Abstract
Endoplasmic reticulum (ER) stress has been implicated in neurodegenerative diseases but its relationship and role in disease progression remain unclear. Using genetic and pharmacological approaches, we showed that mild ER stress (“preconditioning”) is neuroprotective in Drosophila and mouse models of Parkinson disease. In addition, we found that the combination of mild ER stress and apoptotic signals triggers an autophagic response both in vivo and in vitro. We showed that when autophagy is impaired, ER-mediated protection is lost. We further demonstrated that autophagy inhibits caspase activation and apoptosis. Based on our findings, we conclude that autophagy is required for the neuroprotection mediated by mild ER stress, and therefore ER preconditioning has potential therapeutic value for the treatment of neurodegenerative diseases.
Keywords: ER stress, autophagy, Parkinson, apoptosis, Drosophila, mouse
Introduction
The unfolded protein response (UPR) is an evolutionarily conserved adaptive response to perturbations of normal endoplasmic reticulum (ER) physiology,1-3 and is characteristic of several neurodegenerative diseases. Whether ER stress plays a causative role in certain disease conditions is still being debated.4,5 To cope with the aberrant accumulation of unfolded proteins, cells trigger the UPR causing the activation of the ERN1/IRE1, ATF6 and EIF2AK3/PERK pathways.6 Depending on the level of UPR activation and which components of the pathway are activated, ER stress can lead to either cellular death or survival. Specifically, sustained and full-fledged UPR that involves GADD153/CHOP and CASP12/caspase 12 activations is detrimental to the cell.7,8 By contrast, mild ER stress (ER preconditioning) induces selective activation of X box binding protein (XBP1) accompanied by cellular protection.9,10 ER preconditioning induces a cytoprotective response, named ER-hormesis, that protects the cell against a stronger insult.10,11 ER preconditioning has been shown to induce cytoprotection in ischemia/hypoxia models.12 However, in other models of neurodegenerative diseases, the cellular mechanism that elicits ER-mediated cytoprotection remains to be explored. A candidate mechanism for the ER-mediated protection is autophagy. It has been proposed in yeast that UPR activation stimulates autophagy, which in turn acts as a protective mechanism limiting ER expansion.13 Mutations in Drosophila or mouse atg genes lead to spontaneous neurodegeneration, suggesting that basal autophagy is neuroprotective.14-16 In addition, defective autophagic responses are observed in several neurodegenerative diseases including both Alzheimer and Parkinson diseases.17,18
Because the UPR and autophagic responses are evolutionarily conserved,2,3,19,20 we studied the protective mechanisms mediated by mild ER stress in Drosophila and mouse model of Parkinson disease (PD). First, we found that mild ER stress is protective in Drosophila and mouse models of Parkinson disease. In addition, we show that combination of mild ER stress and apoptotic signal induces autophagy, which in turn mediates neuroprotection. We discuss the implications of our findings in the light of the antagonistic relationship between autophagy and apoptosis, as well as the physiological relevance of ER stress and autophagy in neurodegenerative diseases.
Results
Mild ER stress is neuroprotective in Drosophila and mouse models of PD
We have previously shown that mild ER stress inhibits cell death both in vitro and in vivo in Drosophila. Drosophila S2 cells pretreated with a mild dose of tunicamycin (Tm), a chemical inducer of the UPR, exhibited increased resistance to cell death. Similar resistance to cell death was observed in adult Drosophila photoreceptor neurons (PRN) where the UPR was genetically induced by mutations in neither inactivation nor afterpotential A (ninaA), a gene encoding a chaperone specific for the folding of Rhodopsin-1 (Rh1).10 In these experimental conditions, the activation of the Ire1-Xbp1 pathway is well tolerated, does not induce cell lethality but instead increases resistance to exogenous apoptotic insults.
To determine if mild ER stress-mediated protection is effective in Drosophila PD model, we first confirmed that Tm feeding could activate UPR in the Drosophila brain tissues. hsc3/bip expression, a hallmark of UPR activation, was detected by RT-PCR in the heads of flies fed on two doses of Tm (1 μg/ml and 10 μg/ml) (Fig. S1A and S1B). Furthermore, we observed an increase of spliced and unspliced forms of xbp1 (Fig. S1C). In our assays, we chose the weakest dose of Tm (1 μg/ml) to induce a mild ER stress in Drosophila.
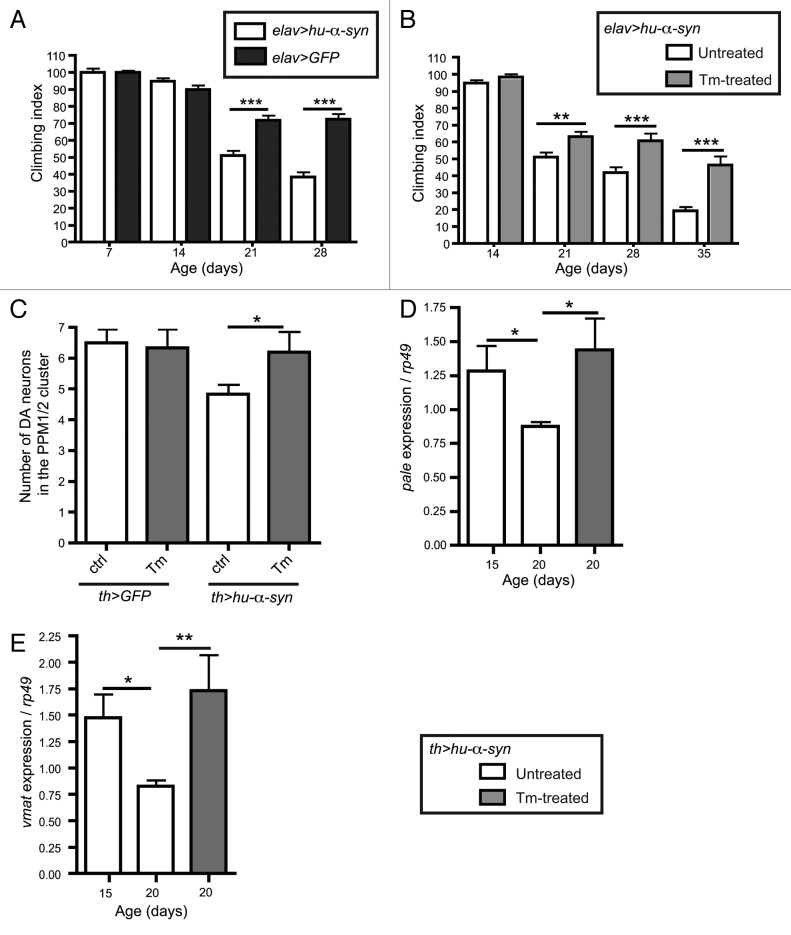
To establish a previously reported Drosophila PD model, we expressed the gene encoding SNCA/human α-synuclein (hu-α-syn) in all neurons with elav-gal4 or selectively in dopaminergic (DA) neurons with th-gal4.21,22 Next, we examined if Tm feeding could improve locomotor functions and dopaminergic neurons viability in hu-α-syn expressing flies. Flies expressing hu-α-syn using the pan-neuronal driver elav exhibited progressive decrease of motility compared with control (Fig. 1A). However, Tm feeding clearly improved climbing ability in 21, 28 and 35 d old flies (Fig. 1B).
Figure 1. Tm is protective in the α-syn Drosophila Parkinson disease model. Flies expressing hu-α-syn in all neurons (A and B) with elav-gal4 driver, or in the dopaminergic neurons (C–E) with the tyrosine hydroxylase driver (th-gal4). (A) Climbing ability of aged matched flies expressing hu-α-syn and control flies expressing GFP in all neurons (n = 100–120 flies). (B) Climbing ability of flies expressing hu-α-syn with or without Tm treatment (n = 100–120 flies). (C) Number of DA neurons in the PPM1/2 brain cluster in hu-α-syn or GFP expressing flies with or without Tm treatment. (D and E) Expression of pale or vmat mRNA normalized to rp49 in flies expressing hu-α-syn with or without Tm (n = 15 flies). *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 in Student’s t-test.
To assess the toxicity of hu-α-syn expression on DA neurons, we measured DA neurons viability and the transcriptional activity of pale, a gene responsible for dopamine metabolism and vesicular monoamine transporter (vmat), a gene specific for dopamine transport.23-25 After 42 d of hu-α-syn expression in DA neurons, we observed 30% loss of DA neurons in the protocerebral posterior medial clusters (PPM1/2) compared with control flies (Fig. 1C). We then detected by RT-qPCR a decrease of pale and vmat expression in th > hu-α-syn flies starting from 20 d onward (Fig. 1D and E). In hu-α-syn expressing flies that were regularly fed on Tm diet, we observed a rescue of DA neuron number in the PPM1/2 cluster (Fig. 1C). Similarly, the expression of pale and vmat was restored following Tm treatment (Fig. 1D and E). Together these results show that Tm feeding induces the UPR in Drosophila brain and is neuroprotective in the hu-α-syn model of Parkinson disease.
To validate these findings in a mammalian PD model, we tested whether mild ER-stress can induce neuroprotection in the 6-OHDA mouse model and in the human SH-SY5Y neuroblastoma cells.26-28 The 6-OHDA mouse model recapitulates the common features of PD, including the loss of dopaminergic (DA) neurons and gradual onset of locomotor dysfunction.29,30 We first assessed if Tm activates the UPR in the mouse brain. We monitored UPR activation by visualizing spliced xbp1 (xbp1s) and bip mRNA (Fig. S1D and S1E) after intraperitoneal (I/P) injection of Tm. Increases in xbp1s and bip mRNA were detected at a low dose of Tm (0.1 mg/kg) in the substantia nigra (SN), where DA neurons of the nigra-striatal pathway are located (Fig. S1D and S1E). No toxic effect or locomotor deficit was observed following chronic injection of Tm (0.1 mg/kg) into mice (Fig. S1H–J). Moreover, following Tm injection at 0.1 mg/kg, the expression of chop, a transcription factor inducing cell death,31 was not increased in the SN of mice (Fig. S1F and S1G). The chop level was only elevated at high doses of Tm (4.5 mg/kg, ED50) whereas xbp1s remained at the basal level at this dose (Fig. S1G and data not shown). These findings show that low doses of Tm activate a nontoxic, mild UPR in the SN.
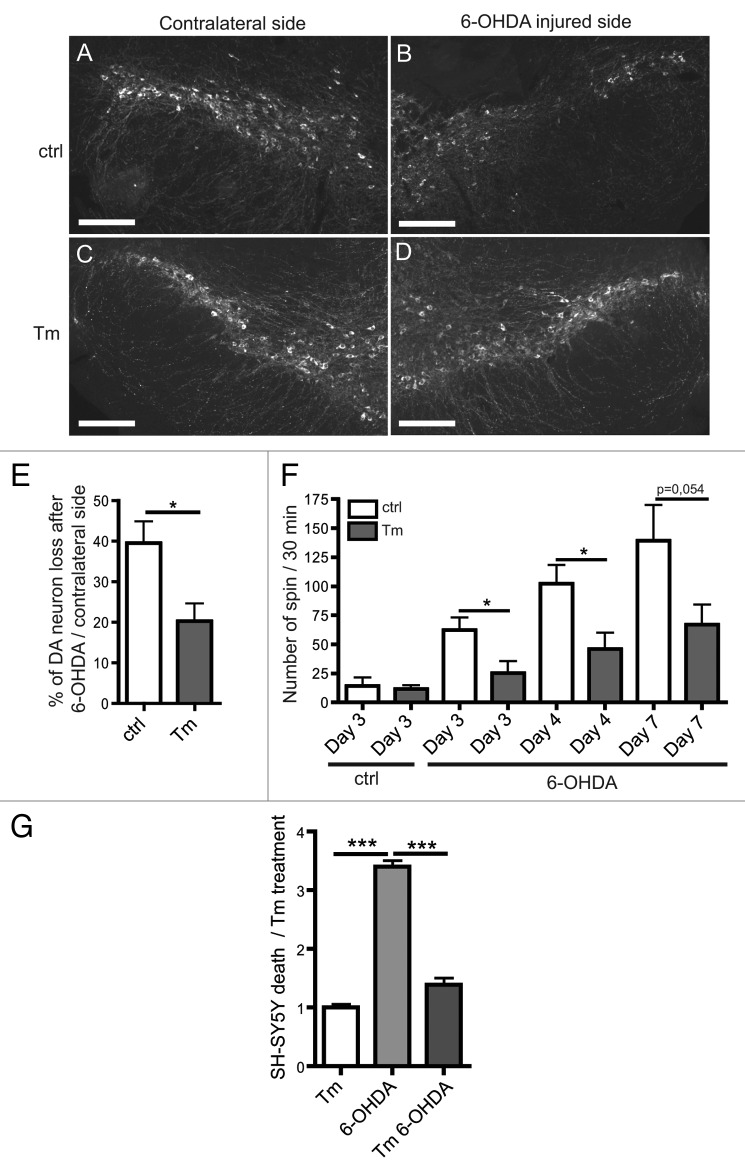
Stereotaxic injection of 6-OHDA into the mouse left striatum induces an asymmetrical loss of DA in the nigra-striatal circuit (Fig. 2A, B, E and ref. 32). To determine if mild ER stress is protective against the 6-OHDA-induced DA loss, we counted TH-positive DA neurons in the bilateral SN (Fig. 2A–E). After 6-OHDA injection, 20% more DA neurons remained in Tm-treated (0.1 mg/kg) than nontreated mice (Fig. 2E). Similarly in the human SH-SY5Y neuroblastoma cell line, we observed that Tm treatment reduced cell death induced by 6-OHDA (Fig. 2G). In addition, in both SN extracts and in SH-SY5Y cells, caspase activation induced by 6-OHDA was significantly inhibited by Tm treatment (Fig. S2A and S2B). These results indicate that Tm treatment-induced ER stress mediates neuron survival by blocking apoptosis both in vitro and in vivo.
Figure 2. Tm is protective in the 6-OHDA mouse Parkinson disease model. (A–D) Sections of the substantia nigra (SN) obtained 4 d after 6-OHDA treatment with or without Tm pre-treatment (0.1 mg/kg). DA neurons are visualized by immunostaining for tyrosine hydroxylase (TH). (E) Quantification of DA neuron loss after 6-OHDA injection normalized to the contralateral side. (F) Rotational behavior of mice after 6-OHDA injection. The graph shows the number of unilateral turns made by mice on days 3, 4 and 7 after the 6-OHDA injection with or without Tm (n = 6–7). (G) In vitro experiments on SH-SY5Y to assess the cell viability after Tm and 6-OHDA treatments. Cell viability was evaluated using trypan blue after Tm and 6-OHDA treatments. Quantification of cell death is normalized to Tm treatment. *p ≤ 0.05, ***p < 0.001 in Student’s t-test. Scale bar: 200 µm.
Next, we studied the effects of Tm treatment on the rotational behavior in the 6-OHDA mouse model (Fig. 2F). The 6-OHDA lesion triggered a progressive increase of unilateral rotational behavior induced by apomorphine treatment. Tm pretreatment markedly reduced the number of turns, indicating that Tm antagonizes 6-OHDA-induced rotational behavior. In summary, our results indicate that Tm treatment protects against the toxic effects of 6-OHDA both in the mouse model and in human neuroblastoma cell line (Fig. 2). These findings are pertinent to Parkinson disease progression and treatment.
Autophagy activation is required for the ER-mediated protection
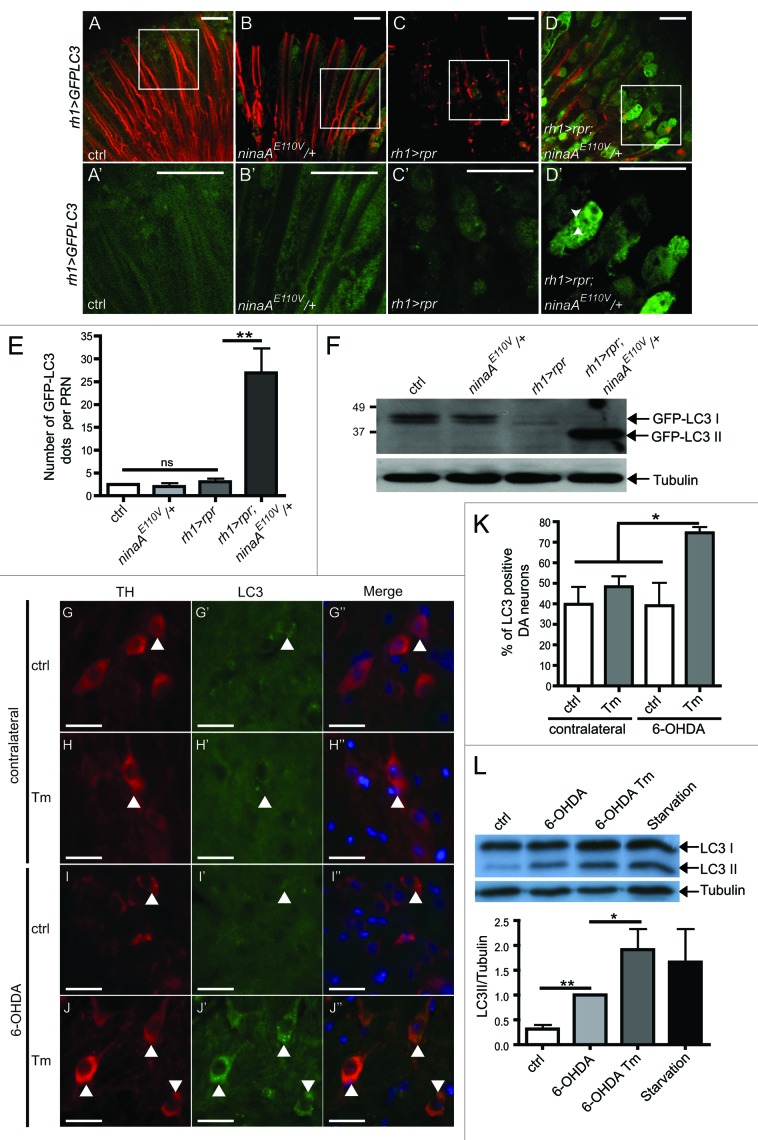
Autophagy and cell death are highly conserved cellular processes during evolution.19,20,33-35 We therefore chose to study the contribution of autophagy in the ER-mediated protection against neuronal cell death in Drosophila. To achieve this goal, we examined autophagy activation in Drosophila retina submitted to genetically-induced ER stress (ninaA mutant) and apoptotic signal (reaper overexpression). In ninaA mutant PRN where apoptosis was induced by the expression of reaper (rpr) under the control of rh1 promoter,10 we coexpressed the GFP-LC3/Atg8 reporter fusion protein construct (referred to as GFP-LC3) and sought GFP-LC3 puncta in dissected whole-mount retina36 (Fig. 3A–D and A′–D′). We found that PRNs subjected to mild ER stress and apoptosis exhibited a marked increase of GFP-LC3 puncta (Fig. 3D, D′ and E). In contrast, diffuse GFP-LC3 staining similar to that in wild-type controls was observed in ninaA mutant PRN and in PRN expressing rpr alone (Fig. 3A–C and 3A′–C′). LC3-I was converted into the active form of LC3 (LC3-II) only in ninaA mutant PRN subjected to rpr expression (Fig. 3F). On the western blot, we could also detect in this latter condition the appearance of free GFP, which is more resistant to lysosomal degradation than LC3 (Fig. S3A). This result suggests that autophagosomes have fused with lysosomes and that autophagic flux is functional. Next, we examined the expression of Ref(2)P/p62, as an indirect mean to evaluate the flux of autophagy in vivo. Ref(2)P/p62 is a multifunctional scaffold protein that is retained in autophagic vacuoles when the process of autophagy is compromised. Ref(2)P accumulation was observed in atg8a mutant as previously described,37 but not in ninaA mutant retina expressing rpr (Fig. S3B). These results show that the autophagy flux is functional in ninaA mutant PRN expressing rpr and indicate that mild ER stress activates autophagy, when combined with an apoptotic signal.
Figure 3. Activation of autophagy by combined ER stress and cell death signals. (A–D′) Whole-mount adult retina from flies expressing GFP-LC3 in PRNs. GFP-LC3 is in green and phalloidin labels PRN rhabdomeres in red. (A′–D′) Higher magnification view of GFP staining in the area surrounded by a white rectangle in (A–D). Scale bar ; 10 µm. (E) Quantification of the number of GFP-LC3 dots per PRN. (F) western blot with anti-GFP antibody showing the conversion of GFP-LC3-I to GFP-LC3-II in Drosophila retina. The western blots shown are representative of three independent experiments. (G–J′′) Substantia nigra sections after Tm and 6-OHDA injections in mice. (G–J) Anti-TH in red. (G′-J′) Anti-LC3 in green. (G′′–J′′) The merge shows TH, LC3 and DAPI (blue) for nuclei. (K) The graph shows the percentage of TH positive cells with LC3 punctates. Between 220 and 250 neurons from each of 3 different mice were assessed. Scale bar 20 µm. (L) western blot and quantification showing the conversion of LC3-I to LC3-II in SH-SY5Y cells treated or not treated with Tm and 6-OHDA in the presence of bafilomycin A1. Cells under starvation are used as a positive control. The western blots shown are representative of three independent experiments. *p ≤ 0,05, **p ≤ 0.01 in Student’s t-test.
In the light of our findings in the Drosophila PRN, we examined autophagy activation of DA neurons in the SN of mice subjected to Tm and 6-OHDA treatments (Fig. 3G–K). Basal LC3 levels were observed in DA neurons in both control mice and mice treated with Tm or 6-OHDA alone (Fig. 3G′–I′). By contrast, punctate LC3 staining was higher in DA neurons in animals subjected to both Tm and 6OHDA treatments (Fig. 3J′ and K). We also examined autophagy activation in SH-SY5Y cells submitted to Tm and 6-OHDA treatments. 6-OHDA induced an increased of LC3II form compared with untreated cells. Moreover, we detected a 2-fold increase in LC3II form by combined treatment with Tm and 6-OHDA compared with 6-OHDA alone (Fig. 3L). The increase of LC3II form was only observed in the presence of bafilomycin A1 that alters the lysosomal pH and prevents lysosomal degradation (Figs. 3L and S3C). This result indicates that the autophagic flux is functional in SH-SY5Y cells submitted to Tm and 6-OHDA treatments. Thus, autophagy is specifically increased in DA and SH-SY5Y neurons in response to a combination of Tm and 6-OHDA and may therefore be responsible for the neuroprotection.
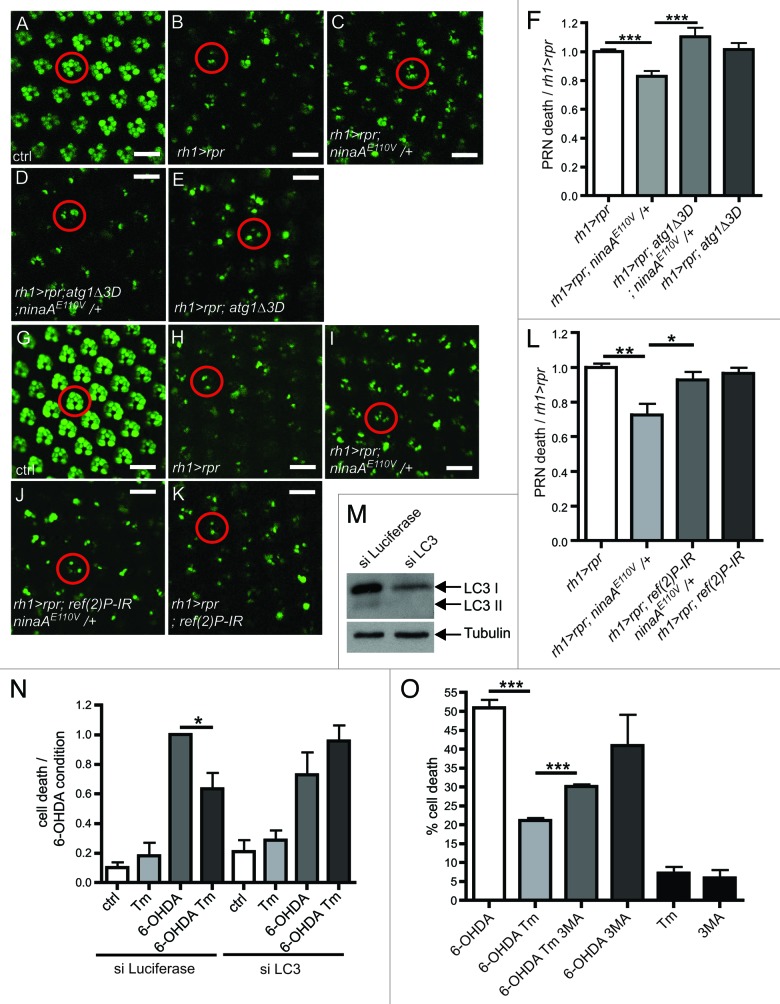
We then investigated whether autophagy activation is required for the ER-mediated protection in Drosophila PRN and in human SH-SY5Y cell line. We first inactivated autophagy in Drosophila PRN with mutations and RNAi knockdown of components of the autophagy pathway. We used the cornea neutralization technique to evaluate PRN viability in living flies.38,39 We found that in the presence of ectopic rpr expression, PRN loss was greater in double-mutant ninaAE110V/atg1Δ3D flies than in flies carrying only ninaAE110V mutation (Fig. 4A–F). Similarly, expression of a transgenic atg6-IR abolished ninaA mutant-mediated protection in Drosophila PRN (Fig. S3D). We then examined the role of Drosophila Ref(2)P/p62, a protein required for the formation of protein aggregates that are eliminated by autophagy in Drosophila brain.37 We found that the expression of transgenic ref(2)P-IR suppressed PRN protection in ninaA mutant (Fig. 4G–L). These results indicate that autophagic clearance contributes to the ER-mediated protection. Next, we examined if Tm-mediated protection required autophagy in SH-SY5Y cell line. To achieve this goal, we performed a siRNA treatment against atg8/LC3, which knocked down LC3 expression on a western blot (Fig. 4M). We found that LC3 knockdown abolished Tm-mediated protection in SH-SY5Y cell submitted to 6-OHDA (Fig. 4N). In addition, we used 3MA which inhibited autophagy induced by starvation or 6-OHDA/Tm treatments (Fig. S3E and S3F). We observed that the 3MA treatment also suppressed Tm-mediated protection in SH-SY5Y cell submitted to 6-OHDA (Fig. 4O). Altogether these results demonstrate that autophagy is required for ER-mediated protection.
Figure 4. Autophagy is required for ER-mediated neuroprotection. (A–E and G–K) Visualization of PRN viability in 16 h-old living flies expressing rh1 > GFP. (A–E) Visualization of PRN in retina overexpressing rpr (rh1 > rpr) and mutant for ninaAE110V/+ and atg1Δ3D. (F) Quantification of PRN loss in the various mutants (B–E) relative to rh1 > rpr (n = 10). (G–K) Visualization of PRN in retina overexpressing rpr (rh1 > rpr), ref(2)P-IR and mutant for ninaAE110V/+. (L) Quantification of PRN loss in the various mutants (H–K) relative to rh1 > rpr (n = 10). (M) western blotting showing LC3I/II levels after siRNA against LC3 in SH-SY5Y cell compared with control (siRNA luciferase). (N) SH-SY5Y cell viability was assessed by trypan blue exclusion after treatments with Tm, 6-OHDA and siRNA against LC3 or luciferase as control (n = 3). (O) SH-SY5Y cell viability was assessed by trypan blue exclusion after Tm and 6-OHDA treatments. 3-MA treatment is used to block autophagy. *p ≤ 0.05, **p < 0.01, ***p < 0.001 in Student’s t-test. Scale bar: 10 µm. The abbreviations used: rh1-gal4; UAS-GFP (rh1 > GFP), rh1-gal4;UAS-rpr (rh1 > rpr).
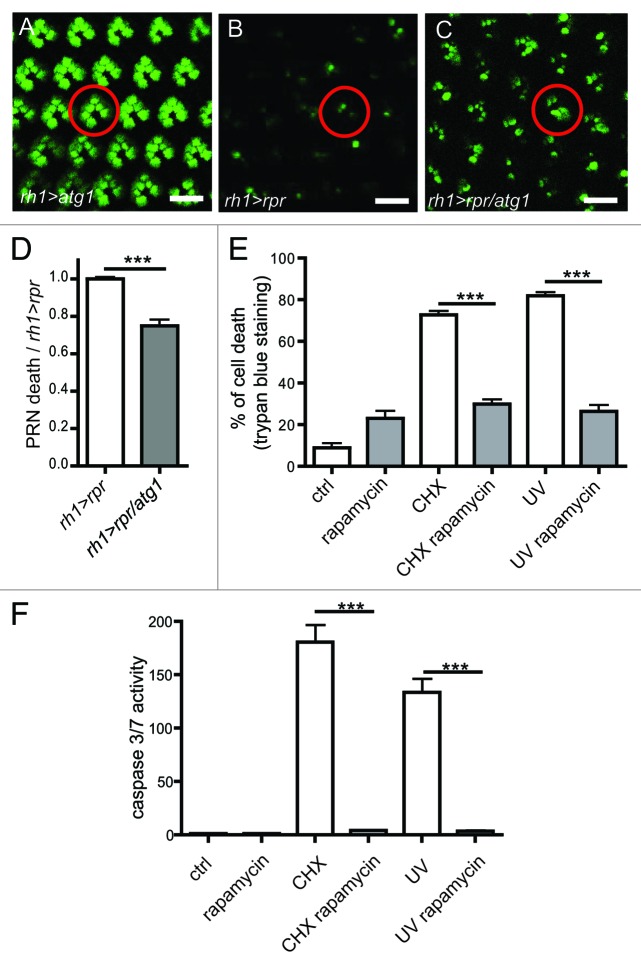
Last, we investigated the controversial issue of whether autophagy inhibits apoptosis.16,40 We found that ectopic atg1 expression rescued the viability of Drosophila PRN from rpr-induced apoptosis (Fig. 5A–D). In addition, rapamycin, an activator of autophagy,41 inhibited Drosophila S2 cell death induced by cycloheximide (CHX) or UVC and suppressed caspase activation (Fig. 5E and F). These results demonstrate that autophagy inhibits apoptosis.
Figure 5. Autophagy inhibits cell death. (A–C) Visualization of PRN viability in 16 h-old living flies expressing GFP. PRN express rpr (rh1 > rpr) and atg1 (rh1 > atg1). (D) Quantification of PRN loss in (B and C) relative to rpr (n = 6–7). (E) In vitro experiments on S2 cells to assess the cell viability after rapamycin and cycloheximide (CHX) treatments and UVC irradiation. Cell death was monitored by FACS analysis after incorporation of propidium iodide. (F) Caspase activity in S2 cells subjected rapamycin and cycloheximide (CHX) treatment and UVC irradiation. Results are expressed as ratio of caspase activity relative to control values (n = 3). Scale bar: 10 µm. ***p < 0.001 in Student’s t-test. The abbreviations used: rh1-gal4;UAS-rpr (rh1 > rpr), rh1-gal4; UAS-atg1 (rh1 > atg1).
Discussion
Our study provides new insight that mild ER stress promotes neuroprotection via the activation of autophagy. We have defined in vitro and in vivo experimental conditions in which the activation of UPR does not induce cell or organism lethality but rather promotes an adaptive response that protects from apoptotic stimuli. We show that a mild dose of tunicamycin (Tm) activates Ire1-Xbp1 and promotes protective autophagy in response to apoptotic stimuli. We have previously proposed that a preferential activation of Ire1-Xbp1 is responsible for protection in Drosophila S2 cells.10 This hypothesis is supported by our new results in which we show that mild dose of Tm induces the Ire1-Xbp1 pathway but not chop expression in mouse brains (Fig. S1). It is also possible that upon mild ER stress, chop is induced with a different kinetic than Ire1-Xbp1 and leads to a partial activation of its transcriptional targets as previously proposed.42 Thus, an adaptive response to mild ER stress may alter chop expression or Ddits/Chop activity and promotes survival. In a recent study, it was shown that adaptive suppression of the Atf4/Chop branch by toll-like receptor engagement, promotes survival in response to prolonged ER stress.43,44 It remains to be demonstrated whether selective activation of Ire1-Xbp1 or suppression of Atf4/Chop promotes neuroprotection in Drosophila and mouse Parkinson disease models.
Several previous studies investigated the link between the UPR and autophagy but a lot remains to be understood on how the UPR activates autophagy and promotes neuroprotection. Yeast cells subjected to severe ER stress manifest an autophagic response, which counterbalances ER expansion.13 In this model, severe ER stress alone induces autophagy, which in turn limits ER expansion. In a recent study, it was shown that mild ER stress promotes cardioprotection against an ischemic/reperfusion injury.45 In this model, autophagy activation could reduce subsequent lethal ischemic reperfusion injury. Another study reports that xbp1 deficiency induces autophagy in a mouse model of the amyotrophic lateral sclerosis.46 The xbp1 deficiency leads to an unexpected rescue of sod1 mutant motor neurons. Although several interpretations have been suggested to explain this result, it is possible that xbp1 deficiency induces an increase in basal ER stress leading to autophagy. From our results, we propose a model in which mild ER stress primes the cells to trigger neuroprotective autophagy upon an apoptotic stimulus. Our results also indicate that autophagy is neuroprotective, and we further delineate a distinct mechanism by which UPR regulates autophagy. The understanding of the complex relationship between UPR, autophagy and apoptosis probably resides in the identification and characterization of key factors that integrate these stress responses. In a recent study, it was shown that these responses are controlled by Bax inhibitor-1 (BI-1). BI-1 is a factor that inhibits IRE1-α, controls autophagy and apoptosis.47 In cells lacking BI-1, IRE1-α is activated and induces autophagy, promoting cell survival. A role of BI-1 remains to be investigated in the control of autophagy in cells submitted to mild ER stress and apoptotic signal.
Previous work has identified a link between apoptosis and autophagy.48 The authors have shown that stimulating cell death with TNFα in the presence of caspase inhibitors induced autophagy in L929 fibroblastic mouse cells.48 Based on their hypothesis, TNFα stimulates an alternative autophagic death program when caspases are inhibited. In our hands, we found that inhibition of caspases with p35 did not induce autophagy in PRN submitted to apoptosis by rpr expression (data not shown). We favor a model in which combined signals of the UPR and apoptosis induce autophagy. In addition, our results differ from the one presented by Lenardo and col.,48 as we demonstrated in several in vivo and in vitro models that autophagy is protective in cells submitted to mild ER stress. As discussed elsewhere, the opposite functions of autophagy on survival and death may depend on cell type and the level of autophagy activation.49
Autophagy has been proposed to inhibit cell death, however its role in the inhibition of apoptosis is a controversial subject.16,40 We have shown that autophagy inhibits caspase activation and apoptosis in several in vitro and in vivo paradigms (Figs. 4 and 5). We have observed a concomitant increase of autophagic markers and decrease of caspase activation in cell submitted to both mild ER stress and apoptosis (Figs. 2, 3 and S2). Our findings suggest that cells switch from an apoptotic to an autophagic response when submitted to both mild ER stress and apoptotic signal. However, how autophagy inhibits apoptosis remains to be uncovered. Autophagosomes could engulf and degrade impaired mitochondria (mitophagy) to prevent the subsequent activation of apoptotic pathway.50 Another hypothesis is that autophagy could directly sequester pro-apoptotic factors, such as caspases, and promote their degradation as previously proposed in a mouse model of Alzheimer disease.51 Further work is required to elucidate this mechanism.
Relevance to pathology
We found that mild ER stress is protective in the Parkinson 6-OHDA mouse model, showing that maintaining UPR at a moderate level could protect against Parkinson disease. After injection in the striatum, 6-OHDA is selectively taken in DA by retrograde transport.52 6-OHDA induces an oxidative burst and caspase activation, which leads to DA death.28 We show that Tm treatment activates mild UPR responses, correlates to reduced DA death and improved locomotor function in mice bearing 6-OHDA lesions. Moreover, mild ER stress protects DA neurons of the SN from 6-OHDA-induced death by limiting caspase activation (Fig. S2) as previously observed in human neuroblastoma cell lines.28 As in the fly paradigm, the increased autophagy in DA submitted to Tm and 6-OHDA suggests that autophagy is an active player of neuroprotection in mice. Our results incite new investigations into therapeutic possibilities to trigger and maintain ER stress at a moderate level, so that the stress response protects against or delay the onset of neurodegeneration, or retard the disease progress.
Materials and Methods
Drosophila genetics
Flies were maintained at 25°C in a 12:12 h light cycle. The wild-type flies used for this study were CantonS strain. The ninaAE110V fly stock is a kind gift from Charles Zuker.53 The rh1-gal4 fly stock is a generous gift from Jessica Treisman.38 UAS-reaper (rpr), UAS-lacZ and atg8KQ70569, elav-gal4 and th-gal4 were obtained from Bloomington stock. UAS-atg1 and atg1Δ3D stocks were a kind gift from Thomas Neufeld,54 UAS-GFP-LC3 was a kindly provided by Harald Stenmark,36 UAS-atg6-IR were kindly obtained from Udai Bhan Pandey and UAS-hu-α-syn was kindly given by Mel Feany. The following genetic combinations were used to express transgenes in adult outer PRN: (1) rh1-gal4; UAS-GFP; UAS-rpr, (2) rh1-gal4; UAS-GFP, (3) rh1-gal4; UAS-GFP-LC3, (4) rh1-gal4; UAS-GFP-LC3; UAS-rpr, (5) rh1-gal4; UAS-atg1, (6) rh1-gal4; ninaAE110V/UAS-GFP-LC3, (7) rh1-gal4; ninaAE110V/UAS-GFP-LC3; UAS-rpr, (8) rh1-gal4; UAS-rpr, (9) rh1-gal4; ninaAE110V/+; UAS-rpr, (10) rh1-gal4;ninaAE110V; UAS-rpr, (11) rh1-gal4; ninaAE110V/UAS-GFP; UAS-rpr, (12) rh1-gal4; ninaAE110V/UAS-GFP; UAS-rpr/atg1Δ3D, (13) rh1-gal4; UAS-GFP/ UAS-lacZ; UAS-atg1, (14) rh1-gal4; UAS-GFP; UAS-atg1/ UAS-rpr. (15) elav-gal4; UAS-hu-α-syn; (16) elav-gal4; UAS-GFP; (17) UAS-hu-α-syn; th-gal4 (18) UAS-lacZ; th-gal4.
Drosophila pharmacological treatments
ER stress was pharmacologically induced using tunicamycin (Tm; Covalab, 11089-65-9), an inhibitor of protein glycosylation. Twenty males and 20 females aged for 24 h were collected and starved for 5 h on 0.8% agarose, 1X PBS medium. Flies were then transferred in vial containing food (0.8% agarose, 10% sucrose, 1X PBS medium) supplemented with Tm (1μg/ml; 10μg/ml) or vehicle solution (Dimethyl sufoxide, 2% Sigma Aldrich, D8418) for 4 h.
Immunostaining on eye whole mount
GFP-LC3 labeling was performed on Drosophila whole-mount retina as previously described.55 Briefly, Drosophila heads were bisected in the middle with a scalpel. Brain tissue was removed to expose retina underneath. The retinae were fixed in 4% PFA for 15min. GFP-LC3 was revealed by immunostaining using a rabbit anti-GFP antibody (1/200, Invitrogen, A-6455) followed by an anti-rabbit secondary antibody (Alexa 488 1/400, Invitrogen, A-21206). Photoreceptor rhabdomeres were visualized using Actin coupled with phalloidin staining (1/400, Sigma Aldrich, 77418-1EA). Retinae were mounted in DAPI mounting media (Vectashield, AbCys, H1500). Fluorescent images were obtained using a Leica SP5 confocal microscope.
Mouse protocol
All animal protocols were approved by the regional ethics committee for animal experiments, Rhônes Alpes (authorization n°153). C57Bl6/J female mice (10 weeks old) were used for this study. Tm (Covalab, 11089-65-9) was administered by intraperitoneal (I/P) injection (0.01 mg/kg, 0.1mg/kg or 4.5 mg/kg). Eighteen hours after Tm treatment, 8 μg of 6-hydroxydopamine (6-OHDA, 4 μg/μl, Tocris, 2547) in 0.02% ascorbic acid was injected stereotaxically into the left striatum of the mouse brain to induce Parkinson disease-like injury.56 Rotational behavior tests were performed on 6-OHDA-treated mice to evaluate alterations of the nigra-striatal pathway. Rotational asymmetry was induced by I/P injection of apomorphine at 0.6 mg/kg (Sigma Aldrich, A4393) on days 3, 4 and 7 as described.30 Motor behavior was tested to assess motricity following Tm injection. Walking distance (cm) was measured over a period of 2 min three times for Tm-treated (n = 7) and control mice (n = 8).
Immunostaining on mice brain sections
Visualization of dopaminergic neurons was performed in mice substantia nigra sections. Mice were sacrificed by lethal I/P injection of pentobarbital, then perfused intracardiacaly with saline solution and 4% PFA for fixation. Brains were extracted, further post-fixed in 4% PFA for 2h, transferred to 30% sucrose solution at 4°C, and serially freezed-sectioned. Fourteen μm-thick floating brain sections were transferred into blocking solution (PBS-triton 0.1%, 4% BSA, 10% normal goat serum) for 1h at RT. DA neurons were visualized using an anti-Tyrosine Hydroxylase antibody (α-TH, 1:2000, Millipore, Ab152) and anti-LC3 antibody (1:800, Cell Signaling, 2775S). Specifically, brain sections were incubated with the primary antibodies at 4°C overnight and with secondary antibodies Alexa 555 (1: 500, Invitrogen, A21424) and Alexa 488 (1: 500, Invitrogen, A-21206) at RT for 2h in the dark. Fluorescent images were taken using an ApoTome Imager M2 with an AxioCam MRm (Zeiss). The loss of DA neurons after 6-OHDA treatment was defined as the percent of TH positive cells in the 6-OHDA injured side compared with the contralateral side. Autophagy level in DA neuron was defined as the percent of TH positive cells with LC3 punctates.
Cell culture
Drosophila S2 cells were cultured in Drosophila Schneider medium (Invitrogen, 21720024) supplemented with 10% fetal bovine serum. Cells were pre-incubated with 0.4 μg/ml of rapamycin for 40 h, then subjected to treatment with 10 µM cycloheximide (Sigma-Aldrich, C1988) or 300 mJ/cm2 UV C (UVC) with a UV irradiator (Vilber Lourmat 254 nm, LBX). After 8 h, the cells were stained with 50µg/ml propidium iodide and analyzed by flow cytometry (FACSCalibur4C) to measure cell death.
SH-SY5Y neuroblastoma cell line were cultured in DMEM:HamF12 (1:1) supplemented with L-Glu plus nonessential amino acid (1%) and 10% FCS (Invitrogen, 10270106). Cells were pre-incubated with 0.5 μg/ml of Tm for 4 h, then subjected to treatment with 50 µM 6-OHDA (Tocris, 2547). After 16 h the cells were stained with 50 μg/ml propidium iodide and analyzed by flow cytometry (FACSCalibur4C) to measure cell death. For starvation, SH-SY5Y were maintained for 24 h in DMEM:HamF12 (1:1) supplemented with L-Glu plus nonessential amino acid (1%) without FCS.
Inhibition of autophagy in SH-SY5Y
Inhibition of autophagy was performed via RNAi as previously described.57 Briefly, 40 nM of small interfering RNA (siRNA) sequences targeting LC3 (5′-GAAGGCGCUUACAGCUCAA-3′) or siRNA targeting luciferase, used as negative control (5′-CGUACGCGGAAUACUUCGA-3′), were introduced in 0,1% lipofectamine 2000 (Invitrogen, 11668019) at day 1. siRNA experiment was repeated 48 h following the first siRNA. Inhibition of autophagy was assessed by western blotting experiment using LC3 antibody (Cell Signaling, 2775S). Autophagy was also inhibited by 3-methyladenine (3-MA; Sigma Aldrich, M9281). Cells were incubated with Tm (0.5 μg/ml) for 4 h, then subjected to 3-MA treatment at 10 µM and 6-OHDA at 50 µM (Tocris, 2547) for 16 h. Cell viability was assessed by trypan blue staining (Sigma Aldrich, T8154). In Figure 3L, autophagy flux was inhibited by adding bafilomycin A1 to the cells at 10 nM for 12 h.
Statistical analysis
Data from mRNA expression, photoreceptor cell survival, autophagy activation, 6-OHDA cytototoxicity, rotatory and motricity behavior assays were analyzed using Student’s t-test (2-group comparison). Level of significance was set at p ≤ 0.05.
Supplementary Material
Acknowledgments
This work was supported by grants from the Fondation pour la Recherche Médicale, from the CNRS (ATIP) to BM and AF and from the cluster 11 HNV (Rhone Alpes, France) for CL. This work was made possible by the DROSO-TOOLS facility and PLATIM facilities of the UMS3444, Biosciences, Lyon, France. We thank Carmen Garrido for technical help, Patrice Codogno, Ioannis Nezis and Dali Ma, for critical reading of the manuscript and our colleagues and Bloomington center for fly stocks and reagents.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Materials
Additional Material and Methods may be found here: www.landesbioscience.com/journals/autophagy/article/19716
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/19716
References
- 1.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–29. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 2.Ryoo HD, Steller H. Unfolded protein response in Drosophila: why another model can make it fly. Cell Cycle. 2007;6:830–5. doi: 10.4161/cc.6.7.4064. [DOI] [PubMed] [Google Scholar]
- 3.Rasheva VI, Domingos PM. Cellular responses to endoplasmic reticulum stress and apoptosis. Apoptosis. 2009;14:996–1007. doi: 10.1007/s10495-009-0341-y. [DOI] [PubMed] [Google Scholar]
- 4.Loewen CA, Feany MB. The unfolded protein response protects from tau neurotoxicity in vivo. PLoS One. 2010;5:5. doi: 10.1371/journal.pone.0013084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matus S, Glimcher LH, Hetz C. Protein folding stress in neurodegenerative diseases: a glimpse into the ER. Curr Opin Cell Biol. 2011;23:239–52. doi: 10.1016/j.ceb.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Malhotra JD, Kaufman RJ. The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol. 2007;18:716–31. doi: 10.1016/j.semcdb.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hetz C, Bernasconi P, Fisher J, Lee AH, Bassik MC, Antonsson B, et al. Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1alpha. Science. 2006;312:572–6. doi: 10.1126/science.1123480. [DOI] [PubMed] [Google Scholar]
- 8.Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 9.Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B, et al. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318:944–9. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mendes CS, Levet C, Chatelain G, Dourlen P, Fouillet A, Dichtel-Danjoy ML, et al. ER stress protects from retinal degeneration. EMBO J. 2009;28:1296–307. doi: 10.1038/emboj.2009.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calabrese V, Cornelius C, Dinkova-Kostova AT, Calabrese EJ, Mattson MP. Cellular stress responses, the hormesis paradigm, and vitagenes: novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid Redox Signal. 2010;13:1763–811. doi: 10.1089/ars.2009.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mao XR, Crowder CM. Protein misfolding induces hypoxic preconditioning via a subset of the unfolded protein response machinery. Mol Cell Biol. 2010;30:5033–42. doi: 10.1128/MCB.00922-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernales S, McDonald KL, Walter P. Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS Biol. 2006;4:e423. doi: 10.1371/journal.pbio.0040423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–9. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 15.Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–4. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- 16.Wang T, Lao U, Edgar BA. TOR-mediated autophagy regulates cell death in Drosophila neurodegenerative disease. J Cell Biol. 2009;186:703–11. doi: 10.1083/jcb.200904090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nixon RA, Yang DS. Autophagy failure in Alzheimer’s disease-locating the primary defect. Neurobiol Dis. 2011;43:38–45. doi: 10.1016/j.nbd.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12:9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–75. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neufeld TP, Baehrecke EH. Eating on the fly: function and regulation of autophagy during cell growth, survival and death in Drosophila. Autophagy. 2008;4:557–62. doi: 10.4161/auto.5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feany MB, Bender WW. A Drosophila model of Parkinson’s disease. Nature. 2000;404:394–8. doi: 10.1038/35006074. [DOI] [PubMed] [Google Scholar]
- 22.Friggi-Grelin F, Coulom H, Meller M, Gomez D, Hirsh J, Birman S. Targeted gene expression in Drosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. J Neurobiol. 2003;54:618–27. doi: 10.1002/neu.10185. [DOI] [PubMed] [Google Scholar]
- 23.Neckameyer WS, Quinn WG. Isolation and characterization of the gene for Drosophila tyrosine hydroxylase. Neuron. 1989;2:1167–75. doi: 10.1016/0896-6273(89)90183-9. [DOI] [PubMed] [Google Scholar]
- 24.Greer CL, Grygoruk A, Patton DE, Ley B, Romero-Calderon R, Chang HY, et al. A splice variant of the Drosophila vesicular monoamine transporter contains a conserved trafficking domain and functions in the storage of dopamine, serotonin, and octopamine. J Neurobiol. 2005;64:239–58. doi: 10.1002/neu.20146. [DOI] [PubMed] [Google Scholar]
- 25.Seugnet L, Galvin JE, Suzuki Y, Gottschalk L, Shaw PJ. Persistent short-term memory defects following sleep deprivation in a drosophila model of Parkinson disease. Sleep. 2009;32:984–92. doi: 10.1093/sleep/32.8.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryu EJ, Harding HP, Angelastro JM, Vitolo OV, Ron D, Greene LA. Endoplasmic reticulum stress and the unfolded protein response in cellular models of Parkinson’s disease. J Neurosci. 2002;22:10690–8. doi: 10.1523/JNEUROSCI.22-24-10690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Renna M, Jimenez-Sanchez M, Sarkar S, Rubinsztein DC. Chemical inducers of autophagy that enhance the clearance of mutant proteins in neurodegenerative diseases. J Biol Chem. 2010;285:11061–7. doi: 10.1074/jbc.R109.072181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hara H, Kamiya T, Adachi T. Endoplasmic reticulum stress inducers provide protection against 6-hydroxydopamine-induced cytotoxicity. Neurochem Int. 2011;58:35–43. doi: 10.1016/j.neuint.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 29.Przedborski S, Levivier M, Kostic V, Jackson-Lewis V, Dollison A, Gash DM, et al. Sham transplantation protects against 6-hydroxydopamine-induced dopaminergic toxicity in rats: behavioral and morphological evidence. Brain Res. 1991;550:231–8. doi: 10.1016/0006-8993(91)91323-S. [DOI] [PubMed] [Google Scholar]
- 30.da Conceição FS, Ngo-Abdalla S, Houzel JC, Rehen SK. Murine model for Parkinson’s disease: from 6-OH dopamine lesion to behavioral test. J Vis Exp. 2010 doi: 10.3791/1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, et al. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–77. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanaka K, Ogawa N, Asanuma M. Molecular basis of 6-hydroxydopamine-induced caspase activations due to increases in oxidative stress in the mouse striatum. Neurosci Lett. 2006;410:85–9. doi: 10.1016/j.neulet.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 33.Mollereau B. Cell death: what can we learn from flies? Editorial for the special review issue on Drosophila apoptosis. Apoptosis. 2009;14:929–34. doi: 10.1007/s10495-009-0383-1. [DOI] [PubMed] [Google Scholar]
- 34.Conradt B. Genetic control of programmed cell death during animal development. Annu Rev Genet. 2009;43:493–523. doi: 10.1146/annurev.genet.42.110807.091533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Z, Klionsky DJ. Eaten alive: a history of macroautophagy. Nat Cell Biol. 2010;12:814–22. doi: 10.1038/ncb0910-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rusten TE, Lindmo K, Juhász G, Sass M, Seglen PO, Brech A, et al. Programmed autophagy in the Drosophila fat body is induced by ecdysone through regulation of the PI3K pathway. Dev Cell. 2004;7:179–92. doi: 10.1016/j.devcel.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 37.Nezis IP, Simonsen A, Sagona AP, Finley K, Gaumer S, Contamine D, et al. Ref(2)P, the Drosophila melanogaster homologue of mammalian p62, is required for the formation of protein aggregates in adult brain. J Cell Biol. 2008;180:1065–71. doi: 10.1083/jcb.200711108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mollereau B, Wernet MF, Beaufils P, Killian D, Pichaud F, Kühnlein R, et al. A green fluorescent protein enhancer trap screen in Drosophila photoreceptor cells. Mech Dev. 2000;93:151–60. doi: 10.1016/S0925-4773(00)00287-2. [DOI] [PubMed] [Google Scholar]
- 39.Gambis A, Dourlen P, Steller H, Mollereau B. Two-color in vivo imaging of photoreceptor apoptosis and development in Drosophila. Dev Biol. 2011;351:128–34. doi: 10.1016/j.ydbio.2010.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim YI, Ryu T, Lee J, Heo YS, Ahnn J, Lee SJ, et al. A genetic screen for modifiers of Drosophila caspase Dcp-1 reveals caspase involvement in autophagy and novel caspase-related genes. BMC Cell Biol. 2010;11:9. doi: 10.1186/1471-2121-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ravikumar B, Berger Z, Vacher C, O’Kane CJ, Rubinsztein DC. Rapamycin pre-treatment protects against apoptosis. Hum Mol Genet. 2006;15:1209–16. doi: 10.1093/hmg/ddl036. [DOI] [PubMed] [Google Scholar]
- 42.Rutkowski DT, Arnold SM, Miller CN, Wu J, Li J, Gunnison KM, et al. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol. 2006;4:e374. doi: 10.1371/journal.pbio.0040374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woo CW, Cui D, Arellano J, Dorweiler B, Harding H, Fitzgerald KA, et al. Adaptive suppression of the ATF4-CHOP branch of the unfolded protein response by toll-like receptor signalling. Nat Cell Biol. 2009;11:1473–80. doi: 10.1038/ncb1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woo CW, Kutzler L, Kimball SR, Tabas I. Toll-like receptor activation suppresses ER stress factor CHOP and translation inhibition through activation of eIF2B. Nat Cell Biol. 2012;14:192–200. doi: 10.1038/ncb2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petrovski G, Das S, Juhasz B, Kertesz A, Tosaki A, Das DK. Cardioprotection by endoplasmic reticulum stress-induced autophagy. Antioxid Redox Signal. 2011;14:2191–200. doi: 10.1089/ars.2010.3486. [DOI] [PubMed] [Google Scholar]
- 46.Hetz C, Thielen P, Matus S, Nassif M, Court F, Kiffin R, et al. XBP-1 deficiency in the nervous system protects against amyotrophic lateral sclerosis by increasing autophagy. Genes Dev. 2009;23:2294–306. doi: 10.1101/gad.1830709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Castillo K, Rojas-Rivera D, Lisbona F, Caballero B, Nassif M, Court FA, et al. BAX inhibitor-1 regulates autophagy by controlling the IRE1α branch of the unfolded protein response. EMBO J. 2011;30:4465–78. doi: 10.1038/emboj.2011.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu L, Alva A, Su H, Dutt P, Freundt E, Welsh S, et al. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004;304:1500–2. doi: 10.1126/science.1096645. [DOI] [PubMed] [Google Scholar]
- 49.Marino G, Madeo F, Kroemer G. Autophagy for tissue homeostasis and neuroprotection. Curr Opin Cell Biol. 2011;23:198–206. doi: 10.1016/j.ceb.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 50.Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang DS, Kumar A, Stavrides P, Peterson J, Peterhoff CM, Pawlik M, et al. Neuronal apoptosis and autophagy cross talk in aging PS/APP mice, a model of Alzheimer’s disease. Am J Pathol. 2008;173:665–81. doi: 10.2353/ajpath.2008.071176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Przedborski S, Levivier M, Jiang H, Ferreira M, Jackson-Lewis V, Donaldson D, et al. Dose-dependent lesions of the dopaminergic nigrostriatal pathway induced by intrastriatal injection of 6-hydroxydopamine. Neuroscience. 1995;67:631–47. doi: 10.1016/0306-4522(95)00066-R. [DOI] [PubMed] [Google Scholar]
- 53.Ondek B, Hardy RW, Baker EK, Stamnes MA, Shieh BH, Zuker CS. Genetic dissection of cyclophilin function. Saturation mutagenesis of the Drosophila cyclophilin homolog ninaA. J Biol Chem. 1992;267:16460–6. [PubMed] [Google Scholar]
- 54.Scott RC, Juhász G, Neufeld TP. Direct induction of autophagy by Atg1 inhibits cell growth and induces apoptotic cell death. Curr Biol. 2007;17:1–11. doi: 10.1016/j.cub.2006.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Domingos PM, Brown S, Barrio R, Ratnakumar K, Frankfort BJ, Mardon G, et al. Regulation of R7 and R8 differentiation by the spalt genes. Dev Biol. 2004;273:121–33. doi: 10.1016/j.ydbio.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 56.Bové J, Serrats J, Mengod G, Cortés R, Tolosa E, Marin C. Neuroprotection induced by the adenosine A2A antagonist CSC in the 6-OHDA rat model of parkinsonism: effect on the activity of striatal output pathways. Exp Brain Res. 2005;165:362–74. doi: 10.1007/s00221-005-2302-1. [DOI] [PubMed] [Google Scholar]
- 57.Plowey ED, Cherra SJ, 3rd, Liu YJ, Chu CT. Role of autophagy in G2019S-LRRK2-associated neurite shortening in differentiated SH-SY5Y cells. J Neurochem. 2008;105:1048–56. doi: 10.1111/j.1471-4159.2008.05217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.