Abstract
Huntington disease (HD) is caused by an extended polyglutamine [poly(Q)] stretch in the Huntingtin (HTT) protein, and is associated with the accumulation of intracellular protein aggregates, onset of progressive chorea, psychiatric symptoms and dementia. Although the mechanism underlying the pathological effects of mutant HTT (mHTT) remains highly controversial, accumulating evidence suggest that protein-folding stress at the endoplasmic reticulum (ER) may contribute to mHTT-mediated degeneration. ER stress is alleviated by the activation of an adaptive reaction known as the unfolded protein response (UPR), whereas chronic ER stress triggers apoptosis by the same pathway. However, most of the studies linking ER stress with HD in vivo are correlative. UPR signaling is initiated by the activation of at least three distinct stress sensors located at the ER membrane known as ERN1/IRE1α, EIF2AK3/PERK and ATF6. These stress sensors control the expression of specialized transcription factors that modulate the upregulation of a variety of target genes involved in folding, protein quality control, autophagy and protein synthesis.
Keywords: Huntington disease, autophagy, XBP1, ER stress, aging, neurodegeneration
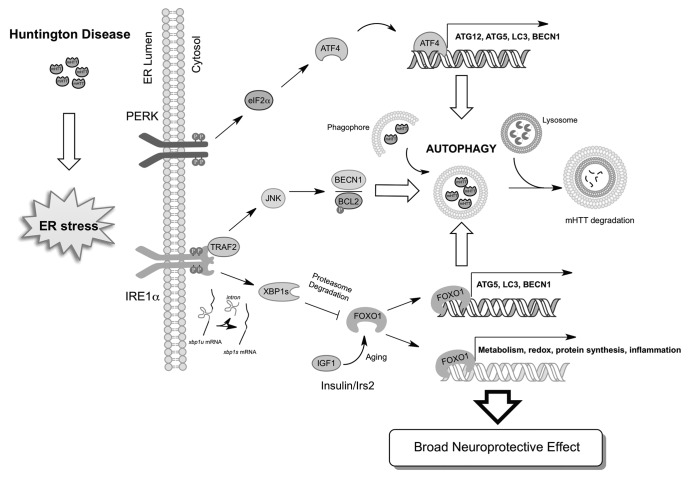
Recent studies indicate that the UPR closely regulates macroautophagy (hereafter referred to as autophagy) levels, which is the main cellular pathway involved in the degradation of abnormal protein aggregates and damaged organelles through the lysosomal pathway. Autophagy is becoming a relevant target for disease intervention in most neurodegenerative diseases. Protein homeostasis is currently viewed as a dynamic network of signaling events that constantly adjust perturbation in every step of protein folding/synthesis and degradation of proteins. This homeostatic balance has been evidenced by many studies in the HD field where manipulation of the UPR has clear consequences in the degradation of mHTT by autophagy. For example, it was recently demonstrated the one role of ERN1 is as a regulator of mHTT clearance. Most of the studies have shown that ERN1 can control autophagy levels by recruiting the adaptor protein TRAF2 and the activation of MAPK8/JNK. MAPK8 in turn activates the BECN1 complex possibly due to the phosphorylation of BCL2, disrupting its interaction with BECN1. However, the authors show that upon expression of mHTT, the ERN1-TRAF2 complex inhibits autophagy flux, enhancing the accumulation of mHTT aggregates (Fig. 1). The authors provide correlative evidence indicating that reducing ERN1 levels in a fly model of HD protects against eye degeneration. Another report indicates that polyQ expression triggers the activation of EIF2AK3 and the phosphorylation of its downstream target EIF2S1/eIF2α, activating autophagy possibly due to the upregulation of ATG12 mRNA, encoding an essential autophagy regulator (Fig. 1). ATF4, the downstream transcription factor of EIF2AK3/EIF2S1 has been recently shown to induce the expression of ATG12, ATG5 and BECN1, providing a direct link between UPR signaling and the control of autophagy. We have previously described another connection between ERN1 signaling and autophagy where targeting the downstream transcription factor XBP1 triggers autophagy, which can provide protection against amyotrophic lateral sclerosis.
Figure 1. Signaling crosstalk between the UPR and autophagy in the nervous system. mHTT pathogenesis triggers ER stress, engaging UPR stress sensors. Activated ERN1/IRE1 induces the TRAF2-MAPK8 pathway, inducing autophagy independent of XBP1. PERK activation and phosphorylation of EIF2S1/eIF2α, leads to upregulation of ATG12 possibly through the transcriptional activity of ATF4. XBP1 negatively regulates the levels of FOXO1, inducing its degradation by the proteasome. Then, inactivation of XBP1 protects against HD due to increased accumulation of FOXO1 and the upregulation of its target genes, which include many autophagy regulators. Signaling events related with aging (i.e., insulin and IGF1 signaling) may intersect in FOXO1 and XBP1 to modulate autophagy levels in HD.
Although the role of the UPR in HD seems to be broadly accepted and is discussed as a relevant factor in the pathogenesis of the disease in many reviews each year, in reality most of the studies are correlative and the field has awaited the genetic tools to definitively prove or disprove this hypothesis. We recently investigated the contribution of ATF4 and XBP1 to HD using mouse models of the disease. Unexpectedly, despite predictions that XBP1 deficiency would enhance the severity of experimental HD, we observed that these mice are markedly more resistant to developing the disease. XBP1 deficiency enhances neuronal survival and improves motor performance of HD mice expressing full-length mHTT. The mechanism of protection appears to be related to the upregulation of autophagy and the degradation of mHTT. We showed using subcellular fractionation and ER studies enhanced targeting of mHTT to autophagosomes in vivo.
Unexpectedly, although XBP1 mRNA splicing is activated in HD mouse models, disease progression is not associated with a clear ER stress response. Consistent with this, targeting ATF4 did not affect mHTT levels in vivo, suggesting that the protective effects of XBP1 deficiency may represent a function independent of ER stress. We explored possible mechanisms underlying the effects of XBP1 on HD and monitored the levels of the transcription factor FOXO1 in our experimental system. This was based on a recent study describing a negative regulation of FOXO1 by XBP1s at the post-translational level in pancreatic β cells by a physical interaction. This regulation is independent of XBP1 transcriptional activity, due to targeting of FOXO1 to proteasome-mediated degradation. FOXO1 is a key factor modulating aging processes in the brain and controls autophagy in neurons. At the molecular level we found a negative regulation of the transcription factor FOXO1 by XBP1 in vivo. In cell culture, we demonstrated that expression of FoxO1 enhances autophagy levels and reduces the levels of mHTT aggregates, providing a novel link between two major stress pathways. Our results are indicative of a critical crosstalk between the ERN1-XBP1 arm of the UPR and FOXO1-autophagy in HD neurons (Fig. 1), suggesting possible therapeutic benefits of targeting this pathway in a disease context. In addition to controlling autophagy, FOXO1 participates in others processes such as mitochondrial metabolism, oxidative stress and the insulin-IGF1 pathway that may contribute to neuroprotective effects observed in XBP1-deficient animals. Interestingly, a recent report showed that manipulation of Insulin signaling (i.e., IRS2) in vivo leads to protection against HD, correlating with enhanced FOXO1 activity and autophagy induction. Finally, genetic studies in C. elegans indicate that XBP1 has a role in aging through the FOXO-insulin-IGF1 signaling pathway. Thus, FOXO transcription factors may represent an interesting signaling intersection between the UPR and autophagy.
The current evidence illuminates how fundamental homeostatic processes such as the UPR pathway and autophagy contribute to handling cellular stress and provides conclusive evidence in favor of a novel physiological function of XBP1 as an adjustor of cellular stress in HD and other protein misfolding disorders. Future therapeutic strategies to manipulate XBP1 levels may have broad beneficial consequences to alleviate degeneration.
Acknowledgments
This article was funded by CHDI Foundation Inc. (C.H.) and FONDECYT no. 3100039 (R.L.V.). In addition we received support from FONDECYT no. 1100176, FONDAP grant no. 15010006, Millennium Institute No. P09-015-F, Muscular Dystrophy Association, ALS Therapy Alliance, North American Spine Society and Alzheimer Disease Foundation (C.H.).
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/20139