Abstract
Recently, we monitored green fluorescent protein (GFP)-expressing monocytes after injection at the entorhinal cortex lesion (ECL) site in mice. We followed their migration out of the central nervous system (CNS) along olfactory nerve fibers penetrating the lamina cribrosa, within the nasal mucosa, and their subsequent appearance within the deep cervical lymph nodes (CLN), with numbers peaking at day 7. This is the same route activated T cells use for reaching the CLN, as we have shown before. Interestingly, GFP cells injected into the brain and subsequently found in the CLN exhibited ramified morphologies, which are typical of microglia and dendritic cells. To gain more insight into immunity and regeneration within the CNS we want to monitor injected monocytes using magnetic resonance imaging (MRI) after labeling with very small superparamagnetic iron oxide particles (VSOP). Due to their small size, nanoparticles have huge potential for magnetic labeling of different cell populations and their MRI tracking in vivo. So far we have verified that incubation with VSOP particles does not alter their migration pattern after ECL.
Keywords: monocytes, microglia, cell trafficking, lymph drainage, MRI, nanoparticles
Due to the lack of classical lymph vessels within brain tissue, cellular emigration out of the CNS parenchyma is either impossible or requires alternate pathways. Knowledge about the dynamics of immune cells within the CNS and the adhesion molecules and chemokines involved is crucial for the development of pharmacological interventions in autoimmune diseases. So far, data has been provided on interstitial and cerebrospinal fluid,1 tracer,2 antigens,3 T-cells4 and monocytes. Most seem to use rather passive means of transport such as an afferent pathway toward the periphery. For monocytes we recently published data5 suggesting active migration out of the CNS as the most likely mode of transport. With the help of GFP we monitored injected monocytes over 21 d after ECL of mice. We observed their migration along the alveus, a myelinated fiber tract, toward the ventral horn of the lateral ventricle. We were also able to track the GFP monocytes around the olfactory bulb, leaving the CNS along olfactory nerve fibers penetrating the lamina cribrosa. Later, we detected GFP cells within the nasal mucosa and established a time frame of their appearance in the CLN. In summary, our injected GFP monocytes were found only in the deep CLN and peaked at 7 d post injection (dpi) with a density of 11 GFP cells/mm2.
Several days after ECL there is an increase in microglia/monocyte cell counts6 due to recruitment of microglia from neighboring sites,7,8 their proliferation6,9 and infiltrating blood-borne monocytes10 adopting the morphology of ramified microglia.10-12 The distinction between microglia and acutely infiltrating macrophages is hampered by the fact that activated microglia express all common macrophage markers13-16 and no labeling technique has yet been developed to resolve this issue. However, after 30 d the total number of microglia/monocyte derivatives in the denervated layers normalizes6,17 without evidence for apoptosis.18 Thus, the fate of microglial cells that have migrated into and proliferated within the parenchyma remains unclear.
Our finding that monocytes peak at 7 d post lesion within the CLN may answer an important question: are microglia able to leave the CNS after a central lesion? We believe our data shows this to be the case. First, monocytes have a half-life of one to three days in the bloodstream before entering various organs and becoming either tissue macrophages19 or microglia in the CNS. Various studies show that macrophages innate to the CNS, namely microglia, originate both in the earliest developmental stages and later on from peripheral blood monocytes.10,11,20,21 Since we found GFP cells with a peak accumulation at 7 dpi in the CLN, the injected monocytes had enough time to transform into microglia. Second, mature blood monocytes typically have a round body and develop processes when transformed into adult tissue macrophages.22 The GFP cells found in the peripheral CLN possessed multiple ramified processes.
Currently, we are focusing on another possibility for monitoring the GFP monocytes. An elegant way to study monocyte/microglia involvement in lesion models is using VSOP. Very promising are new citrate-coated VSOP with a diameter of only 7–10 nm. Experimental and clinical data suggest that electrostatically stabilized citrate-coated VSOP may have some advantages over conventional sterically stabilized ultrasmall superparamagnetic iron oxide (USPIO) particles.23,24 In addition, VSOP are used for marking stabilized cells and subsequent detection of in vivo cell migration.25-27 It has been possible to monitor implanted VSOP-labeled neuronal progenitor cells over six weeks.28,29 However, other immune-competent cells can also be sufficiently labeled. Neutrophil granule cells and T cells were labeled ex vivo with nanoparticles, injected and traced via MRI at inflammatory sites.30-32
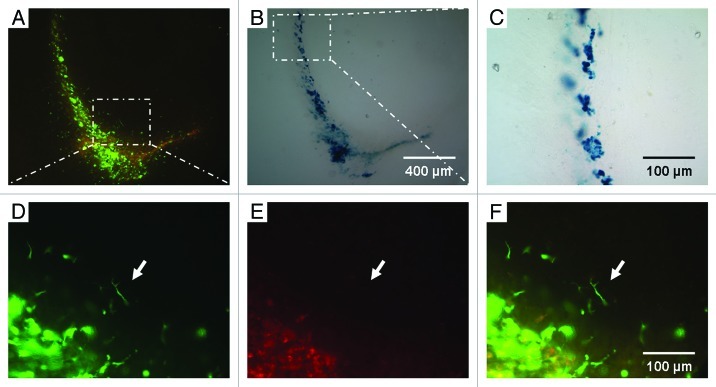
With the help of MRI, iron oxide-loaded monocytes could be easily monitored over a long period of time. After incubating the blood-derived GFP monocytes additionally with 3 mM electrostatically stabilized citrate-coated VSOP for 12 h we were able to detect the cells under the fluorescent microscope (Fig. 1A) as well as in the same sections after Prussian blue staining (Fig. 1B). Labeling the bloodborne GFP monocytes with VSOP did not alter their migration route (Fig. 1A); we still detected GFP monocytes migrating along the alveus. GFP cells could also be correlated with Prussian blue-stained iron oxide cells (Fig. 1B and C). Some of the monocytes revealed their ramified morphology within brain tissue (Fig. 1D–F).
Figure 1. VSOP-labeled GFP monocytes after ECL. (A) Overview of a mouse sacrificed 48 h after ECL and injection of VSOP-labeled monocytes in double fluorescence demonstrating the specificity of the GFP signal (magnification 100x). (B) Same detail with iron oxide Prussian blue staining. (C) Higher magnification shows the intracellular iron oxide (magnification 400x). In (D–F) (magnification 400x) the specific GFP signal is indicated. (D) Under green fluorescence the GFP monocytes are clearly visible. (E) Under red fluorescence only some background fluorescence can be seen. (F) Green/red fluorescence merged. Note the ramified monocyte indicated by the arrow.
We now want to monitor the VSOP-loaded GFP monocytes using MRI. First, however, a substantial assessment of cytotoxic effects is mandatory since iron oxide particles contain biodegraded iron, which can be utilized in cell cycle iron metabolism, leaving open the possibility of iron-catalyzed generation of reactive oxygen species.33 Our research focuses on identifying the in vivo migration paths and distribution of blood monocytes in zones of axonal degeneration induced by ECL. Our long-term aim is to influence immunity and regeneration in the CNS by specific application of genetically modified microglial progenitor cells.
Approaching of targeting factors and receptors involved in the transport of monocytes in central lesion processes has been accelerated in recent years. For instance, a long-term blockade of mononuclear cells through CCR2 antagonists shows a potent reduction in the severity of central injury34 due to a decreased accumulation of monocytes at the lesion site. In another approach, the humanized monoclonal antibody natalizumab blocked the α integrin of monocytes and thereby inhibited their migration into the CNS tissue in multiple sclerosis.35 Furthermore antigen presentation of APC (e.g., macrophages) toward regulatory T cells (Tregs) plays an essential role preventing autoimmunological processes: In translational studies the modulation of macrophages by glatirameracetate initiates the differentiation of naïve T cells into Tregs, reducing the invasion of leukocytes into the CNS and thereby the inflammatory process.36
Bone marrow-derived microglia could have tremendous clinical implications for the treatment of many neuroimmunological diseases of the human CNS, since specific microglial precursors could be used as Trojan horses to deliver neuroprotective or immune-relevant genes into the CNS37 and lead to innovative approaches in curing neuroimmune diseases.
Acknowledgments
M.K. is a scholar of the German National Academic Foundation and recipient of a stipend from the Gerhard C. Starck Foundation. J.G. is supported by DFG grant KFO 213 and I.B. by DFG grant FOR 1336. Kimberly Mason carefully revised this manuscript.
Glossary
Abbreviations:
- CLN
cervical lymph nodes
- CNS
central nervous system
- dpi
days post injection
- ECL
entorhinal cortex lesion
- GFP
green fluorescent protein
- MRI
magnetic resonance imaging
- MS
multiple sclerosis
- Tregs
regulatory T cells
- USPIO
ultrasmall superparamagnetic iron oxide
- VSOP
very small superparamagnetic iron oxide particles
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/20281
References
- 1.Weller RO. Mechanisms of cerebrospinal fluid absorption. Dev Med Child Neurol. 1974;16:85–7. doi: 10.1111/j.1469-8749.1974.tb02718.x. [DOI] [PubMed] [Google Scholar]
- 2.Boulton M, Flessner M, Armstrong D, Mohamed R, Hay J, Johnston M. Contribution of extracranial lymphatics and arachnoid villi to the clearance of a CSF tracer in the rat. Am J Physiol. 1999;276:R818–23. doi: 10.1152/ajpregu.1999.276.3.R818. [DOI] [PubMed] [Google Scholar]
- 3.Harling-Berg CJ, Park TJ, Knopf PM. Role of the cervical lymphatics in the Th2-type hierarchy of CNS immune regulation. J Neuroimmunol. 1999;101:111–27. doi: 10.1016/S0165-5728(99)00130-7. [DOI] [PubMed] [Google Scholar]
- 4.Goldmann J, Kwidzinski E, Brandt C, Mahlo J, Richter D, Bechmann I. T cells traffic from brain to cervical lymph nodes via the cribroid plate and the nasal mucosa. J Leukoc Biol. 2006;80:797–801. doi: 10.1189/jlb.0306176. [DOI] [PubMed] [Google Scholar]
- 5.Kaminski M, Bechmann I, Pohland M, Kiwit J, Nitsch R, Glumm J. Migration of monocytes after intracerebral injection at entorhinal cortex lesion site. J Leukoc Biol. 2012;92:31–9. doi: 10.1189/jlb.0511241. [DOI] [PubMed] [Google Scholar]
- 6.Hailer NP, Grampp A, Nitsch R. Proliferation of microglia and astrocytes in the dentate gyrus following entorhinal cortex lesion: a quantitative bromodeoxyuridine-labelling study. Eur J Neurosci. 1999;11:3359–64. doi: 10.1046/j.1460-9568.1999.00808.x. [DOI] [PubMed] [Google Scholar]
- 7.Gehrmann J, Schoen SW, Kreutzberg GW. Lesion of the rat entorhinal cortex leads to a rapid microglial reaction in the dentate gyrus. A light and electron microscopical study. Acta Neuropathol. 1991;82:442–55. doi: 10.1007/BF00293378. [DOI] [PubMed] [Google Scholar]
- 8.Jensen MB, Finsen B, Zimmer J. Morphological and immunophenotypic microglial changes in the denervated fascia dentata of adult rats: correlation with blood-brain barrier damage and astroglial reactions. Exp Neurol. 1997;143:103–16. doi: 10.1006/exnr.1996.6337. [DOI] [PubMed] [Google Scholar]
- 9.Gall C, Rose G, Lynch G. Proliferative and migratory activity of glial cells in the partially deafferented hippocampus. J Comp Neurol. 1979;183:539–49. doi: 10.1002/cne.901830306. [DOI] [PubMed] [Google Scholar]
- 10.Bechmann I, Goldmann J, Kovac AD, Kwidzinski E, Simbürger E, Naftolin F, et al. Circulating monocytic cells infiltrate layers of anterograde axonal degeneration where they transform into microglia. FASEB J. 2005;19:647–9. doi: 10.1096/fj.04-2599fje. [DOI] [PubMed] [Google Scholar]
- 11.Priller J, Flügel A, Wehner T, Boentert M, Haas CA, Prinz M, et al. Targeting gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. Nat Med. 2001;7:1356–61. doi: 10.1038/nm1201-1356. [DOI] [PubMed] [Google Scholar]
- 12.Prodinger C, Bunse J, Krüger M, Schiefenhövel F, Brandt C, Laman JD, et al. CD11c-expressing cells reside in the juxtavascular parenchyma and extend processes into the glia limitans of the mouse nervous system. Acta Neuropathol. 2011;121:445–58. doi: 10.1007/s00401-010-0774-y. [DOI] [PubMed] [Google Scholar]
- 13.Aloisi F. Immune function of microglia. Glia. 2001;36:165–79. doi: 10.1002/glia.1106. [DOI] [PubMed] [Google Scholar]
- 14.Becher B, Bechmann I, Greter M. Antigen presentation in autoimmunity and CNS inflammation: how T lymphocytes recognize the brain. J Mol Med (Berl) 2006;84:532–43. doi: 10.1007/s00109-006-0065-1. [DOI] [PubMed] [Google Scholar]
- 15.Carson MJ, Reilly CR, Sutcliffe JG, Lo D. Mature microglia resemble immature antigen-presenting cells. Glia. 1998;22:72–85. doi: 10.1002/(SICI)1098-1136(199801)22:1<72::AID-GLIA7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 16.Popovich PG, Hickey WF. Bone marrow chimeric rats reveal the unique distribution of resident and recruited macrophages in the contused rat spinal cord. J Neuropathol Exp Neurol. 2001;60:676–85. doi: 10.1093/jnen/60.7.676. [DOI] [PubMed] [Google Scholar]
- 17.Jensen MB, González B, Castellano B, Zimmer J. Microglial and astroglial reactions to anterograde axonal degeneration: a histochemical and immunocytochemical study of the adult rat fascia dentata after entorhinal perforant path lesions. Exp Brain Res. 1994;98:245–60. doi: 10.1007/BF00228413. [DOI] [PubMed] [Google Scholar]
- 18.Bechmann I, Lossau S, Steiner B, Mor G, Gimsa U, Nitsch R. Reactive astrocytes upregulate Fas (CD95) and Fas ligand (CD95L) expression but do not undergo programmed cell death during the course of anterograde degeneration. Glia. 2000;32:25–41. doi: 10.1002/1098-1136(200010)32:1<25::AID-GLIA30>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 19.Ziegler-Heitbrock HW. Definition of human blood monocytes. J Leukoc Biol. 2000;67:603–6. doi: 10.1002/jlb.67.5.603. [DOI] [PubMed] [Google Scholar]
- 20.Davoust N, Vuaillat C, Androdias G, Nataf S. From bone marrow to microglia: barriers and avenues. Trends Immunol. 2008;29:227–34. doi: 10.1016/j.it.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 21.Greter M, Heppner FL, Lemos MP, Odermatt BM, Goebels N, Laufer T, et al. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat Med. 2005;11:328–34. doi: 10.1038/nm1197. [DOI] [PubMed] [Google Scholar]
- 22.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–64. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 23.Taupitz M, Wagner S, Schnorr J, Kravec I, Pilgrimm H, Bergmann-Fritsch H, et al. Phase I clinical evaluation of citrate-coated monocrystalline very small superparamagnetic iron oxide particles as a new contrast medium for magnetic resonance imaging. Invest Radiol. 2004;39:394–405. doi: 10.1097/01.rli.0000129472.45832.b0. [DOI] [PubMed] [Google Scholar]
- 24.Wagner S, Schnorr J, Pilgrimm H, Hamm B, Taupitz M. Monomer-coated very small superparamagnetic iron oxide particles as contrast medium for magnetic resonance imaging: preclinical in vivo characterization. Invest Radiol. 2002;37:167–77. doi: 10.1097/00004424-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Fleige G, Seeberger F, Laux D, Kresse M, Taupitz M, Pilgrimm H, et al. In vitro characterization of two different ultrasmall iron oxide particles for magnetic resonance cell tracking. Invest Radiol. 2002;37:482–8. doi: 10.1097/00004424-200209000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Schulze E, Ferrucci JT, Jr., Poss K, Lapointe L, Bogdanova A, Weissleder R. Cellular uptake and trafficking of a prototypical magnetic iron oxide label in vitro. Invest Radiol. 1995;30:604–10. doi: 10.1097/00004424-199510000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Weissleder R, Cheng HC, Bogdanova A, Bogdanov A., Jr Magnetically labeled cells can be detected by MR imaging. J Magn Reson Imaging. 1997;7:258–63. doi: 10.1002/jmri.1880070140. [DOI] [PubMed] [Google Scholar]
- 28.Bulte JW, Douglas T, Witwer B, Zhang SC, Strable E, Lewis BK, et al. Magnetodendrimers allow endosomal magnetic labeling and in vivo tracking of stem cells. Nat Biotechnol. 2001;19:1141–7. doi: 10.1038/nbt1201-1141. [DOI] [PubMed] [Google Scholar]
- 29.Lewin M, Carlesso N, Tung CH, Tang XW, Cory D, Scadden DT, et al. Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nat Biotechnol. 2000;18:410–4. doi: 10.1038/74464. [DOI] [PubMed] [Google Scholar]
- 30.Krieg FM, Andres RY, Winterhalter KH. Superparamagnetically labelled neutrophils as potential abscess-specific contrast agent for MRI. Magn Reson Imaging. 1995;13:393–400. doi: 10.1016/0730-725X(94)00111-F. [DOI] [PubMed] [Google Scholar]
- 31.Yeh TC, Zhang W, Ildstad ST, Ho C. Intracellular labeling of T-cells with superparamagnetic contrast agents. Magn Reson Med. 1993;30:617–25. doi: 10.1002/mrm.1910300513. [DOI] [PubMed] [Google Scholar]
- 32.Yeh TC, Zhang W, Ildstad ST, Ho C. In vivo dynamic MRI tracking of rat T-cells labeled with superparamagnetic iron-oxide particles. Magn Reson Med. 1995;33:200–8. doi: 10.1002/mrm.1910330209. [DOI] [PubMed] [Google Scholar]
- 33.Hoepken HH, Korten T, Robinson SR, Dringen R. Iron accumulation, iron-mediated toxicity and altered levels of ferritin and transferrin receptor in cultured astrocytes during incubation with ferric ammonium citrate. J Neurochem. 2004;88:1194–202. doi: 10.1046/j.1471-4159.2003.02236.x. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Cui L, Gonsiorek W, Min SH, Anilkumar G, Rosenblum S, et al. CCR2 and CXCR4 regulate peripheral blood monocyte pharmacodynamics and link to efficacy in experimental autoimmune encephalomyelitis. J Inflamm (Lond) 2009;6:32. doi: 10.1186/1476-9255-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–21. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 36.Weber MS, Hohlfeld R, Zamvil SS. Mechanism of action of glatiramer acetate in treatment of multiple sclerosis. Neurotherapeutics. 2007;4:647–53. doi: 10.1016/j.nurt.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prinz M, Mildner A. Microglia in the CNS: immigrants from another world. Glia. 2011;59:177–87. doi: 10.1002/glia.21104. [DOI] [PubMed] [Google Scholar]