Abstract
Lipocalin-type prostaglandin D synthase (L-PGDS) is one of the most abundant proteins in the cerebrospinal fluid. Nevertheless, its role in the central nervous system is far from clear. Here, we present evidence that L-PGDS induces glial cell migration and morphological changes in vitro and in vivo. We also identified myristoylated alanine-rich C-kinase substrate (MARCKS), heat shock proteins and actin as L-PGDS-binding proteins, demonstrating that MARCKS/Akt/Rho/Jnk pathways are involved in the L-PGDS actions in glia. We further show that the cell migration-promoting activity of L-PGDS is independent of PGD2 production. The results suggest a novel non-enzymatic function of L-PGDS protein in brain inflammation, and may have an impact on glial cell biology and brain pathology related with reactive gliosis. L-PGDS is a potential drug target that can be exploited for therapeutic intervention of glia-driven neuroinflammation and related diseases.
Keywords: glia, cell migration, morphology, L-PGDS, MARCKS, neuroinflammation, cerebrospinal fluid
Lipocalin-type prostaglandin D synthase (L-PGDS) is a member of the lipocalin family, which binds and transports small hydrophobic molecules. L-PGDS is a glutathione (GSH)-independent brain-type enzyme that catalyzes conversion of PGH2 to PGD2.1,2 L-PGDS is distinct from hematopoietic PGDS (H-PGDS), which is a GSH-dependent spleen-type enzyme. L-PGDS has been implicated in diverse cellular processes, such as cellular apoptosis/survival,3-5 cell differentiation,6 cell cycle progression,7 inflammation8,9 and metabolic syndrome.7,10 L-PGDS is expressed in the central nervous system (CNS) in addition to a variety of peripheral tissues. In fact, L-PGDS is one of the most abundant proteins in the cerebrospinal fluid, and has been previously called a β-trace.11,12 Nonetheless, little is known about its functional role in the CNS. A recent study showed that L-PGDS is a regulator of glial cell migration and morphology.13 The study further demonstrated that the effects of L-PGDS on glial migration were independent of PGD2 production. This result is somewhat surprising because PGDS has long been thought as an enzyme that catalyzes the isomerization of PGH2 to produce PGD2. The PGD2-independent effects of L-PGDS were, however, demonstrated by several ways: (1) PGD2 receptor antagonists did not significantly influence the L-PGDS-induced glial cell migration; (2) recombinant L-PGDS protein treatment exerted little effect on the PGD2 levels in glial culture medium; (3) a variant of L-PGDS protein (C65A mutant without the ability to catalyze the conversion of PGH2 to PGD2) also promoted glial cell migration; and finally (4) L-PGDS and PGD2 used different intracellular signaling pathways to promote glial cell migration. These results suggest that secreted L-PGDS protein indeed acts as a lipocalin, rather than PGD2-producing enzyme, to regulate glial cell phenotypes. L-PGDS protein present in cerebrospinal fluid or locally secreted in the inflammatory site may modulate glial recruitment into the injury site in the CNS.
In an attempt to elucidate the molecular mechanisms underlying the L-PGDS-induced glial cell migration, L-PGDS-interacting proteins were identified by coimmunoprecipitation followed by liquid chromatography and tandem mass spectrometry (LC-MS/MS) analysis. In order to identify the L-PGDS-binding proteins on or near the cell surface, intact glial cells were treated with L-PGDS protein, and thoroughly washed prior to formaldehyde-mediated crosslinking. L-PGDS-treated glial cells were then lysed and immunoprecipitated using the anti-L-PGDS antibody. The proteins coimmunoprecipitated with L-PGDS were separated by PAGE and visualized by silver staining, which were then identified with LC-MS/MS analysis. MARCKS, actin and a group of heat shock proteins were identified as the major L-PGDS-binding proteins (Table 1). The interaction between L-PGDS and MARCKS was confirmed by a separate immunoprecipitation and western blot analysis. An important role of MARCKS in the L-PGDS-induced glial migration was demonstrated by knocking down MARCKS expression using siRNA. MARCKS knockdown partially abrogated the L-PGDS effects on glial cell migration. Further studies using pharmacological inhibitors, dissection of intracellular signal transduction pathways, and morphological analysis revealed that L-PGDS induced glial cell migration via MARCKS/Akt/Rho/Jnk pathways, leading to augmented formation of actin filaments and focal adhesion (Fig. 1). The list of proteins co-immunoprecipitated with L-PGDS also included heat shock proteins, actin, α-enolase and reticulon-4. However, mechanistic involvement of these proteins in the L-PGDS actions in glia remains to be determined. Heat shock proteins and actin may indirectly interact with L-PGDS through membrane-anchoring adaptor proteins (Fig. 1), suggesting that stress response and actin cytoskeleton may be associated with L-PGDS effects on glial cell morphology and motility. α-enolase is a glycolytic enzyme that is expressed in most tissues.14 α-enolase has been identified as an autoantigen in Hashimoto encephalopathy, asthma and Behcet disease. It is also known as the Myc-binding protein-1 (MBP1), which regulates c-myc activity. The interaction between L-PGDS and α-enolase has to be confirmed and deserves further investigation. Reticulon-4, also known as Nogo, is an inhibitor of neurite outgrowth in CNS.15 Reticulon-4 is associated with endoplasmic reticulum, and has a potent inhibitory effect on neurite outgrowth, which blocks the CNS regeneration. Membrane-associated reticulon-4 binds to its receptor (NgR) to inhibit axon outgrowth. Blockade of reticulon-4 during neuronal damage is thought to enhance restoration of damaged neurons. As the neurite outgrowth requires local movement of cellular processes, interaction between L-PGDS and reticulon-4 may regulate axonal outgrowth. The precise role of reticulon-4 in the L-PGDS actions may need to be clarified by further investigation.
Table 1. List of proteins coimmunoprecipitated with L-PGDS.
| Accession number* | Protein name | Symbol | Peptide hit** |
|---|---|---|---|
| IPI00554929 |
Heat shock protein HSP 90-β |
Hsp90ab1 |
31 |
| IPI00110850 |
Actin, cytoplasmic 1 |
Actb |
25 |
| IPI00323357 |
Heat shock cognate 71 kDa protein |
Hspa8 |
12 |
| IPI00319992 |
78 kDa glucose-regulated protein |
Hspa5 |
7 |
| IPI00462072 |
α-enolase |
Eno1 |
7 |
| IPI00129526 |
Hsp90b1 endoplasmin |
Hsp90b1 |
5 |
| IPI00275539 |
Reticulon 4 |
Rtn4 |
4 |
| IPI00229534 |
Myristoylated alanine-rich C-kinase substrate |
Marcks |
4 |
| IPI00111560 |
Isoform 1 of protein SET |
Set |
3 |
| IPI00115679 |
Isoform 2 of neutral α-glucosidase AB |
Ganab |
3 |
| IPI00133903 |
Stress-70 protein, mitochondrial |
Hspa9 |
2 |
| IPI00604969 | Titin isoform N2-A | Ttn | 2 |
SWISS-PROT accession numbers are listed. **Number of peptide hit identified by LC-MS/MS analysis. In brief, NIH3T3 fibroblast cells were treated with the recombinant L-PGDS protein (1 μg/ml), and then crosslinked with 1% formaldehyde for 1 h and rinsed twice with PBS. Cells were lysed in triple-detergent lysis buffer (50 mM TRIS-HCl, pH 8.0; 150 mM NaCl; 0.02% sodium azide; 0.1% SDS; 1% Nonidet P-40; 0.5% sodium deoxycholate; and 1 mM phenylmethyl sulfonyl fluoride). The lysates were centrifuged for 20 min at 4°C, and the supernatants were collected. The protein concentration in the cell lysates was determined using the Quant-iT Protein Assay kit (Molecular Probes). To remove nonspecific binding proteins in the lysates, the samples were incubated in a ~30 μl packed volume of recombinant protein G-agarose (PGA) for 1 h at 4°C. After a brief centrifugation, supernatants were collected and then incubated with anti-L-PGDS antibody (1 μg/ml; Cayman Chemical) for 4 h at 4°C. PGA (30 μl) was then added and incubated for 4 h. Afterwards, L-PGDS-Ab-PGA complexes were washed three times with wash buffer (50 mM HEPES, 150 mM NaCl, 0.1% Triton X-100 and 10% glycerol). For the identification of coimmunoprecipitated proteins, the immunoprecipitation samples were separated by electrophoresis on a 10% polyacrylamide gel and visualized by silver staining. The protein band of interest was excised from the silver-stained gel for in-gel tryptic digestion. The excised gel slices were destained and shrunk by dehydration in acetonitrile and dried in a vacuum centrifuge. Proteins within the shrunken gel slices were then digested overnight with trypsin at a substrate/enzyme ratio of 10:1 (wt/wt) in 25 mM ammonium bicarbonate (pH 8.0). The enzyme reaction was terminated by the addition of 0.1% formic acid in water. Peptides from gel pieces were extracted by sonication for 10 min and supernatants containing the peptides were transferred to new tubes. Peptides were analyzed using a liquid chromatography (LC) and tandem mass spectrometry (MS/MS) system with reverse-phase LC, which consisted of a Surveyor MS pump (Thermo Electron), a Spark autosampler (Spark Holland), and a Finnigan LTQ linear ion-trap mass spectrometer (Thermo Electron) equipped with nanospray ionization sources. All MS/MS data were searched against the IPI mouse protein database (version 3.16) using the SEQUEST algorithm (Thermo Electron) incorporated into BioWorks software (version 3.2).
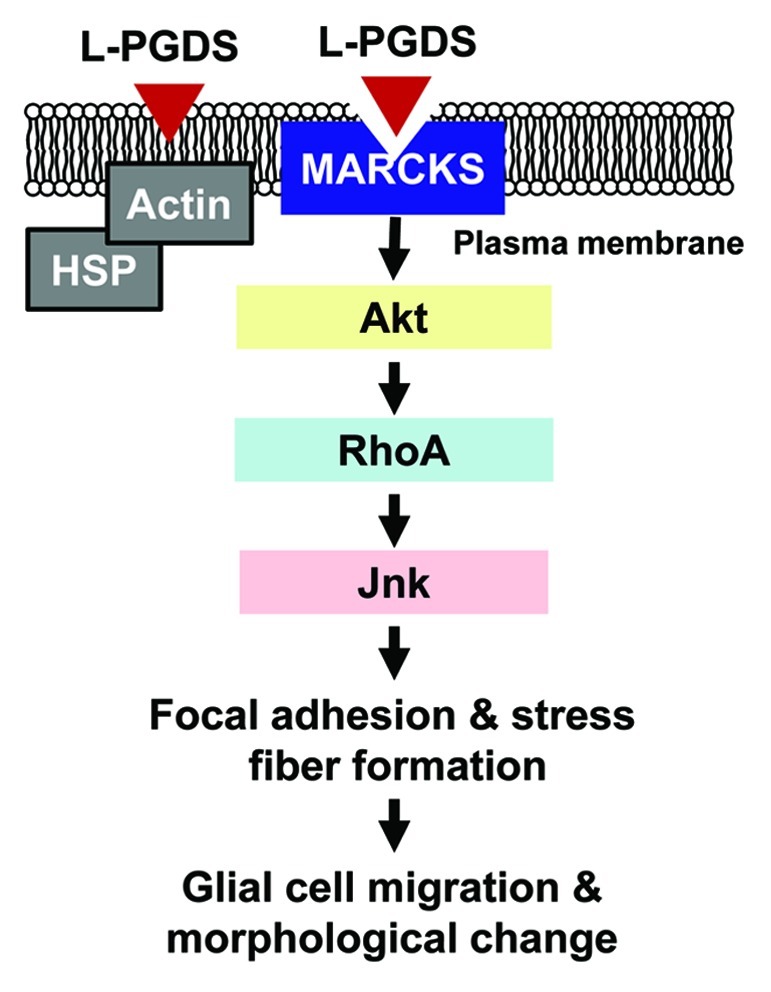
Figure 1. Schematic diagram depicting the L-PGDS-induced glial cell migration and morphological changes. Secreted L-PGDS protein promotes glial cell migration and morphological change by binding to MARCKS protein in plasma membrane. L-PGDS-MARCKS complex may sequentially activate Akt, RhoA, and Jnk, which in turn induces actin polymerization and focal adhesion formation. The effects of L-PGDS under this condition seem to be independent of its enzymatic activity to produce PGD2. Alternatively, L-PGDS may indirectly interact with heat shock proteins (HSP) or actin through membrane-anchoring adaptor proteins. The role of these proteins, however, needs to be further investigated in the future. Adapted from Lee et al.13
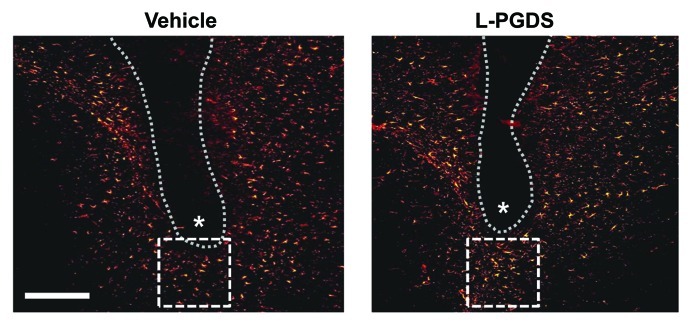
Although much of the findings in the study by Lee et al.13 were based on in vitro experiments, the effect of L-PGDS on glial migration was also determined in vivo.13 When L-PGDS protein was stereotaxically injected into the specific regions of the mouse brain such as striatum or cortex, the number of GFAP-positive astrocytes in the peri-region of the L-PGDS-injected site was significantly higher compared with the vehicle-injection (Fig. 2). The results are based on immunofluorescence detection of GFAP-positive astrocytes from the six independent tissue sections per animal. Astrocyte count obtained from three different animals per group (vehicle vs. L-PGDS-injected mice) showed statistically significant differences. These results support that L-PGDS enhances glial migration and accumulation in brain. These phenotypic changes of glia are well known to be associated with reactive gliosis. Therefore, the recent report by Lee et al.13 suggests a novel non-enzymatic role of L-PGDS in brain inflammation, and has an impact on glial cell biology and brain pathology that is related with reactive gliosis. Since cell migration plays a pivotal role in development, wound healing, immune/inflammatory responses and tumor metastasis,16-18 the effects of L-PGDS on cell migration and morphology identified in the study by Lee et al.13 may broaden our understanding of these biological processes. In conclusion, L-PGDS is a potential drug target that can be exploited for therapeutic intervention of glia-driven neuroinflammation and related diseases.
Figure 2. L-PGDS induces astrocyte migration in mouse brain. Anesthetized mice (body weight 30 g) were positioned in a stereotaxic apparatus (Stoelting). Body temperature of the mice was maintained at 37°C during surgery using a homeothermic heat blanket (Harvard Apparatus Co.). A skin incision was made to expose the skull, and a small hole was drilled through the skull. The vehicle or L-PGDS protein (1 μl; 1 mg/ml) was stereotaxically injected into the striatum of the mouse brain at the stereotaxic coordinates of 1 mm anterior to the bregma, 2 mm lateral to the bregma and 4 mm below the skull using a 26-gauge needle. The flow rate of the injection was 0.1 μl/min maintained by a microsyringe pump (Harvard Apparatus Co.). After removing the needle, the skin was sutured with 6.0 mm silk thread. After 48 h, the mice were sacrificed, and brain sections were prepared for immunohistochemical analysis of astrocytes. The tissues were permeabilized in 0.1% Triton X-100 and blocked with 1% BSA and 5% normal donkey serum. After washing with PBS, the sections were incubated at 4°C overnight with the mouse monoclonal antibody against glial fibrillary acidic protein (GFAP) (1:500 dilution; BD Biosciences). The sections were then incubated with donkey Cy3-conjugated anti-mouse IgG antibody (1:200 dilution; Jackson Immunoresearch Laboratories). The sections were mounted on DAPI-containing gelatin solution. Data acquisition and immunohistological intensity measurement of GFAP staining was performed with a NIH image J program (NIH Image). Composite images of the stained sections were FFT band-pass filtered to eliminate low-frequency drifts (> 20 pixels = 50 μm) and high-frequency noises (< 1 pixel = 2.5 μm). The images were binary thresholded at 50% of the background level, and the particles were then converted to a sub-threshold image area with a size less than 300 and larger than 5 pixels, which was judged as GFAP-positive cells. The range (5–300 pixels) was obtained from the analyzed size of GFAP-positive cells from six sections of each animal. Dotted line and asterisks indicate the guide cannula and protein injection sites, respectively. Immunofluorescence analysis revealed that the GFAP-positive astrocytes were recruited into the peri-injection site indicated by dotted square box following the injection of L-PGDS protein. The number of astrocytes recruited in the dotted square (200 μm × 200 μm) was counted and statistically analyzed from the six independent tissue sections: vehicle, 32 ± 6; L-PGDS, 58 ± 7 (values are mean ± SD). A representative microscopic image for each condition is shown. Scale bar, 200 μm. Adapted from Lee et al.13
Acknowledgments
This work was supported by Basic Science Research Program through the National Research Foundation (NRF) funded by the Ministry of Education, Science and Technology (MEST) of Korean government (2010-0029460). This study was also supported by a grant of the Korea Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (A111345, A100176).
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/20251
References
- 1.Urade Y, Fujimoto N, Hayaishi O. Purification and characterization of rat brain prostaglandin D synthetase. J Biol Chem. 1985;260:12410–5. [PubMed] [Google Scholar]
- 2.Urade Y, Hayaishi O. Prostaglandin D synthase: structure and function. Vitam Horm. 2000;58:89–120. doi: 10.1016/S0083-6729(00)58022-4. [DOI] [PubMed] [Google Scholar]
- 3.Ragolia L, Palaia T, Frese L, Fishbane S, Maesaka JK. Prostaglandin D2 synthase induces apoptosis in PC12 neuronal cells. Neuroreport. 2001;12:2623–8. doi: 10.1097/00001756-200108280-00008. [DOI] [PubMed] [Google Scholar]
- 4.Maesaka JK, Palaia T, Frese L, Fishbane S, Ragolia L. Prostaglandin D(2) synthase induces apoptosis in pig kidney LLC-PK1 cells. Kidney Int. 2001;60:1692–8. doi: 10.1046/j.1523-1755.2001.00989.x. [DOI] [PubMed] [Google Scholar]
- 5.Ragolia L, Palaia T, Paric E, Maesaka JK. Elevated L-PGDS activity contributes to PMA-induced apoptosis concomitant with downregulation of PI3-K. Am J Physiol Cell Physiol. 2003;284:C119–26. doi: 10.1152/ajpcell.00247.2002. [DOI] [PubMed] [Google Scholar]
- 6.Xin X, Huber A, Meyer P, Flammer J, Neutzner A, Miller NR, et al. L-PGDS (betatrace protein) inhibits astrocyte proliferation and mitochondrial ATP production in vitro. J Mol Neurosci. 2009;39:366–71. doi: 10.1007/s12031-009-9214-7. [DOI] [PubMed] [Google Scholar]
- 7.Ragolia L, Palaia T, Hall CE, Maesaka JK, Eguchi N, Urade Y. Accelerated glucose intolerance, nephropathy, and atherosclerosis in prostaglandin D2 synthase knock-out mice. J Biol Chem. 2005;280:29946–55. doi: 10.1074/jbc.M502927200. [DOI] [PubMed] [Google Scholar]
- 8.Schuligoi R, Grill M, Heinemann A, Peskar BA, Amann R. Sequential induction of prostaglandin E and D synthases in inflammation. Biochem Biophys Res Commun. 2005;335:684–9. doi: 10.1016/j.bbrc.2005.07.130. [DOI] [PubMed] [Google Scholar]
- 9.Joo M, Kwon M, Cho YJ, Hu N, Pedchenko TV, Sadikot RT, et al. Lipopolysaccharide-dependent interaction between PU.1 and c-Jun determines production of lipocalin-type prostaglandin D synthase and prostaglandin D2 in macrophages. Am J Physiol Lung Cell Mol Physiol. 2009;296:L771–9. doi: 10.1152/ajplung.90320.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka R, Miwa Y, Mou K, Tomikawa M, Eguchi N, Urade Y, et al. Knockout of the l-pgds gene aggravates obesity and atherosclerosis in mice. Biochem Biophys Res Commun. 2009;378:851–6. doi: 10.1016/j.bbrc.2008.11.152. [DOI] [PubMed] [Google Scholar]
- 11.Hoffmann A, Conradt HS, Gross G, Nimtz M, Lottspeich F, Wurster U. Purification and chemical characterization of beta-trace protein from human cerebrospinal fluid: its identification as prostaglandin D synthase. J Neurochem. 1993;61:451–6. doi: 10.1111/j.1471-4159.1993.tb02145.x. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe K, Urade Y, Mäder M, Murphy C, Hayaishi O. Identification of beta-trace as prostaglandin D synthase. Biochem Biophys Res Commun. 1994;203:1110–6. doi: 10.1006/bbrc.1994.2297. [DOI] [PubMed] [Google Scholar]
- 13.Lee S, Jang E, Kim JH, Lee WH, Suk K. Lipocalin-type prostaglandin D2 synthase regulates glial cell migration and morphology through marcks: prostaglandin D2-independent effects. J Biol Chem. 2012;287:9414–28. doi: 10.1074/jbc.M111.330662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Subramanian A, Miller DM. Structural analysis of alpha-enolase. Mapping the functional domains involved in down-regulation of the c-myc protooncogene. J Biol Chem. 2000;275:5958–65. doi: 10.1074/jbc.275.8.5958. [DOI] [PubMed] [Google Scholar]
- 15.GrandPré T, Nakamura F, Vartanian T, Strittmatter SM. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature. 2000;403:439–44. doi: 10.1038/35000226. [DOI] [PubMed] [Google Scholar]
- 16.Vicente-Manzanares M, Webb DJ, Horwitz AR. Cell migration at a glance. J Cell Sci. 2005;118:4917–9. doi: 10.1242/jcs.02662. [DOI] [PubMed] [Google Scholar]
- 17.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, et al. Cell migration: integrating signals from front to back. Science. 2003;302:1704–9. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 18.Franz CM, Jones GE, Ridley AJ. Cell migration in development and disease. Dev Cell. 2002;2:153–8. doi: 10.1016/S1534-5807(02)00120-X. [DOI] [PubMed] [Google Scholar]