Abstract
Integrin receptors play important roles in cell adhesion and tumor metastasis. The coupling of mechanical sensing and biochemical ligation is known to collectively regulate the activation of integrin receptors. Recently, oligomerization of activated integrins has been considered as the primordial signature of cytoskeletal remodeling and the initiation of various downstream signals, such as focal and fibrillar adhesions. However, spatio-temporal reorganization of activated integrins and associated proteins remains poorly understood. Here, we summarized the recent discovery of sequential biophysical events of integrin activation during early adhesion formation. Using the cyclic Arg-Gly-Asp (RGD) peptide as a mobile ligand on supported lipid membranes, a series of previously unreported events were observed following integrin αvβ3 clustering and cell spreading, including a long-range lateral translocation of the integrin clusters. With initial clustering, localized actin polymerization occurred in a Src family kinase dependent manner. Clustering of liganded integrins recruits various adaptor proteins and serves as a reaction core for mechanobiological activities. In addition, there are future possibilities to investigate the role of other synergetic interactions with the activated integrin receptors.
Keywords: integrin, RGD peptide, supported lipid membrane, Src family kinase, actin polymerization, tumor metastasis, synergy receptor
Introduction
Integrin-mediated cell adhesion regulates a variety of biological processes, such as metastasis in cancer, differentiation, and the immune response.1-6 Composed of distinct α and β subunit chains, integrin heterodimers span the plasma membrane and can serve as molecular transducers to decipher signals from the extracellular matrix (ECM) in conjunction with cell-cell membrane junctions.7-10 The outside-in activation of integrin receptors is initiated by ligand binding to integrin extracellular domains and involves a multi-component assembly process, including chemical triggering, mechanical sensing, and cytoskeletal reorganization.11,12 For example, the extracellular domain of the integrin, αvβ3, binds to the ECM protein fibronectin and mediates cell-matrix adhesions. This binding causes conformational changes in integrin cytoplasmic domain that results in recruitment of various cytosolic proteins, such as talin and paxillin13-15 and multicomponent linkages to the actin cytoskeletal network. On the other hand, overexpression of integrin αvβ3 is often associated with highly metastatic cancer.16 Investigation of the molecular organization of activated integrin complexes has become an important target for cancer therapeutic studies.17,18
The Arg-Gly-Asp (RGD) amino acid sequence in the tenth fibronectin type III domain has been biochemically identified as the major binding ligand for integrin αvβ3.19,20 However, recent biophysical studies indicate that mechanical properties of an RGD-tagged substrate are also critical to support adhesion formation.21 With identical ligand density, rigidity of the adhesive substrate causes different cell spreading behavior and stem cell differentiation.22,23 Soft adhesive substrates causes stem cells to spread less and to differentiate into adipocyte-like lineages, while rigid substrates cause larger spread areas and differentiation into osteocytes. More interestingly, the spatial arrangement of the RGD motif can affect downstream assembly of the adhesion machinery.24 A minimum adhesion unit, as little as four liganded integrins, can support stable adhesion formation.25 There is an emerging interest in understanding how integrin receptors spatially organize during early activation. Nevertheless, most previous studies were based on randomly immobilized ligands, and the random variations in ligand density hinder the observation of fine steps in initial reorganization of activated integrin receptors. Here, we have summarized findings from new approaches that have defined the molecular events during integrin activation by biochemical and mechanical signals.
Functionalized Supported Membrane with Nano-Partitions
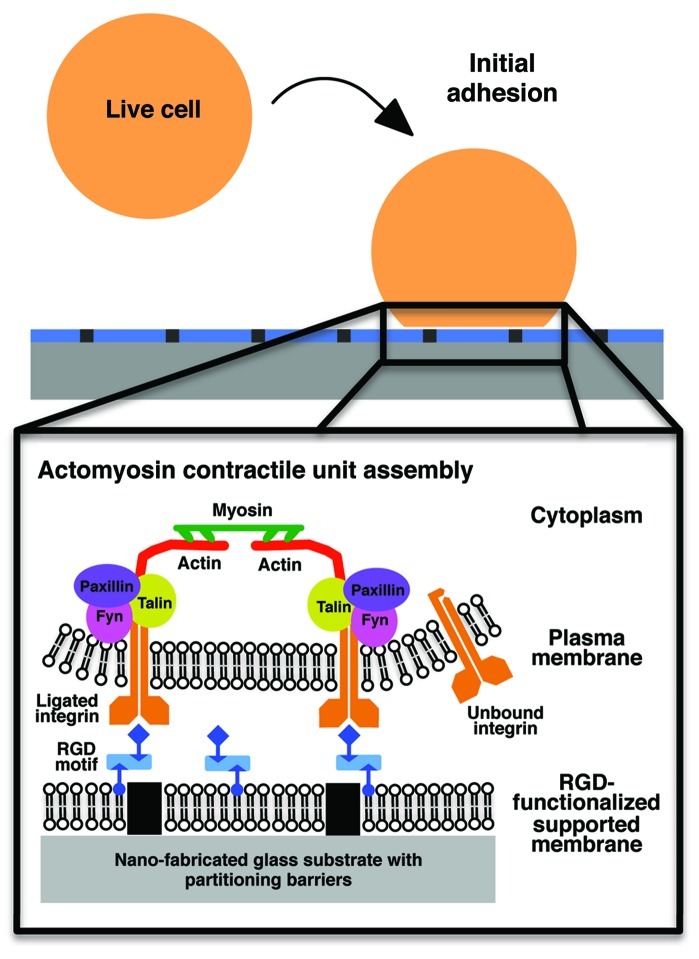
Dynamic assembly of integrin aggregates after ligand activation can be studied using various engineered biomaterials, such as supported lipid membranes. Supported membranes contain a continuous single lipid bilayer on a solid substrate and have been widely used as a model system to unravel key questions in cell biology, such as phenomena in the formation of immunological synapses26,27 and ephrin-mediated cell junctions.28,29 Formed by spontaneous fusion of small phospholipid vesicles on clean, planar glass substrates, self-assembled supported lipid membranes exhibit many characteristics of normal lipid bilayers including two-dimensional mobility of membrane components but are compatible with high-resolution microscopic techniques.30,31 More importantly, the density of functional ligands and membrane composition can be controlled.32 For example, biotinylated cyclic RGD peptides can be stably attached to lipids via Cascade Blue-labeled neutravidin and diffuse at lipid rates, laterally.10 The presentation of mobile RGD ligand at the cellular interface provides a flexible tool to explore the spatial-temporal regulation of integrin activation (Fig. 1).
Figure 1. Use mobile RGD ligand on nano-patterned supported membrane to study early events of integrin activation. Cyclic RGD peptide with fluorescent label illustrated activated integrin αvβ3 and formed multi-component clusters on fluid supported lipid bilayer membranes. Various cytoplasmic proteins, including talin, paxillin and Fyn, were found at initial RGD-integrin clusters. Actin polymerization at RGD-integrin clusters was observed during early adhesion formation in a Src family kinase-dependent manner. Nano-patterned metal lines embedded in RGD-membranes acted as partitioning barriers and provide force resistance in fluid membrane environment. Newly assembled actomyosin contractile network pulls RGD-integrin clusters toward each other against nano-partitioning barriers, prior to active expansion in the cell spreading process. When barriers are present, 80% of cells underwent spreading. When barriers are absent, only 20% of cells spread.
To determine if force on integrin-ligand complexes affects their function, partitioning barriers can be placed in supported membranes by prefabricated periodic parallel metal lines over glass substrates (Fig. 1). While continuous supported membranes behave as force-free substrates, periodic partitioning barriers embedded in supported membranes can serve as nucleation sites for force-dependent processes to occur. Previously, we have formed partitioning barriers with thin chromium lines of 5 nm in height and 100 nm in line width with 1 to 4 μm gap spacing.10,31 Partitioning barriers composed of nano-patterned metal lines can be fabricated by electron beam33,34 or nano-imprint lithography.10,35,36 As cells bind to lipid-linked RGDs in supported membranes, the nano-patterned metal lines passively restrict lateral movement of RGD-integrin complexes and provide local rigidity for adhesion formation.
Initial Integrin Clustering and Actin Polymerization
With nano-partitioned RGD-membranes, the molecular events following integrin activation can be investigated from a different perspective. Interestingly, mobile RGD ligand binding to integrins resulted in prompt clustering of RGD-integrin complexes as imaged by live fluorescence microscopy.10 Initial clustering of integrin receptors has been modeled as diffusion to a trap in a two-dimensional surface. Similar to adhesion formation on immobile fibronectin-coated substrates,37-39 a number of focal adhesion proteins rapidly accumulated in proportion to RGD-integrin cluster size, including talin, paxillin and focal adhesion kinase (FAK). The temporal recruitment of these functional proteins at RGD-integrin clusters occurred even when myosin was inhibited by blebbistatin and thus, revealed that integrin activation during early cell adhesion can be force independent. RGD binding to integrin is sufficient to trigger conformational changes of integrin cytoplasmic domains and form molecular linkages to the actin cytoskeleton network.
A critical new finding was that the clustering of liganded integrins caused local actin polymerization that expanded outwards from the cluster.10 As RGD-integrin complexes continued to develop after initial clustering, contractile activity of myosin in the actin network caused aggregation of activated clusters. Myosin pulling on adjacent F-actin arrays from integrin clusters caused newly formed RGD-integrin clusters to actively move toward each other to form larger clusters. When the fabricated barriers separated individual integrin clusters that were contracting, components piled up against the metal lines (Fig. 1). The nano-patterned metal lines embedded in fluidic RGD membrane provided local sites of mechanical resistance for subsequent force-dependent signaling events.
Nano-Partitions in RGD-Membranes Support Stable Adhesion
Intriguingly, actomyosin activity that produced contractile movement between integrin clusters was needed for activation of further spreading of the cells. Eighty percent of cells that developed contractile assembly of integrin clusters were able to spread out after initial adhesion. Without contractile assembly of integrin clusters, only 20% of cells spread on the bilayers. While initial clustering of RGD-integrin complexes was force independent, activation of cell spreading has an additional force-dependent step that was not observed previously because integrin ligands were normally bound to immobile substrates.
After activation of cell spreading, myosin-II contraction of actin filaments behind the leading edge of the spreading plasma membrane caused a retraction of the whole cell edge. When there were no barriers in the RGD-membrane, cells often contracted to a very small area (50–100 μm2). Prefabricated arrays of metal lines with different gap spacing resulted in an increase in the final cell spreading area in proportion with the line density. With gap spacing of 1 μm that had the highest density of lines, cells spread to a relatively large area (800 μm2). However, cell spreading area decreased to 50–100 μm2 with gap spacing of 4 μm. The higher barrier density meant that there would be more adhesion sites. Alternatively, the distance of 4 µm may be greater than the length of the contractile units that are needed for rigidity sensing, that leads to focal adhesion formation (rigidity sensing appears to occur through a localized contraction of 1–3 µm to a constant displacement40). In either case, a higher density of adhesions will result in a larger spread area.
Another interesting observation was the spatial-temporal recruitment of vinculin. The association of vinculin at initial RGD-integrin clusters was low, and then increased as cells entered contractile phase after spreading. As RGD-integrin clusters experienced larger contractile forces and moved inwards, vinculin recruitment at the clusters increased. The increase of vinculin at adhesion site under higher force could be as a result of mechanically stretched talins41-43 that provided more binding domains for vinculins.
The Role of Src Family Kinases
Src family kinases play an important role in signaling cascades of integrin activation during cell spreading44 as well in several cancers and cell transformation. Because inhibition of Src family kinases blocks cell spreading, we wondered if Src family kinases were involved in the early steps that were seen on the supported bilayers. Previously, it was shown that integrin activation could promote Rac1GTP-mediated cell spreading by activating FAK and the Src family kinase Fyn,44 in particular. In other words, Fyn activation could support the outward translocation of integrin clusters by directly promoting Rac1GTP-mediated actin protrusion. Indeed, when 10 μM of 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine45 (PP2) was added to inhibit Src family kinase activity, both initial contractile movement and long-ranged outward movement of RGD-integrin complexes were abolished. Further, actin polymerization from the clustered integrins was blocked, indicating that the Src family kinases were involved in the earliest steps of activation of actin polymerization from clustered integrins.
Differential actin polymerization of Src-Yes-Fyn (SYF, membrane-associated tyrosine kinases) knockout cells46 on RGD membrane also revealed the role of SYF in integrin-mediate cell adhesion.44,47 While the detail mechanism of Fyn-mediated activation of actin polymerization is still under investigation, it is generally believed that Fyn acts as a specific tyrosine kinase48,49 at activated integrin clusters to upregulate proteins that locally promote actin polymerization.
Synergy in Activation of Integrin Clusters
Different integrin heterodimers often respond to specific ECM components50,51 (Table 1). For example, integrin α2β1 is a collagen I receptor,52 and α5β1 binds to a second fibronectin site through an interaction of α5 the ninth fibronectin type III domain.53 The combinatorial effect from multiple ECM components leads to a complexity of signal transduction in vivo. Many recent studies have shown that decellularized tissues provide an excellent matrix for the differentiation of mesenchymal stem cells into the original tissue type.54,55 Thus, the combination of different matrices appears to have a major effect on the differentiation process. It will be important to use a variety of different matrix ligands to determine how the synergy of different integrin binding components occurs.
Table 1. Functional sequences for various integrin and synergy receptors.
| Integrin receptors | Functional amino acid sequence in ECM |
|---|---|
| αvβ3 |
RGD in fibronectin10 |
| α5β1 |
PHSRN in fibronectin53 |
| α2β1 | DGEA in collagen I52 |
There are various orthogonal methods to independently attach functional molecules to headgroup-modified phospholipids30,31 (Table 2). By linking different fluorescent-labeled functional ligand peptides onto mobile supported membranes, this combinational approach can provide new insights to clarify convoluted cross-interactions between integrin superfamily members. In addition, other membrane receptors, such as syndecan-4, also play supportive roles in integrin activation.56-58 Auxiliary synergy ligands on nano-patterned RGD-membranes will allow the spatial arrangement of different ligand-receptor complexes to be controlled to reveal their cooperative nature in downstream adhesion signaling.
Table 2. Biocompatible techniques to attach functional peptides onto lipid membrane.
| Type | Lipid headgroup | Linker | Protein/peptide tag |
|---|---|---|---|
| Covalent bond |
Maleimide |
N/A |
Cysteine residue |
| Multiple chelation |
Nickel(II)-NTA |
N/A |
Multiple histidine residues |
| Strong ligation |
Biotin motif |
Neutravidin |
AviTag or biotin motif |
| GPI anchoring | N/A | N/A | Glycosylphosphatidylinositol (GPI) tails |
Summary
Integrin-mediated cell adhesion involves clustering of integrin receptors and is closely regulated by both chemical and mechanical cues. This coordinated reorganization of membrane proteins into multi-component large-scale patterns has been an emerging theme of cellular signaling,31,59,60 and bioengineered support membranes with nano-partitioning barriers provide new opportunities to investigate their functional roles and compositional stoichiometry. Previously, it was impracticable to probe integrin clustering dynamics and cytoskeletal remodeling due to the unknown distribution of fixed-ligand configurations. Mobile RGD ligands on supported membranes have revealed several new features of early integrin adhesion formation, including the role of clustering in activating actin polymerization through Src-family kinase stimulation. Without substrate restraints, the clustering of activated integrins can provide an understanding of the basic adhesion complex assembly process. This integrated approach creates more possibilities to investigate the detailed mechanisms of force-dependent and force-independent cellular processes that are critical in cancer where the cells ignore microenvironmental cues and grow inappropriately.
Various integrin heterodimers have been identified as pharmacological targets to suppress the development of tumor invasion and immune disorder.17,18 For examples, soluble cyclic RGD motif has been implemented as potent inhibitors for integrin αvβ3 to treat glioblastoma multiforme.61,62 The ability to visualize the dynamic reorganization of activated integrins will deepen our knowledge of cell-matrix adhesion and bring new insights of mechano-biological downstream signaling in adhesion turnover, cell migration, and tumor invasion.
Acknowledgments
The authors acknowledge helpful discussions with Dr Alexander Bershadsky, Dr Naila Alieva, Kevin Hartman and Dr Jay T. Groves. C.H.Y. acknowledges support from National Science Council of Taiwan, Grant NSC98-2917-I-564-165.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/20753
References
- 1.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673–7. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–9. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kinashi T. Intracellular signalling controlling integrin activation in lymphocytes. Nat Rev Immunol. 2005;5:546–59. doi: 10.1038/nri1646. [DOI] [PubMed] [Google Scholar]
- 5.Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol. 2006;7:265–75. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- 6.Evans R, Patzak I, Svensson L, De Filippo K, Jones K, McDowall A, et al. Integrins in immunity. J Cell Sci. 2009;122:215–25. doi: 10.1242/jcs.019117. [DOI] [PubMed] [Google Scholar]
- 7.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/S0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 8.Luo B-H, Springer TA. Integrin structures and conformational signaling. Curr Opin Cell Biol. 2006;18:579–86. doi: 10.1016/j.ceb.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zamir E, Katz BZ, Aota S, Yamada KM, Geiger B, Kam Z. Molecular diversity of cell-matrix adhesions. J Cell Sci. 1999;112:1655–69. doi: 10.1242/jcs.112.11.1655. [DOI] [PubMed] [Google Scholar]
- 10.Yu CH, Law JB, Suryana M, Low HY, Sheetz MP. Early integrin binding to Arg-Gly-Asp peptide activates actin polymerization and contractile movement that stimulates outward translocation. Proc Natl Acad Sci U S A. 2011;108:20585–90. doi: 10.1073/pnas.1109485108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore SW, Roca-Cusachs P, Sheetz MP. Stretchy proteins on stretchy substrates: the important elements of integrin-mediated rigidity sensing. Dev Cell. 2010;19:194–206. doi: 10.1016/j.devcel.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vogel V, Sheetz MP. Cell fate regulation by coupling mechanical cycles to biochemical signaling pathways. Curr Opin Cell Biol. 2009;21:38–46. doi: 10.1016/j.ceb.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laukaitis CM, Webb DJ, Donais K, Horwitz AF. Differential dynamics of alpha 5 integrin, paxillin, and alpha-actinin during formation and disassembly of adhesions in migrating cells. J Cell Biol. 2001;153:1427–40. doi: 10.1083/jcb.153.7.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moser M, Legate KR, Zent R, Fässler R. The tail of integrins, talin, and kindlins. Science. 2009;324:895–9. doi: 10.1126/science.1163865. [DOI] [PubMed] [Google Scholar]
- 15.Wiesner S, Legate KR, Fässler R. Integrin-actin interactions. Cell Mol Life Sci. 2005;62:1081–99. doi: 10.1007/s00018-005-4522-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varner JA, Cheresh DA. Integrins and cancer. Curr Opin Cell Biol. 1996;8:724–30. doi: 10.1016/S0955-0674(96)80115-3. [DOI] [PubMed] [Google Scholar]
- 17.Cox D, Brennan M, Moran N. Integrins as therapeutic targets: lessons and opportunities. Nat Rev Drug Discov. 2010;9:804–20. doi: 10.1038/nrd3266. [DOI] [PubMed] [Google Scholar]
- 18.Cabodi S, del Pilar Camacho-Leal M, Di Stefano P, Defilippi P. Integrin signalling adaptors: not only figurants in the cancer story. Nat Rev Cancer. 2010;10:858–70. doi: 10.1038/nrc2967. [DOI] [PubMed] [Google Scholar]
- 19.Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 20.Haas TA, Plow EF. Integrin-ligand interactions: a year in review. Curr Opin Cell Biol. 1994;6:656–62. doi: 10.1016/0955-0674(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 21.Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol. 2009;10:21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- 22.Fu J, Wang YK, Yang MT, Desai RA, Yu X, Liu Z, et al. Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nat Methods. 2010;7:733–6. doi: 10.1038/nmeth.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen CS. Mechanotransduction - a field pulling together? J Cell Sci. 2008;121:3285–92. doi: 10.1242/jcs.023507. [DOI] [PubMed] [Google Scholar]
- 24.Arnold M, Hirschfeld-Warneken VC, Lohmüller T, Heil P, Blümmel J, Cavalcanti-Adam EA, et al. Induction of cell polarization and migration by a gradient of nanoscale variations in adhesive ligand spacing. Nano Lett. 2008;8:2063–9. doi: 10.1021/nl801483w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schvartzman M, Palma M, Sable J, Abramson J, Hu X, Sheetz MP, et al. Nanolithographic control of the spatial organization of cellular adhesion receptors at the single-molecule level. Nano Lett. 2011;11:1306–12. doi: 10.1021/nl104378f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hartman NC, Nye JA, Groves JT. Cluster size regulates protein sorting in the immunological synapse. Proc Natl Acad Sci U S A. 2009;106:12729–34. doi: 10.1073/pnas.0902621106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu CH, Wu HJ, Kaizuka Y, Vale RD, Groves JT. Altered actin centripetal retrograde flow in physically restricted immunological synapses. PLoS One. 2010;5:e11878. doi: 10.1371/journal.pone.0011878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nair PM, Salaita K, Petit RS, Groves JT. Using patterned supported lipid membranes to investigate the role of receptor organization in intercellular signaling. Nat Protoc. 2011;6:523–39. doi: 10.1038/nprot.2011.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salaita K, Nair PM, Petit RS, Neve RM, Das D, Gray JW, et al. Restriction of receptor movement alters cellular response: physical force sensing by EphA2. Science. 2010;327:1380–5. doi: 10.1126/science.1181729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin W-C, Yu C-H, Triffo S, Groves JT. Supported Membrane Formation, Characterization, Functionalization, and Patterning for Application in Biological Science and Technology. John Wiley & Sons, Inc., 2010. [DOI] [PubMed] [Google Scholar]
- 31.Yu CH, Groves JT. Engineering supported membranes for cell biology. Med Biol Eng Comput. 2010;48:955–63. doi: 10.1007/s11517-010-0634-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galush WJ, Nye JA, Groves JT. Quantitative fluorescence microscopy using supported lipid bilayer standards. Biophys J. 2008;95:2512–9. doi: 10.1529/biophysj.108.131540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeMond AL, Mossman KD, Starr T, Dustin ML, Groves JT. T cell receptor microcluster transport through molecular mazes reveals mechanism of translocation. Biophys J. 2008;94:3286–92. doi: 10.1529/biophysj.107.119099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mossman KD, Campi G, Groves JT, Dustin ML. Altered TCR signaling from geometrically repatterned immunological synapses. Science. 2005;310:1191–3. doi: 10.1126/science.1119238. [DOI] [PubMed] [Google Scholar]
- 35.Fengxiang Z, Hong Yee L. Ordered three-dimensional hierarchical nanostructures by nanoimprint lithography. Nanotechnology. 2006;17:1884. doi: 10.1088/0957-4484/17/8/013. [DOI] [Google Scholar]
- 36.Guo LJ. Recent progress in nanoimprint technology and its applications. J Phys D Appl Phys. 2004;37:R123. doi: 10.1088/0022-3727/37/11/R01. [DOI] [Google Scholar]
- 37.Critchley DR, Gingras AR. Talin at a glance. J Cell Sci. 2008;121:1345–7. doi: 10.1242/jcs.018085. [DOI] [PubMed] [Google Scholar]
- 38.Deakin NO, Turner CE. Paxillin comes of age. J Cell Sci. 2008;121:2435–44. doi: 10.1242/jcs.018044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schaller MD. Cellular functions of FAK kinases: insight into molecular mechanisms and novel functions. J Cell Sci. 2010;123:1007–13. doi: 10.1242/jcs.045112. [DOI] [PubMed] [Google Scholar]
- 40.Ghassemi S, Meacci G, Liu S, Gondarenko AA, Mathur A, Roca-Cusachs P, et al. Cells test substrate rigidity by local contractions on submicrometer pillars. Proc Natl Acad Sci U S A. 2012;109:5328–33. doi: 10.1073/pnas.1119886109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hytönen VP, Vogel V. How force might activate talin’s vinculin binding sites: SMD reveals a structural mechanism. PLoS Comput Biol. 2008;4:e24. doi: 10.1371/journal.pcbi.0040024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Margadant F, Chew LL, Hu X, Yu H, Bate N, Zhang X, et al. Mechanotransduction in vivo by repeated talin stretch-relaxation events depends upon vinculin. PLoS Biol. 2011;9:e1001223. doi: 10.1371/journal.pbio.1001223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, Sheetz MP. Stretching single talin rod molecules activates vinculin binding. Science. 2009;323:638–41. doi: 10.1126/science.1162912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kostic A, Sheetz MP. Fibronectin rigidity response through Fyn and p130Cas recruitment to the leading edge. Mol Biol Cell. 2006;17:2684–95. doi: 10.1091/mbc.E05-12-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, et al. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 46.Klinghoffer RA, Sachsenmaier C, Cooper JA, Soriano P. Src family kinases are required for integrin but not PDGFR signal transduction. EMBO J. 1999;18:2459–71. doi: 10.1093/emboj/18.9.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S, et al. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127:1015–26. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koka S, Minick GT, Zhou Y, Westendorf JJ, Boehm MB. Src regulates the activity of the mammalian formin protein FHOD1. Biochem Biophys Res Commun. 2005;336:1285–91. doi: 10.1016/j.bbrc.2005.08.257. [DOI] [PubMed] [Google Scholar]
- 49.Young KG, Copeland JW. Formins in cell signaling. Biochim Biophys Acta. 2010;1803:183–90. doi: 10.1016/j.bbamcr.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 50.Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010;339:269–80. doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Humphries MJ. Integrin structure. Biochem Soc Trans. 2000;28:311–39. doi: 10.1042/0300-5127:0280311. [DOI] [PubMed] [Google Scholar]
- 52.Staatz WD, Fok KF, Zutter MM, Adams SP, Rodriguez BA, Santoro SA. Identification of a tetrapeptide recognition sequence for the alpha 2 beta 1 integrin in collagen. J Biol Chem. 1991;266:7363–7. [PubMed] [Google Scholar]
- 53.Burrows L, Clark K, Mould AP, Humphries MJ. Fine mapping of inhibitory anti-alpha5 monoclonal antibody epitopes that differentially affect integrin-ligand binding. Biochem J. 1999;344:527–33. doi: 10.1042/0264-6021:3440527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Godier-Furnémont AF, Martens TP, Koeckert MS, Wan L, Parks J, Arai K, et al. Composite scaffold provides a cell delivery platform for cardiovascular repair. Proc Natl Acad Sci U S A. 2011;108:7974–9. doi: 10.1073/pnas.1104619108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Singelyn JM, Christman KL. Injectable materials for the treatment of myocardial infarction and heart failure: the promise of decellularized matrices. J Cardiovasc Transl Res. 2010;3:478–86. doi: 10.1007/s12265-010-9202-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix--cytoskeleton crosstalk. Nat Rev Mol Cell Biol. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- 57.Couchman JR. Transmembrane signaling proteoglycans. Annu Rev Cell Dev Biol. 2010;26:89–114. doi: 10.1146/annurev-cellbio-100109-104126. [DOI] [PubMed] [Google Scholar]
- 58.Morgan MR, Humphries MJ, Bass MD. Synergistic control of cell adhesion by integrins and syndecans. Nat Rev Mol Cell Biol. 2007;8:957–69. doi: 10.1038/nrm2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Groves JT. Learning the chemical language of cell-surface interactions. Sci STKE. 2005;2005:pe45. doi: 10.1126/stke.3012005pe45. [DOI] [PubMed] [Google Scholar]
- 60.Hartman NC, Groves JT. Signaling clusters in the cell membrane. Curr Opin Cell Biol. 2011;23:370–6. doi: 10.1016/j.ceb.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stupp R, Hegi ME, Neyns B, Goldbrunner R, Schlegel U, Clement PM, et al. Phase I/IIa study of cilengitide and temozolomide with concomitant radiotherapy followed by cilengitide and temozolomide maintenance therapy in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28:2712–8. doi: 10.1200/JCO.2009.26.6650. [DOI] [PubMed] [Google Scholar]
- 62.Reardon DA, Fink KL, Mikkelsen T, Cloughesy TF, O’Neill A, Plotkin S, et al. Randomized phase II study of cilengitide, an integrin-targeting arginine-glycine-aspartic acid peptide, in recurrent glioblastoma multiforme. J Clin Oncol. 2008;26:5610–7. doi: 10.1200/JCO.2008.16.7510. [DOI] [PubMed] [Google Scholar]
- 63.Woods A, Longley RL, Tumova S, Couchman JR. Syndecan-4 binding to the high affinity heparin-binding domain of fibronectin drives focal adhesion formation in fibroblasts. Arch Biochem Biophys. 2000;374:66–72. doi: 10.1006/abbi.1999.1607. [DOI] [PubMed] [Google Scholar]
- 64.Saito Y, Imazeki H, Miura S, Yoshimura T, Okutsu H, Harada Y, et al. A peptide derived from tenascin-C induces beta1 integrin activation through syndecan-4. J Biol Chem. 2007;282:34929–37. doi: 10.1074/jbc.M705608200. [DOI] [PubMed] [Google Scholar]