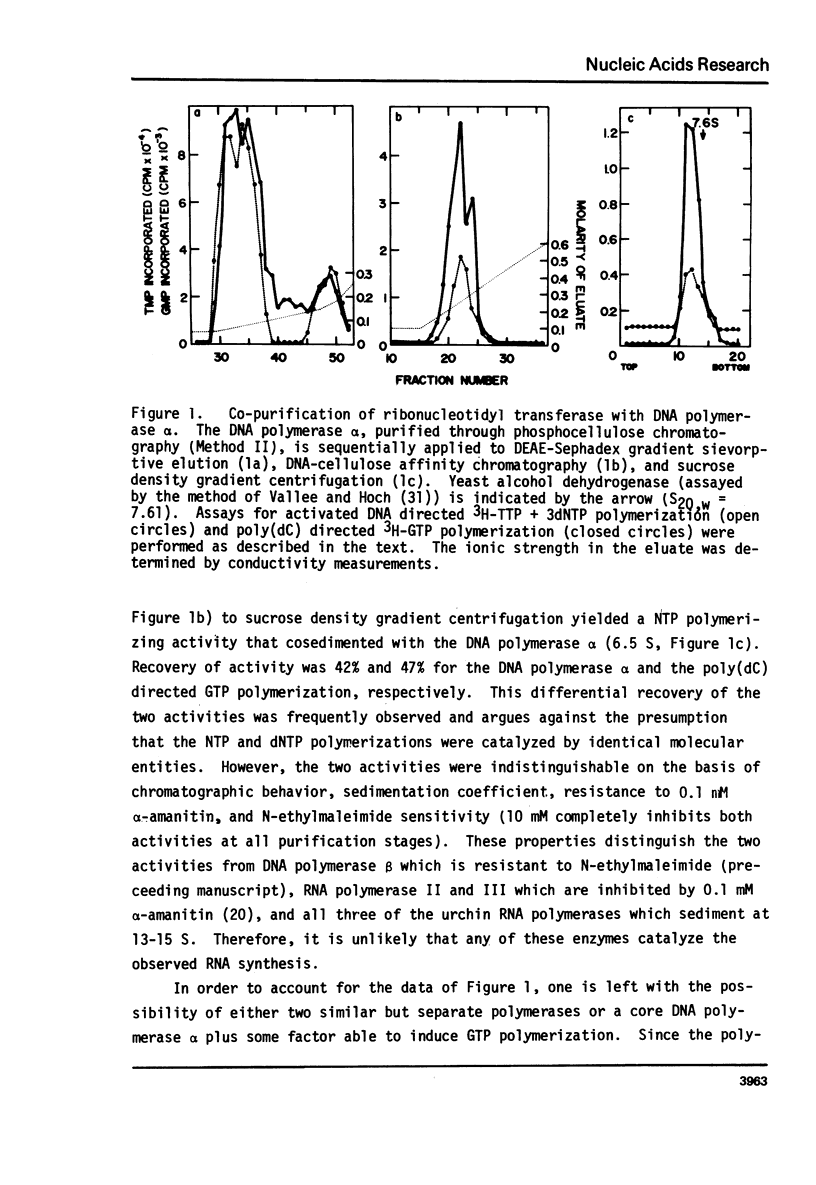
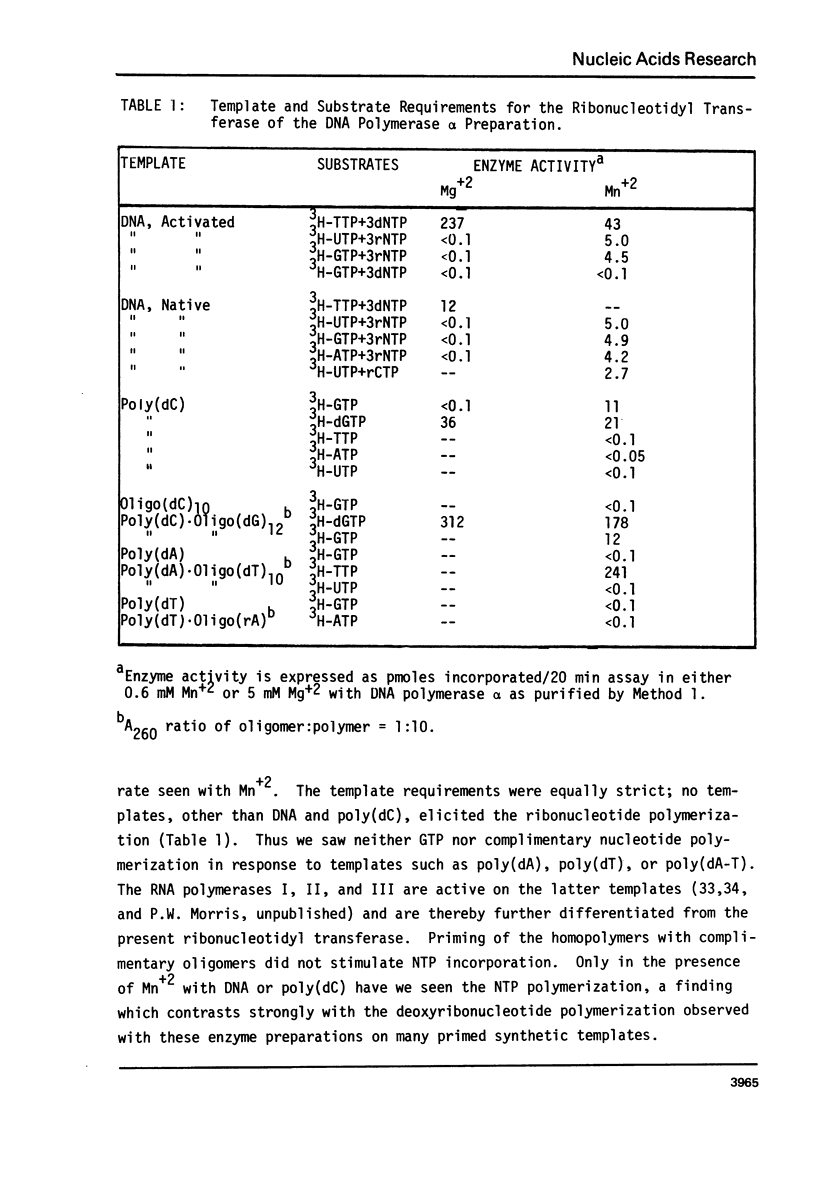
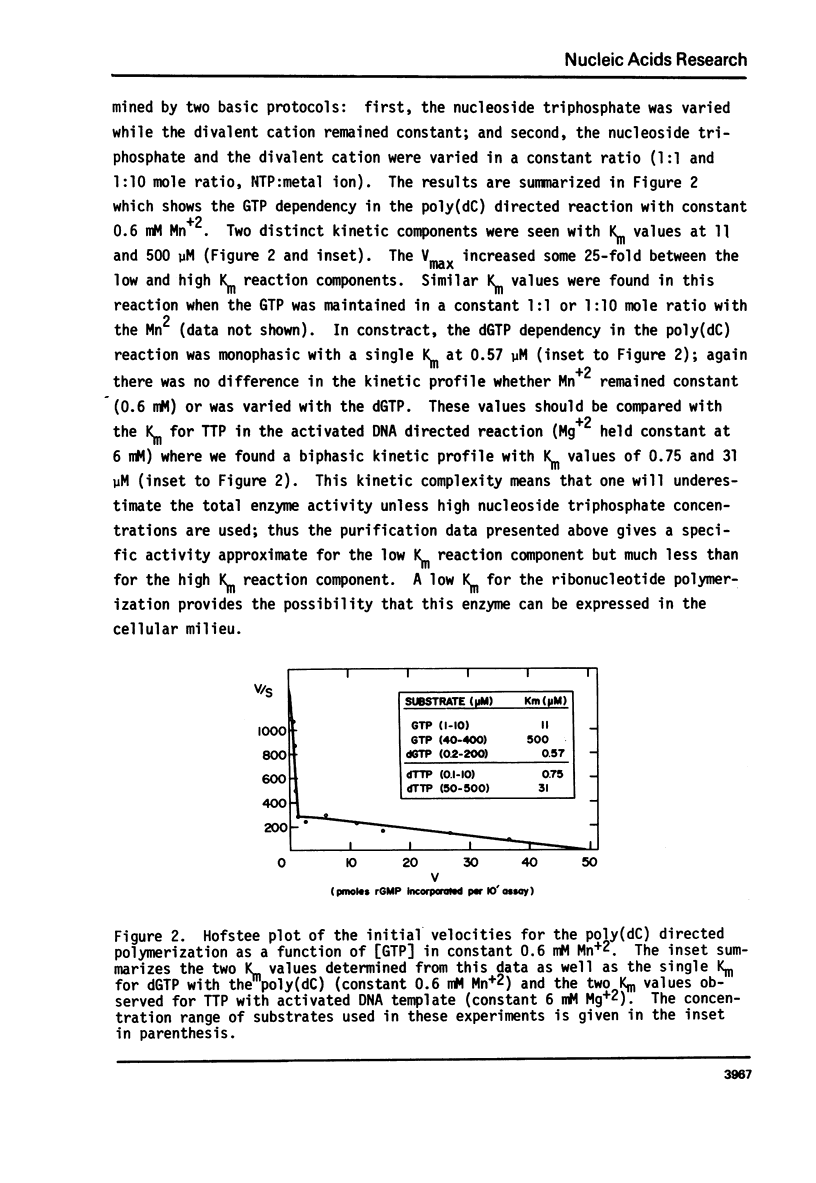
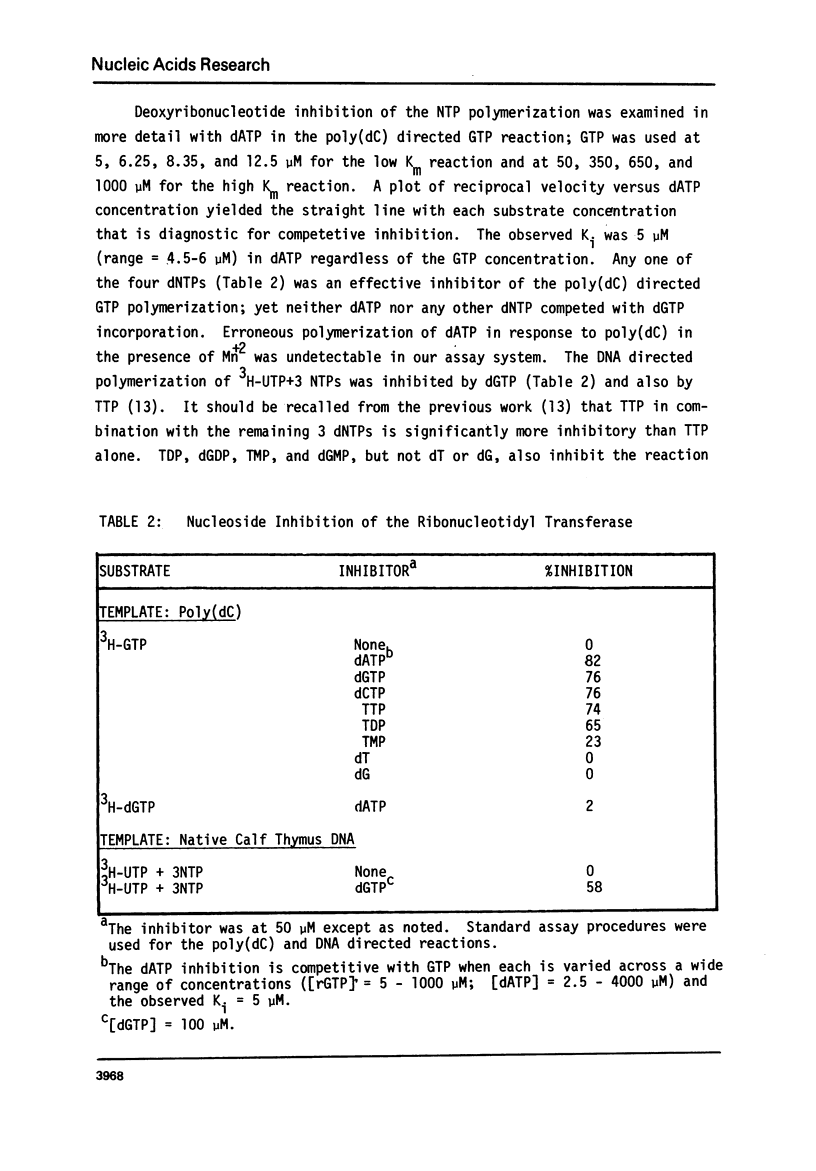
Abstract
Three ribonucleotidyl transferase types have been described in the sea urchin: riboadenylate trnasferase, the DNA dependent RNA polymerases, and a DNA polymerase associated ribonucleotidyl transferase (Biochemistry 15:3106-3113, 1976). In the present work this latter ribonucleotidyl transferase was found to purify with DNA polymerase alpha through phosphocellulose, DEAE-Sephadex and DNA cellulose and to cosediment at 6.5 S. This ribonucleotidyl transferase was active with Mn+2, but not Mg+2, on calf thymus DNA and poly(dC). Other synthetic templates elicited DNA polymerase alpha but no ribonucleotidyl transferase activity. From alkaline hydrolysates of the poly(dC) directed GTP polymerization, we found Goh and Gp in a ratio of 1:16 indicating an average chain length of 17 residues after a 20 min reaction. Co-polymerization of GTP (5 micrometer) and dGTP (10 micrometer) yielded a non-random distribution of the ribonucleotide in the deoxyribonucleotide. The properties of this urchin ribonucleotidyl transferase are unlike any previously described eukaryotic transferase and the data is discussed with reference to the known properties of E. coli DNA polymerase I and the primase.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOLLUM F. J. Calf thymus polymerase. J Biol Chem. 1960 Aug;235:2399–2403. [PubMed] [Google Scholar]
- Bouché J. P., Rowen L., Kornberg A. The RNA primer synthesized by primase to initiate phage G4 DNA replication. J Biol Chem. 1978 Feb 10;253(3):765–769. [PubMed] [Google Scholar]
- Brutlag D., Schekman R., Kornberg A. A possible role for RNA polymerase in the initiation of M13 DNA synthesis. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2826–2829. doi: 10.1073/pnas.68.11.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasson R., Reichard P. Primase initiates Okazaki pieces during polyoma DNA synthesis. Nature. 1978 Mar 9;272(5649):184–185. doi: 10.1038/272184a0. [DOI] [PubMed] [Google Scholar]
- Fisher P. A., Korn D. DNA polymerase-alpha. Purification and structural characterization of the near homogeneous enzyme from human KB cells. J Biol Chem. 1977 Sep 25;252(18):6528–6535. [PubMed] [Google Scholar]
- Hunter T., Francke B. Letter: In vitro polyoma DNA synthesis: involvement of RNA in discontinuous chain growth. J Mol Biol. 1974 Feb 15;83(1):123–130. doi: 10.1016/0022-2836(74)90427-6. [DOI] [PubMed] [Google Scholar]
- INMAN R. B. TRANSITIONS OF DNA HOMOPOLYMERS. J Mol Biol. 1964 Sep;9:624–637. doi: 10.1016/s0022-2836(64)80171-6. [DOI] [PubMed] [Google Scholar]
- Kirkegaard L. H. Gradient sievorptive chromatography. A focusing system for the separation of cellular components. Biochemistry. 1973 Sep 11;12(19):3627–3632. doi: 10.1021/bi00743a009. [DOI] [PubMed] [Google Scholar]
- Kirkegaard L. H., Johnson T. J., Bock R. M. Ion filtration chromatography: a powerful new technique for enzyme purification applied to E. coli alkaline phosphatase. Anal Biochem. 1972 Nov;50(1):122–138. doi: 10.1016/0003-2697(72)90492-7. [DOI] [PubMed] [Google Scholar]
- Loeb L. A. Purification and properties of deoxyribonucleic acid polymerase from nuclei of sea urchin embryos. J Biol Chem. 1969 Apr 10;244(7):1672–1681. [PubMed] [Google Scholar]
- Longacre S. S., Rutter W. J. Nucleotide polymerases in the developing avian erythrocyte. J Biol Chem. 1977 Jan 10;252(1):273–283. [PubMed] [Google Scholar]
- Matsukage A., Sivarajan M., Wilson S. H. Studies on DNA alpha-polymerase of mouse myeloma: partial purification and comparison of three molecular forms of the enzyme. Biochemistry. 1976 Nov 30;15(24):5305–5314. doi: 10.1021/bi00669a017. [DOI] [PubMed] [Google Scholar]
- Morris P. W., Rutter W. J. Nucleic acid polymerizing enzymes in developing Strongylocentrotus franciscanus embryos. Biochemistry. 1976 Jul 13;15(14):3106–3113. doi: 10.1021/bi00659a026. [DOI] [PubMed] [Google Scholar]
- Morris P. W., Venton D. L., Kelley K. M. Biochemistry of the amatoxins: preparation and characterization of a stably iodinated alpha-amanitin. Biochemistry. 1978 Feb 21;17(4):690–698. doi: 10.1021/bi00597a021. [DOI] [PubMed] [Google Scholar]
- Rougeon F., Brun G., Chapeville F. Comparative studies on the template-initiator requirements of the chick-embryo DNA polymerase I and the avian-myeloblastosis-virus DNA polymerase. Eur J Biochem. 1974 Jan 16;41(2):253–261. doi: 10.1111/j.1432-1033.1974.tb03266.x. [DOI] [PubMed] [Google Scholar]
- Rowen L., Kornberg A. A ribo-deoxyribonucleotide primer synthesized by primase. J Biol Chem. 1978 Feb 10;253(3):770–774. [PubMed] [Google Scholar]
- Rowen L., Kornberg A. Primase, the dnaG protein of Escherichia coli. An enzyme which starts DNA chains. J Biol Chem. 1978 Feb 10;253(3):758–764. [PubMed] [Google Scholar]
- Schekman R., Weiner A., Kornberg A. Multienzyme systems of DNA replication. Science. 1974 Dec 13;186(4168):987–993. doi: 10.1126/science.186.4168.987. [DOI] [PubMed] [Google Scholar]
- Schekman R., Weiner A., Kornberg A. Multienzyme systems of DNA replication. Science. 1974 Dec 13;186(4168):987–993. doi: 10.1126/science.186.4168.987. [DOI] [PubMed] [Google Scholar]
- Tseng B. Y., Goulian M. Evidence for covalent association of RNA with nascent DNA in human lymphocytes. J Mol Biol. 1975 Dec 5;99(2):339–346. doi: 10.1016/s0022-2836(75)80150-1. [DOI] [PubMed] [Google Scholar]
- Tseng B. Y., Goulian M. Initiator RNA of discontinuous DNA synthesis in human lymphocytes. Cell. 1977 Oct;12(2):483–489. doi: 10.1016/0092-8674(77)90124-6. [DOI] [PubMed] [Google Scholar]
- Tsiapalis C. M., Dorson J. W., Bollum F. J. Purification of terminal riboadenylate transferase from calf thymus gland. J Biol Chem. 1975 Jun 25;250(12):4486–4496. [PubMed] [Google Scholar]
- Vallee B. L., Hoch F. L. ZINC, A COMPONENT OF YEAST ALCOHOL DEHYDROGENASE. Proc Natl Acad Sci U S A. 1955 Jun 15;41(6):327–338. doi: 10.1073/pnas.41.6.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Sande J. H., Loewen P. C., Khorana H. G. Studies on polynucleotides. 118. A further study of ribonucleotide incorporation into deoxyribonucleic acid chains by deoxyribonucleic acid polymerase I of Escherichia coli. J Biol Chem. 1972 Oct 10;247(19):6140–6148. [PubMed] [Google Scholar]
- Weissbach A., Poonian M. Nucleic acids attached to solid matrices. Methods Enzymol. 1974;34:463–475. doi: 10.1016/s0076-6879(74)34057-8. [DOI] [PubMed] [Google Scholar]
- Wickner S. DNA or RNA priming of bacteriophage G4 DNA synthesis by Escherichia coli dnaG protein. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2815–2819. doi: 10.1073/pnas.74.7.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner S., Hurwitz J. Conversion of phiX174 viral DNA to double-stranded form by purified Escherichia coli proteins. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4120–4124. doi: 10.1073/pnas.71.10.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]



