Abstract
Proper degradation of aged and damaged mitochondria through mitophagy is essential to ensure mitochondrial integrity and function. Translocation of PARK2/Parkin onto damaged mitochondria induces mitophagy in many non-neuronal cell types. However, direct evidence showing PARK2-mediated mitophagy in mature neurons is controversial, leaving unanswered questions as to how, where, and by what time course PARK2-mediated mitophagy occurs in neurons following mitochondrial depolarization. We applied long time-lapse imaging in live mature cortical neurons to monitor the slow but dynamic and spatial PARK2 translocation onto damaged mitochondria and subsequent degradation through the autophagy-lysosomal pathway. In comparison with non-neuronal cells, our study reveals unique features of PARK2-mediated mitophagy in mature neurons, which will advance our understanding of pathogenesis of several major neurodegenerative diseases characterized by damaged mitochondria or a dysfunctional autophagy-lysosomal system.
Keywords: mitochondria, Parkin, PARK2, lysosome, autophagosome, autophagy, depolarization, mitochondrial mobility, neuronal mitophagy, mitochondrial membrane potential, mitochondrial quality control
Mitochondria are essential organelles for neuronal function, development and survival. Throughout a neuron’s lifetime, aged and damaged mitochondria undergo dynamic recycling via fusion/fission or are ultimately eliminated via mitophagy, an autophagic pathway specific for mitochondrial degradation. Dysfunctional mitochondria not only produce energy less efficiently, but also release harmful reactive oxygen species and initiate apoptotic signaling cascades, which have been linked to the pathogenesis of several major neurodegenerative diseases. Proper sequestration of damaged mitochondria into autophagosomes and subsequent degradation within the lysosomal system constitute a key cellular pathway in mitochondrial quality control mechanisms. Mutations in PINK1 and PARK2 are linked to autosomal recessive early onset Parkinson disease. Recent studies demonstrate that the PINK1-PARK2 pathway mediates mitophagy, and that PARK2 translocation onto mitochondria induces mitochondrial degradation via mitophagy in several non-neuronal cell types where PARK2 is overexpressed, such as in HEK293, HeLa, MEF and neuroblastoma cells. However, evidence from live mature neurons showing dynamic PARK2 translocation and degradation of depolarized mitochondria via the lysosomal pathway is still missing or controversial, thus raising questions concerning whether neuronal mitophagy has some unique features or is controlled by unknown mechanisms. We have recently focused on addressing (1) whether PARK2 translocates to mitochondria in live neurons; (2) whether this translocation is selective for depolarized mitochondria; (3) whether mitochondrial mobility is altered during the mitophagic process; and (4) where PARK2-targeted mitochondria are spatially distributed in neurons to facilitate their elimination through the autophagy-lysosomal system.
First, we speculate that the quality of neuronal culture is critical for allowing us to observe PARK2 translocation following CCCP-induced dissipation of mitochondrial membrane potential (Δψm). If the optimal conditions are not met, the uncoupled mitochondria will quickly trigger apoptosis before PARK2 translocation can be observed. We established a high quality culturing condition of primary cortical neurons such that they survive long enough to exhibit PARK2 translocation. CCCP treatment for 24 h induces 26.67% of neurons to undergo PARK2 translocation onto depolarized mitochondria. Co-treatment with CCCP and lysosomal inhibitors (LIs) results in a doubling in the percentage (55.87%) of neurons with PARK2 translocation. Thus, proper lysosomal function is critical to avoid the accumulation of PARK2-associated mitochondria in neurons upon dissipating Δψm. Second, we examined PARK2 translocation kinetics by imaging neurons at various time points during CCCP treatment. PARK2 translocation between 0.5–6 h was exceptionally rare, occasionally observed as early as 12 h, and became increasingly frequent after 18 h of CCCP treatment. We further imaged the dynamic recruitment of endogenous PARK2 to mitochondria in mature neurons following CCCP/LIs treatment. To confirm this, we alternatively isolated the mitochondria-enriched membrane fraction from mature cortical neurons following the same treatment. While the majority of PARK2 is present in the cytosolic fraction, mitochondrial depolarization results in the association of substantial levels of endogenous PARK2, along with autophagic markers LC3-II and SQSTM1/p62 with the mitochondrial membrane. These results consistently indicate the recruitment of both endogenous and exogenous PARK2 onto depolarized mitochondria in mature cortical neurons.
Next, we utilized a Δψm-sensitive dye, TMRE, to examine whether PARK2-targeted mitochondria display dissipated Δψm. Healthy mitochondria will sequester and accumulate TMRE, thus resulting in a higher TMRE fluorescence intensity. In control neurons, the majority of mitochondria are labeled by TMRE, reflecting their electrochemically active status. In contrast, PARK2-targeted mitochondria display significantly decreased or absent TMRE staining, suggesting that PARK2 selectively targets to depolarized mitochondria. We then examined whether endogenous PARK2 is required for eliminating damaged mitochondria. Knocking down PARK2 in neurons impairs the elimination of dysfunctional mitochondria, resulting in the accumulation of mitochondria with reduced TMRE intensity. Thus, our results support the hypothesis that PARK2-mediated mitophagy is one of the neuronal mechanisms maintaining mitochondrial quality.
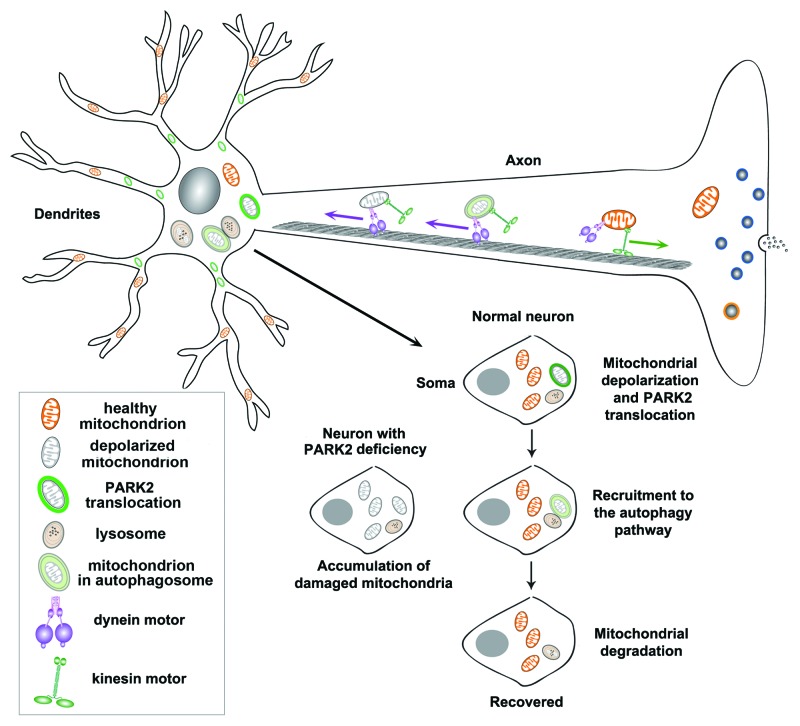
Intriguingly, PARK2 forms typical ring-like structures surrounding the fragmented mitochondria in the soma and proximal dendritic regions, but it is hardly detectable in axons and the distal dendrites. We further observed that in neurons with PARK2 translocation, anterograde axonal transport of mitochondria is reduced, whereas retrograde transport is relatively increased. Thus, altered mitochondrial mobility may be attributed to the unique distribution pattern. PARK2-tagged depolarized mitochondria are restricted to the soma for degradation, where lysosomes are predominately located, while healthy mitochondria are distributed distally to support synaptic functions. To test this hypothesis, we utilized syntaphilin, an axonal mitochondria docking protein, to artificially immobilize mitochondria in distal processes. To our surprise, we found that PARK2 is recruited to stationary mitochondria anchored by syntaphilin in distal processes. Therefore, the PARK2-mediated process prevents dysfunctional mitochondria from traveling peripherally, leading to their accumulation in somatodendritic regions. In addition, our long time-lapse imaging exhibits dynamic PARK2 recruitment and degradation of depolarized mitochondria within the autophagy-lysosomal system. CCCP exposure results in LC3-labeled ring-like structures surrounding fragmented mitochondria in the somadendritic regions and enhances the recruitment of mitochondria to lysosomes for degradation in live cortical neurons.
In summary, our study reveals several unique features of PARK2-mediated mitophagy in mature cortical neurons. First, PARK2 is selectively recruited to depolarized mitochondria to form ring-like structures, a process occurring more slowly than in non-neuronal cells. Second, following 24 h CCCP incubation, PARK2 translocation only occurs in a small percentage of neurons. Third, PARK2 translocation is restricted to the somatodendritic regions, where mature lysosomes are predominantly located. This spatial and dynamic process allows neurons to efficiently eliminate dysfunctional mitochondria via the autophagy-lysosomal pathway (Fig. 1).
Figure 1. A proposed model of neuronal mitochondrial transport and quality control via PARK2-mediated mitophagy. PARK2 is selectively recruited to depolarized mitochondria to form a ring-like structure predominantly accumulated in the soma and proximal dendritic regions. PARK2 recruitment is hardly detectable in axons and the distal dendritic processes. In neurons with PARK2 translocation, anterograde axonal transport of mitochondria is reduced, whereas retrograde transport is relatively increased. Altered mitochondrial mobility is attributed to a unique distribution pattern: while healthy mitochondria are distributed distally to support synaptic function, PARK2-targeted depolarized mitochondria are restricted to the somatodendritic regions, where mature lysosomes are predominantly located. This spatial and dynamic process allows neurons to efficiently eliminate damaged mitochondria via the autophagy-lysosomal pathway for neuronal recovery. In contrast, PARK2 deficiency impairs the elimination of dysfunctional mitochondria.
Acknowledgments
This work was supported by the Intramural Research Program of NINDS, NIH (Z.-H.S.), the NIH Pathway to Independence Award K99 (Q.C.) and HHMI-NIH Research Scholars Program (H.M.Z.).
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/20218