Abstract
Since its first description in wound granulation tissue, the myofibroblast has been recognized to be a key actor in the epithelial-mesenchymal cross-talk that plays a crucial role in many physiological and pathological situations, such as regulation of prostate development, ventilation-perfusion in lung alveoli or organ fibrosis. The presence of myofibroblasts in the stroma reaction to epithelial tumors is well established and many data are accumulating which suggest that the stroma compartment is an active participant in tumor onset and/or evolution. In this review we summarize the evidence in favor of this concept, the main mechanisms that regulate myofibroblast differentiation and function, as well as the biophysical and biochemical factors possibly involved in epithelial-stroma interactions, using liver carcinoma as main model, in view of achieving a better understanding of tumor progression mechanisms and of tools directed toward stroma as eventual therapeutic target.
Keywords: fibrosis, wound healing, transforming growth factor-beta, extracellular matrix, mechanosensing
Introduction
Tissue-resident stromal cells, often loosely defined under the term “fibroblast,” form the extracellular matrix (ECM) supportive scaffold of connective tissues during development and maintain the stroma in the adult organism. In conditions of disturbed tissue homeostasis, such as occurring after injury during wound healing, chronic inflammation or upon transformation of adjacent epithelium in cancer, stromal cells become activated to proliferate and upregulate ECM production. A central feature of activated stroma cells is the acquisition of smooth muscle (SM) features, most notably neo-formation of contractile stress fibers and expression of α-SM actin (α-SMA); hence the name of myofibroblast. The transient acquisition of this phenotype is beneficial for normal tissue repair processes when myofibroblast remodeling activities restore and preserve tissue integrity. However, persistence of myofibroblasts result in tissue stiffening and deformation.1 In fibrosis, stiff scar tissue alters normal organ function; during the stroma reaction to epithelial tumors, the mechanical and chemical conditions generated by myofibroblasts promote tumor progression. The resemblance between tumor formation and normal wound healing has often been stressed.2,3 We here develop that, in fact, the stroma environment altered by tumors resembles fibrotic tissue in many aspects. Most evidently, similar to fibrotic contractures, “healing” does not stop in the tumor environment. After elaborating on similar features between these two pathological conditions, we use liver cancers as an example to highlight that fibrosis is not only associated with tumor development but can be a neoplastic risk factor.
The Stroma of Normal Organs
The stromal ECM
Traditionally, the stroma of different organs is described as a structural scaffold dominated by ECM with sparsely embedded cells. The stroma ECM includes different types of collagens, elastin, fibronectin, hyaluronic acid, proteoglycans and glycoproteins.4 In addition to playing important roles during development5 and in maintaining tissue architecture, ECM components can modify the activity of growth factors and cytokines. The ECM can act as a reservoir for growth factors that are deposited and can be rapidly released upon cellular request.6,7 ECM molecules protect growth factors from degradation and provide biological latency.8 For example, latency and activation of transforming growth factor-β1 (TGFβ1) is orchestrated by binding to the fibrillin protein family member latent TGFβ1 binding protein-1 (LTBP-1), decorin or thrombospondin-1.9-11 Vascular endothelial growth factor (VEGF) also binds to heparin or proteoglycans, and release of VEGF by plasmin or heparanase triggers endothelial cell proliferation.12 The importance of maintaining ECM homeostasis becomes evident during aging when ECM synthesis and degradation get out of balance. With age, structural and functional changes in components of the dermal-epidermal junction result in a less effective epidermal anchoring system.13 The synthesis of type I and III collagen is decreased in fibroblasts of the elderly;14 this is accompanied by decreased levels of TGFβ1 and the downstream mediator connective tissue growth factor (CTGF/CCN2). It has been suggested that reduced activity of the TGFβ1/Smad/CCN2 axis mediates reduced type I procollagen expression in aged human skin.15 In contrast, fibroblasts of aged connective tissue produce higher levels of collagen-degrading enzymes than those in young tissue. Since fibroblasts cannot properly adhere to fragmented collagen, they seem to lose the ability to mechano-sense the properties of their scaffold ECM.16
Origins and roles of cells in the stroma of normal organs: all fibroblasts?
Organs rely on the migration, proliferation and secretory activity of stromal cells that generate the ECM composition essential for normal tissue homeostasis and physiology.17 To maintain these functions in the adult organism, connective tissue cells must be renewed. Traditionally, the term fibroblast is often used synonymously for stromal cells; however, it becomes increasingly clear that the stromal cell population is tremendously heterogeneous and of multiple origins. Local and circulating stem cells are among a variety of progenitor cells that have been identified to regenerate the stroma.18,19 Local mesenchymal stem cells (MSCs), also called mesenchymal stromal cells,20 are present in most human adult tissues.21,22 The dermis of the skin for example is hosting resident MSCs;23 adipose precursor cells regulate skin stem cell activity.24 Multipotent adult stem cells with the ability to generate both neural and mesodermal progeny, coined skin-derived precursors, have also been identified and isolated from the skin.25,26 Skin-derived precursors are neural crest-related precursors that migrate into the skin during embryogenesis, persist in a dermal niche, and play key roles in physiology and potentially pathology of the skin.27 In addition, perivascular cells, generally referred to as pericytes, which are associated with blood vessels, also provide a reservoir of multipotent progenitor cells.28 Pericytes obtained from various human organs, including skeletal muscle, pancreas, adipose tissue, umbilical cord and placenta, exhibit osteogenic, chondrogenic and adipogenic potentials, i.e., features of MSCs.29,30
Another possible but debated source for the local stroma cell population is epithelial cells that undergo epithelial-to-mesenchymal transition (EMT). EMT is defined as the process through which epithelial cells, including endothelial cells, lose their traits and gain features associated with mesenchymal cells.31 Epithelial cells, which form the lining of glandular organs, normally exist as sheets of polarized cells with tight intercellular and cell-basement membrane junctions. During EMT, epithelial cells dissociate these junctions, start to migrate independently and invade the ECM.32 EMT facilitates a number of physiological processes, such as normal wound healing, as well as of pathological situations, such as fibrosis and cancer metastasis.33-36 However, the role of EMT in contributing to normal stroma turnover is unclear.
In addition to local stem cells, bone marrow (BM)-derived and blood-circulating progenitors can participate in the physiological renewal of the stroma.37 BM-derived stromal cell progenitors include fibrocytes and MSCs. Fibrocytes are characterized by the co-expression of the fibroblast markers collagen type I and II, fibronectin (FN), together with monocyte markers CD13 and CD11b, and hematopoietic progenitor markers CD34, CD45 and CD105.38,39 They exhibit ECM remodeling properties including the ability to participate in collagen turnover. Due to apparent differences in the collagen and proteoglycan expression profiles between fibrocytes and fibroblasts, it has been proposed that fibrocytes predominantly fulfill an ECM-stabilizing function.40 Circulating BM-derived MSCs are generally identified by the combined expression of specific cell surface proteins such as CD105, CD90, CD44, CD73, CD166, CD29, CD106 and Stro-1.41-43 In contrast to fibrocytes, MSCs are negative for the monocyte surface proteins CD13 and CD11b and the hematopoietic markers CD34 and CD45.44,45 Adult MSCs isolated from BM and expanded in culture are able to differentiate into a number of mesenchymal phenotypes, including those that form bone, cartilage, muscle, fat and other connective tissues.46 It has been shown that adipocytes can originate from BM-derived circulating progenitor cells, indicating mesenchymal transition as a contributor to homeostatic stroma maintenance.47 Whereas the contribution of MSCs in pathological situations is beginning to be understood, their contribution and specific roles in maintaining homeostasis of normal stroma remain large elusive.
The Cancer Stroma: Similarities with Physiological and Pathological Tissue Repair
Loss of epithelial homeostasis is a common trigger for the stroma reaction against tumors and for normal wound healing
To describe and understand the cellular and molecular processes involved in cancer development, the analogy with healing wounds is often stressed.2,3 In wounded skin and epithelialized organs, disruption of the epithelium is often accompanied by dysfunction of the underlying connective tissue.48 This effect of lost homeostasis is analogous to the influence that neoplastic epithelium has on the surrounding stromal cells. The disturbed interaction between neoplastic cells and the surrounding connective tissue confers to the stroma component its peculiar features.49 Schauer and Rowley, among others, have recently supported the concept that injury changes the priority of cell behavior: “…it is becoming very clear that the stromal compartment response to acute wound repair in adult tissue is governed by a biology of priority, beginning when the epithelial layer is breached. Host response mechanisms within repairing tissue follow a repair-centric biology of priority, as compared with a developmental biology of priority, as factors, mechanisms and the cell/tissue biology is different in many ways.”50
An important element in cancer progression is the cross-talk between the transformed epithelium and stromal cells. Examples from embryonic development highlight the influence that the stroma or mesenchyme has on epithelial behavior. During development of the prostate, the urogenital sinus mesenchyme fulfills a number of regulatory functions: it specifies prostatic epithelial identity, induces epithelial bud formation, elicits prostatic bud growth and regulates ductal branching, promotes differentiation of a secretory epithelium, and specifies the types of secretory proteins expressed.51,52 Reciprocally, prostatic epithelium induces SM differentiation in the mesenchyme. The central role of mesenchyme in prostatic epithelium development has been elegantly demonstrated by analyzing tissue recombinants composed of androgen-receptor-positive wild-type mesenchyme and androgen-receptor-negative epithelium.53 These studies revealed that ductal morphogenesis, differentiation, apoptosis and proliferation of epithelial cells are all regulated by stromal and not epithelial androgen receptors.53,54
The mammary gland is another excellent model to understand the function of stromal-epithelial interactions. It has been shown that TGFβ1, a major inducer of ECM deposition, acts during mammary gland development but also in a variety of developing tissues to mediate epithelial-mesenchymal interactions.55 This notion is supported by the immunohistochemical localization of TGFβ1 in numerous mouse embryonic tissues at sites where epithelial-mesenchymal interactions is occurring.56 Moreover, the normal human mammary gland undergoes a well-defined sequence of histological changes in both epithelial and stromal compartments during the menstrual cycle. In this process, the ECM surrounding individual cells plays a central role in modulating cell proliferation, differentiation and gene expression. Hence, stroma-derived ECM molecules may act as mediators in the hormonal control of the mammary gland in addition to their structural role.57 It has also been shown that mammary epithelial-mesenchymal interactions influence the alternative splicing of FN, which plays a key role in cell adhesive and migratory behavior related to fundamental processes such as embryogenesis and maintenance of tissue integrity, but also wound healing and malignancy.58,59 Interestingly, in the mammary gland, strategically positioned cells of the myoepithelial lineage are implicated in the phenomenon of menstrual cycle-related growth spurts in the adult resting breast. These myoepithelial cells mediate the complex signaling pathways involved in local epithelial-mesenchymal interactions, and which are crucial for both orderly growth during development and maintenance of homeostasis in the adult.60 In the adult organism, epithelial-mesenchymal interactions play a role in tissue homeostasis by reciprocally maintaining epithelial and stromal differentiation and growth-quiescence.61
Parallels between the evolutions of cancer and wound healing
Acute injuries heal in a highly regulated sequence of overlapping processes that require the coordinated completion of a variety of cellular activities.62,63 Wound healing phases include (1) vascular constriction and fibrin clot formation; (2) invasion of inflammatory cells into the wound and release of pro-inflammatory cytokines and growth factors;64 (3) a proliferative phase which is characterized by granulation tissue formation and re-epithelialization; and (4) the ECM remodeling phase during which the remaining inflammatory, vascular and fibroblastic cells die by apoptosis.
In normal wound healing, platelets are a major source of pro-inflammatory growth factors and establish the provisional fibrin ECM. A recent study suggested that platelets prime metastasizing tumor cells toward a mesenchymal phenotype already in the circulation by providing them with active TGFβ1 and by physical interaction.65 In the local tumor stroma, platelets and other blood-derived hematopoietic cells are rich sources for a variety of growth factors. In addition, these cells66 together with tumor cells release plasma-membrane-derived microparticles which contribute to the activation of cancer-associated myofibroblasts.67 Although vessels are generally not damaged in cancer, they become hyperpermeable and permit leakage of plasma proteins, fibrinogen and FN into the stroma.68 The cross-linked fibrin ECM is rich in plasma- and inflammatory cell-derived growth factors; this is strikingly similar to the ECM of developing cancer.3,69 The predominant factors are cytokines of the fibroblast growth factor (FGF) family, platelet-derived growth factor (PDGF), tumor necrosis factor (TNF)-α, epidermal growth factor (EGF), hepatocyte growth factor (HGF), TGFβ1 and VEGF.70,71 In acute wounds, the formation of reactive stroma starts approximately four days after injury, when macrophages, endothelial and fibroblastic cells invade the wound space. It is clear that chronic inflammation and different inflammatory cells contribute to cancer development.72,73 Macrophages augment inflammatory responses and additionally secrete VEGF and FGF, which promote angiogenesis.74 The formation of numerous new capillaries confers to the stroma a granular appearance. Neovascularization is essential for the synthesis, deposition, and organization of a new ECM. Moreover, FGF, TGFβ1 and PDGF cause fibroblast infiltration and activation into contractile myofibroblasts, which dominate the proliferation and remodeling phases of wound healing.
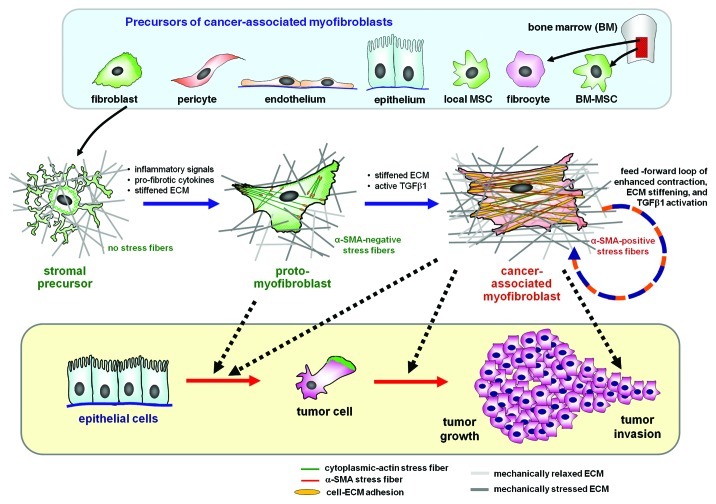
After the inflammatory phases, the wound repair process is characterized by significant migration into the injured zone, proliferation and activation of quiescent precursor cells and their activation into myofibroblasts, which then leads to wound contraction and ECM remodeling (Fig. 1).74 Initially, fibroblastic cells utilize the fibrin cross-linked fibers formed by the end of the inflammatory phase to migrate across the wound, subsequently adhering to FN. Fibroblastic cells then progressively transform in myofibroblasts which deposit collagen (mainly collagen type III) into the wound bed, leading to the formation of a sophisticated ECM network to which they can adhere, permitting their migration, and allowing the final formation of the granulation tissue. Both their precursor cells and activated myofibroblasts are responsible for the production of ECM components, including collagens and elastin, which give the ECM strength and resilience. They also synthesize highly hydrated molecules, such as proteoglycans and glycosaminoglycans. This ECM regulates cellular functions via cell adhesion and migration, also providing a storage and diffusion system for signaling molecules, ions, hormones, nutrients and waste products.63
Figure 1. The myofibroblast in the tumor stroma. Myofibroblasts associated with the tumor stroma can be activated from a variety of different progenitor cells, including locally residing fibroblasts, epithelial and endothelial cells via epithelial-to-mesenchymal transition, pericytes and bone marrow-derived circulating fibrocytes and mesenchymal stem cells (MSCs). In the intact tissue, the precursor cells are stress-shielded by a functional extracellular matrix (ECM); they do not develop contractile features and cell-matrix adhesions. Upon injury and loss of tissue homeostasis inflammatory signals activate stromal cells to spread and remodel the initially soft ECM. The gradual increase in ECM stiffness permits the formation of contractile microfilament bundles of cytoplasmic actins (stress fibers) that characterize the proto-myofibroblast. Transforming growth factor-β1 (TGFβ1) in conjunction with the stiff ECM stimulates proto-myofibroblasts to express and incorporate α-smooth muscle actin (α-SMA) into stress fibers. The force generated by α-SMA-containing stress fibers leads to further ECM contraction, thereby establishing a mechanical feedback loop. The chemical and mechanical environment created by proto-myofibroblasts and differentiated myofibroblasts support epithelial cell transformation, invasion and tumor growth.
When normal healing wounds are closed and re-epithelialized, cellularity within the granulation tissue decreases due in part to myofibroblast and endothelial cell apoptosis.75 The additive effect of reduced growth factor expression, increased ECM turnover, and nitric oxide generation may result in the myofibroblast and vascular cell apoptosis seen during the rapid remodeling of this tissue.76 Beside normal scar formation, hypertrophic scarring occurs in some circumstances, such as in young burn patients. Hypertrophic scarring leads to severe functional and aesthetic defects77 caused by the numerous α-SMA expressing and contracting myofibroblasts.78 Interestingly, in fetal wounds, healing can occur without scarring or contracture.79 This ability is lost in late gestation. In vivo, early fetal wounds show markedly fewer α-SMA-positive myofibroblasts than in late fetal wounds or in adult wounds where they are abundant.80
For wounds to heal successfully, all phases must occur in the proper sequence. Any factors interfering with one or several phases of this highly regulated process can cause impaired (chronic wounds) or overly (fibrosis and hypertrophic scarring) wound healing.81 Tumor growth induces an abnormal and prolonged stromal reaction leading to excessive scarring not unlike hypertrophic cutaneous scars observed after severe acute injury such as severe burns.49 One of the major players in creating hypertrophic scars or connective tissue deformations in general is the myofibroblast, the predominant phenotype of cancer-associated fibroblasts.
The Role of Myofibroblasts in the Cancer Stroma
Definition of the myofibroblast
At the time of their discovery in normally healing wound granulation tissue, myofibroblasts have been described as fibroblasts that are specialized for ECM secretion and contraction.82 The term myofibroblast was chosen to acknowledge the co-existence of fibroblast morphological features, such as a developed endoplasmic reticulum and SM features such as contractile actin microfilament bundles.82 This functional and morphological definition is still valid as of today as their most basic but also most essential characterization.83 Myofibroblast activation comprises two phases, each of which is characterized by specific cytoskeletal features. (1) De novo acquisition of contractile bundles that generate sufficient forces to promote cell migration and to initially remodel the ECM.84 For these lower contractile cells, we have introduced the term “proto-myofibroblast.”1 (2) In the presence of mechanical stress and TGFβ1, proto-myofibroblasts can further differentiate into myofibroblasts that neo-express α-SMA in stress fibers.1 It is the incorporation of α-SMA into stress fibers that renders myofibroblasts highly contractile (Fig. 1).85 By strict definition, cells that express α-SMA but do not incorporate it into contractile microfilament bundles cannot be functional myofibroblasts. In standard cell culture, during which fibroblastic cells almost inevitably form stress fibers, the term “myofibroblast” generally describes cells expressing α-SMA in contractile microfilament bundles.
Neo-expression of α-SMA is the most widely used criterion to identify tissue myofibroblasts and to diagnose myofibroblast-related diseases. Using α-SMA expression as marker, myofibroblasts have been shown to be the predominant sub-population of cancer-associated fibroblasts in the tumor stroma.86,87 In the complex environments of healing wounds and tumors, α-SMA cannot be used as a unique myofibroblast identifier. Vascular SM cells populating the tumor stroma and wound granulation tissue are also α-SMA positive. However, SM cells express late muscle differentiation markers that are rarely expressed by myofibroblasts, including SM myosin heavy chain, h-caldesmon and smoothelin.88 The muscle intermediate filament protein desmin is another reliable exclusion criterion because it is expressed in myofibroblasts only in exceptional situations.88 It is equally difficult to distinguish between myofibroblasts and pericytes, which can express vimentin and desmin, are α-SMA positive and SM myosin negative but which generally lack contractile features in vivo, i.e., stress fibers.89,90 The fact that the myofibroblast shares features and markers with many other cell types raises the interesting question whether it should be regarded as a cell type or a phenotype. Indeed, myofibroblasts derive from a variety of different precursor cells (Fig. 1) further indicating that “myofibroblast” designates a collection of cells that may be as heterogeneous as the “fibroblast.” We will use the general term “cancer-associated myofibroblast” to summarize this heterogeneous population.
Origins of myofibroblasts in cancer and fibrosis
Many elegant cell tracing studies have been performed to identify the source(s) for α-SMA positive myofibroblasts in different conditions of injury, fibrosis and tumor development.91 To date, it is probably safe to state that our body opportunistically activates myofibroblasts from whatever sources are available in a given condition. In response to injury and disturbed tissue homeostasis, local fibroblasts have been shown undergo myofibroblast activation in skin,92 liver,93-95 lung,96-98 heart,99-101 kidney102 and in the stroma reaction to epithelial tumors.103-106 EMT is another mechanism of myofibroblast generation from local epithelial and endothelial precursors during tumor development,107-109 as well as in kidney fibrosis110 and lung fibrosis.111,112 EMT has been demonstrated to contribute to fibrosis of the heart113 and liver.114,115 In fibrotic liver, hepatic stellate cells (HSCs) are another important source of myofibroblasts.93,95 In addition, de-differentiation of SM cells into ECM synthesizing cells contributes to the myofibroblast population.116 In the tumor stroma, systemic sclerosis, vessel repair and dermal scarring, pericytes have been suggested to acquire contractile myofibroblast features.89,117
Additional possible sources for cancer-associated myofibroblasts are BM-derived, blood-circulating fibrocytes and MSCs, which are recruited to inflamed and remodeled tissue. BM-derived cells have been associated in varying numbers with myofibroblast-containing lesions in the context of tumor development118,119 and in animals subjected to fibrotic stimuli in a variety of different organs.118,120-131 Other studies seem to exclude that BM-derived fibrocytes contribute to myofibroblast formation in liver124 and lung fibrosis132 and the definitive answer to the question of fibrocyte-to-myofibroblast differentiation remains open. Circulating and transplanted MSCs have been shown to target to the stroma environment of epithelial tumors.117,133-136 This tumor homing property has been suggested to be exploited for the tumor-specific delivery of anti-cancer drugs, cytokines and viruses137,138 and to suppress tumor development.139 However, the tumor environment seems to activate MSCs to myofibroblasts similar to other precursor cells.117,140,141 BM-MSCs cultured in tumor-conditioned medium differentiate into α-SMA-positive myofibroblasts.142 Recent studies evaluating the interaction between MSCs and epithelial tumor cells in vivo and in vitro indicate that acquisition of the myofibroblast phenotype by MSCs can reduce the success of an envisaged MSC therapy and may even amplify the disease. MSCs that have become activated by mildly invasive human breast carcinoma cells in culture enhance the metastatic potential of the cancer cells when injected subcutaneously; this effect is mediated in a paracrine feedback loop.143 In contrast, other studies did not confirm myofibroblast activation of BM-MSCs in the tumor environment117,144 and the contribution of BM-derived MSCs to the myofibroblast population remains a matter of debate.145,146
Mechanisms of myofibroblast activation: The role of TGFβ1
Independent from the identity of their precursors, myofibroblast activation is promoted by few essential factors. We will here focus on TGFβ1, the most potent myofibrogenic growth factor, and on mechanical stress. Both factors have substantial influence on tumor development and progression and recent studies have demonstrated their close interdependence.147 TGFβ1 has long been recognized as a key molecule implied both in wound repair and tumor formation.148 Although many biological effects of TGFβ1 are per se beneficial for the organism, the same actions are used and diverted by tumor cells to spread the disease.149 Beneficial functions of TGFβ1 in normal tissues include maintaining homeostasis of adult tissues by controlling proliferation of epithelial cells, endothelial cells, immune cells and fibroblasts.150-152 The fact that cancer cells become insensitive to the growth-arresting action of TGFβ1, together with its pro-angiogenic effects and its potential to inducing EMT of cancer cells, allocates to TGFβ1 a central role in tumor development.153-155 TGFβ1 causes the disruption of cell-cell junctions between vascular endothelial cells and thereby facilitates extravasation of metastasizing tumor cells.156 Blocking the TGFβ1 responsiveness of tumor cells has metastasis-suppressing effects.157,158 TGFβ1 further exerts pro-fibrotic and pro-tumorigenic activities by mediating the inflammatory response, causing excessive ECM production, decreasing synthesis of matrix metalloproteinases (MMPs), and increasing the synthesis of tissue inhibitors of MMPs (TIMPs).159 TGFβ1 further promotes myofibroblast activation and survival92,160-165 and enhances the retention of hyaluronan in the ECM that positively feeds back on myofibroblast activation.166,167 It appears that the temporal and spatial availability of TGFβ1 to tumor and stromal cells determines whether it acts as a suppressor or promoter of cancer progression and metastasis.65,152,155
The time and local availability of TGFβ1 partly depend on the cellular source. In the circulation, platelets have recently been shown to supply metastasizing tumor cells with bioactive TGFβ1.65 Other prominent sources of TGFβ1 in the tumor environment are the transformed epithelium itself, inflammatory, vascular and fibroblastic stroma cells.155,168 Myofibroblasts strongly contribute to the production and activation of TGFβ1 in the activated stroma and thereby generate the autocrine feed-forward loop that is characteristic for persisting myofibroblast activities,169 similar to those observed in fibrosis. Given the pleiotrophic effects of TGFβ1, different strategies are currently pursued to inhibit TGFβ1 action in a cell-specific manner in addition to general blocking approaches.170,171 The recent findings of a contraction- and integrin-mediated TGFβ1 activation mechanism acting in myofibroblasts may provide novel tools to achieve this goal.172 TGFβ1 is synthesized together with its pro-peptide, the latency-associated peptide (LAP) which is intracellularly cleaved but remains associated with TGFβ1 to produce the small latent complex (SLC).173 Myofibroblasts and other cells secrete SLC in a large latent complex with the covalently bound LTBP-1 (Fig. 2).173,174 LTBP-1 is a member of the fibrillin protein family and stores latent TGFβ1 in the ECM where it is accessible for cell-mediated activation.8,10,11 Activation of latent TGFβ1 from ECM deposits involves dissociation from LAP and is promoted by a variety of different mechanisms that depend on the cell type and physiological context.9,173,175,176 Transmembrane integrins have been identified as major players in the activation of latent TGFβ1, which contains RGD motifs for integrin binding in the LAP portion.177-179 Binding comprises all αv integrins (αvβ1, αvβ3, αvβ5, αvβ6 and αvβ8), integrins α5β1, α8β1 and αIIbβ3.176,180,181 Whereas αvβ8 integrin seems to guide proteases to the latent complex for proteolytic TGFβ1 activation,175,180,182 integrins αvβ5, αvβ6 and αvβ3 were shown to activate latent TGFβ1 independently from proteolytic action. Notably, all these integrins are upregulated in different fibrotic conditions and in the tumor environment.176,181
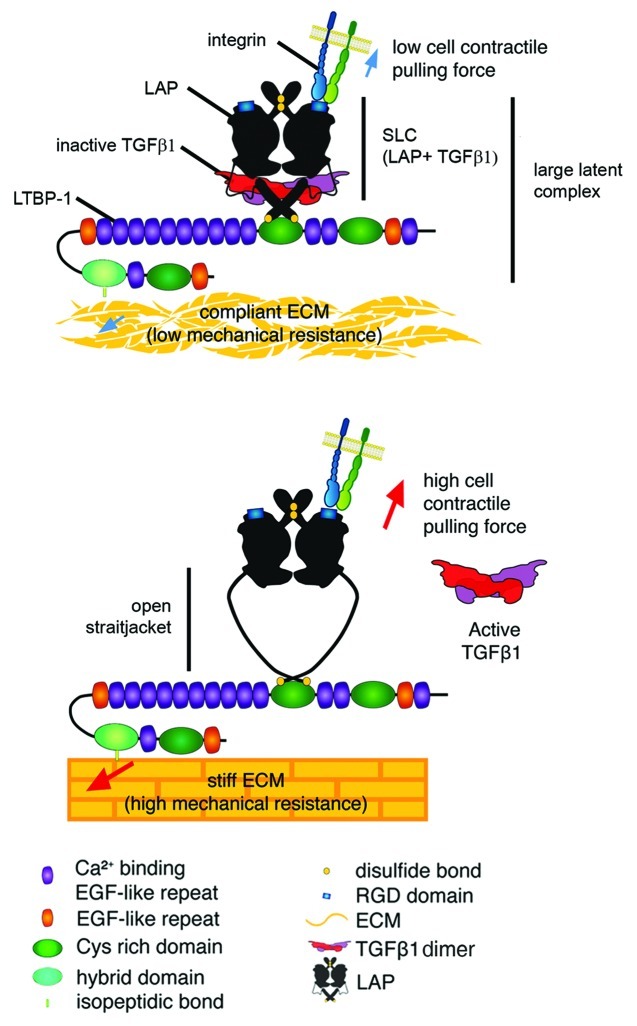
Figure 2. Integrin-mediated TGFβ1 activation by cell contraction. Structural and force spectroscopy data suggest that the transforming growth factor-β1 (TGFβ1) and latent TGFβ1 binding protein-1 (LTBP-1) binding domains of the latency-associated peptide (LAP) act as a sensor in a mechanical model of integrin-mediated TGFβ1 activation. Mechanical stretch, applied through cell integrins will open the latent TGFβ1 complex to release TGFβ1. Flexible domains in the LAP that are prone to unfolding lie within a domain that has been coined “straitjacket.”179 In the context of a poorly organized and compliant matrix and when cells develop only low contractile activity, the lack of sufficient mechanical tension will prevent the integrin-mediated conformational changes that are required to activate TGFβ1 from the latent complex. Conversely, on a stiff matrix the transmission of contractile forces via integrins to the LAP will favor unfolding of the straitjacket region, resulting in TGFβ1 release. Reproduced with permission.184
Different recent studies have demonstrated force-dependent activation of latent TGFβ1.179,183-188 Together they support the concept that cells, in particular contractile myofibroblasts, can exert tensile forces on ECM-stored latent TGFβ1 via specific integrins to activate the latent cytokine. Consistently, inhibition of myofibroblast integrin binding to LAP using RGD- and LAP-competitive peptides blocks activation of latent TGFβ1 in vitro.183 A similar effect has been achieved by inhibiting contraction of myofibroblast183 and epithelial cells.188 It appears that traction force exerted to the large latent complex induces a conformational change in LAP that liberates active TGFβ1. We have recently measured the mechanical stress involved in TGFβ1 activation at the molecular level and demonstrated TGFβ1 release upon force transmission to LAP.184 The recently published three-dimensional structure of the SLC179 and force spectroscopy of large latent complex unfolding184 support the following mechanism of TGFβ1 activation from the large latent complex (Fig. 2): (1) Mechanical force applied to LAP by single integrins can unfold a flexible portion of LAP that traps TGFβ1 in a “straitjacket” conformation. (2) Complete opening of the straitjacket is only possible when LAP is bound to LTBP-1, which must be anchored with the ECM. The ECM needs to be sufficiently stiff to mechanically resist the cell pulling forces. (3) TGFβ1 is released in an all-or-nothing fashion.
Of all protease-independent TGFβ1-activating integrins, the epithelial integrin αvβ6 has been most extensively studied, predominantly in the context of lung fibrosis.189 Deletion or inhibition of β6 integrin in vivo efficiently protects from the development of experimentally induced lung fibrosis with no apparent side effects.190,191 The role of this integrin in tumor development is far less clear but it has been suggested that overexpression of αvβ6 stimulates tumor progression by supporting EMT.192,193 Whether this effect is related to its function in TGFβ1 activation remains elusive. The contribution of integrins that are expressed by myofibroblasts and mesenchymal cells to the activation of latent TGFβ1 in the tumor environment has not been addressed. However, inhibition of the “pro-angiogenic” integrins αvβ3 and αvβ5 has already entered clinical trials as one strategy to interfere with tumor angiogenesis.194 It will be interesting to investigate if targeting these integrins will also intercept the autocrine loop of myofibroblast differentiation and TGFβ1 activation in the tumor.
Mechanisms of myofibroblast activation: The role of tissue stiffness
The second main permissive factor for myofibroblast activation and persistence is mechanical stress that arises from active remodeling activities in the stroma but also from the resulting mechanical properties of the ECM.1,83 We will here focus on the role of ECM stiffness in myofibroblast activation. It has to be noted that cells actively probe ECM stiffness by exerting pulling forces; higher resistance of their environment to pulling results in higher intracellular stress.1,83 Stiff ECM promotes myofibroblast phenotypic conversion at several levels,83 notably by improving the efficiency of latent TGFβ1 activation. Whereas compliant substrates appear to absorb the large deformations generated by myofibroblast contraction and thus protect the latent complex against conformational changes, the complex is stretchable on stiff substrates.183 It is a well established fact that the cancer-associated stroma is stiffer than the surrounding normal soft connective tissue.195-199 This mechanical condition is clinically exploited to detect tumors by palpation and/or elastography.200-202 The migratory activity of activated stromal cells and the high contractile activity of myofibroblasts are instrumental in gradually increasing ECM stiffness in more advanced stages of the stroma reaction.83 Initial stiffening however seems to be promoted by other processes. Small changes in tissue stiffness occur during the inflammatory response in fibrosis and tumor development and seem to be induced by cross-linking of collagen by lysyl oxidases (LOX) and LOX-like enzymes; inhibition of these enzymes has tumor-suppressing effects.203-206 Another cause for “pre-stiffening” in the tumor environment can be high interstitial fluid pressure and increased lymphatic flow.207,208 Using three-dimensional collagen co-cultured of fibroblasts and tumor cells, it has been shown recently that even very low interstitial fluid flow can lead to ECM and fibroblast alignment and subsequent myofibroblast differentiation.209 This process is associated with upregulated levels of TGFβ1.210
Tissue stiffness is measured as Young’s modulus in units of pascal and high stress is required to deform materials with high Young’s modulus. The stiffness of normal soft organs usually ranges between 2–15 kPa when measured at the cellular level using atomic force microscopy; skin and mammary gland tissue are situated at the lower stiffness range with 0.2–5 kPa.211,212 During the remodeling processes occurring in normal repair, fibrosis and tumor development, ECM stiffness gradually increases and can reach Young’s moduli of 50–100 kPa in mature scar tissue. Notably, fibrotic scar tissue is always stiffer than the texture of the normal organ.213-216
A great variety of different in vitro systems have been developed to study stiffness-dependent cell behavior in two and three dimensions.83,217-222 Since this field of mechanobiology literally exploded over the last decade, we will here only briefly summarize studies addressing myofibroblast activation by exposure to stiff substrates. By growing fibroblasts on very soft two-dimensional polyacrylamide gels and in three-dimensional soft collagen gels, development of stress fibers and acquisition of the proto-myofibroblast phenotype is suppressed; α-SMA stress fibers are beginning to be developed in cells grown isolated on culture substrates exhibiting an elastic modulus of at least 3 kPa.221,223 Stiffer culture substrates with a Young’s modulus of > 20 kPa are required to permit further myofibroblast differentiation and expression of α-SMA.213-215 In three-dimensional setups, myofibroblasts express α-SMA in attached stressed collagen gel cultures but not in free-floating relaxed gels.224-226 In mechanically challenged rat skin wounds, expression of α-SMA is accelerated; releasing wounds from mechanical tension has the opposite effect.84 Similar results have been obtained by stretching human burn scar tissue in situ.227 The level of stress exerted by the stiff ECM and/or dynamic changes in stress are also important signals for myofibroblast survival and thus their persistence. Increased apoptotic figures have been reported for fibroblasts in stress-released collagen gels228 and experimentally relaxed wounds.229 Conversely, mechanically stressing dermal wounds in mice causes hypertrophic scarring, presumably by decreasing the rate of apoptotic myofibroblasts.230
Consequences of myofibroblast activation in the tumor stroma
Activation of stromal cells into cancer-associated myofibroblasts has many consequences for tumor development, progression and cancer metastasis. Although their myofibroblast phenotype has not always been considered in the respective studies, activated cancer-associated fibroblasts have been reported to produce a variety of growth factors and cytokines that promote tumor progression. Secreted stroma-derived factors include TGFβ1, HGF, EGF, VEGF, stroma-derived factor-1, basic FGF and the pro-inflammatory cytokines CXCL14, IL-1, IL-6 and IL-8.105,231,232 Moreover, cancer-associated myofibroblasts contribute to ECM remodeling and foster cancer cell invasion by producing MMPs and TIMPs, such as MMP-1, MMP-2, MMP-3, MMP-9, MMP-13 and MMP-14.233 Further, cancer-associated myofibroblasts are the main producers of the tumor-surrounding ECM by secreting the matricellular protein CCN2, collagens, tenascin C, FN and elastins.107,155
In addition to altering the chemical composition of the stroma, the mechanical responsiveness of myofibroblasts and their role in ECM remodeling and stiffening has important implications for tumor progression.199 Enhanced tissue stiffness not only feeds back positively on the activation of cancer-associated myofibroblast but seems to increase the risk and invasiveness of tumors. For breast cancers, a clear correlation exists between the degree of mammographic densities (compact and stiff tissue) and the risk of cancer formation.234,235 Cell culture studies demonstrated that substrate stiffness directly governs the behavior and differentiation of murine mammary epithelial cells. On culture substrates that mimic the softness of normal mammary gland tissue (0.2 kPa), these cells form polarized acini surrounded by an intact basement membrane. This structure resembling the in vivo structure of the normal breast ducts is increasingly disturbed with increasing substrate stiffness. On substrates with a stiffness of 5 kPa, reproducing the mechanical conditions of the tumor stroma, lumen formation is inhibited and no surrounding basement membrane is established. As a consequence of the higher substrate stiffness, epithelial cells lose cell-cell adherens junctions and cell polarity to attain a mesenchymal phenotype with distinct stress fibers and increased migratory activity.195 Physiologically soft ECM was shown to suppress the mitogenic activity of epithelial (and mesenchymal) cells; stiffer substrates induce mitogenesis.217,236,237 Hence, the stiff ECM generated by the cancer-associated fibroblast/myofibroblast remodeling activities not only impacts the stromal compartment but drives epithelial cells into an invasive and proliferative phenotype, at least in culture. Physical cues by the ECM are not necessarily created by the mechanical properties but can be of topographic nature. Lines of tension and stroma cell-generated ECM tracks serve as guidance cues for collective cancer invasion.238-242 The pattern and alignment of collagen fibers in the tumor stroma may even find clinical application to assess the predict of human breast cancer patient long-term survival.243
The Tumor Stroma in Liver Cancers
In the following section we present two examples of liver cancer that are frequently associated with fibrotic conditions. In these cases stromal changes and myofibroblast activation clearly enhance the risk of cancerogenesis.
Hepatocellular carcinoma
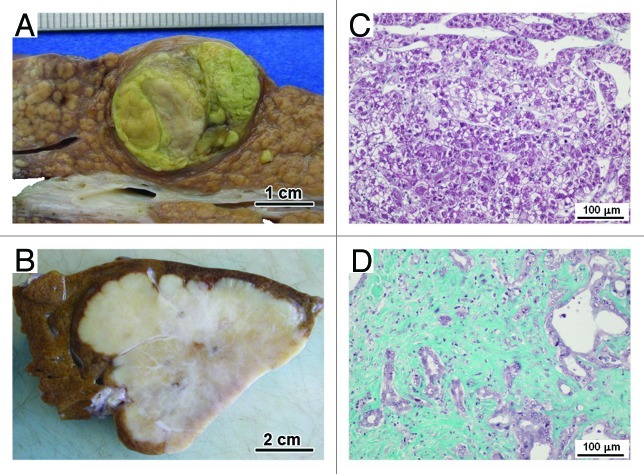
Hepatocellular carcinomas have numerous etiologies, notably chronic B and C virus infection or chronic alcohol abuse. Often, hepatocellular carcinomas arise in livers showing cirrhosis (Fig. 3A). Irrespective of the cause, it is in itself a precancerous condition. Depending on the degree of differentiation, the tumor is composed of large anastomosing plates and acini of tumor hepatocytes surrounded by capillaries. Except in rare forms of scirrhous or fibrolamellar hepatocellular carcinoma, tumor stroma is scanty (Fig. 3C). Sometimes the tumor is encapsulated. Often, the tumor stroma is mixed with the fibrous stroma of the surrounding cirrhosis. Microscopically, at low magnification, tumor stroma seems to be organized like the connective tissue surrounding the normal liver parenchyma. At high magnification or ultrastructurally, there are important modifications. The vessels surrounding the tumoral plates are not sinusoids, but continuous capillaries with a continuous basement membrane. They contain fewer Kupffer cells than normal liver.244 The space between endothelial cells and the tumoral hepatocytes does not contain HSCs, but cells expressing myofibroblastic features including expression of α-SMA. The ECM is remodeled with more deposition of collagen, notably collagen type I,245 fibrillin-1 and de novo expression of elastin.246 Myofibroblasts are involved in the remodeling of the ECM; they both synthesize ECM245-247 and degrade ECM with proteinases, like urokinase or MMPs.248-250 Interactions between malignant hepatocytes and stromal cells are complex. Tumor cells can recruit cancer-associated myofibroblasts locally or by migration from the transdifferentiated stromal cells through multiple mechanisms. Some growth factors or cytokines are involved in activation and recruitment of stromal cells, like TGFβ1 or PDGF.245 As shown by Leyland and coworkers using non-tumor hepatocytes, degradation of the ECM by the tumor cell proteinases can indirectly activate stromal cells.251 Conversely, cancer-associated myofibroblasts can enhance tumor invasiveness, notably by expression of HGF252,253 or by secretion of MMPs.254
Figure 3. Hepatocellular carcinoma and cholangiocarcinoma. Gross appearances of a typical case of hepatocellular carcinoma undergoing on cirrhosis (A) and of a typical case of intrahepatic cholangiocarcinoma showing important hyaline changes (B). The matrix of the tumor stroma is scanty in the hepatocellular carcinoma (C) while in the center of the intrahepatic cholangiocarcinoma, the tumor stroma is fibrous and the tumor cells are inconspicuous (D) (Masson’s trichrome histochemistry).
Cholangiocarcinoma
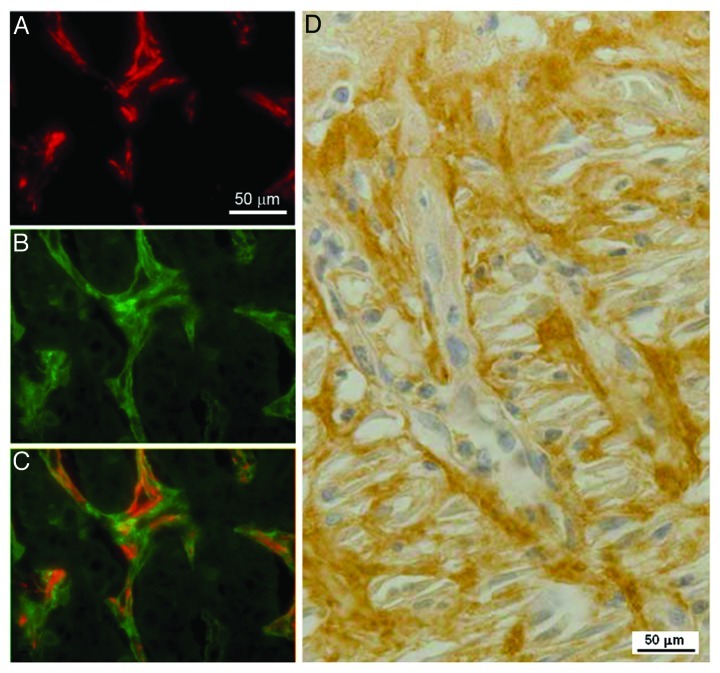
Bile-duct carcinoma (cholangiocarcinoma) is less common than hepatocellular carcinoma. The tumor is composed of tubular adenocarcinoma set in an abundant fibrous stroma. Three main forms are distinguished: intrahepatic or peripheral bile-duct carcinoma (cholangiocarcinoma), hilar adenocarcinoma (Klatskin tumor) and carcinoma of the extra-hepatic bile ducts. Unlike scanty stroma reaction inside hepatocellular carcinoma tumors, the stroma in cholangiocarcinoma is abundant, sclerous, sometimes with calcification and may be extensive, and submerges the scanty tumoral tubules in cholangiocarcinoma (Fig. 3B and D). Inside cholangiocarcinoma tumors, stroma and tumor cells are mixed and there are no clear boundaries between the periphery of the lesion (invasion front) and the inner tumor. The number of cancer-associated myofibroblasts is elevated in the stroma and is correlated with the degree of tumor fibrosis.255 Around the tumor, α-SMA expressing cells seem in direct continuity with cancer-associated myofibroblasts.255 These cells are involved in deposition and remodeling of ECM.256 Stromal cells express MMP-1, MMP-2, MMP-3, MMP-9 or tissue inhibitors of MMPs, TIMP-1 and TIMP-2.257 This expression of MMPs and TIMPs is stronger in cholangiocarcinomas with severe invasion.257 Our lab has recently used a proteomic approach to identify differentially expressed proteins in peripheral cholangiocarcinoma cases and compared expression with paired non-tumor liver tissue from the same patients.258 Overall, of the approximately 2,400 protein spots visualized in each gel, 172 protein spots showed significant differences in expression level between tumor and non-tumor tissue with p < 0.01. Of these, 100 spots corresponding to 138 different proteins were identified by mass spectrometry: 70 proteins were overexpressed whereas 68 proteins were under-expressed in tumor samples compared with non-tumor samples. Among the overexpressed proteins, immunohistochemistry studies confirmed an increased expression of 14-3-3 protein in tumor cells while α-SMA, as expected, and periostin were shown to be overexpressed in the cancer-associated myofibroblasts surrounding tumor cells (Fig. 4). 14-3-3 proteins have been shown to have roles in suppressing apoptosis or regulating cell survival259 and may represent a novel indicator of cancer transformation in hepatocytes or biliary epithelium. Periostin is a secreted protein that has integrin binding sites and has been shown to be expressed by tumor cells and tumor stroma in melanoma and colon cancer.260 Recent examination of fibroblasts isolated from cholangiocarcinoma has shown that these cells are capable of producing periostin and that high levels of periostin production correlate with poor prognosis.261 Periostin has been shown to induce cell migration and also EMT in pancreatic cancer cells,262 thus it may represent a novel mediator of tumor growth in cholangiocarcinoma. The detection of periostin or other overexpressed proteins such as 14-3-3 proteins in serum may therefore prove to be useful markers for detection of malignancy.263
Figure 4. Expression of α-SMA and of periostin in the cholangiocarcinoma tissue and in rat granulation tissue. In the cholangiocarcinoma tissue, there is a strong staining for α-smooth muscle actin (α-SMA) in the stromal cells within the tumor (A). A similar distribution is seen with periostin staining (B). Merge image for the two proteins (C) shows that α-SMA and periostin are colocalized in stromal cells, though periostin (green) has a slightly wider distribution than α-SMA (red) as it is both intracellular in myofibroblasts and deposited in the matrix. (D) In the rat granulation tissue, a strong expression for periostin is observed in myofibroblasts, underlining the similarity between the stroma reaction and the granulation tissue.
Comparison between hepatocellular carcinoma and cholangiocarcinoma
The question arises why the stroma reaction is scanty in hepatocellular carcinomas and abundant in cholangiocarcinomas. It is conceivable that the cells contributing to the myofibroblastic population and which participate in stroma reaction are different. Myofibroblasts are absent from normal liver. Precursor cells of the myofibroblast in the liver, HSCs and portal fibroblasts, are differently distributed in the hepatic lobule.264,265 HSCs resemble pericytes and are located along the sinusoids, in the Disse space between the endothelium and the hepatocytes.266,267 In contrast, portal fibroblasts are embedded in the portal tract connective tissue around portal structures such as vessels and biliary structures. Differences have been reported between these two fibrogenic cell populations, concerning the mechanisms leading to myofibroblastic differentiation, activation and “deactivation.”266-268 It is now widely accepted that the different types of lesions leading to liver fibrosis, e.g., lesions caused by alcohol abuse and viral hepatitis, involve specific fibrogenic cell subpopulations.95 Concerning the stroma reaction to hepatic tumors, we suggest that HSC-derived myofibroblasts and portal fibroblast-derived myofibroblasts are involved in the stroma reactions encountered in hepatocellular carcinomas and cholangiocarcinomas, respectively. Both types of cancer-associated myofibroblasts promote tumor progression.
Interestingly, scirrhous hepatocellular carcinoma, a rare variant of hepatocellular carcinoma, is characterized by abundant fibrous stroma and has been known to express several liver stem/progenitor cell markers.269 It has been recently shown that scirrhous hepatocellular carcinomas harbor traits intermediate between hepatocellular carcinoma and cholangiocarcinoma including stem cell traits, which are similar to those of cholangiocarcinoma-like hepatocellular carcinoma.270 It will be interesting to characterize the stromal cells involved in this particular hepatocellular carcinoma; we suggest that tumor cells derive from precursor cells able to acquire hepatocyte or cholangiocyte phenotype and located in Hering canals (see below) at the border between parenchyma and portal area.
The contribution of liver MSCs to the cancer-associated myofibroblast population is as yet unclear. Association of MSCs with the well characterized epithelial stem cells located in Hering canals, may constitute a niche and this concept is currently explored.271 Several studies have demonstrated that, following injury, a significant proportion of myofibroblasts may originate from BM,272 but the contribution of these cells to collagen production is not known.273 Lineage tracing studies fail to demonstrate the generation of myofibroblasts from hepatic epithelial cells (hepatocytes and cholangiocytes) by EMT.274 However, the controversy about the ability of hepatic epithelial cells to become myofibroblasts remains.275 Thus, the possibility that MSCs, BM-derived cells and EMT represent alternative sources of cancer-associated myofibroblasts cannot be excluded at this time. Irrespectively, stroma-myofibroblast interactions represent an interesting tumor differentiation-independent target for therapy of cancers, particularly for hepatocellular carcinoma and cholangiocarcinoma.
Because of the lack of a good animal model, the pathogenesis of the stroma reaction in human hepatocellular carcinoma remains unclear. Different experimental models have been proposed to study hepatocellular carcinoma development. However, in most models, stroma reaction is practically absent. Using a model associating diethylnitrosamine exposure and N-nitrosomorpholine treatment, Taras and coworkers have observed that hepatocellular carcinoma cells were surrounded by a stroma reaction resembling that observed in humans.276 In cholangiocarcinoma, models using thioacetamide have been developed, mimicking correctly the multi-stage progression of human cholangiocarcinoma.277,278 Finally, it is important to underline that the tumor stroma, beside representing a therapeutic target, is also a source of many potential new tumor biomarkers, and little is known regarding how changes in stromal gene expression affect cancer progression. Recently, the expression of stroma-related genes was analyzed in 122 hepatocellular carcinoma samples, and the results were related to patient prognosis;279 the signature reveals the strong prognostic capacity of immune responses, angiogenic activity and ECM remodeling, highlighting the importance of stromal biology in hepatocellular carcinoma progression.
Perspectives
Numerous studies have shown that the stroma regulates epithelial cell transformation, survival, cancer growth and metastasis.232 Conversely, factors derived from the tumor activate local and circulating precursor cells in the stroma to generate this tumor-permissive microenvironment. It becomes increasingly clear that both tumor cells and stromal cells comprise tremendously heterogeneous cell populations. However, many activated stroma cells share myofibroblastic features irrespective of their origin. Functional cancer-associated myofibroblasts, i.e., ECM secreting and contracting stromal cells exhibiting α-SMA-positive stress fibers, play a central role in the detrimental cross-talk between tumor and stroma. Hence, anti-cancer strategies are conceivable with the aim to specifically target this phenotype in the tumor stroma. Such strategies can possibly target the contractile apparatus of the cancer-associated myofibroblast to reduce ECM stiffening,280 which contributes to the persistence and progression of the tumor. Other approaches can include the inhibition of specific myofibroblast integrins that are important in cell mechanosensing and transmission of intracellular force to the ECM.281
Myofibroblast integrins are also possibly involved in activating the pro-tumorigenic factor TGFβ1. Due to its central role in acting on tumor and stroma cells, targeting TGFβ1 and TGFβ1 pathways is already clinically tested in therapeutic approaches.170 Anti-TGFβ1 strategies include the use of small-molecule inhibitors of the TGFβ1 receptor type I kinase domain, TGFβ1-specific neutralizing antibodies and oligonucleotide antisense compounds.170,282 Inhibiting the amplification loop operated by TGFβ1 on tumor cells leads to a decrease of tumor neovascularization and in the end to limit the tumor metastasis impairing with the dissemination of cells by the blood tract and acting directly on the cells. During this process it has been shown that Smad7 and I-Smad that inhibits TGFβ1 and bone morphogenetic protein signaling, efficiently inhibits lung and liver metastasis of mouse breast cancer JygMC(A) cells.156 Smad7 also inhibited the migration and invasion of cells, indicating that Smad7 leads to prevention of the EMT process.156 All these strategies principally aim in inhibiting later effects of the already active TGFβ1 during tumor formation and preventing metastasis of advanced cancers. However, intercepting already during the activation process of TGFβ1 mediated by a specific set of integrins emerges as alternative, possibly more targeted approach.171,180,189 Another advantage of targeting integrins is that it will interfere with tumor development at multiple levels. Notably, anti-integrin strategies are already well developed to inhibit angiogenesis with the aim to cut off the cancer supply with oxygen and nutrients.194
Acknowledgments
M.O. holds a doctoral fellowship from Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro. The research leading to this work has been further supported by the Canadian Institutes of Health Research grants #210820 and #219974 (B.H.), the Collaborative Health Research Programme NSERC/CIHR grant #1004005, the Heart and Stroke Foundation Ontario grant #NA7086 (B.H.), the Swiss National Science Foundation grant #3200-067254 (B.H.), and grants from the University of Limoges and from the French Ministry of Research (A.D.). We are very grateful to P. Bioulac-Sage and S. Lepreux (Service d’Anatomie Pathologique, Hôpital Pellegrin, CHU Bordeaux, France) for the illustrations of pathological cases and to I.A. Darby (Cancer and Tissue Repair Laboratory, School of Medical Sciences, RMIT University, Bundoora, VIC Australia) for the images concerning periostin immunostaining.
Glossary
Abbreviations:
- α-SMA
α-smooth muscle actin
- BM
bone marrow
- CGTF/CCN2
connective tissue growth factor
- ECM
extracellular matrix
- EGF
epidermal growth factor
- EMT
epithelial-to-mesenchymal transition
- FGF
fibroblast growth factor
- FN
fibronectin
- HSC
hepatic stellate cell
- HGF
hepatocyte growth factor
- LAP
latency-associated protein
- LOX
lysyl oxidase
- LTBP-1
latent TGFβ1 binding protein 1
- MMP
matrix metalloproteinase
- MSC
mesenchymal stem cell
- PDGF
platelet-derived growth factor
- SLC
small latent complex
- SM
smooth muscle
- TGFβ
transforming growth factor-beta
- TIMP
tissue inhibitor of MMP
- TNF
tumor necrosis factor
- VEGF
vascular endothelial growth factor
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/20377
References
- 1.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–63. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 2.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–9. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 3.Schäfer M, Werner S. Cancer as an overhealing wound: an old hypothesis revisited. Nat Rev Mol Cell Biol. 2008;9:628–38. doi: 10.1038/nrm2455. [DOI] [PubMed] [Google Scholar]
- 4.Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123:4195–200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fata JE, Werb Z, Bissell MJ. Regulation of mammary gland branching morphogenesis by the extracellular matrix and its remodeling enzymes. Breast Cancer Res. 2004;6:1–11. doi: 10.1186/bcr634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009;17:153–62. doi: 10.1111/j.1524-475X.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- 7.Mott JD, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol. 2004;16:558–64. doi: 10.1016/j.ceb.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–9. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sweetwyne MT, Murphy-Ullrich JE. Thrombospondin1 in tissue repair and fibrosis: TGF-β-dependent and independent mechanisms. Matrix Biol. 2012;31:178–86. doi: 10.1016/j.matbio.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Todorovic V, Rifkin DB. LTBPs, more than just an escort service. J Cell Biochem. 2012;113:410–8. doi: 10.1002/jcb.23385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramirez F, Rifkin DB. Extracellular microfibrils: contextual platforms for TGFbeta and BMP signaling. Curr Opin Cell Biol. 2009;21:616–22. doi: 10.1016/j.ceb.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson KL, Marasco SF, Street AM. Practical management of anticoagulation, bleeding and blood product support for cardiac surgery. Part one: bleeding and anticoagulation issues. Heart Lung Circ. 2001;10:142–53. doi: 10.1046/j.1444-2892.2001.00096.x. [DOI] [PubMed] [Google Scholar]
- 13.Le Varlet B, Chaudagne C, Saunois A, Barré P, Sauvage C, Berthouloux B, et al. Age-related functional and structural changes in human dermo-epidermal junction components. J Investig Dermatol Symp Proc. 1998;3:172–9. doi: 10.1038/jidsymp.1998.34. [DOI] [PubMed] [Google Scholar]
- 14.Dumas M, Chaudagne C, Bonté F, Meybeck A. In vitro biosynthesis of type I and III collagens by human dermal fibroblasts from donors of increasing age. Mech Ageing Dev. 1994;73:179–87. doi: 10.1016/0047-6374(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 15.Quan T, Qin Z, Xia W, Shao Y, Voorhees JJ, Fisher GJ. Matrix-degrading metalloproteinases in photoaging. J Investig Dermatol Symp Proc. 2009;14:20–4. doi: 10.1038/jidsymp.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisher GJ, Varani J, Voorhees JJ. Looking older: fibroblast collapse and therapeutic implications. Arch Dermatol. 2008;144:666–72. doi: 10.1001/archderm.144.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cox TR, Erler JT. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Model Mech. 2011;4:165–78. doi: 10.1242/dmm.004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson KA, Majka SM, Wulf GG, Goodell MA. Stem cells: a minireview. J Cell Biochem Suppl. 2002;38:1–6. doi: 10.1002/jcb.10045. [DOI] [PubMed] [Google Scholar]
- 19.Kolonin MG, Evans KW, Mani SA, Gomer RH. Alternative origins of stroma in normal organs and disease. Stem Cell Res. 2012;8:312–23. doi: 10.1016/j.scr.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salem HK, Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells. 2010;28:585–96. doi: 10.1002/stem.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–13. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 22.Hunt GC, Schwarzbauer JE. Tightening the connections between cadherins and fibronectin matrix. Dev Cell. 2009;16:327–8. doi: 10.1016/j.devcel.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Jahoda CA, Whitehouse J, Reynolds AJ, Hole N. Hair follicle dermal cells differentiate into adipogenic and osteogenic lineages. Exp Dermatol. 2003;12:849–59. doi: 10.1111/j.0906-6705.2003.00161.x. [DOI] [PubMed] [Google Scholar]
- 24.Festa E, Fretz J, Berry R, Schmidt B, Rodeheffer M, Horowitz M, et al. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell. 2011;146:761–71. doi: 10.1016/j.cell.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunt DP, Jahoda C, Chandran S. Multipotent skin-derived precursors: from biology to clinical translation. Curr Opin Biotechnol. 2009;20:522–30. doi: 10.1016/j.copbio.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Toma JG, Akhavan M, Fernandes KJ, Barnabé-Heider F, Sadikot A, Kaplan DR, et al. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3:778–84. doi: 10.1038/ncb0901-778. [DOI] [PubMed] [Google Scholar]
- 27.Fernandes KJ, Toma JG, Miller FD. Multipotent skin-derived precursors: adult neural crest-related precursors with therapeutic potential. Philos Trans R Soc Lond B Biol Sci. 2008;363:185–98. doi: 10.1098/rstb.2006.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamanishi H, Fujiwara S, Soma T. Perivascular localization of dermal stem cells in human scalp. Exp Dermatol. 2012;21:78–80. doi: 10.1111/j.1600-0625.2011.01407.x. [DOI] [PubMed] [Google Scholar]
- 29.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–13. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Paquet-Fifield S, Schlüter H, Li A, Aitken T, Gangatirkar P, Blashki D, et al. A role for pericytes as microenvironmental regulators of human skin tissue regeneration. J Clin Invest. 2009;119:2795–806. doi: 10.1172/JCI38535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–8. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shook D, Keller R. Mechanisms, mechanics and function of epithelial-mesenchymal transitions in early development. Mech Dev. 2003;120:1351–83. doi: 10.1016/j.mod.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Yang L, Moses HL. Transforming growth factor beta: tumor suppressor or promoter? Are host immune cells the answer? Cancer Res. 2008;68:9107–11. doi: 10.1158/0008-5472.CAN-08-2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 2007;67:10123–8. doi: 10.1158/0008-5472.CAN-07-3127. [DOI] [PubMed] [Google Scholar]
- 35.Potenta S, Zeisberg E, Kalluri R. The role of endothelial-to-mesenchymal transition in cancer progression. Br J Cancer. 2008;99:1375–9. doi: 10.1038/sj.bjc.6604662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–84. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roufosse CA, Direkze NC, Otto WR, Wright NA. Circulating mesenchymal stem cells. Int J Biochem Cell Biol. 2004;36:585–97. doi: 10.1016/j.biocel.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 38.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 39.Mattoli S, Bellini A, Schmidt M. The role of a human hematopoietic mesenchymal progenitor in wound healing and fibrotic diseases and implications for therapy. Curr Stem Cell Res Ther. 2009;4:266–80. doi: 10.2174/157488809789649232. [DOI] [PubMed] [Google Scholar]
- 40.Bianchetti L, Barczyk M, Cardoso J, Schmidt M, Bellini A, Mattoli S. Extracellular matrix remodelling properties of human fibrocytes. J Cell Mol Med. 2012;16:483–95. doi: 10.1111/j.1582-4934.2011.01344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991;78:55–62. [PubMed] [Google Scholar]
- 42.Minguell JJ, Erices A, Conget P. Mesenchymal stem cells. Exp Biol Med (Maywood) 2001;226:507–20. doi: 10.1177/153537020122600603. [DOI] [PubMed] [Google Scholar]
- 43.Pittenger MF. Mesenchymal stem cells from adult bone marrow. Methods Mol Biol. 2008;449:27–44. doi: 10.1007/978-1-60327-169-1_2. [DOI] [PubMed] [Google Scholar]
- 44.He Q, Wan C, Li G. Concise review: multipotent mesenchymal stromal cells in blood. Stem Cells. 2007;25:69–77. doi: 10.1634/stemcells.2006-0335. [DOI] [PubMed] [Google Scholar]
- 45.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 46.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 47.Crossno JT, Jr., Majka SM, Grazia T, Gill RG, Klemm DJ. Rosiglitazone promotes development of a novel adipocyte population from bone marrow-derived circulating progenitor cells. J Clin Invest. 2006;116:3220–8. doi: 10.1172/JCI28510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Werner S, Krieg T, Smola H. Keratinocyte-fibroblast interactions in wound healing. J Invest Dermatol. 2007;127:998–1008. doi: 10.1038/sj.jid.5700786. [DOI] [PubMed] [Google Scholar]
- 49.Sarrazy V, Billet F, Micallef L, Coulomb B, Desmoulière A. Mechanisms of pathological scarring: role of myofibroblasts and current developments. Wound Repair Regen. 2011;19(Suppl 1):s10–5. doi: 10.1111/j.1524-475X.2011.00708.x. [DOI] [PubMed] [Google Scholar]
- 50.Schauer IG, Rowley DR. The functional role of reactive stroma in benign prostatic hyperplasia. Differentiation. 2011;82:200–10. doi: 10.1016/j.diff.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hayward SW, Rosen MA, Cunha GR. Stromal-epithelial interactions in the normal and neoplastic prostate. Br J Urol. 1997;79(Suppl 2):18–26. doi: 10.1111/j.1464-410x.1997.tb16917.x. [DOI] [PubMed] [Google Scholar]
- 52.Risbridger GP, Taylor RA. Minireview: regulation of prostatic stem cells by stromal niche in health and disease. Endocrinology. 2008;149:4303–6. doi: 10.1210/en.2008-0465. [DOI] [PubMed] [Google Scholar]
- 53.Cunha GR, Cooke PS, Kurita T. Role of stromal-epithelial interactions in hormonal responses. Arch Histol Cytol. 2004;67:417–34. doi: 10.1679/aohc.67.417. [DOI] [PubMed] [Google Scholar]
- 54.Risbridger G, Wang H, Young P, Kurita T, Wang YZ, Lubahn D, et al. Evidence that epithelial and mesenchymal estrogen receptor-alpha mediates effects of estrogen on prostatic epithelium. Dev Biol. 2001;229:432–42. doi: 10.1006/dbio.2000.9994. [DOI] [PubMed] [Google Scholar]
- 55.Silberstein GB, Strickland P, Coleman S, Daniel CW. Epithelium-dependent extracellular matrix synthesis in transforming growth factor-beta 1-growth-inhibited mouse mammary gland. J Cell Biol. 1990;110:2209–19. doi: 10.1083/jcb.110.6.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heine U, Munoz EF, Flanders KC, Ellingsworth LR, Lam HY, Thompson NL, et al. Role of transforming growth factor-beta in the development of the mouse embryo. J Cell Biol. 1987;105:2861–76. doi: 10.1083/jcb.105.6.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferguson JE, Schor AM, Howell A, Ferguson MW. Changes in the extracellular matrix of the normal human breast during the menstrual cycle. Cell Tissue Res. 1992;268:167–77. doi: 10.1007/BF00338066. [DOI] [PubMed] [Google Scholar]
- 58.Blaustein M, Pelisch F, Coso OA, Bissell MJ, Kornblihtt AR, Srebrow A. Mammary epithelial-mesenchymal interaction regulates fibronectin alternative splicing via phosphatidylinositol 3-kinase. J Biol Chem. 2004;279:21029–37. doi: 10.1074/jbc.M314260200. [DOI] [PubMed] [Google Scholar]
- 59.Singh P, Carraher C, Schwarzbauer JE. Assembly of fibronectin extracellular matrix. Annu Rev Cell Dev Biol. 2010;26:397–419. doi: 10.1146/annurev-cellbio-100109-104020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jolicoeur F, Gaboury LA, Oligny LL. Basal cells of second trimester fetal breasts: immunohistochemical study of myoepithelial precursors. Pediatr Dev Pathol. 2003;6:398–413. doi: 10.1007/s10024-003-1125-y. [DOI] [PubMed] [Google Scholar]
- 61.Coulomb B, Lebreton C, Dubertret L. Influence of human dermal fibroblasts on epidermalization. J Invest Dermatol. 1989;92:122–5. doi: 10.1111/1523-1747.ep13071335. [DOI] [PubMed] [Google Scholar]
- 62.Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res. 2009;37:1528–42. doi: 10.1177/147323000903700531. [DOI] [PubMed] [Google Scholar]
- 63.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–21. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 64.Campos AC, Groth AK, Branco AB. Assessment and nutritional aspects of wound healing. Curr Opin Clin Nutr Metab Care. 2008;11:281–8. doi: 10.1097/MCO.0b013e3282fbd35a. [DOI] [PubMed] [Google Scholar]
- 65.Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20:576–90. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Siljander PR. Platelet-derived microparticles—an updated perspective. Thromb Res. 2011;127(Suppl 2):S30–3. doi: 10.1016/S0049-3848(10)70152-3. [DOI] [PubMed] [Google Scholar]
- 67.Webber J, Steadman R, Mason MD, Tabi Z, Clayton A. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res. 2010;70:9621–30. doi: 10.1158/0008-5472.CAN-10-1722. [DOI] [PubMed] [Google Scholar]
- 68.Dvorak HF, Weaver VM, Tlsty TD, Bergers G. Tumor microenvironment and progression. J Surg Oncol. 2011;103:468–74. doi: 10.1002/jso.21709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–70. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 71.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 72.Ruffell B, Au A, Rugo HS, Esserman LJ, Hwang ES, Coussens LM. Leukocyte composition of human breast cancer. Proc Natl Acad Sci U S A. 2012;109:2796–801. doi: 10.1073/pnas.1104303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tan TT, Coussens LM. Humoral immunity, inflammation and cancer. Curr Opin Immunol. 2007;19:209–16. doi: 10.1016/j.coi.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 74.Shaw TJ, Martin P. Wound repair at a glance. J Cell Sci. 2009;122:3209–13. doi: 10.1242/jcs.031187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Desmoulière A, Redard M, Darby I, Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol. 1995;146:56–66. [PMC free article] [PubMed] [Google Scholar]
- 76.Darby IA, Bisucci T, Pittet B, Garbin S, Gabbiani G, Desmoulière A. Skin flap-induced regression of granulation tissue correlates with reduced growth factor and increased metalloproteinase expression. J Pathol. 2002;197:117–27. doi: 10.1002/path.1074. [DOI] [PubMed] [Google Scholar]
- 77.Kwan P, Hori K, Ding J, Tredget EE. Scar and contracture: biological principles. Hand Clin. 2009;25:511–28. doi: 10.1016/j.hcl.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 78.Ehrlich HP, Desmoulière A, Diegelmann RF, Cohen IK, Compton CC, Garner WL, et al. Morphological and immunochemical differences between keloid and hypertrophic scar. Am J Pathol. 1994;145:105–13. [PMC free article] [PubMed] [Google Scholar]
- 79.Buchanan EP, Longaker MT, Lorenz HP. Fetal skin wound healing. Adv Clin Chem. 2009;48:137–61. doi: 10.1016/S0065-2423(09)48006-5. [DOI] [PubMed] [Google Scholar]
- 80.Estes JM, Vande Berg JS, Adzick NS, MacGillivray TE, Desmoulière A, Gabbiani G. Phenotypic and functional features of myofibroblasts in sheep fetal wounds. Differentiation. 1994;56:173–81. doi: 10.1046/j.1432-0436.1994.5630173.x. [DOI] [PubMed] [Google Scholar]
- 81.Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89:219–29. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gabbiani G, Ryan GB, Majne G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971;27:549–50. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- 83.Hinz B. The myofibroblast: paradigm for a mechanically active cell. J Biomech. 2010;43:146–55. doi: 10.1016/j.jbiomech.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 84.Hinz B, Mastrangelo D, Iselin CE, Chaponnier C, Gabbiani G. Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am J Pathol. 2001;159:1009–20. doi: 10.1016/S0002-9440(10)61776-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell. 2001;12:2730–41. doi: 10.1091/mbc.12.9.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Orimo A, Weinberg RA. Heterogeneity of stromal fibroblasts in tumors. Cancer Biol Ther. 2007;6:618–9. doi: 10.4161/cbt.6.4.4255. [DOI] [PubMed] [Google Scholar]
- 87.Sugimoto H, Mundel TM, Kieran MW, Kalluri R. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol Ther. 2006;5:1640–6. doi: 10.4161/cbt.5.12.3354. [DOI] [PubMed] [Google Scholar]
- 88.Arnoldi R, Chaponnier C, Gabbiani G, Hinz B. Heterogeneity of smooth muscle. In: Hill J, ed. Muscle: Fundamental Biology and Mechanisms of Disease: Elsevier Inc, 2012. [Google Scholar]
- 89.Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 90.Dore-Duffy P, Cleary K. Morphology and properties of pericytes. Methods Mol Biol. 2011;686:49–68. doi: 10.1007/978-1-60761-938-3_2. [DOI] [PubMed] [Google Scholar]
- 91.Hinz B, Phan SH, Thannickal VJ, Prunotto M, Desmoulière A, Varga J, et al. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol. 2012;180:1340–55. doi: 10.1016/j.ajpath.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526–37. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- 93.Friedman SL. Evolving challenges in hepatic fibrosis. Nat Rev Gastroenterol Hepatol. 2010;7:425–36. doi: 10.1038/nrgastro.2010.97. [DOI] [PubMed] [Google Scholar]
- 94.Dranoff JA, Wells RG. Portal fibroblasts: Underappreciated mediators of biliary fibrosis. Hepatology. 2010;51:1438–44. doi: 10.1002/hep.23405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Desmoulière A. Hepatic stellate cells: the only cells involved in liver fibrogenesis? A dogma challenged. Gastroenterology. 2007;132:2059–62. doi: 10.1053/j.gastro.2007.03.075. [DOI] [PubMed] [Google Scholar]
- 96.Hardie WD, Glasser SW, Hagood JS. Emerging concepts in the pathogenesis of lung fibrosis. Am J Pathol. 2009;175:3–16. doi: 10.2353/ajpath.2009.081170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Araya J, Nishimura SL. Fibrogenic reactions in lung disease. Annu Rev Pathol. 2010;5:77–98. doi: 10.1146/annurev.pathol.4.110807.092217. [DOI] [PubMed] [Google Scholar]
- 98.Hoyles RK, Derrett-Smith EC, Khan K, Shiwen X, Howat SL, Wells AU, et al. An essential role for resident fibroblasts in experimental lung fibrosis is defined by lineage-specific deletion of high-affinity type II transforming growth factor β receptor. Am J Respir Crit Care Med. 2011;183:249–61. doi: 10.1164/rccm.201002-0279OC. [DOI] [PubMed] [Google Scholar]
- 99.van den Borne SW, Diez J, Blankesteijn WM, Verjans J, Hofstra L, Narula J. Myocardial remodeling after infarction: the role of myofibroblasts. Nat Rev Cardiol. 2010;7:30–7. doi: 10.1038/nrcardio.2009.199. [DOI] [PubMed] [Google Scholar]
- 100.Leask A. Potential therapeutic targets for cardiac fibrosis: TGFbeta, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ Res. 2010;106:1675–80. doi: 10.1161/CIRCRESAHA.110.217737. [DOI] [PubMed] [Google Scholar]
- 101.Porter KE, Turner NA. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol Ther. 2009;123:255–78. doi: 10.1016/j.pharmthera.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 102.Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol. 2011;7:684–96. doi: 10.1038/nrneph.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.De Wever O, Demetter P, Mareel M, Bracke M. Stromal myofibroblasts are drivers of invasive cancer growth. Int J Cancer. 2008;123:2229–38. doi: 10.1002/ijc.23925. [DOI] [PubMed] [Google Scholar]
- 104.Anderberg C, Pietras K. On the origin of cancer-associated fibroblasts. Cell Cycle. 2009;8:1461–2. doi: 10.4161/cc.8.10.8557. [DOI] [PubMed] [Google Scholar]
- 105.Cirri P, Chiarugi P. Cancer-associated-fibroblasts and tumour cells: a diabolic liaison driving cancer progression. Cancer Metastasis Rev. 2012;31:195–208. doi: 10.1007/s10555-011-9340-x. [DOI] [PubMed] [Google Scholar]
- 106.Guyot C, Lepreux S, Darby IA, Desmoulière A. The biology of tumor stroma. The cancer handbook, 2nd edn 2007; 1:155–67. [Google Scholar]
- 107.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 108.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–54. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 109.Zeisberg EM, Potenta SE, Sugimoto H, Zeisberg M, Kalluri R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol. 2008;19:2282–7. doi: 10.1681/ASN.2008050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kizu A, Medici D, Kalluri R. Endothelial-mesenchymal transition as a novel mechanism for generating myofibroblasts during diabetic nephropathy. Am J Pathol. 2009;175:1371–3. doi: 10.2353/ajpath.2009.090698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chapman HA. Epithelial-mesenchymal interactions in pulmonary fibrosis. Annu Rev Physiol. 2011;73:413–35. doi: 10.1146/annurev-physiol-012110-142225. [DOI] [PubMed] [Google Scholar]
- 112.Piera-Velazquez S, Li Z, Jimenez SA. Role of endothelial-mesenchymal transition (EndoMT) in the pathogenesis of fibrotic disorders. Am J Pathol. 2011;179:1074–80. doi: 10.1016/j.ajpath.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–61. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 114.Zeisberg M, Yang C, Martino M, Duncan MB, Rieder F, Tanjore H, et al. Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J Biol Chem. 2007;282:23337–47. doi: 10.1074/jbc.M700194200. [DOI] [PubMed] [Google Scholar]
- 115.Pinzani M. Epithelial-mesenchymal transition in chronic liver disease: fibrogenesis or escape from death? J Hepatol. 2011;55:459–65. doi: 10.1016/j.jhep.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 116.Coen M, Gabbiani G, Bochaton-Piallat ML. Myofibroblast-mediated adventitial remodeling: an underestimated player in arterial pathology. Arterioscler Thromb Vasc Biol. 2011;31:2391–6. doi: 10.1161/ATVBAHA.111.231548. [DOI] [PubMed] [Google Scholar]
- 117.Kidd S, Spaeth E, Watson K, Burks J, Lu H, Klopp A, et al. Origins of the tumor microenvironment: quantitative assessment of adipose-derived and bone marrow-derived stroma. PLoS One. 2012;7:e30563. doi: 10.1371/journal.pone.0030563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ishii G, Sangai T, Sugiyama K, Ito T, Hasebe T, Endoh Y, et al. In vivo characterization of bone marrow-derived fibroblasts recruited into fibrotic lesions. Stem Cells. 2005;23:699–706. doi: 10.1634/stemcells.2004-0183. [DOI] [PubMed] [Google Scholar]
- 119.Direkze NC, Hodivala-Dilke K, Jeffery R, Hunt T, Poulsom R, Oukrif D, et al. Bone marrow contribution to tumor-associated myofibroblasts and fibroblasts. Cancer Res. 2004;64:8492–5. doi: 10.1158/0008-5472.CAN-04-1708. [DOI] [PubMed] [Google Scholar]
- 120.Forbes SJ, Russo FP, Rey V, Burra P, Rugge M, Wright NA, et al. A significant proportion of myofibroblasts are of bone marrow origin in human liver fibrosis. Gastroenterology. 2004;126:955–63. doi: 10.1053/j.gastro.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 121.Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol. 2003;171:380–9. doi: 10.4049/jimmunol.171.1.380. [DOI] [PubMed] [Google Scholar]
- 122.Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, et al. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114:438–46. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sakai N, Wada T, Yokoyama H, Lipp M, Ueha S, Matsushima K, et al. Secondary lymphoid tissue chemokine (SLC/CCL21)/CCR7 signaling regulates fibrocytes in renal fibrosis. Proc Natl Acad Sci U S A. 2006;103:14098–103. doi: 10.1073/pnas.0511200103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kisseleva T, Uchinami H, Feirt N, Quintana-Bustamante O, Segovia JC, Schwabe RF, et al. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J Hepatol. 2006;45:429–38. doi: 10.1016/j.jhep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 125.Haudek SB, Xia Y, Huebener P, Lee JM, Carlson S, Crawford JR, et al. Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proc Natl Acad Sci U S A. 2006;103:18284–9. doi: 10.1073/pnas.0608799103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Andersson-Sjöland A, de Alba CG, Nihlberg K, Becerril C, Ramírez R, Pardo A, et al. Fibrocytes are a potential source of lung fibroblasts in idiopathic pulmonary fibrosis. Int J Biochem Cell Biol. 2008;40:2129–40. doi: 10.1016/j.biocel.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 127.Wada T, Sakai N, Matsushima K, Kaneko S. Fibrocytes: a new insight into kidney fibrosis. Kidney Int. 2007;72:269–73. doi: 10.1038/sj.ki.5002325. [DOI] [PubMed] [Google Scholar]
- 128.Moeller A, Gilpin SE, Ask K, Cox G, Cook D, Gauldie J, et al. Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179:588–94. doi: 10.1164/rccm.200810-1534OC. [DOI] [PubMed] [Google Scholar]
- 129.Mori L, Bellini A, Stacey MA, Schmidt M, Mattoli S. Fibrocytes contribute to the myofibroblast population in wounded skin and originate from the bone marrow. Exp Cell Res. 2005;304:81–90. doi: 10.1016/j.yexcr.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 130.Direkze NC, Forbes SJ, Brittan M, Hunt T, Jeffery R, Preston SL, et al. Multiple organ engraftment by bone-marrow-derived myofibroblasts and fibroblasts in bone-marrow-transplanted mice. Stem Cells. 2003;21:514–20. doi: 10.1634/stemcells.21-5-514. [DOI] [PubMed] [Google Scholar]
- 131.Fathke C, Wilson L, Hutter J, Kapoor V, Smith A, Hocking A, et al. Contribution of bone marrow-derived cells to skin: collagen deposition and wound repair. Stem Cells. 2004;22:812–22. doi: 10.1634/stemcells.22-5-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest. 2004;113:243–52. doi: 10.1172/JCI18847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hall B, Dembinski J, Sasser AK, Studeny M, Andreeff M, Marini F. Mesenchymal stem cells in cancer: tumor-associated fibroblasts and cell-based delivery vehicles. Int J Hematol. 2007;86:8–16. doi: 10.1532/IJH97.06230. [DOI] [PubMed] [Google Scholar]
- 134.Studeny M, Marini FC, Dembinski JL, Zompetta C, Cabreira-Hansen M, Bekele BN, et al. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst. 2004;96:1593–603. doi: 10.1093/jnci/djh299. [DOI] [PubMed] [Google Scholar]
- 135.Hung SC, Deng WP, Yang WK, Liu RS, Lee CC, Su TC, et al. Mesenchymal stem cell targeting of microscopic tumors and tumor stroma development monitored by noninvasive in vivo positron emission tomography imaging. Clin Cancer Res. 2005;11:7749–56. doi: 10.1158/1078-0432.CCR-05-0876. [DOI] [PubMed] [Google Scholar]
- 136.Menon LG, Picinich S, Koneru R, Gao H, Lin SY, Koneru M, et al. Differential gene expression associated with migration of mesenchymal stem cells to conditioned medium from tumor cells or bone marrow cells. Stem Cells. 2007;25:520–8. doi: 10.1634/stemcells.2006-0257. [DOI] [PubMed] [Google Scholar]
- 137.Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ, Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62:3603–8. [PubMed] [Google Scholar]
- 138.Komarova S, Kawakami Y, Stoff-Khalili MA, Curiel DT, Pereboeva L. Mesenchymal progenitor cells as cellular vehicles for delivery of oncolytic adenoviruses. Mol Cancer Ther. 2006;5:755–66. doi: 10.1158/1535-7163.MCT-05-0334. [DOI] [PubMed] [Google Scholar]
- 139.Khakoo AY, Pati S, Anderson SA, Reid W, Elshal MF, Rovira II, et al. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi’s sarcoma. J Exp Med. 2006;203:1235–47. doi: 10.1084/jem.20051921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mishra PJ, Mishra PJ, Glod JW, Banerjee D. Mesenchymal stem cells: flip side of the coin. Cancer Res. 2009;69:1255–8. doi: 10.1158/0008-5472.CAN-08-3562. [DOI] [PubMed] [Google Scholar]
- 141.Opalenik SR, Davidson JM. Fibroblast differentiation of bone marrow-derived cells during wound repair. FASEB J. 2005;19:1561–3. doi: 10.1096/fj.04-2978fje. [DOI] [PubMed] [Google Scholar]
- 142.Mishra PJ, Mishra PJ, Humeniuk R, Medina DJ, Alexe G, Mesirov JP, et al. Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res. 2008;68:4331–9. doi: 10.1158/0008-5472.CAN-08-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–63. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 144.Barisic-Dujmovic T, Boban I, Clark SH. Fibroblasts/myofibroblasts that participate in cutaneous wound healing are not derived from circulating progenitor cells. J Cell Physiol. 2010;222:703–12. doi: 10.1002/jcp.21997. [DOI] [PubMed] [Google Scholar]
- 145.Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19:180–92. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 146.Caplan AI. Why are MSCs therapeutic? New data: new insight. J Pathol. 2009;217:318–24. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hinz B. Tissue stiffness, latent TGF-beta1 activation, and mechanical signal transduction: implications for the pathogenesis and treatment of fibrosis. Curr Rheumatol Rep. 2009;11:120–6. doi: 10.1007/s11926-009-0017-1. [DOI] [PubMed] [Google Scholar]
- 148.Oft M, Heider KH, Beug H. TGFbeta signaling is necessary for carcinoma cell invasiveness and metastasis. Curr Biol. 1998;8:1243–52. doi: 10.1016/S0960-9822(07)00533-7. [DOI] [PubMed] [Google Scholar]
- 149.Akhurst RJ, Derynck R. TGF-beta signaling in cancer--a double-edged sword. Trends Cell Biol. 2001;11:S44–51. doi: 10.1016/s0962-8924(01)02130-4. [DOI] [PubMed] [Google Scholar]
- 150.ten Dijke P, Arthur HM. Extracellular control of TGFbeta signalling in vascular development and disease. Nat Rev Mol Cell Biol. 2007;8:857–69. doi: 10.1038/nrm2262. [DOI] [PubMed] [Google Scholar]
- 151.Feng XH, Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev Biol. 2005;21:659–93. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- 152.Wakefield LM, Stuelten C. Keeping order in the neighborhood: new roles for TGFbeta in maintaining epithelial homeostasis. Cancer Cell. 2007;12:293–5. doi: 10.1016/j.ccr.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 153.Bierie B, Moses HL. Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 2006;6:506–20. doi: 10.1038/nrc1926. [DOI] [PubMed] [Google Scholar]
- 154.Pardali K, Moustakas A. Actions of TGF-beta as tumor suppressor and pro-metastatic factor in human cancer. Biochim Biophys Acta. 2007;1775:21–62. doi: 10.1016/j.bbcan.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 155.Ikushima H, Miyazono K. TGFbeta signalling: a complex web in cancer progression. Nat Rev Cancer. 2010;10:415–24. doi: 10.1038/nrc2853. [DOI] [PubMed] [Google Scholar]
- 156.Azuma H, Ehata S, Miyazaki H, Watabe T, Maruyama O, Imamura T, et al. Effect of Smad7 expression on metastasis of mouse mammary carcinoma JygMC(A) cells. J Natl Cancer Inst. 2005;97:1734–46. doi: 10.1093/jnci/dji399. [DOI] [PubMed] [Google Scholar]
- 157.Padua D, Zhang XH, Wang Q, Nadal C, Gerald WL, Gomis RR, et al. TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell. 2008;133:66–77. doi: 10.1016/j.cell.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Guasch G, Schober M, Pasolli HA, Conn EB, Polak L, Fuchs E. Loss of TGFbeta signaling destabilizes homeostasis and promotes squamous cell carcinomas in stratified epithelia. Cancer Cell. 2007;12:313–27. doi: 10.1016/j.ccr.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Meulmeester E, Ten Dijke P. The dynamic roles of TGF-β in cancer. J Pathol. 2011;223:205–18. doi: 10.1002/path.2785. [DOI] [PubMed] [Google Scholar]
- 160.Desmoulière A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–11. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhang HY, Phan SH. Inhibition of myofibroblast apoptosis by transforming growth factor beta(1) Am J Respir Cell Mol Biol. 1999;21:658–65. doi: 10.1165/ajrcmb.21.6.3720. [DOI] [PubMed] [Google Scholar]
- 162.Taipale J, Saharinen J, Keski-Oja J. Extracellular matrix-associated transforming growth factor-beta: role in cancer cell growth and invasion. Adv Cancer Res. 1998;75:87–134. doi: 10.1016/S0065-230X(08)60740-X. [DOI] [PubMed] [Google Scholar]
- 163.Ruiz-Ortega M, Rodríguez-Vita J, Sanchez-Lopez E, Carvajal G, Egido J. TGF-beta signaling in vascular fibrosis. Cardiovasc Res. 2007;74:196–206. doi: 10.1016/j.cardiores.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 164.Grainger DJ. TGF-beta and atherosclerosis in man. Cardiovasc Res. 2007;74:213–22. doi: 10.1016/j.cardiores.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 165.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18:816–27. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 166.Meran S, Thomas D, Stephens P, Martin J, Bowen T, Phillips A, et al. Involvement of hyaluronan in regulation of fibroblast phenotype. J Biol Chem. 2007;282:25687–97. doi: 10.1074/jbc.M700773200. [DOI] [PubMed] [Google Scholar]
- 167.Webber J, Meran S, Steadman R, Phillips A. Hyaluronan orchestrates transforming growth factor-beta1-dependent maintenance of myofibroblast phenotype. J Biol Chem. 2009;284:9083–92. doi: 10.1074/jbc.M806989200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Joshi A, Cao D. TGF-beta signaling, tumor microenvironment and tumor progression: the butterfly effect. Front Biosci. 2010;15:180–94. doi: 10.2741/3614. [DOI] [PubMed] [Google Scholar]
- 169.Kojima Y, Acar A, Eaton EN, Mellody KT, Scheel C, Ben-Porath I, et al. Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc Natl Acad Sci U S A. 2010;107:20009–14. doi: 10.1073/pnas.1013805107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hawinkels LJ, Ten Dijke P. Exploring anti-TGF-β therapies in cancer and fibrosis. Growth Factors. 2011;29:140–52. doi: 10.3109/08977194.2011.595411. [DOI] [PubMed] [Google Scholar]
- 171.Varga J, Pasche B. Transforming growth factor beta as a therapeutic target in systemic sclerosis. Nat Rev Rheumatol. 2009;5:200–6. doi: 10.1038/nrrheum.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hinz B, Gabbiani G. Fibrosis: recent advances in myofibroblast biology and new therapeutic perspectives. F1000 Biol Rep. 2010;2:78. doi: 10.3410/B2-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116:217–24. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 174.Hyytiäinen M, Penttinen C, Keski-Oja J. Latent TGF-beta binding proteins: extracellular matrix association and roles in TGF-beta activation. Crit Rev Clin Lab Sci. 2004;41:233–64. doi: 10.1080/10408360490460933. [DOI] [PubMed] [Google Scholar]
- 175.Jenkins G. The role of proteases in transforming growth factor-beta activation. Int J Biochem Cell Biol. 2008;40:1068–78. doi: 10.1016/j.biocel.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 176.Wipff PJ, Hinz B. Integrins and the activation of latent transforming growth factor beta1—an intimate relationship. Eur J Cell Biol. 2008;87:601–15. doi: 10.1016/j.ejcb.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 177.Derynck R, Jarrett JA, Chen EY, Goeddel DV. The murine transforming growth factor-beta precursor. J Biol Chem. 1986;261:4377–9. [PubMed] [Google Scholar]
- 178.Worthington JJ, Klementowicz JE, Travis MA. TGFβ: a sleeping giant awoken by integrins. Trends Biochem Sci. 2011;36:47–54. doi: 10.1016/j.tibs.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 179.Shi M, Zhu J, Wang R, Chen X, Mi L, Walz T, et al. Latent TGF-β structure and activation. Nature. 2011;474:343–9. doi: 10.1038/nature10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Nishimura SL. Integrin-mediated transforming growth factor-beta activation, a potential therapeutic target in fibrogenic disorders. Am J Pathol. 2009;175:1362–70. doi: 10.2353/ajpath.2009.090393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sheppard D. Integrin-mediated activation of latent transforming growth factor beta. Cancer Metastasis Rev. 2005;24:395–402. doi: 10.1007/s10555-005-5131-6. [DOI] [PubMed] [Google Scholar]
- 182.Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, et al. The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J Cell Biol. 2002;157:493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol. 2007;179:1311–23. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Buscemi L, Ramonet D, Klingberg F, Formey A, Smith-Clerc J, Meister JJ, et al. The single-molecule mechanics of the latent TGF-β1 complex. Curr Biol. 2011;21:2046–54. doi: 10.1016/j.cub.2011.11.037. [DOI] [PubMed] [Google Scholar]
- 185.Zhou Y, Hagood JS, Lu B, Merryman WD, Murphy-Ullrich JE. Thy-1-integrin alphav beta5 interactions inhibit lung fibroblast contraction-induced latent transforming growth factor-beta1 activation and myofibroblast differentiation. J Biol Chem. 2010;285:22382–93. doi: 10.1074/jbc.M110.126227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Maeda T, Sakabe T, Sunaga A, Sakai K, Rivera AL, Keene DR, et al. Conversion of mechanical force into TGF-β-mediated biochemical signals. Curr Biol. 2011;21:933–41. doi: 10.1016/j.cub.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ahamed J, Burg N, Yoshinaga K, Janczak CA, Rifkin DB, Coller BS. In vitro and in vivo evidence for shear-induced activation of latent transforming growth factor-beta1. Blood. 2008;112:3650–60. doi: 10.1182/blood-2008-04-151753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Giacomini MM, Travis MA, Kudo M, Sheppard D. Epithelial cells utilize cortical actin/myosin to activate latent TGF-β through integrin α(v)β(6)-dependent physical force. Exp Cell Res. 2012;318:716–22. doi: 10.1016/j.yexcr.2012.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Aluwihare P, Munger JS. What the lung has taught us about latent TGF-beta activation. Am J Respir Cell Mol Biol. 2008;39:499–502. doi: 10.1165/rcmb.2008-0003ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–28. doi: 10.1016/S0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 191.Horan GS, Wood S, Ona V, Li DJ, Lukashev ME, Weinreb PH, et al. Partial inhibition of integrin alpha(v)beta6 prevents pulmonary fibrosis without exacerbating inflammation. Am J Respir Crit Care Med. 2008;177:56–65. doi: 10.1164/rccm.200706-805OC. [DOI] [PubMed] [Google Scholar]
- 192.Bates RC, Bellovin DI, Brown C, Maynard E, Wu B, Kawakatsu H, et al. Transcriptional activation of integrin beta6 during the epithelial-mesenchymal transition defines a novel prognostic indicator of aggressive colon carcinoma. J Clin Invest. 2005;115:339–47. doi: 10.1172/JCI23183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ramos DM, Dang D, Sadler S. The role of the integrin alpha v beta6 in regulating the epithelial to mesenchymal transition in oral cancer. Anticancer Res. 2009;29:125–30. [PubMed] [Google Scholar]
- 194.Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med. 2011;17:1359–70. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- 195.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–54. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 196.Kumar S, Weaver VM. Mechanics, malignancy, and metastasis: the force journey of a tumor cell. Cancer Metastasis Rev. 2009;28:113–27. doi: 10.1007/s10555-008-9173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Beacham DA, Cukierman E. Stromagenesis: the changing face of fibroblastic microenvironments during tumor progression. Semin Cancer Biol. 2005;15:329–41. doi: 10.1016/j.semcancer.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 198.Andersen ES, Christensen PB, Weis N. Transient elastography for liver fibrosis diagnosis. Eur J Intern Med. 2009;20:339–42. doi: 10.1016/j.ejim.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 199.DuFort CC, Paszek MJ, Weaver VM. Balancing forces: architectural control of mechanotransduction. Nat Rev Mol Cell Biol. 2011;12:308–19. doi: 10.1038/nrm3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Khaled W, Reichling S, Bruhns OT, Ermert H. Ultrasonic strain imaging and reconstructive elastography for biological tissue. Ultrasonics. 2006;44(Suppl 1):e199–202. doi: 10.1016/j.ultras.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 201.Glaser KJ, Felmlee JP, Manduca A, Kannan Mariappan Y, Ehman RL. Stiffness-weighted magnetic resonance imaging. Magn Reson Med. 2006;55:59–67. doi: 10.1002/mrm.20748. [DOI] [PubMed] [Google Scholar]
- 202.Garra BS. Imaging and estimation of tissue elasticity by ultrasound. Ultrasound Q. 2007;23:255–68. doi: 10.1097/ruq.0b013e31815b7ed6. [DOI] [PubMed] [Google Scholar]
- 203.Santhanam AN, Baker AR, Hegamyer G, Kirschmann DA, Colburn NH. Pdcd4 repression of lysyl oxidase inhibits hypoxia-induced breast cancer cell invasion. Oncogene. 2010;29:3921–32. doi: 10.1038/onc.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Georges PC, Hui JJ, Gombos Z, McCormick ME, Wang AY, Uemura M, et al. Increased stiffness of the rat liver precedes matrix deposition: implications for fibrosis. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1147–54. doi: 10.1152/ajpgi.00032.2007. [DOI] [PubMed] [Google Scholar]
- 205.Barker HE, Chang J, Cox TR, Lang G, Bird D, Nicolau M, et al. LOXL2-mediated matrix remodeling in metastasis and mammary gland involution. Cancer Res. 2011;71:1561–72. doi: 10.1158/0008-5472.CAN-10-2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Heldin CH, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure—an obstacle in cancer therapy. Nat Rev Cancer. 2004;4:806–13. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- 208.Swartz MA, Fleury ME. Interstitial flow and its effects in soft tissues. Annu Rev Biomed Eng. 2007;9:229–56. doi: 10.1146/annurev.bioeng.9.060906.151850. [DOI] [PubMed] [Google Scholar]
- 209.Ng CP, Hinz B, Swartz MA. Interstitial fluid flow induces myofibroblast differentiation and collagen alignment in vitro. J Cell Sci. 2005;118:4731–9. doi: 10.1242/jcs.02605. [DOI] [PubMed] [Google Scholar]
- 210.Shieh AC, Rozansky HA, Hinz B, Swartz MA. Tumor cell invasion is promoted by interstitial flow-induced matrix priming by stromal fibroblasts. Cancer Res. 2011;71:790–800. doi: 10.1158/0008-5472.CAN-10-1513. [DOI] [PubMed] [Google Scholar]
- 211.Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer. 2009;9:108–22. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Yu H, Mouw JK, Weaver VM. Forcing form and function: biomechanical regulation of tumor evolution. Trends Cell Biol. 2011;21:47–56. doi: 10.1016/j.tcb.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Goffin JM, Pittet P, Csucs G, Lussi JW, Meister JJ, Hinz B. Focal adhesion size controls tension-dependent recruitment of alpha-smooth muscle actin to stress fibers. J Cell Biol. 2006;172:259–68. doi: 10.1083/jcb.200506179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Liu F, Mih JD, Shea BS, Kho AT, Sharif AS, Tager AM, et al. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol. 2010;190:693–706. doi: 10.1083/jcb.201004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Olsen AL, Bloomer SA, Chan EP, Gaça MD, Georges PC, Sackey B, et al. Hepatic stellate cells require a stiff environment for myofibroblastic differentiation. Am J Physiol Gastrointest Liver Physiol. 2011;301:G110–8. doi: 10.1152/ajpgi.00412.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Bissell MJ, Hines WC. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med. 2011;17:320–9. doi: 10.1038/nm.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Assoian RK, Klein EA. Growth control by intracellular tension and extracellular stiffness. Trends Cell Biol. 2008;18:347–52. doi: 10.1016/j.tcb.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Chan MW, Hinz B, McCulloch CA. Mechanical induction of gene expression in connective tissue cells. Methods Cell Biol. 2010;98:178–205. doi: 10.1016/S0091-679X(10)98008-4. [DOI] [PubMed] [Google Scholar]
- 219.Buxboim A, Ivanovska IL, Discher DE. Matrix elasticity, cytoskeletal forces and physics of the nucleus: how deeply do cells ‘feel’ outside and in? J Cell Sci. 2010;123:297–308. doi: 10.1242/jcs.041186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Grinnell F, Petroll WM. Cell motility and mechanics in three-dimensional collagen matrices. Annu Rev Cell Dev Biol. 2010;26:335–61. doi: 10.1146/annurev.cellbio.042308.113318. [DOI] [PubMed] [Google Scholar]
- 221.Janmey PA, Winer JP, Murray ME, Wen Q. The hard life of soft cells. Cell Motil Cytoskeleton. 2009;66:597–605. doi: 10.1002/cm.20382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Swartz MA, Lund AW. Lymphatic and interstitial flow in the tumour microenvironment: linking mechanobiology with immunity. Nat Rev Cancer. 2012;12:210–9. doi: 10.1038/nrc3186. [DOI] [PubMed] [Google Scholar]
- 223.Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, et al. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton. 2005;60:24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- 224.Grinnell F. Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol. 1994;124:401–4. doi: 10.1083/jcb.124.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Arora PD, Narani N, McCulloch CA. The compliance of collagen gels regulates transforming growth factor-beta induction of alpha-smooth muscle actin in fibroblasts. Am J Pathol. 1999;154:871–82. doi: 10.1016/S0002-9440(10)65334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Hinz B. Masters and servants of the force: the role of matrix adhesions in myofibroblast force perception and transmission. Eur J Cell Biol. 2006;85:175–81. doi: 10.1016/j.ejcb.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 227.Junker JP, Kratz C, Tollbäck A, Kratz G. Mechanical tension stimulates the transdifferentiation of fibroblasts into myofibroblasts in human burn scars. Burns. 2008;34:942–6. doi: 10.1016/j.burns.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 228.Grinnell F, Zhu M, Carlson MA, Abrams JM. Release of mechanical tension triggers apoptosis of human fibroblasts in a model of regressing granulation tissue. Exp Cell Res. 1999;248:608–19. doi: 10.1006/excr.1999.4440. [DOI] [PubMed] [Google Scholar]
- 229.Carlson MA, Longaker MT, Thompson JS. Wound splinting regulates granulation tissue survival. J Surg Res. 2003;110:304–9. doi: 10.1016/S0022-4804(02)00098-7. [DOI] [PubMed] [Google Scholar]
- 230.Aarabi S, Bhatt KA, Shi Y, Paterno J, Chang EI, Loh SA, et al. Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis. FASEB J. 2007;21:3250–61. doi: 10.1096/fj.07-8218com. [DOI] [PubMed] [Google Scholar]
- 231.Räsänen K, Vaheri A. Activation of fibroblasts in cancer stroma. Exp Cell Res. 2010;316:2713–22. doi: 10.1016/j.yexcr.2010.04.032. [DOI] [PubMed] [Google Scholar]
- 232.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–52. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011;3:a005058. doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227–36. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 235.Wolfe JN. Risk for breast cancer development determined by mammographic parenchymal pattern. Cancer. 1976;37:2486–92. doi: 10.1002/1097-0142(197605)37:5<2486::AID-CNCR2820370542>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 236.Winer JP, Janmey PA, McCormick ME, Funaki M. Bone marrow-derived human mesenchymal stem cells become quiescent on soft substrates but remain responsive to chemical or mechanical stimuli. Tissue Eng Part A. 2009;15:147–54. doi: 10.1089/ten.tea.2007.0388. [DOI] [PubMed] [Google Scholar]
- 237.Klein EA, Yin L, Kothapalli D, Castagnino P, Byfield FJ, Xu T, et al. Cell-cycle control by physiological matrix elasticity and in vivo tissue stiffening. Curr Biol. 2009;19:1511–8. doi: 10.1016/j.cub.2009.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Gritsenko PG, Ilina O, Friedl P. Interstitial guidance of cancer invasion. J Pathol. 2012;226:185–99. doi: 10.1002/path.3031. [DOI] [PubMed] [Google Scholar]
- 239.Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall JF, Harrington K, et al. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol. 2007;9:1392–400. doi: 10.1038/ncb1658. [DOI] [PubMed] [Google Scholar]
- 240.Condeelis J, Segall JE. Intravital imaging of cell movement in tumours. Nat Rev Cancer. 2003;3:921–30. doi: 10.1038/nrc1231. [DOI] [PubMed] [Google Scholar]
- 241.Ilina O, Bakker GJ, Vasaturo A, Hofmann RM, Friedl P. Two-photon laser-generated microtracks in 3D collagen lattices: principles of MMP-dependent and -independent collective cancer cell invasion. Phys Biol. 2011;8:015010. doi: 10.1088/1478-3975/8/1/015010. [DOI] [PubMed] [Google Scholar]
- 242.Friedl P, Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell. 2011;147:992–1009. doi: 10.1016/j.cell.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 243.Conklin MW, Eickhoff JC, Riching KM, Pehlke CA, Eliceiri KW, Provenzano PP, et al. Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am J Pathol. 2011;178:1221–32. doi: 10.1016/j.ajpath.2010.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Liu K, He X, Lei XZ, Zhao LS, Tang H, Liu L, et al. Pathomorphological study on location and distribution of Kupffer cells in hepatocellular carcinoma. World J Gastroenterol. 2003;9:1946–9. doi: 10.3748/wjg.v9.i9.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Faouzi S, Le Bail B, Neaud V, Boussarie L, Saric J, Bioulac-Sage P, et al. Myofibroblasts are responsible for collagen synthesis in the stroma of human hepatocellular carcinoma: an in vivo and in vitro study. J Hepatol. 1999;30:275–84. doi: 10.1016/S0168-8278(99)80074-9. [DOI] [PubMed] [Google Scholar]
- 246.Dubuisson L, Lepreux S, Bioulac-Sage P, Balabaud C, Costa AM, Rosenbaum J, et al. Expression and cellular localization of fibrillin-1 in normal and pathological human liver. J Hepatol. 2001;34:514–22. doi: 10.1016/S0168-8278(00)00048-9. [DOI] [PubMed] [Google Scholar]
- 247.Le Bail B, Faouzi S, Boussarie L, Guirouilh J, Blanc JF, Carles J, et al. Osteonectin/SPARC is overexpressed in human hepatocellular carcinoma. J Pathol. 1999;189:46–52. doi: 10.1002/(SICI)1096-9896(199909)189:1<46::AID-PATH392>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 248.Monvoisin A, Neaud V, De Lédinghen V, Dubuisson L, Balabaud C, Bioulac-Sage P, et al. Direct evidence that hepatocyte growth factor-induced invasion of hepatocellular carcinoma cells is mediated by urokinase. J Hepatol. 1999;30:511–8. doi: 10.1016/S0168-8278(99)80113-5. [DOI] [PubMed] [Google Scholar]
- 249.Dubuisson L, Monvoisin A, Nielsen BS, Le Bail B, Bioulac-Sage P, Rosenbaum J. Expression and cellular localization of the urokinase-type plasminogen activator and its receptor in human hepatocellular carcinoma. J Pathol. 2000;190:190–5. doi: 10.1002/(SICI)1096-9896(200002)190:2<190::AID-PATH511>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 250.Monvoisin A, Bisson C, Si-Tayeb K, Balabaud C, Desmoulière A, Rosenbaum J. Involvement of matrix metalloproteinase type-3 in hepatocyte growth factor-induced invasion of human hepatocellular carcinoma cells. Int J Cancer. 2002;97:157–62. doi: 10.1002/ijc.1595. [DOI] [PubMed] [Google Scholar]
- 251.Leyland H, Gentry J, Arthur MJ, Benyon RC. The plasminogen-activating system in hepatic stellate cells. Hepatology. 1996;24:1172–8. doi: 10.1002/hep.510240532. [DOI] [PubMed] [Google Scholar]
- 252.Neaud V, Faouzi S, Guirouilh J, Le Bail B, Balabaud C, Bioulac-Sage P, et al. Human hepatic myofibroblasts increase invasiveness of hepatocellular carcinoma cells: evidence for a role of hepatocyte growth factor. Hepatology. 1997;26:1458–66. doi: 10.1002/hep.510260612. [DOI] [PubMed] [Google Scholar]
- 253.Neaud V, Hisaka T, Monvoisin A, Bedin C, Balabaud C, Foster DC, et al. Paradoxical pro-invasive effect of the serine proteinase inhibitor tissue factor pathway inhibitor-2 on human hepatocellular carcinoma cells. J Biol Chem. 2000;275:35565–9. doi: 10.1074/jbc.M006101200. [DOI] [PubMed] [Google Scholar]
- 254.Yamamoto H, Itoh F, Adachi Y, Sakamoto H, Adachi M, Hinoda Y, et al. Relation of enhanced secretion of active matrix metalloproteinases with tumor spread in human hepatocellular carcinoma. Gastroenterology. 1997;112:1290–6. doi: 10.1016/S0016-5085(97)70143-4. [DOI] [PubMed] [Google Scholar]
- 255.Terada T, Makimoto K, Terayama N, Suzuki Y, Nakanuma Y. Alpha-smooth muscle actin-positive stromal cells in cholangiocarcinomas, hepatocellular carcinomas and metastatic liver carcinomas. J Hepatol. 1996;24:706–12. doi: 10.1016/S0168-8278(96)80267-4. [DOI] [PubMed] [Google Scholar]
- 256.Okamura N, Yoshida M, Shibuya A, Sugiura H, Okayasu I, Ohbu M. Cellular and stromal characteristics in the scirrhous hepatocellular carcinoma: comparison with hepatocellular carcinomas and intrahepatic cholangiocarcinomas. Pathol Int. 2005;55:724–31. doi: 10.1111/j.1440-1827.2005.01891.x. [DOI] [PubMed] [Google Scholar]
- 257.Terada T, Okada Y, Nakanuma Y. Expression of immunoreactive matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases in human normal livers and primary liver tumors. Hepatology. 1996;23:1341–4. doi: 10.1002/hep.510230608. [DOI] [PubMed] [Google Scholar]
- 258.Darby IA, Vuillier-Devillers K, Pinault E, Sarrazy V, Lepreux S, Balabaud C, et al. Proteomic analysis of differentially expressed proteins in peripheral cholangiocarcinoma. Cancer Microenviron. 2010;4:73–91. doi: 10.1007/s12307-010-0047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Li Z, Zhao J, Du Y, Park HR, Sun SY, Bernal-Mizrachi L, et al. Down-regulation of 14-3-3zeta suppresses anchorage-independent growth of lung cancer cells through anoikis activation. Proc Natl Acad Sci U S A. 2008;105:162–7. doi: 10.1073/pnas.0710905105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Tilman G, Mattiussi M, Brasseur F, van Baren N, Decottignies A. Human periostin gene expression in normal tissues, tumors and melanoma: evidences for periostin production by both stromal and melanoma cells. Mol Cancer. 2007;6:80. doi: 10.1186/1476-4598-6-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Utispan K, Thuwajit P, Abiko Y, Charngkaew K, Paupairoj A, Chau-in S, et al. Gene expression profiling of cholangiocarcinoma-derived fibroblast reveals alterations related to tumor progression and indicates periostin as a poor prognostic marker. Mol Cancer. 2010;9:13. doi: 10.1186/1476-4598-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Kanno A, Satoh K, Masamune A, Hirota M, Kimura K, Umino J, et al. Periostin, secreted from stromal cells, has biphasic effect on cell migration and correlates with the epithelial to mesenchymal transition of human pancreatic cancer cells. Int J Cancer. 2008;122:2707–18. doi: 10.1002/ijc.23332. [DOI] [PubMed] [Google Scholar]
- 263.Fujimoto K, Kawaguchi T, Nakashima O, Ono J, Ohta S, Kawaguchi A, et al. Periostin, a matrix protein, has potential as a novel serodiagnostic marker for cholangiocarcinoma. Oncol Rep. 2011;25:1211–6. doi: 10.3892/or.2011.1194. [DOI] [PubMed] [Google Scholar]
- 264.Guyot C, Lepreux S, Combe C, Doudnikoff E, Bioulac-Sage P, Balabaud C, et al. Hepatic fibrosis and cirrhosis: the (myo)fibroblastic cell subpopulations involved. Int J Biochem Cell Biol. 2006;38:135–51. doi: 10.1016/j.biocel.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 265.Forbes SJ, Parola M. Liver fibrogenic cells. Best Pract Res Clin Gastroenterol. 2011;25:207–17. doi: 10.1016/j.bpg.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 266.Guyot C, Combe C, Balabaud C, Bioulac-Sage P, Desmoulière A. Fibrogenic cell fate during fibrotic tissue remodelling observed in rat and human cultured liver slices. J Hepatol. 2007;46:142–50. doi: 10.1016/j.jhep.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 267.Guyot C, Lepreux S, Combe C, Sarrazy V, Billet F, Balabaud C, et al. Fibrogenic cell phenotype modifications during remodelling of normal and pathological human liver in cultured slices. Liver Int. 2010;30:1529–40. doi: 10.1111/j.1478-3231.2010.02342.x. [DOI] [PubMed] [Google Scholar]
- 268.Bosselut N, Housset C, Marcelo P, Rey C, Burmester T, Vinh J, et al. Distinct proteomic features of two fibrogenic liver cell populations: hepatic stellate cells and portal myofibroblasts. Proteomics. 2010;10:1017–28. doi: 10.1002/pmic.200900257. [DOI] [PubMed] [Google Scholar]
- 269.Theise N, Nakashima O, Park Y, Nakanuma Y. Combined hepatocellular-cholangiocarcinoma. In: Bosman FT CF, Hruban RH, Theise ND, ed. WHO classification of tumors of the digestive system. Lyon (France): IARC Press, 2010:225-7. [Google Scholar]
- 270.Seok JY, Na DC, Woo HG, Roncalli M, Kwon SM, Yoo JE, et al. A fibrous stromal component in hepatocellular carcinoma reveals a cholangiocarcinoma-like gene expression trait and EMT. Hepatology. 2012;55:1776–86. doi: 10.1002/hep.25570. [DOI] [PubMed] [Google Scholar]
- 271.Greenbaum LE, Wells RG. The role of stem cells in liver repair and fibrosis. Int J Biochem Cell Biol. 2011;43:222–9. doi: 10.1016/j.biocel.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Fujimiya T, Liu J, Kojima H, Shirafuji S, Kimura H, Fujimiya M. Pathological roles of bone marrow-derived stellate cells in a mouse model of alcohol-induced fatty liver. Am J Physiol Gastrointest Liver Physiol. 2009;297:G451–60. doi: 10.1152/ajpgi.00055.2009. [DOI] [PubMed] [Google Scholar]
- 273.Higashiyama R, Moro T, Nakao S, Mikami K, Fukumitsu H, Ueda Y, et al. Negligible contribution of bone marrow-derived cells to collagen production during hepatic fibrogenesis in mice. Gastroenterology. 2009;137:1459–66, e1. doi: 10.1053/j.gastro.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 274.Chu AS, Diaz R, Hui JJ, Yanger K, Zong Y, Alpini G, et al. Lineage tracing demonstrates no evidence of cholangiocyte epithelial-to-mesenchymal transition in murine models of hepatic fibrosis. Hepatology. 2011;53:1685–95. doi: 10.1002/hep.24206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Popov Y, Schuppan D. Epithelial-to-mesenchymal transition in liver fibrosis: dead or alive? Gastroenterology. 2010;139:722–5. doi: 10.1053/j.gastro.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 276.Taras D, Blanc JF, Rullier A, Dugot-Senant N, Laurendeau I, Bièche I, et al. Halofuginone suppresses the lung metastasis of chemically induced hepatocellular carcinoma in rats through MMP inhibition. Neoplasia. 2006;8:312–8. doi: 10.1593/neo.05796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Jan YY, Yeh TS, Yeh JN, Yang HR, Chen MF. Expression of epidermal growth factor receptor, apomucins, matrix metalloproteinases, and p53 in rat and human cholangiocarcinoma: appraisal of an animal model of cholangiocarcinoma. Ann Surg. 2004;240:89–94. doi: 10.1097/01.sla.0000129492.95311.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Yeh CN, Maitra A, Lee KF, Jan YY, Chen MF. Thioacetamide-induced intestinal-type cholangiocarcinoma in rat: an animal model recapitulating the multi-stage progression of human cholangiocarcinoma. Carcinogenesis. 2004;25:631–6. doi: 10.1093/carcin/bgh037. [DOI] [PubMed] [Google Scholar]
- 279.Gao Q, Wang XY, Qiu SJ, Zhou J, Shi YH, Zhang BH, et al. Tumor stroma reaction-related gene signature predicts clinical outcome in human hepatocellular carcinoma. Cancer Sci. 2011;102:1522–31. doi: 10.1111/j.1349-7006.2011.01981.x. [DOI] [PubMed] [Google Scholar]
- 280.Hinz B, Gabbiani G, Chaponnier C. The NH2-terminal peptide of alpha-smooth muscle actin inhibits force generation by the myofibroblast in vitro and in vivo. J Cell Biol. 2002;157:657–63. doi: 10.1083/jcb.200201049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Hinz B, Gabbiani G. Cell-matrix and cell-cell contacts of myofibroblasts: role in connective tissue remodeling. Thromb Haemost. 2003;90:993–1002. doi: 10.1160/TH03-05-0328. [DOI] [PubMed] [Google Scholar]
- 282.Uchio K, Graham M, Dean NM, Rosenbaum J, Desmoulière A. Down-regulation of connective tissue growth factor and type I collagen mRNA expression by connective tissue growth factor antisense oligonucleotide during experimental liver fibrosis. Wound Repair Regen. 2004;12:60–6. doi: 10.1111/j.1067-1927.2004.012112.x-1. [DOI] [PubMed] [Google Scholar]