cell kinases and serves as a receptor for another EPEC adhesin called intimin.20 The process leads to intimate attachment of the bacterium to the host cell surface, microvilli effacement, and the formation of an actin-rich pedestal. The A/E effect is dependent on a 35 kbp chromosomal pathogenicity island, called the locus of enterocyte effacement (LEE), which harbors 41 genes organized in several operons, and encodes the T3SS, T3SS effectors, and related proteins.21,22 The emerging theme suggests that many of these effectors act as a complex network of interactions to subvert key intracellular-signaling pathways, innate immune responses, membrane traffic and cytoskeletal elements.23 Alterations in epithelial ion and water transport, loss of apical microvilli and the disruption of epithelial tight junctions (TJs) are thought to contribute to the diarrheal effect.19,24-26
The complete and functional BFP is encoded by a ~95 kb EPEC adherence factor plasmid containing an operon of 14 genes.27,28 The basic pilin subunit comprising the BFP, also called bundlin or BfpA, is encoded by diverse bfpA alleles divided into two bundlin allele groups, 〈 and ®.29-31 Recent studies have shown that BFP composed of 〈-bundlin variants mediate specific adherence of EPEC to the host cell by recognizing an N-acetyllactosamine (LacNac) moiety in HEp-2,32 and human intestinal33 cells. Binding of LacNac has been shown to induce pili retraction and to regulate virulence-associated gene expression.34 Nonetheless, ®-bundlin variants do not bind LacNac and the specific BFP host cell receptors containing the LacNac moiety have not been identified yet.
BFP recruit other EPEC cells into aggregates, resulting in the formation and expansion of bacterial microcolonies. This process is hypothesized to be mediated by interactions among extended pilus fibers due to biogenesis and subsequent oligomerization of bundlin subunits. Bacterial aggregates can disaggregate and disperse—a process that may contribute to bacterial dissemination. Two ATPases, BfpD and BfpF, drive these opposing processes: BfpD mediates pilus assembly (extension), while BfpF is required for BFP retraction. Disruption of any of these genes leads to anomalies in BFP functions. For instance, a bfpF mutant forms bacterial microcolonies, but fails to disperse them over time.1 Moreover, studies performed on volunteers showed that EPEC bfpF mutants (i.e., hyperpiliated bacteria unable to retract pili) were capable of causing human disease, but 200-fold more bacteria were required in comparison to wild type.4 Hence, these studies provide a clear link between BFP retraction and the induction of the EPEC disease. However, how pilus retraction contributes to EPEC pathogenesis at the cellular level remains largely unexplored.
Bundle Forming pilus Retraction Facilitates Effective Disruption of Tight Junctions by Enhancing Effector Translocation
To investigate the effects of BFP retraction on epithelial host cells, we genetically engineered an 〈-bundlin expressing EPEC whose pilus retraction is initiated upon exposure to an inducer.1 Briefly, EPEC-bfpF, in which the Walker A box motifs (required for ATP hydrolysis presumed to energize the retraction of the pilus filament) were replaced with a scar sequence, was complemented with a plasmid containing an intact bfpF gene under the control of an arabinose-inducible promoter. This strain behaves like a bfpF mutant until inducer is added, at which time it behaves like the wild type strain.1
Infection of polarized epithelial Madin Darby canine kidney (MDCK) or Caco-2 cells with EPEC under conditions whereby BfpF expression was suppressed did not disrupt the TJs barrier functions, and had no effect on the continuous staining of the cell-cell junctions compared with controls. In contrast, cell infection with the same EPEC strain but under conditions that allowed induced BfpF expression (i.e., in the presence of arabinose) resulted in a disruption of TJ barrier functions and the appearance of fragmented staining of both tight junctional markers. Taken together our observations suggested that BFP retraction contributes to the capacity of EPEC to disrupt the structure and barrier function of TJs.1
Using a real-time analysis of effector translocation assay,35 we were able to show that EPEC-bfpF mutants fail to efficiently translocate EspF, an effector protein suggested to play a central role in the disruption of TJs,36 into host cells. Interestingly, inefficient translocation was also observed for Tir, a result that was consistent with reduced Tir tyrosine phosphorylation and inability of EPEC-bfpF mutants to induce the formation of fully mature pedestals. A model was proposed whereby in the absence of pili retraction, bacteria are essentially coated with long pili that separate the microbe’s and host cell surfaces, thus precluding efficient insertion of the T3SS injectisome and the subsequent introduction of bacterial effectors into the host cell. Pili retraction, however, promptly and effectively closes this gap, facilitating efficient introduction of the bacterial effectors into the host cells, which enhances pedestal biogenesis and TJ disruption.1 These observations may now provide partial explanation for the reduced capacity a bfpF mutant to cause a disease in humans.4
The Mechanisms by which Tfp Retraction Contributes to EPEC Infection
While it is clear that retraction of Tfp affects the host cell, the molecular basis for these effects is not yet understood. The mere force generated by Tfp retraction on the host cell plasma membrane may elicit signaling pathways.37 Tfp retraction also brings additional surface bacterial adhesins (e.g., intimin) in contact with the host cell surface and increases the efficiency with which bacterial protein effectors are introduced into the cell.38 These combined effects prompt the clustering of host cell plasma membrane proteins and lipids into a gigantic lipid raft-like membrane domain.39,40 This newly generated domain is enriched with cytoskeletal and other host cell signaling elements, which in the case of EPEC, will eventually transform into an actin-rich pedestal, and be involved in the disruption of the TJ barrier functions. The mechanism of TJ disruption could involve enforced mobilization of junctional protein complexes from their normal location (the TJs) to the pedestal domains, as indeed was demonstrated for the tight junctional protein, ZO-1,41 dephosphorylation and dissociation of occludin42 and the contraction of the perijunctional actomyosin ring likely due to myosin light chain phosphorylation.43 The recruitment of other junctional components, such as the Par3/Par6/PKCζ polarity complex, as demonstrated for meningococcal Tfp44 may also be postulated. In either scenario, targeted adherence of bacteria precisely to intercellular junctional regions is expected to facilitate the execution of these processes.
Tfp Dynamics Control Microcolony Behavior
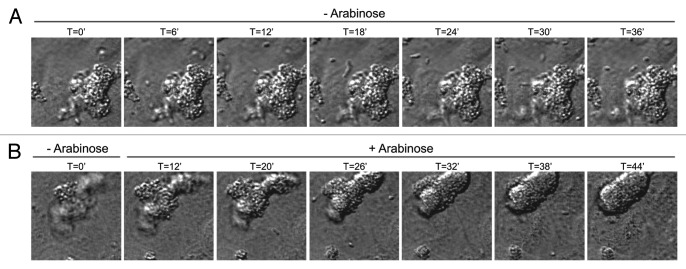
Using live cell imaging confocal microscopy we report here that EPEC that have been grown in tissue culture media often adhere to polarized epithelial monolayers as preformed microcolonies, despite the fact that both individual organisms and preformed colonies are present in the inoculum. This tendency does not depend on the ability to retract BFP, or on the T3SS, as EPEC-escV, which have non-functional T3SSs and EPEC-bfpF, which cannot retract its pili, exhibit similar behavior.1 However, using the bacteria in which pilus retraction can be controlled by addition of an inducer revealed that the microcolony undergoes significant morphological changes only when retraction is initiated, adopting a more rounded and compact shape as exemplified in Figure 1 (Vids. S1 and S2). These BfpF-dependent changes imply that Tfp retraction could have a profound impact on the process by which the microbe establishes an intimate contact between the microcolony and the host cell plasma membrane. We envision that similar effects occur in the human intestine, and that microcolony compactness greatly facilitates efficient introduction of the type III protein effectors into the host cells at high local concentrations, which is obviously essential for maximizing their subversive effects.
Figure 1.
Tfp retraction induces significant changes in microcolony shape and compactness. Time-lapse imaging was performed on MDCK cells infected with EPEC whose bfpF gene expression is under the control of an arabinose-inducible promoter1 (Vids. S1 and S2). A close-up of a single microcolony is shown. (A) In the absence of arabinose (- Arabinose), pili cannot be retracted. Under these conditions, and following attachment to the host cell, the EPEC microcolony seems to preserve a highly irregular structure over time. Bacteria comprising the microcolony are seen as distinctive individuals. (B) In a parallel experiment, bacteria were allowed to attach to host cells in the absence of arabinose (- Arabinose), and at a certain time point arabinose was added to the culture (+ Arabinose). Under these conditions BfpF is expressed, pili retraction is induced, the microcolony transforms from irregular to an oval-like shape and the appearance of individual bacteria becomes vague, most likely due to increase in their compactness within the microcolony.
Finally, we have observed that the majority of EPEC microcolonies adhere to polarized monolayers precisely over the intercellular junctions (Fig. 2). This preferential adherence, reminiscent of the previously noted adherence of enterohemorrhagic E. coli as “log jams” to epithelial junctions,45 does not require the T3SS and is usually the result of initial binding. As EPEC have been demonstrated by numerous investigators1,46-48 to radically alter tight junction function, this observation suggests that EPEC and other enteric pathogens may have evolved a common mechanism to efficiently target this site for maximal effect. Further studies will be required to investigate the role of BFP dynamics and other adhesins in this process.
Figure 2.
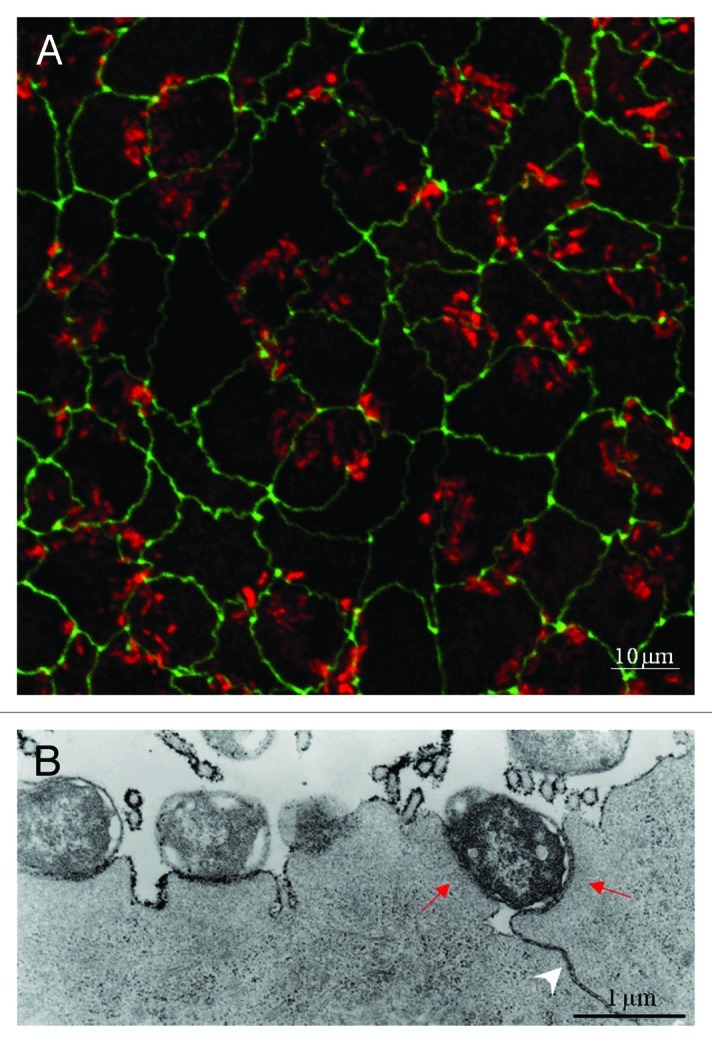
EPEC adheres to cell-cell junctions in polarized MDCK cells. (A) Confocal microscopy; EPEC-wt was allowed to interact with polarized MDCK cells expressing the tight junctional protein GFP-ZO-1 (green) for 3.5 h at 37°C. Cells were stained with Texas-red phalloidin to visualize the actin-rich pedestals at infection sites (red). Images (x-y optical sections) were taken by confocal fluorescence microscopy. The results show that EPEC-wt microcolonies adhere preferentially to ZO-1-labeled regions of the monolayer. (B) Transmission electron microscopy: Cells were stained with osmium and lanthanum post-fixation. Lanthanum is a useful marker for extracellular materials such as polysaccharides and glycoproteins, and it does not penetrate the lipid bilayer. Lanthanum applied to the apical surface of intact MDCK monolayers will not pass the junctional barrier, unless EPEC disrupts the TJs. The transmission electron micrographs clearly demonstrate that TJs of EPEC-treated MDCK cells became leaky to lanthanum (an arrowhead denotes electron dense lanthanum labeling of lateral membranes). Red arrows point toward a bacterium forming two pedestals in adjacent cells immediately above the cell-cell junctions. This image suggests that the bacterium is capable of injecting subversive effectors simultaneously into two neighboring cells, in the vicinity of tight junctions.
Conclusions
Tfp are the most prevalent type of pilus known, found in numerous pathogenic as well as non-pathogenic bacteria and archaea. The ability of these pili to undergo cycles of extension and retraction provides bacteria that produce them with the capacity of surface translational movement, and also with the potential to sense and respond to external cues at a distance from their host. We have shown that pilus retraction provides the bacteria with another advantage by allowing more efficient injection of T3SS toxins and receptors into host cells.1 We suggest that this effect occurs, not only at the level of the individual bacterium, but also at the level of the microcolony. By concentrating the bacteria into compact clusters, Tfp retraction allows a highly coordinated assault by a horde of bacteria against one or a few host cells. The nature of signals that permits this coordination will provide clues as to how it can be averted.
Supplementary Material
Acknowledgments
We are grateful to Ilan Rosenshine, Eitan Zahavi, Naomi Melamed-Book and Joshua A. Lieberman for contributions to the original manuscript. This work was supported in part by Public Health Service Award AI32074 from the National Institutes of Health (M.S.D.) and by the Israel Science Foundation 1167/08 (B.A.).
Supplemental Material
Supplemental materials can be found here: www.landesbioscience.com/journals/gutmicrobes/article/19814/
Footnotes
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/19814
References
- Zahavi EE, Lieberman JA, Donnenberg MS, Nitzan M, Baruch K, Rosenshine I, et al. Bundle-forming pilus retraction enhances enteropathogenic Escherichia coli infectivity. Mol Biol Cell. 2011;22:2436–47. doi: 10.1091/mbc.E11-01-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- 1.Zahavi EE, Lieberman JA, Donnenberg MS, Nitzan M, Baruch K, Rosenshine I, et al. Bundle-forming pilus retraction enhances enteropathogenic Escherichia coli infectivity. Mol Biol Cell. 2011;22:2436–47. doi: 10.1091/mbc.E11-01-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Proft T, Baker EN. Pili in Gram-negative and Gram-positive bacteria - structure, assembly and their role in disease. Cell Mol Life Sci. 2009;66:613–35. doi: 10.1007/s00018-008-8477-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Craig L, Pique ME, Tainer JA. Type IV pilus structure and bacterial pathogenicity. Nat Rev Microbiol. 2004;2:363–78. doi: 10.1038/nrmicro885. [DOI] [PubMed] [Google Scholar]
- 4.Bieber D, Ramer SW, Wu CY, Murray WJ, Tobe T, Fernandez R, et al. Type IV pili, transient bacterial aggregates, and virulence of enteropathogenic Escherichia coli. Science. 1998;280:2114–8. doi: 10.1126/science.280.5372.2114. [DOI] [PubMed] [Google Scholar]
- 5.Chamot-Rooke J, Mikaty G, Malosse C, Soyer M, Dumont A, Gault J, et al. Posttranslational modification of pili upon cell contact triggers N. meningitidis dissemination. Science. 2011;331:778–82. doi: 10.1126/science.1200729. [DOI] [PubMed] [Google Scholar]
- 6.Jin F, Conrad JC, Gibiansky ML, Wong GC. Bacteria use type-IV pili to slingshot on surfaces. Proc Natl Acad Sci U S A. 2011;108:12617–22. doi: 10.1073/pnas.1105073108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gibiansky ML, Conrad JC, Jin F, Gordon VD, Motto DA, Mathewson MA, et al. Bacteria use type IV pili to walk upright and detach from surfaces. Science. 2010;330:197. doi: 10.1126/science.1194238. [DOI] [PubMed] [Google Scholar]
- 8.Merz AJ, So M, Sheetz MP. Pilus retraction powers bacterial twitching motility. Nature. 2000;407:98–102. doi: 10.1038/35024105. [DOI] [PubMed] [Google Scholar]
- 9.Skerker JM, Berg HC. Direct observation of extension and retraction of type IV pili. Proc Natl Acad Sci U S A. 2001;98:6901–4. doi: 10.1073/pnas.121171698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pelicic V. Type IV pili: e pluribus unum? Mol Microbiol. 2008;68:827–37. doi: 10.1111/j.1365-2958.2008.06197.x. [DOI] [PubMed] [Google Scholar]
- 11.Craig L, Li J. Type IV pili: paradoxes in form and function. Curr Opin Struct Biol. 2008;18:267–77. doi: 10.1016/j.sbi.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maier B. Using laser tweezers to measure twitching motility in Neisseria. Curr Opin Microbiol. 2005;8:344–9. doi: 10.1016/j.mib.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Maier B, Potter L, So M, Long CD, Seifert HS, Sheetz MP. Single pilus motor forces exceed 100 pN. Proc Natl Acad Sci U S A. 2002;99:16012–7. doi: 10.1073/pnas.242523299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biais N, Higashi DL, Brujic J, So M, Sheetz MP. Force-dependent polymorphism in type IV pili reveals hidden epitopes. Proc Natl Acad Sci U S A. 2010;107:11358–63. doi: 10.1073/pnas.0911328107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ochoa TJ, Contreras CA. Enteropathogenic escherichia coli infection in children. Curr Opin Infect Dis. 2011;24:478–83. doi: 10.1097/QCO.0b013e32834a8b8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Girón JA, Ho AS, Schoolnik GK. An inducible bundle-forming pilus of enteropathogenic Escherichia coli. Science. 1991;254:710–3. doi: 10.1126/science.1683004. [DOI] [PubMed] [Google Scholar]
- 17.Scaletsky IC, Silva ML, Trabulsi LR. Distinctive patterns of adherence of enteropathogenic Escherichia coli to HeLa cells. Infect Immun. 1984;45:534–6. doi: 10.1128/iai.45.2.534-536.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frankel G, Phillips AD. Attaching effacing Escherichia coli and paradigms of Tir-triggered actin polymerization: getting off the pedestal. Cell Microbiol. 2008;10:549–56. doi: 10.1111/j.1462-5822.2007.01103.x. [DOI] [PubMed] [Google Scholar]
- 19.Guttman JA, Finlay BB. Subcellular alterations that lead to diarrhea during bacterial pathogenesis. Trends Microbiol. 2008;16:535–42. doi: 10.1016/j.tim.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Phillips N, Hayward RD, Koronakis V. Phosphorylation of the enteropathogenic E. coli receptor by the Src-family kinase c-Fyn triggers actin pedestal formation. Nat Cell Biol. 2004;6:618–25. doi: 10.1038/ncb1148. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt MA. LEEways: tales of EPEC, ATEC and EHEC. Cell Microbiol. 2010;12:1544–52. doi: 10.1111/j.1462-5822.2010.01518.x. [DOI] [PubMed] [Google Scholar]
- 22.Dean P, Kenny B. The effector repertoire of enteropathogenic E. coli: ganging up on the host cell. Curr Opin Microbiol. 2009;12:101–9. doi: 10.1016/j.mib.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong AR, Pearson JS, Bright MD, Munera D, Robinson KS, Lee SF, et al. Enteropathogenic and enterohaemorrhagic Escherichia coli: even more subversive elements. Mol Microbiol. 2011;80:1420–38. doi: 10.1111/j.1365-2958.2011.07661.x. [DOI] [PubMed] [Google Scholar]
- 24.Guttman JA, Finlay BB. Tight junctions as targets of infectious agents. Biochim Biophys Acta. 2009;1788:832–41. doi: 10.1016/j.bbamem.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 25.Hodges K, Gill R. Infectious diarrhea: Cellular and molecular mechanisms. Gut Microbes. 2010;1:4–21. doi: 10.4161/gmic.1.1.11036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viswanathan VK, Hodges K, Hecht G. Enteric infection meets intestinal function: how bacterial pathogens cause diarrhoea. Nat Rev Microbiol. 2009;7:110–9. doi: 10.1038/nrmicro2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donnenberg MS, Kaper JB. Enteropathogenic Escherichia coli. Infect Immun. 1992;60:3953–61. doi: 10.1128/iai.60.10.3953-3961.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milgotina EDM. The bundlin-forming pilus and other Type IV pili. Chapter 3 In: Jarrell KF, Ed. Pili and Flagella: Current Research and Future Trends Norfolk (UK): Caister Academic Press 2009. [Google Scholar]
- 29.Blank TE, Lacher DW, Scaletsky IC, Zhong H, Whittam TS, Donnenberg MS. Enteropathogenic Escherichia coli O157 strains from Brazil. Emerg Infect Dis. 2003;9:113–5. doi: 10.3201/eid0901.020072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blank TE, Zhong H, Bell AL, Whittam TS, Donnenberg MS. Molecular variation among type IV pilin (bfpA) genes from diverse enteropathogenic Escherichia coli strains. Infect Immun. 2000;68:7028–38. doi: 10.1128/IAI.68.12.7028-7038.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernandes PJ, Guo Q, Donnenberg MS. Functional consequences of sequence variation in bundlin, the enteropathogenic Escherichia coli type IV pilin protein. Infect Immun. 2007;75:4687–96. doi: 10.1128/IAI.00009-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hyland RM, Sun J, Griener TP, Mulvey GL, Klassen JS, Donnenberg MS, et al. The bundlin pilin protein of enteropathogenic Escherichia coli is an N-acetyllactosamine-specific lectin. Cell Microbiol. 2008;10:177–87. doi: 10.1111/j.1462-5822.2007.01028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hyland RM, Beck P, Mulvey GL, Kitov PI, Armstrong GD. N-acetyllactosamine conjugated to gold nanoparticles inhibits enteropathogenic Escherichia coli colonization of the epithelium in human intestinal biopsy specimens. Infect Immun. 2006;74:5419–21. doi: 10.1128/IAI.00739-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Humphries RM, Griener TP, Vogt SL, Mulvey GL, Raivio T, Donnenberg MS, et al. N-acetyllactosamine-induced retraction of bundle-forming pili regulates virulence-associated gene expression in enteropathogenic Escherichia coli. Mol Microbiol. 2010;76:1111–26. doi: 10.1111/j.1365-2958.2010.07192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mills E, Baruch K, Charpentier X, Kobi S, Rosenshine I. Real-time analysis of effector translocation by the type III secretion system of enteropathogenic Escherichia coli. Cell Host Microbe. 2008;3:104–13. doi: 10.1016/j.chom.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 36.McNamara BP, Koutsouris A, O’Connell CB, Nougayréde JP, Donnenberg MS, Hecht G. Translocated EspF protein from enteropathogenic Escherichia coli disrupts host intestinal barrier function. J Clin Invest. 2001;107:621–9. doi: 10.1172/JCI11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Howitt MR, Lee JY, Lertsethtakarn P, Vogelmann R, Joubert LM, Ottemann KM, et al. ChePep controls Helicobacter pylori Infection of the gastric glands and chemotaxis in the Epsilonproteobacteria. MBio. 2011;2:98–111. doi: 10.1128/mBio.00098-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lafont F, van der Goot FG. Bacterial invasion via lipid rafts. Cell Microbiol. 2005;7:613–20. doi: 10.1111/j.1462-5822.2005.00515.x. [DOI] [PubMed] [Google Scholar]
- 39.Boettcher JP, Kirchner M, Churin Y, Kaushansky A, Pompaiah M, Thorn H, et al. Tyrosine-phosphorylated caveolin-1 blocks bacterial uptake by inducing Vav2-RhoA-mediated cytoskeletal rearrangements. PLoS Biol. 2010;8:1000457. doi: 10.1371/journal.pbio.1000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sason H, Milgrom M, Weiss AM, Melamed-Book N, Balla T, Grinstein S, et al. Enteropathogenic Escherichia coli subverts phosphatidylinositol 4,5-bisphosphate and phosphatidylinositol 3,4,5-trisphosphate upon epithelial cell infection. Mol Biol Cell. 2009;20:544–55. doi: 10.1091/mbc.E08-05-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hanajima-Ozawa M, Matsuzawa T, Fukui A, Kamitani S, Ohnishi H, Abe A, et al. Enteropathogenic Escherichia coli, Shigella flexneri, and Listeria monocytogenes recruit a junctional protein, zonula occludens-1, to actin tails and pedestals. Infect Immun. 2007;75:565–73. doi: 10.1128/IAI.01479-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simonovic I, Rosenberg J, Koutsouris A, Hecht G. Enteropathogenic Escherichia coli dephosphorylates and dissociates occludin from intestinal epithelial tight junctions. Cell Microbiol. 2000;2:305–15. doi: 10.1046/j.1462-5822.2000.00055.x. [DOI] [PubMed] [Google Scholar]
- 43.Zolotarevsky Y, Hecht G, Koutsouris A, Gonzalez DE, Quan C, Tom J, et al. A membrane-permeant peptide that inhibits MLC kinase restores barrier function in in vitro models of intestinal disease. Gastroenterology. 2002;123:163–72. doi: 10.1053/gast.2002.34235. [DOI] [PubMed] [Google Scholar]
- 44.Coureuil M, Mikaty G, Miller F, Lécuyer H, Bernard C, Bourdoulous S, et al. Meningococcal type IV pili recruit the polarity complex to cross the brain endothelium. Science. 2009;325:83–7. doi: 10.1126/science.1173196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McKee ML, O’Brien AD. Investigation of enterohemorrhagic Escherichia coli O157:H7 adherence characteristics and invasion potential reveals a new attachment pattern shared by intestinal E. coli. Infect Immun. 1995;63:2070–4. doi: 10.1128/iai.63.5.2070-2074.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Canil C, Rosenshine I, Ruschkowski S, Donnenberg MS, Kaper JB, Finlay BB. Enteropathogenic Escherichia coli decreases the transepithelial electrical resistance of polarized epithelial monolayers. Infect Immun. 1993;61:2755–62. doi: 10.1128/iai.61.7.2755-2762.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Philpott DJ, McKay DM, Sherman PM, Perdue MH. Infection of T84 cells with enteropathogenic Escherichia coli alters barrier and transport functions. Am J Physiol. 1996;270:G634–45. doi: 10.1152/ajpgi.1996.270.4.G634. [DOI] [PubMed] [Google Scholar]
- 48.Spitz J, Yuhan R, Koutsouris A, Blatt C, Alverdy J, Hecht G. Enteropathogenic Escherichia coli adherence to intestinal epithelial monolayers diminishes barrier function. Am J Physiol. 1995;268:G374–9. doi: 10.1152/ajpgi.1995.268.2.G374. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.