Abstract
Autophagy plays a protective role during many viral and bacterial infections. Predictably, evolution has led to several viruses developing mechanisms by which to evade the inhibitory effects of the pathway. However, one family of viruses, the picornaviruses, has gone one step further, by actively exploiting autophagy. Using mice in which Atg5 has been conditionally deleted in pancreatic acinar cells, we have studied the outcome of infection by coxsackievirus B3 (CVB3), a member of the enterovirus genus and picornavirus family. Two key findings emerged: disruption of autophagy (1) dramatically compromised virus replication in vivo, and (2) significantly limited pancreatic disease.
Keywords: autophagy, pancreatitis, Atg5, enterovirus, coxsackievirus, pancreas, acinar, in vivo, picornavirus, CVB3
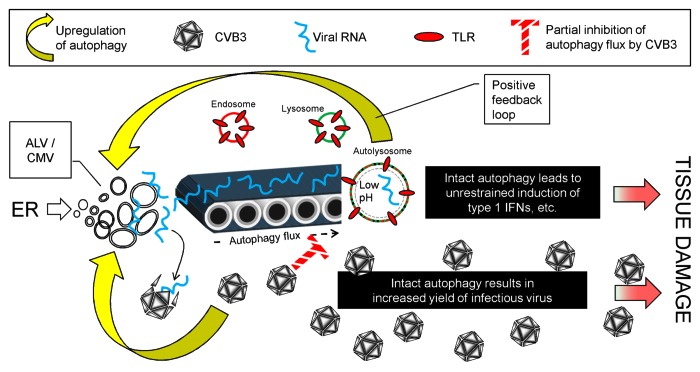
Over the past few years, several in vitro studies using immortalized cells have shown a direct relationship between autophagy and picornavirus titers; increasing or decreasing autophagy causes parallel changes in virus yield. The observed effects were modest, ranging from 10-fold to as little as 1.3-fold, but we considered it important to evaluate the interactions between picornaviruses and autophagy in an in vivo model. Using a Cre/lox approach, a mouse line was developed in which an exon from the Atg5 gene was deleted specifically in pancreatic acinar cells. Similar mice have been generated by others, and were reported to be largely free from overt pancreatic dysfunction. We confirmed this observation, showing that—despite a clear deficit in pancreatic autophagy—the mice were generally healthy, and signs of pancreatic disease were minimal. Thus, basal autophagy appears to be dispensable for pancreatic acinar cells. However, CVB3 infection reveals two striking differences between Atg5f/f/Cre+ mice and littermate controls. First, virus titers are dramatically reduced in the Atg5-deficient pancreata. The reduction was present at all time points studied, but is greatest early in infection (~2000-fold lower at day 1 post infection). Virus replication is largely restored in acinar cells containing a single intact copy of Atg5. Hence, in vivo CVB3 replication benefits from, but does not absolutely require, functional Atg5 protein in pancreatic acinar cells. Second, we observed markedly less CVB3-induced pancreatic pathology in the Atg5-deficient mice: there was less destruction of acinar cells, fewer inflammatory infiltrates, and a minimal rise in serum amylase. Figure 1 presents, in diagrammatic form, our hypotheses regarding these two major in vivo observations.
Figure 1. CVB3 and autophagy: Proposed interactions, and their pathogenic effects. CVB3 infection upregulates autophagy (lower yellow arrow), increasing the abundance of small autophagy-like vesicles (ALVs) which may provide a replication platform for the virus, allowing it to replicate to high titers, causing extensive disease (lower red arrow). Some of the viral RNA is captured by the autophagy pathway (conveyor belt) and ultimately encounters and activates TLRs in the autolysosome. This causes further upregulation of autophagy, thereby increasing the delivery of viral RNA to the TLRs; i.e., we propose that a positive feedback loop may exist (top yellow arrow). Viral proteins substantially, but incompletely, block autophagy flux (cross-hatched red T). These blocking effects may delay, but cannot prevent, the explosive innate response, which causes immunopathological damage, exacerbating disease (upper red arrow). In the absence of an intact autophagy pathway (Atg5f/f/Cre+ mice) both viral replication and TLR activation are reduced, thereby limiting disease.
The Effects of Autophagy on CVB3 Replication In Vivo
Picornavirus infection induces a plethora of small membranous structures [autophagy-like vesicles (ALVs; a.k.a. compound membrane vesicles, CMVs)] that appear to be related to autophagy. It has been proposed that these may act as two-dimensional surfaces upon which viral replication takes place. CVB3 appears to increase the abundance of these small vesicles in two ways; by upregulating autophagy, and by significantly inhibiting autophagy flux, preventing the normal flux-related consumption of these structures. ALVs/CMVs are undoubtedly induced by picornavirus infection, but their role in virus replication remains unproven. Using electron microscopy to analyze CVB3-infected acinar cells from normal mice, we observed large highly-geometric arrays comprising closely-packed ~8-nm diameter subunits that may represent individual viral RNA polymerase proteins. We have suggested that these lattices may be sites of active viral RNA synthesis and, consistent with this, they are rarely detected in infected acinar cells from Atg5f/f/Cre+ mice, in which both virus RNA levels and infectious virus yield, are reduced. These putative replication lattices are invariably adjacent to rough endoplasmic reticulum, and do not show any clear colocalization with ALVs/CMVs; thus, the relative importance of ALVs and lattices in CVB3 replication remains to be determined. Compared with normal cells, Atg5f/f/Cre+ acinar cells are equally susceptible to infection, and show no defect in the processing of viral proteins, nor in viral packaging or egress. However, both viral genome replication and infectious virus yield are reduced ~10-fold in isolated Atg5-deficient acini that are infected in tissue culture. We speculate that the ~2000-fold difference in pancreatic titer may result from (1) this relatively small (10-fold) difference being amplified during multiple rounds of infection in vivo, and (2) the in vivo nature of the experiment, in which both virus and acinar cells are in the natural environments.
The Effects of Autophagy on Pathogenesis and Disease
Pancreatitis is markedly reduced in the CVB3-infected Atg5f/f/Cre+ mice, indicating that an intact autophagy pathway is deleterious to the host. This outcome may be related solely to virus titer: intact autophagy leads to increased CVB3 replication, with a consequent exacerbation of pancreatitis. However, we speculate that autophagy may contribute to disease in at least one additional way. Others have shown that an intact autophagy pathway facilitates the delivery of RNA virus replication intermediates to lysosomes, where they encounter toll-like receptor-7 (TLR7), triggering the innate cellular response. It is also known that TLR7-mediated signals upregulate autophagy. Hence, we hypothesize that viral infection may initiate an autophagy-dependent positive feedback loop, in which the pathway delivers viral single-stranded RNA to the autolysosome, and the consequent TLR7 triggering promotes increased autophagy flux. We suggest that this may represent an evolutionary strategy to maximize innate signaling by an infected cell, alerting other components of the immune system. However, this unconstrained innate response—although intended to protect—may have immunopathological consequences, providing a second route by which the autophagy pathway exacerbates disease.
Autophagy participates in another form of pancreatitis, induced by cerulein, a pancreatic secretagogue. Cerulein-induced pancreatitis is mediated, in part, by the intracellular activation of trypsinogen, a proenzyme that is abundant in acinar cells. Following administration of cerulein, mice lacking Atg5 expression in pancreatic acinar cells show reduced trypsinogen activation, and are resistant to the development of pancreatitis, suggesting that—as we have reported for CVB3 infection—autophagy may sometimes be detrimental. It will be interesting to determine if CVB3 infection, too, activates trypsinogen in acinar cells. If this occurs, and is autophagy-dependent, it would represent a third mechanism by which an intact autophagy pathway contributes to the severity of virus-induced pancreatitis. More importantly, it also would support the hypothesis that autophagy may be a final common pathway in pancreatitis, regardless of its etiology; if so, this would mark autophagy as a therapeutic target for this frequently serious disease.
Acknowledgments
This work was supported by NIH grant R01 grants AI042314 and HL093177 to J.L.W. This is manuscript number 21651 from the Scripps Research Institute.
Glossary
Abbreviations:
- ALV
autophagy-like vesicles
- CMV
compound membrane vesicles
- CVB3
coxsackievirus B3
- TLR
toll-like receptor
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/20160