Abstract
Emerging evidence is shedding light on a large and complex network of epigenetic modifications at play in human stem cells. This “epigenetic landscape” governs the fine-tuning and precision of gene expression programs that define the molecular basis of stem cell pluripotency, differentiation and reprogramming. This review will focus on recent progress in our understanding of the processes that govern this landscape in stem cells, such as histone modification, DNA methylation, alterations of chromatin structure due to chromatin remodeling and non-coding RNA activity. Further investigation into stem cell epigenetics promises to provide novel advances in the diagnosis and treatment of a wide array of human diseases.
Keywords: embryonic stem cells, epigenetics, chromatin, histone modification, post translational modification, Polycomb, Trithorax, non-coding RNA, hydroxymethylation
Introduction
Stem cells are defined by two fundamental properties: self-renewal and pluripotency or multipotency. Until recently, the analysis of stem cells and their lineages has largely focused on transcriptional regulation. Current data, however, suggests that the genome undergoes major epigenetic alterations during embryonic stem cell (ES cell) differentiation in mammalian development.1 Exciting progress in multiple studies of specific epigenetic features of human and mouse stem cells has provided insights into the unique properties of pluripotent and niche-restricted stem cells, and outlined the importance of the molecular mechanisms that control these epigenetic events. Complex regulations of the self-renewal and differentiation states of embryonic and adult stem cells and their “stemness” not only heavily rely on transcriptional factor networks, but also on the properties of the chromatin within the cells, the so-called “epigenetic landscape.” This heritable landscape is indispensable for establishing different degrees of chromatin compaction and conveying specialized gene expression patterns, which define the molecular basis of pluripotency, reprogramming and early human development. The importance of epigenetic regulation in maintaining gene expression and, therefore, cell fate determination is well established.
Epigenetic mechanisms operating within the cell include: post-translational modifications of histones (histone PTMs) and incorporation of histone variants, changes in DNA methylation, ATP-dependent chromatin remodeling and the implementation of RNAi pathways and non-protein coding RNAs. There is a highly orchestrated and collaborative action between different epigenetic pathways to establish unique epigenetic states and to drive the final outcome of the transcriptional hierarchy mediated by transcriptional factors. This synergistic regulation allows for alterations of gene expression without changes to the DNA sequence. Perturbation of these epigenetic components may result in changes to local chromatin configuration and nuclear architecture within the stem cell, collapsing the self-renewal circuitry and triggering loss of stemness by promoting differentiation.2-5 Somatic cell nuclear transfer experiments have also unambiguously demonstrated that reprogramming to a pluripotent state requires large-scale epigenetic changes within the cell.6-8 By examining the abundance of modified histones and binding patterns of their modifying complexes, such as Polycomb group (PcG) and Trithorax group (TrxG) proteins, as well as replication timing and chromatin accessibility, new studies have revealed that stem cells manage their status through multiple layers of epigenetic events that impose flexible but precise control over the expression of important regulatory genes.8-11 For instance, this complex regulation promotes the expression of pluripotency-associated factors, such as OCT3/4 (POU5F1) and NANOG, while transiently prohibiting activation of the genes that drive cellular differentiation along specific differentiation pathways.
In this review, we will discuss recent progress that points to an active role of epigenetic regulation in pluripotency and stemness, as well as in driving cell fate specification. We will also discuss recent discoveries that have shaped the emerging viewpoints in the field, focusing on the following questions: (1) How are epigenetic pathways involved in retaining stem cell potential?; (2) How does a stem cell rapidly transition into a morphologically and molecularly distinct cell type, and is this event driven by epigenetic alterations?; (3) Is this process reversible?
Stem Cells and Epigenetics
According to accepted terminology, stem cells are immature cells with the ability to self-renew and differentiate into multiple cell types. They can be classified, based on their relevance to developmental events, as embryonic or adult stem cells. Induced pluripotent stem (iPS) cells represent an additional class of stem cells, artificially derived from non-pluripotent cells (e.g., adult somatic or stem cells).12 Stem cells of all types are characterized by stable, heritable states, allowing for multiple developmental pathways. During development, the potency of stem cells is reduced over time from totipotent (morula) to pluripotent (embryonic stem cells) to multipotent (fetal and adult stem cells) to omnipotent (precursor cells) due to progressive gene silencing. Genes active in earlier progenitors are gradually silenced at later stages during development, and subsets of cell type-specific genes are turned on. This progression is the result of selective expression of transcription factors and orchestrated action of the classic “corner stones” of epigenetics: chromatin remodeling and chromatin modifications, DNA methylation of CpG dinucleotides and activity of non-coding RNA.13-15 An important property of the epigenetic changes within the cell is that they are heritable. Once established, epigenetic modifications can be maintained and propagated through cellular division. Establishment of specific epigenetic signatures within the cell requires coordinated action of numerous enzymatic machineries responsible for deposition (writers) and removal (erasers) of epigenetic modifications, as well as protein complexes that recognize these modifications (readers).
Multiple levels of epigenetic regulation converge in the chromatin to establish transcriptionally permissive, less condensed euchromatin, and highly condensed and often repressed heterochromatin.16 Such complex nuclear architecture of stem cells is important for regulating transcriptional outcomes. Several recent studies suggest that nuclear structure experiences tremendous morphological alterations when ES cells progress along the differentiation axis. These alterations range from changes in nuclear lamina,17 size and shape of the nuclei, nucleolus, nuclear speckles (domains enriched in splicing factors) and Cajal bodies.18 As a result of epigenetic events, the level of chromatin compaction and its accessibility and positioning within specialized nuclear domains undergoes dynamic changes upon stem cell differentiation, as shown by changes in chromatin organization components, such as heterochromatin,19 promyelocytic leukemia bodies (PML NBs)18 and centromere positioning.20 The first line of evidence for these remarkable dynamics came from visualization of chromatin in mouse ES cells using electron microscopy. The chromatin of pluripotent stem cells was noticeably devoid of heterochromatin, though prevalent in differentiated cells, suggesting that ES cells have an open or “loose” chromatin structure. Later, it was proven that chromatin of pluripotent ES cells is characterized by high rates of histone protein exchange, coupled with dispersed and very dynamic localization of heterochromatic markers, such as heterochromatic histone modifications like trimethylation on lysine 9 of histone H3 (H3K9me3) and chromatin bound protein HP1.19 Open chromatin correlates with a globally permissive transcriptional state, and has been proposed to contribute to the developmental plasticity, or pluripotency, of ES cells.21
As differentiation advances, cells undergo global chromatin reorganization,22 leading to accumulation of more rigid heterochromatin, driven by compaction of major satellite repeats and the pericentric regions of some chromosomes, resulting in concentrated heterochromatic foci detectable upon cytological analysis.19 This suggests that the pluripotent nature of the ES cell genome becomes more transcriptionally restrained, due to chromatin condensation and maturation of heterochromatin upon differentiation. It has also been experimentally confirmed that the pluripotency-specific genes OCT3/4 and NANOG, as well as lineage specific genes, undergo changes in their relative positioning between the transcriptionally-restrictive nuclear periphery and the transcriptionally-permissive nuclear interior,14,20 thus suggesting that three dimensional nuclear architecture is an important regulator of gene transcriptional activity in stem cells.
Understanding how this nuclear organization is established and how it influences gene expression might subsequently allow for a better understanding of pluripotency as a cellular state.
Stem Cell Chromatin Composition: Nucleosome Composition and Histone Variants
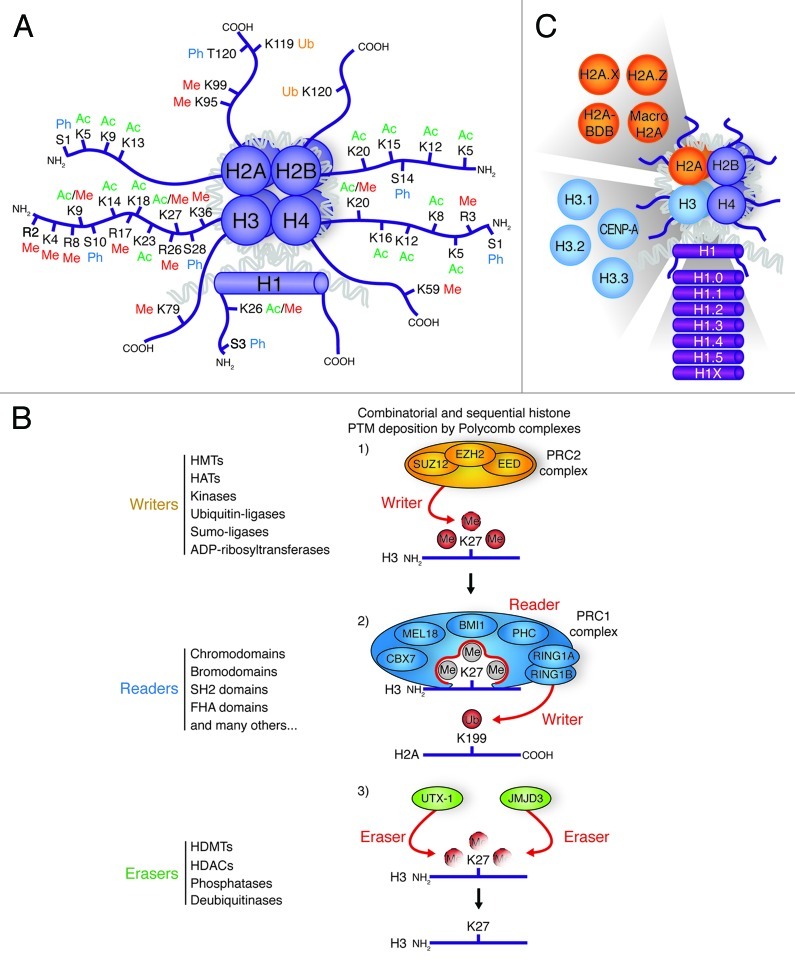
Before we take a dive into the ocean of epigenetic players and mechanisms that control pluripotency, self-renewal and differentiation, it is important to discuss the basics of chromatin composition. Early work on the nuclear packaging of chromosomal DNA has defined the basic unit of the DNA/protein complex known as chromatin. This fundamental unit, the nucleosome, is comprised of two copies of the histone proteins H3, H4, H2A and H2B creating a bead-like structure. Then, 146 bp of DNA is wrapped around the surface of this structure formed by these core histone proteins. The linker histone, H1, binds the nucleosome at the entry and exit sites of the DNA wrapped around the nucleosome core particle, thus locking the nucleosomal particle in place (Fig. 1A).
Figure 1. Histone post-translational modifications and variants. (A) Schematic drawing of a nucleosome with the four canonical histones (H2A, H2B, H3 and H4) and the linker histone H1. The covalent PTMs [methylation (Me), acetylation (Ac), ubiquitination (Ub), and phosphorylation (Ph)] are highlighted on the N- and C-terminal tails of each histone. (B) Graphical representation of histone PTM writers, which add covalent PTMs to histone tails, readers, which recognize and bind histone PTMs, and erasers, which remove histone PTMs. Protein families associated with these steps are listed on the left. In this example, the PRC2 complex adds (writes) tri-methylation on lysine 27 of histone H3. This is then recognized by the reader complex PRC1. The RING1A and RING1B subunits of PRC1 (writers) subsequently act to ubiquitinate lysine 199 of histone H2A. UTX-1 and JMJD3 can act to remove (erase) histone H3 K27 tri-methylation. (C) The known histone variants in ESCs are represented next to the canonical histones they replace.
In the context of chromatin, nucleosomes can be: (1) covalently modified by chromatin modifying complexes, which provide histone tail and globular domain modifications or; (2) repositioned by chromatin remodeling complexes, which cause an alteration of DNA-histone contacts. This occurs in a highly combinatorial and, sometimes, mutually exclusive fashion. Nucleosomal packaging and histone modifications dictate the different degrees of primary chromatin compaction, e.g., six nucleosomes per 11 nm in the euchromatic chromatin fiber vs. 12–15 nucleosomes per 11 nm in heterochromatin, which is achieved by additional chromatin structural proteins.23
The core histones are subjected to numerous different PTMs including acetylation, methylation, phosphorylation, poly-ADP-rybosylation, ubiquitination and sumoylation (Fig. 1A).24 Different chromatin states are defined by combinatorial patterns of these histone modifications that are often referred to as the “histone code.”25 Each histone modification can induce or inhibit subsequent PTMs, and such cross talk can operate on the same nucleosome or can be established between nucleosomes.26 To add to this complexity, the chromatin structure is also influenced by effector or “reader” proteins that recognize single or multiple histone PTMs (Fig. 1B). Moreover, this recognition can occur with PTMs on a single nucleosome, or several nucleosomes that can be either present on the same or different chromatin fibers (inter-chromosomal interactions). We will address some of these specific reader proteins at a later point.
The majority of histone PTMs have been shown to be reversible (Fig. 1B). The balance between enzymatic machineries responsible for establishment, maintenance and removal of histone PTMs significantly contributes to chromatin dynamics in stem cells and is indispensable for driving cell-type specific biological outcomes.
In addition to the four canonical histone proteins (H2A, H2B, H3 and H4), many variant forms of histones exist in different organisms. H1.0, H1.1, H1.2, H1.3, H1.4, H1.5 and H1X are the variants of H1; H2A.X, H2A.Z, H2A-BDB and Macro H2A are replacement variants for H2A; and H3.1, H3.2, H3.3 and CENP-A are variants of the core histone H3 (Fig. 1C, for review see ref. 27). The variants are usually present as single-copy genes that are not restricted in their expression to the S-phase, but are expressed throughout the cell cycle. Unlike the major subtypes, the variant histone genes contain introns and their transcripts are often polyadenylated. These features are thought to be important in the post-transcriptional regulation of these proteins.28 Some variants exchange with the pre-existing histones during development and differentiation, and are therefore referred to as replacement histones. Currently, the majority of studies aimed at elucidating the functions of histone variants are based on correlation between the localization of variants and the transcriptional activity of certain loci, or on analyses of phenotypes associated with the loss of the variant.
The large number of histone variants leads to the question of how many different nucleosomal structures exist, and whether structural alterations can account for differences in function and localization of these nucleosomes. Use of fluorescent recovery after photobleaching (FRAP) technology in recent investigations of chromatin in mouse ES cells and their differentiated progeny has allowed for the assessment of nucleosomal structure.29 The published study has demonstrated that ES cells preserve their differentiation potential by maintaining a loosely bound fraction of histones and other chromatin-associated proteins, which through free exchange with bound histones and chromatin generate a state of active, “breathing” chromatin. Consistent with high levels of transcription in ES cells, the same group reported that the only structural chromatin protein lacking hyperdynamic behavior is histone variant H3.3. The histone variant H3.3 preferentially associates with transcriptionally active regions.30 This finding is also consistent with the observed accelerated differentiation of cells lacking histone chaperone HIRA (TUP1). Hira−/− cells are marked by a drastic reduction of H3.3 incorporated into open chromatin, and are prediscposed to the formation of heterochromatin, thus promoting cellular differentiation. Ultimately, histone variant H3.3 deposition into the chromatin of actively transcribed genes can contribute to the cellular memory phenomena. Experiments performed by Ng and Gurdon in Xenopus laevis provide the first documented evidence of the persistence of epigenetic memory of a transcriptionally active state and propose the role of histone variant H3.3 in this process,31 thus further highlighting the important role of histone variants in the regulation of stem cell epigenetics.
DNA Methylation Status as Major Epigenetic Player in Stem Cells
Although there is a strong correlation between transcriptionally favorable states of chromatin and the pluripotency of stem cells, the chain of molecular events, as well as the full spectrum of molecular players providing for chromatin plasticity, remains largely unknown. Recent discoveries indicate that chromatin structural proteins and chromatin modifying activities, such as histone acetyl transferases (HATs), histone deacetylases (HDACs), histone methyltransferases (HMTs) and histone demethylases (HDMs), as well as DNA methyltransferases (DNTMs), are essential regulators of open chromatin state and pluripotency.
DNA methylation
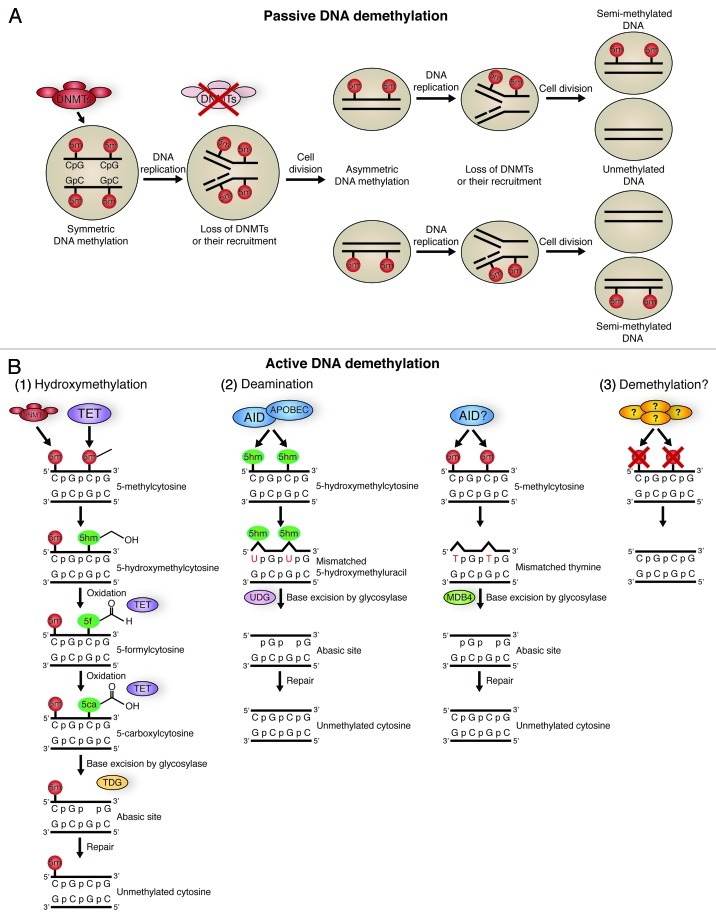
DNA methylation is a classic example of epigenetic inheritance of gene expression during development, and therefore it is not surprising that recent evidence indicates its key role in stem cell function. DNA methylation patterns are directed and preserved by the action of the DNA methyl transferase (DNMT) family, whereas the effects of DNA methylation are mediated by recruitment of the “reader” methyl-CpG-binding domain (MBD) family,32 containing proteins such as MBD2, MBD3, MBD4, MeCP2 and KAISO or, alternatively, by blocking the binding of transcriptional factors to their cognate response elements. In most eukaryotes, methylation of the 5′site of cytosines (5-mC) represses transcription through association with MBD proteins, which in turn are part of chromatin remodeling complexes.32-34 Since removal of the methyl group from 5′methylated cytosine is a thermodynamically unfavorable event, the existence of a bona fide DNA demethylase has been a subject of debate.35 Until recently, DNA methylation was regarded as stable and irreversible. However, new evidence indicates that DNA demethylation can occur passively, when DNA methylation enzymes and/or their complexes are denied access to the newly replicated DNA (Fig. 2A), or actively through the selective recruitment of various enzymes, in both animals and plants.36,37 For example, in plants, the DNA glycosylase enzymes ROS1 and DEMETER are two well-characterized DNA demethylases.38,39 In animals, DNA demethylation is performed by cytidine deaminases in concert with DNA glycosylases (Fig. 2B).40,41
Figure 2. Pathways of DNA methylation and demethylation. (A) Passive DNA demethylation. DNA in a cell is 5′ methylated at CpG islands in a symmetrical fashion by DNA methyl transferases (DNMTs). If there is a loss of DNMT function, followed by DNA replication and cell division, cells containing asymmetric DNA methylation will arise. If there is continued loss of DNMT function and further DNA replication and cell division, this will give rise to cells with unmethylated DNA. This action is passive, as it relies on DNA replication and cellular division. (B) Active DNA methylation. Several pathways can lead to active demethylation of DNA without the need for DNA replication, and in the presence of DNMTs. Hydroxymethylation (1) of 5-methylcytosines (5-mC) is performed by TET proteins. 5-hydroxymethylcytosine (5-hmC) may act as a substrate for further modifications, or may itself be sufficient to prevent factors that interact with methyl-cytosine from having an effect. 5-hmC can be further oxidized by TET proteins to 5-formylcytosine (5fC) and 5-carbonylcytosine (5caC).54 5caC is a substrate for TDG glycosylase, creating an abasic site through base excision, which is then repaired with an unmethylated cytosine.54,55 Both 5-mC and 5-hmC can act as substrates for deaminases (2). The deaminases AID/APOBEC can convert 5-hmC to 5-hydroxymethyl-uracil (5hmU).57 This is then repaired by mismatch-repair pathways, beginning with base excision by the glycosylase UDG. AID may also be able to convert 5-mC to thymine, which is repaired beginning with the glycosylase MDB4. The presence of a bona fide DNA demethylase (3) is a controversial topic, but several groups have proposed candidates.35
In animal germ cells, genes that control cell differentiation are methylated and transcriptionally inactive.42 However, there are at least two developmental periods, both in germ cells and in pre-implantation embryos, during which methylation patterns are reprogrammed genome-wide, generating cells with a broad developmental potential. After fertilization, the parental genome undergoes a rapid loss of DNA methylation in the first several rounds of cellular division, suggesting active DNA demethylation events.40,41 Contrary to this, the maternal genome is gradually demethylated and this demethylation appears to be mediated through passive mechanisms.43
As a consequence of this genome-wide demethylation during embryonic development, genes that are essential for stem cell renewal are activated, suggesting that existing DNA methylation must be erased, especially at the promoters of genes that are essential for pluripotency, such as NANOG and OCT3/4.44 Studies have indicated that mouse Nanog promoter methylation is erased by active and passive demethylation after fertilization, before expression commences in the morula. In mouse ES cells, the normally active Nanog promoter is silenced when targeted by de novo methylation.45 Interestingly, DNA methylation at genes that are essential for stem cell renewal are primarily associated with coding sequences, not gene promoters. Furthermore, nearly one-quarter of all methylation identified in embryonic stem cells was in a non-CG context, suggesting that embryonic stem cells may use different methylation mechanisms to affect gene regulation.46,47
DNA hydroxymethylation
Several lines of evidence demonstrate that Ten-11 translocation family proteins, TET1–3, have the capacity to convert 5-mC to 5-hydroxymethyl-cytosine (5-hmC) (Fig. 2B).48,49 It has been reported that ES cells deficient in the three enzymes that are involved in de novo DNA methylation and its maintenance (TKO cells) are also deficient in 5-hmC, thus suggesting that 5-hmC arises from the processing of pre-existing 5-mC within the gene body during transcription.50,51 Hydroxymethylation of cytosines may lead to passive demethylation during cell division, as 5-hmC is a poor substrate for DNMT1 recognition (Fig. 2A).52 However, an emerging consensus in the DNA methylation field is that hydroxymethylation leads to active replacement of methylated cytosines via DNA repair pathways, in the absence of cell division (Fig. 2B).53 These pathways rely, in part, on the further enzymatic modification of 5-hmC to 5-formyl-cytosine (5-fC) and, subsequently, 5-carboxyl-cytosine (5-caC). 5-caC can then be removed by the base-excision repair pathway, leading to its replacement with unmodified cytosine (Fig. 2B).54,55 Recent studies have identified enrichment of 5-fC and 5-caC in the genomic DNA of mES cells and mouse organs, suggesting that this pathway is active in stem cells.56 Another pathway of active DNA demethylation involves the base excision repair of 5-hmC by the activation-induced deaminase (AID)/apolipoprotein B mRNA-editing enzyme complex (APOBEC) family of cytidine deaminases (Fig. 2B).54,57
These observations raise the possibility that 5-hmC may act as a distinct epigenetic state contributing to dynamic changes in DNA methylation and transcriptional regulation during embryonic development. This possibility is supported by the finding that Tet1 is highly expressed in mouse ES cells, which is concurrent with elevated 5-hmC levels relative to differentiated cells.50,58 Ito et al. first suggested that cytosine hydroxymethylation might be involved in the maintenance of pluripotency in stem cells through their observation that Tet1 knock-down in mES cells correlates with downregulation of Nanog and methylation of the Nanog promoter, thus supporting a role for TET1 in regulating DNA methylation status.56 The levels of TET1 and TET2 are dramatically downregulated upon differentiation of ES cells and embryoid body (EB) formation. Declining levels of TETs during differentiation are associated with a decrease in hydroxymethylation at the promoters of pluripotency genes. This event is coupled with increased 5-mC methylation and gene silencing. It was also reported that 5-hmC is mostly associated with euchromatin and, whereas 5-mC is under-represented at gene promoters and CpG islands, 5-hmC is enriched in gene bodies and is associated with increased transcriptional levels.50,51,59 Most, if not all, 5-hmC in the genome depends on pre-existing 5-mC, and the balance between these two modifications varies depending on the genomic region. In initial studies, knockdown of Tet1 and Tet2 causes downregulation of a group of genes that includes pluripotency-related genes (including Esrrb, Prdm14, Dppa3, Klf2, Tcl1 and Zfp42).50 Concomitant with this event, it has been shown that increased methylation of pluripotency gene promoters shifts ES cell to extra-embryonic lineage differentiation.
However, these observations have recently been challenged. Several groups have reported that although TET1 and TET2 levels are high in ES cells, these proteins may regulate lineage-specific genes, rather than pluripotency factors such as NANOG.50,53,60 Newly reported data suggest that TET1 is dispensable for ES cell maintenance, and that its loss is compatible with embryonic development and postnatal survival.61 This conclusion is drawn from the observation that Tet1 −/− mES cells: (1) express markers of pluripotency, such as OCT3/4, SOX2 and NANOG; (2) remain in an undifferentiated state and; (3) can support normal development of the embryo proper in a tetraploid complementation assay.61 Furthermore, TET1 homozygous mutant mice are both viable and fertile. Taken together, this suggests that TET1 is not essential for postnatal survival. This is most likely due to a level of functional redundancy between TET family members. Although Tet2 expression in mES cells is 5-fold lower than Tet1, and that there is no observed increase in Tet2 expression in Tet1−/− mES cells, it is likely that expression of Tet2 can compensate for Tet1 loss.61 This is consistent with the observation that Tet1 knockout mES cells exhibit only a 35% reduction in 5-hmC levels; however, a 60% knockdown of Tet2 on this background further reduces 5-hmC levels.61 It will be necessary to develop double and triple knockouts of the Tet genes in order to fully understand the roles of TET proteins and 5-hmC in vivo.
The studies outlined in this section have made great progress into understanding the function of TET proteins and have answered a number of fundamental questions regarding the mechanisms of DNA demethylation. However, it is as yet unclear what the precise functions of these proteins are in ES cells. The body of evidence discussed herein suggests that the roles played by TET proteins in ES cells may not be trivial, and further studies will be required to understand the precise role of hydroxymethylation in these cells.
Histone Post-Translational Modifications and Chromatin Modifying Activities in the Regulation of the Stem Cell Chromatin Landscape
Chromatin states: euchromatin, heterochromatin and bivalent chromatin
Recent genome–wide studies have indicated that chromatin in ES cells has a specific histone PTM profile, characterized by an abundance of histone modifications associated with “open” and transcriptionally permissive euchromatin, such as H3K4me3, H3K9ac and H4ac (Fig. 3A). “Silent” transcriptionally repressive heterochromatin is associated with PTMs such as H3K9me3 and H3K27me3 (Fig. 3B).
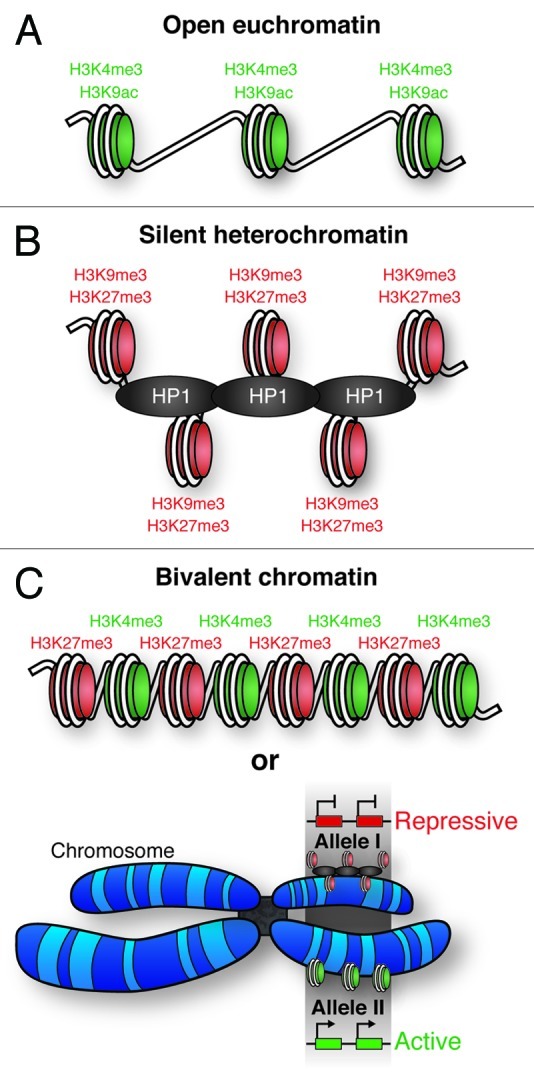
Figure 3. – Chromatin states. (A) Open, active euchromatin is characterized by histone H3 lysine 4 methylation (H3K4Me3) and lysine 9 acetylation (H3K9Ac). (B) Silent, repressive heterochromatin is characterized by methylation of histone H3 lysine 9 (H3K9Me3) and lysine 27 (H3K27Me3). Heterochromatic DNA is more densely packed than euchromatin, facilitated by heterochromatin protein 1 (HP1). (C) Regions of the genome have been identified with both activating (H3K4me3) and repressive (H3K27Me3) histone marks; termed bivalent chromatin. It is possible that these PTMs occur on neighboring nucleosomes, however, it is also possible that one allele of the gene is associated with heterochromatin, and the other euchromatin. This would give the appearance of bivalent chromatin.
In stem cell chromatin, less accessible lineage-specific genes are maintained in a silent but transcriptionally available (poised) state, which is characterized by the simultaneous presence of both the repressive H3K27me3 and the activating H3K4me3, creating so-called bivalent domains (Fig. 3C). Such bivalent domains are thought to mark a subset of key developmentally regulated genes in ES cells that are kept at a low transcriptional rate.9,11 The enrichment of the bivalent marks at conserved domains overlapping with sites for the recruitment of the core pluripotency factors OCT3/4, SOX2 and NANOG originally suggested a functional relationship between such bivalent domains and pluripotency. The initially proposed model of bivalent domains implied that their formation and/or maintenance might be regulated through these “master regulators.” However, it has recently been reported that bivalency is not a unique feature of pluripotent cells. Bivalent chromatin is not limited to ES cells; multipotent adult hematopoietic stem (HS) and progenitor cells, as well as neural progenitors and terminal neurons also exhibit bivalent domains at specific sites targeted by Polycomb group proteins.62-64 Interestingly, during cellular differentiation, the active mark within bivalent chromatin, H3K4me3, is lost and de novo DNA methylation locks genes controlled by this type of chromatin in a silent state.63 It cannot be excluded that several other bivalent or multivalent chromatin modification states exist in cells. It is important to keep in mind that only a small fraction of the known histone PTMs have been examined in current studies, and a more comprehensive analysis of histone PTMs, together with their relationship to gene expression, may help to decipher the enigma of bivalent chromatin. It is also important to mention that the combinatorial patterns of bivalency might simply reflect allelic differences in chromatin modifications, explaining the apparent coexistence of both histone marks at the same loci, as shown in Figure 3C.
Chromatin bivalency poses an interesting hypothesis tailored to explain the molecular mechanisms involved in the regulation of accessibility and transcriptional competence of differentiation-specific genes in ES and adult stem and progenitor cells; however, several examples bypass the need for bivalency in pluripotent cells, such as epigenetic regulation of some loci during B-cell development.65 In this study, the intergenic and cis-acting element in the mouse Lambda5-VpreB1 locus does not exhibit bivalency. On the contrary, it is marked by histone H3 acetylation and histone H3 lysine 4 methylation at a discrete site in ES cells. The epigenetic modifications spread from this site toward the VpreB1 and Lambda5 genes at later stages in B-cell development, and a large, active chromatin domain is established in pre-B cells when the genes are fully expressed. These results suggest that localized and unambiguous rather than bivalent epigenetic marking is important for establishing the region of transcriptional competence for the Lambda5 and VpreB1 genes as early as the pluripotent ES cell stage.
Future investigations will be needed to demonstrate the significance of chromatin bivalency in the stem cells pluripotency, lineage commitment and control of the developmental regulators.
Histone acetyltransferases and deacetylases
The acetylation of histones H3 and H4 are catalyzed by histone acetyltransferases (HATs/KAT) and the removal of acetyl-group is achieved by action of histone deacetylases (HDACs).66 In the past 10 years, multiple studies have indicated an interplay between HATs and HDACs, and research has advanced significantly as these enzymatic activities have become more amenable to molecular and biochemical analysis. Since both HATs and HDACs are integral components of transcriptional co-activator and co-repressor complexes respectively, it is no surprise that, despite their opposing activities, both acetylation and deacetylation are required for proper ES cell differentiation and adult stem cell function.67 For instance, inhibition of HDACs prevents the differentiation of ES cells,68 thus suggesting histone deacetylation events are part of cell-type specification. Similarly, the histone acetyltransferase KAT3B (p300) is required for differentiation of ES cells, but is dispensable to maintain their self-renewal properties.69 Since HDACs and HATs are also involved in a wide array of cellular events, including transcriptional and post-transcriptional regulation, and even post-translational modifications, their regulatory role might extend not only to modification of histones, but also to dynamic acetylation/deacetylation of key regulatory ES cell differentiation modulators like members of the SOX family, TGF-β family, WNT and NOTCH.
Histone methyltransferases
Historically, cellular inheritance was explained by the methylation of promoter DNA; however, a new wave of published data argues that DNA methylation is not the only mechanism utilized by the cell to impart epigenetic memory. How is the epigenetic memory of silent chromatin handled?
In multiple organisms, genes encoding developmental regulators are tightly controlled. Such control is mediated not only through transcriptional factors, which participate in auto- and cross-regulation self-renewal circuitry (for review, see ref. 70), but also by a handful of chromatin regulators catalyzing histone methylation and demethylation. Multiprotein repressive Polycomb group (PcG) and activating trithorax (TrxG) chromatin modifying complexes have long been known for their significance in the regulation of the lineage–specific genes during Drosophila development. Current research suggests that both of these complexes regulate the nuclear organization of their target genes, and mechanistically cross-talk with noncoding RNAs and the RNAi machinery.71 Next, we will discuss the role of these complexes and their integral chromatin modifying activities in the stem cell biology.
Polycomb group (PcG) protein complexes
A series of recent studies have revealed that, in order to maintain pluripotency, mouse and human ES cells deploy mechanisms for dynamic repression of genes regulating developmental pathways in such a way that this repression can be epigenetically maintained through cell division.72 The epigenetic modifier PcG complex proteins can perform this function.10,73-76 The PcG complex is an evolutionary conserved family of chromatin regulators known best for their role in establishing and maintaining the silent state of homeotic gene expression during embryonic development.77 Mammalian PcG proteins assemble at least three biochemically distinct complexes PRC1, PRC2 and PhoRC. Polycomb repressive complex 2 (PRC2) acts to stabilize repressive chromatin structure through the function of chromatin modifiers, such as enhancer of zeste (EZH2), embryonic ectoderm development protein (EED), and suppressor of zeste 12 (SUZ12), all of which are histone methyltransferases responsible for depositing H3K27me2 and H3K27me3 marks onto chromatin (Fig. 1B).78,79 By performing ChIP-on-CHIP analysis for SUZ12 and EED proteins in ES cells, Lee and coworkers have demonstrated that genome-wide binding of these modifiers overlaps with the chromosomal region of H3K27me3 deposition within the highly evolutionary-conserved genomic segments in the vicinity of transcriptionally silent genes.74 The 1,800 genes identified as targets include the majority of OCT3/4, NANOG and SOX2 regulated genes in human ES cells,80 such as the regulators of differentiation GATA4 and CDX2. These results, together with the observation that EZH2 is required for maintaining the proper H3K27me3 marks in pluripotent epiblast cells,81,82 suggest that PRC2 could be potentially viewed as a component of epigenetic memory strategies required for ES cell maintenance.
Nucleosomes containing H3K27me3 provide a binding platform for the recruitment of the PRC1 complex containing PHC, CBX, BMI1 and RING1A, RING1B (RNF2) and MEL-18 (PCGF2) via the affinity of chromodomain containing proteins to these PTMs.10 The activity of the PRC1 complex was implicated in the establishing of high-order chromatin structure.83 How does PRC1 complex compact chromatin? Evidence indicates that the E3-ligase activity of the RING1A and RING1B proteins present in the PRC1 complex can mono-ubiquitinate H2AK199.84 This activity appears to be stimulated by the BMI1 and MEL-18 (PCGF2) subunits of PRC1 (Fig. 1B).
This logical interdependency between PRC2 and PRC1 functions does not, however, fully explain the phenotypes of ES cell models that are deficient for one of the PRC2 components (e.g., EED). Mutant ES cells demonstrate gross loss of H3K27 methylation, but still retain their ability to self-renew and maintain normal morphology.10,85 This occurs despite the fact that several neuron-specific genes, and GATA4 and GATA6 factors, are transcriptionally upregulated on the background of Eed deficiency. The mutant ES cells simply manifest high level of spontaneous differentiation10 and are still capable of producing all the three germ layers upon injection in blastocysts.85-87 Similar to this, SUZ12−/− ES cells do not demonstrate a full requirement for PRC2 in maintenance of ES cell pluripotency.74
It is probable that both H3K27 methylating enzymes and DNA methylation regulate the timing of differentiation and maintenance of cell-type identity, in a coordinated and semi-redundant fashion.88-90 As a result, cellular inheritance could be achieved by means of methylation on the DNA in the absence of a functional PRC2 complex. It is known that PcG proteins can directly control DNA methylation,91 thus providing another important role as connectors between key epigenetic events. Future analysis of the functional links between DNA methylation status and the context-dependent action of PcG complexes in ES cell models will extend our understanding of molecular memory of a silent state and its link to pluripotency.
Trithorax group (TrxG) protein complexes
As we have discussed above, maintenance of ES cell self-renewal relies upon two equally important events: repression of lineage-specific genes mediated by PcG complexes and the transcriptional activity of self-renewal genes, such as OCT3/4, SOX2 and KLF, which is mediated by the trithorax group of histone modifiers. In contrast to PcG complexes, TrxG complexes mediate deposition of histone PTMs that mark active transcription, such as H3K4me3.77 Until now, little was known about TrxG-associated members in the context of ES cell self-renewal and pluripotency maintenance, or somatic cell reprogramming. The SET/MLL (mixed-lineage leukemia) histone methyltransferase family are mammalian homologs of Drosophila TrxG, which function as conserved, multisubunit ensembles that catalyze methylation of H3K4. SET/MLL histone methyltransferases alone are catalytically inept and require the core subunits ASH2L, RBBP5 and WDR5 for HMT activity. RBBP5 and ASH2L form a heterodimer to provide for HMT activity of the MLL1 complex,92 and ASH2L has been reportedly required for mouse embryogenesis.93 On the other hand, WDR5, which recognizes and interacts with H3K4me2, has been shown to be indispensible for SET/MLL complex assembly and for the transition of H3K4me2 to the trimethylated state (H3K4me3).94 This unique ability of WDR5 to bind unmethylated or dimethylated H3K4 is indicative of its participation in both reading and writing of H3K4 methylation. Although the exact logistics and chain of molecular events of this process need to be fully unraveled, new evidence indicates that WDR5 expression levels positively correlate with the undifferentiated ES cell state, thus suggesting a specific WDR5 function in ES and iPS cell maintenance.8 WDR5 knockdown in ES cells induces changes in stem cell morphology and increased ectodermal and trophectodermal gene expression, suggesting that WDR5 depletion induces the collapse of the transcriptional network of ES cells.8 Genome-wide analysis indicates that WDR5 is critical for maintenance of global and localized H3K4me3, as well as for transcriptional activation of specific targets in ES cells. Interestingly, WDR5 directly interacts with the master pluripotency factor OCT3/4, and this interaction is stabilized upon formation of multimeric complexes, even in the absence of ASH2L or RBBP5,8 thus suggesting that the function of the WDR5-OCT3/4 partnership might extend beyond H3K4 methylation. Nevertheless, WDR5 and OCT3/4 share overlapping gene regulatory functions, where both of these factors co-localize with RBBP5 and H3K4me3 in genome-wide mapping experiments.8 This work represents the first unbiased, high-resolution mapping of core TrxG members, and demonstrates their interconnectivity with the core transcriptional network, which is required for maintenance of ES cell self-renewal. Of interest, WDR5 has also recently been shown to function as a subunit of other nuclear complexes, such as the histone acetyltransferase ATAC295 and human chromodomain helicase DNA binding protein 8 (CHD8), important for ATP-dependent chromatin remodeling.96 It remains unclear, however, whether these two complexes contribute to the observed WDR5 phenotype in ES cells.
Histone demethylases
In a similar manner to stemness, the differentiation of pluripotent stem cells into tissue-specific lineages has proven to be controlled by epigenetic components. The exit from the self-renewing state is accompanied by changes in the covalent modifications of histones, for example, an increase in the silencing-associated histone H3K9me2 and H3K9me3 marks on the chromatin and removal of H3K27me3. DNA sequence-specific factors can act as a landing pad for the recruitment of specialized enzymatic machineries that either deposit75 or remove the PTMs on chromatin (for details of a multitude of histone PTMs and the substrate-specificity of enzymes responsible for their deposition see references24,97 and Figure 1). Whist it has been known for a number of years that histone acetylation and phosphorylation are reversible, only relatively recently has it been shown that methyl-groups can be enzymatically removed from lysine residues, and demethylation enzymes have been identified.24,98-101 The debate as to whether H3K27me3 can be actively removed has been settled by a series of papers identifying two related jumonji-family proteins, JMJD3 and UTX, which specifically demethylate H3K27me3 (Fig. 1B).102-106 Both of these demethylases are members of MLL protein complexes, which antagonize PcG-mediated gene silencing. Interestingly, JMJD3 is a direct gene target of silencing mediator of retinoic acid and thyroid hormone receptors (SMRT), which, through its interaction with retinoic acid receptors (RARs), represses JMJD3 expression to maintain a neural stem cell state.105 Retinoic acid (RA) treatment of neural progenitors results in upregulation of JMJD3 and decreases in H3K27me3 levels on the promoter of the DLX5 gene, a marker of differentiated neurons.
It has also been show that the H3K9me2 and H3K9me3 demethylase genes, JMJD1A and JMJD2C, are positively regulated by the ES cell transcription factor, OCT3/4. Interestingly, JMJD1A or JMJD2C depletion leads to ES cell differentiation and is accompanied by a reduction in expression of pluripotency genes, favoring induction of lineage marker genes. JMJD1A demethylates H3K9me2 at the promoter regions of TCL1, TCFCP2L1 and ZFP57, leading to upregulation of the expression of these pluripotency-associated genes. JMJD2C also acts as a positive regulator for NANOG.4
In general, histone demethylases are tightly integrated in the transcriptional factor regulatory networks in ES cells.107,108 To give one example, the high-ranking gene JARID2 is the target for seven core regulators of ES cells. JARID2, also known as Jumonji (JMJ), is highly expressed in ES cells; however, it is downregulated in the whole embryonic body at the onset of differentiation. Later during cellular differentiation events, the compartments where JMJ is expressed expand gradually, with expression detectable in almost all adult tissues, although the intensities vary among cell types.108,109
These observations suggest that histone demethylases link core transcription factor networks to the regulation of chromatin status during cellular differentiation. Once again, the actions of histone methylases and demethylases seem to be interconnected with DNA methylation. For instance, TET1 binds a significant proportion of Polycomb and Trithorax group target genes. Remarkably, 5-hmC is significantly enriched predominantly at two groups of promoters. First, at inactive promoters, many of which contain bivalent chromatin domains with both activating H3K4me3, and repressive H3K27me3.51,110 In contrast, in the second group of promoters, TET1 is associated with active histone marks, including H3K4me3, H3K4me1 and H3K36me, a mark associated with transcriptional elongation.51,59,111 These data indicate that 5-hmC can be associated with both actively transcribed and repressed target genes. The relationship of DNA methyltransferases and hydroxymethylases with histone demethylases awaits further investigation, but one can speculate that their combinatorial action provides for the balancing act of histone PTMs in stem cells.
ATP-Dependent Chromatin Remodeling Complexes in the Regulation of the Stem Cell Chromatin Landscape
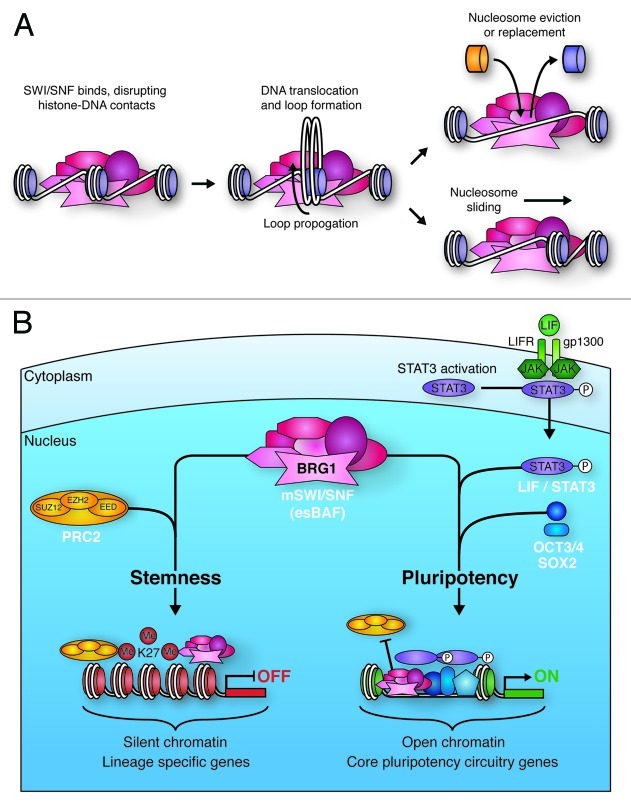
When it comes to the discussion of the chromatin landscape and its regulation, it is important to emphasize the role played by chromatin remodeling complexes, which change the chromatin architecture by modulating interaction between nucleosomal particles and DNA. These chromatin remodeling complexes are enzymes that transiently disrupt the association between DNA and histones in an ATP-dependent manner. This allows for nucleosome sliding, translocation and eviction, as well as changes in nucleosome composition through the exchange of canonical histones with histone variants. This in turn may induce conformational changes in nucleosomes and control different degrees of the condensation state of chromatin (Fig. 4A).112
Figure 4. Mechanisms of SWI/SNF-mediated chromatin remodeling. (A) SWI/SNF binding to chromatin disrupts histone-DNA contacts. This allows for the creation of a loop of DNA that propagates around the nucleosome, ultimately resulting in repositioning of the DNA with respect to the nucleosome (sliding). This sliding can lead to eviction of adjacent nucleosomes. DNA looping also facilitates histone replacement with histone variants. (B) BRG1 containing mSWI/SNF in ES cells (esBAF) plays vital roles in the pathways promoting ES cell stemness and pluripotency. esBAF enforces repressive H3K27me3 marks deposited by the PRC2 complex at many lineage specific genes that would otherwise promote differentiation. In contrast, esBAF antagonizes PRC2 action at LIF targets, such as core pluripotency circuitry genes. By creating more accessible chromatin at these locations, esBAF prepares the chromatin environment for the cooperative action of phospho-STAT3 and master pluripotency regulators, such as OCT3/4 and SOX2.
Chromatin remodeling complexes are numerous and highly abundant, with each complex displaying distinct patterns of activity. These complexes can be divided into families based on their subunit composition and biochemical activity.15 The precise subunit composition of these complexes can be fine-tuned to confer functional specificity within the cell. For the purpose of this review, we shall discuss two of the most well studied ATP-dependent chromatin remodelers: SWI/SNF and CHD1. For a thorough review all ATP-dependent chromatin remodeling complexes, see a recent review by Hargreaves and Crabtree.113
SWI/SNF chromatin remodeling activity
Perhaps one of the most well studied examples of ATP-dependent chromatin remodelers is the SWI/SNF protein complex (mSWI/SNF in mammals, also known as BAF). The 9–12 subunits of mSWI/SNF are gene families and are combinatorialy assembled, with one of two mutually exclusive catalytic ATPase subunits, brahma homolog (BRM, also known as SMARCA2) or BRM/SWI2-related gene 1 (BRG1, also known as SMARCA4). Variations in mSWI/SNF subunit composition contribute to targeting, assembly and regulation of lineage-specific functions during ES cell differentiation.114,115
Unlike the yeast SWI/SNF complex, the mammalian SWI/SNF complex is not monomorphic. mSWI/SNF subunit exchange assists the transition of stem cells from pluripotency to a multipotent state, and further to terminal differentiation.116-119 For example, ES cells express the subunits BRG1, BAF155 and BAF60A; however, upon differentiation, switch in subunit expression occurs, and these are replaced by BRM, BAF170 and BAF60C, respectively.118,120 Moreover, differential usage of the mSWI/SNF subunits BAF53A and BAF53B has also been shown upon transitioning of neural precursors to terminally differentiated neurons.117 Inactivation of mouse SWI/SNF subunits such as BRG1, BAF47, BAF57, BAF60, BAF155, BAF180 and BAF250A leads to embryonic lethality and BRG1, BAF47 and BAF155 to the failure of formation of pluripotent cells (individual phenotypes outlined in more detail in Table 1).15,119,121-126 In human ES cells, the BRG-containing mSWI/SNF complex is required for the ability of ES cells to maintain self-renewal and remain pluripotent (Fig. 4B).118,127
Table 1. Roles of mSWI/SNF complex components in mammalian development.
| Name | Alias | Lethality | Observed phenotype | Reference(s) |
|---|---|---|---|---|
| BAF250a |
ARID1A |
Embryonic lethal at E6.5 |
Inhibition of self-renewal in ES cells. Absence/impaired differentiation of mesoderm. Promotes primitive ectoderm and endoderm. |
114 |
| BAF47 |
INI1, SNF5 |
Embryonic lethal at E3.5 to E5.5: peri-implantation lethal |
Peri-implantation defects. Nervous system and soft tissue sarcomas in heterozygotes. |
119, 121 |
| BAF60c |
SMARCD3 |
- |
Heart defects; defects in establishment of left-right asymmetry. |
117, 164 |
| BAF155 |
SRG3 |
Trophoblast stage |
Defects in formation of inner cell mass. Heterozygotes display brain organization problems (exencephaly) due to failure of neural tube closure. |
118 |
| BAF53a |
- |
- |
Required for neuronal stem-cell proliferation. |
115 |
| BAF53b |
- |
- |
Required for activity-dependent dendritic outgrowth. |
165 |
| BAF57 |
Smarce1 |
- |
Dominant-negative mutations prevent T-cell development. |
166 |
| BAF180 |
PBRM1 |
- |
Impaired epithelial-to-mesenchymal-transition (EMT) and arrested maturation of epicardium at E11.5. Leads to defects in coronary vessel formation. |
167 |
| BRG1 |
- |
Arrest at two-cell state (ZGA) |
Required for Zygotic Genome Activation. H3K4me2 reduced. Required for differentiation of neurones, lymphocytes, and adipose and heart tissues. Required for β-globin expression activation during embryonic erythropoiesis. |
115, 117, 120, 168, 169, 170 |
| BRM | - | None | Greater body mass. | 171 |
Genome-wide analysis of mSWI/SNF binding in mouse ES cells conducted by Ho et al. suggests that the complexes bind roughly 3% of the genome, with a 2.1 kb footprint.127 The majority of mSWI/SNF binding is not at transcriptional start sites, but rather at distal enhancer and silencer sites.127 BRG1 binding overlaps more closely with H3K4me1 than H3K4me3, suggesting it occupies enhancers and regulatory elements rather than sites of active transcription.113,127 This is in contrast to yeast SWI/SNF that activates its targets by binding promoters.15 Further studies by Ho et al. have shown that the mSWI/SNF complex binds to the enhancers and promoters of genes encoding important pluripotency regulators, and cooperates with the master regulators of pluripotency, such as OCT3/4 and SOX2 for control of stemness circuitry (Fig. 4B).118 Such interaction suggests a functional overlap between mSWI/SNF and the core pluripotency pathways.118 In parallel, mSWI/SNF complexes also act as transcriptional repressors on a number of differentiation specific genes in ES cells (Fig. 4B).118 It has been shown that addition of the mSWI/SNF complex to the master pluripotency cocktail used to reprogram somatic cells into induced pluripotent stem cells (iPS cells) increases reprogramming efficiency dramatically.128
The mechanism by which mSWI/SNF acts to repress or activate transcription is not clear in mammals; however, it is proposed to function in a similar manner to yeast SWI/SNF activity, by mobilizing nucleosome sliding along DNA and by catalyzing the insertion and eviction of histone octamers.129 This activity is probably indispensable for denying or allowing access of transcriptional factors to their cognate DNA binding sites.129 Importantly, SWI/SNF complexes are also capable of recruiting histone deacetylases, which remove activating acetyl marks from histone tails, further promoting the repressive state.130
CHD1 adaptor protein
Another example of a protein with chromatin remodeling activity required for mediating cellular stemness is chromodomain helicase DNA binding protein 1 (CHD1), which functions as substrate recognition component of the transcription regulatory histone acetylation (HAT) complex SAGA. Evidence indicates that CHD1 can be used as a molecular adaptor, bringing SNF2, the FACT complex and the PAF complex to H3K4me2/3.131 It has been suggested that such an adaptor may be required to maintain open chromatin in ES cells, thus providing for pluripotency. In fact, it is a target gene for OCT3/4, SOX2, NANOG, SMAD1, ZFX and E2F1.132 Chd1 RNAi mouse ES cells have decreased self-renewal but maintain expression of markers of the undifferentiated state, such as alkaline phosphatase, SSEA1 and OCT3/4, as shown by immunofluorescence experiments.5 Since CHD1 is classically associated with active transcription, one would expect drastic downregulation of stemness genes at the transcriptional level upon knockdown. Surprisingly, however, experimental evidence indicates that only a few genes, other than Oct3/4, are downregulated. This could be due to residual levels of CHD1 in RNAi mutants, which could be sufficient for maintenance of the ES cell transcriptome. However, Chd1 RNAi ES cells have skewed formation of primitive endoderm upon differentiation to embryoid bodies, which consequently leads to a loss of cardiac mesoderm differentiation and abnormally high levels of neural differentiation.5 Subsequent genome-wide location analysis for CHD1 indicated that CHD1 binding strongly correlates with RNAP II and H3K4me3. Surprisingly, bivalent domains were largely devoid of Chd1, and major H3K9 methyltransferases and demethylases are expressed in Chd1 RNAi ES cells at similar levels to control ES cells. However, rapid exchange of histone H1 is compromised in Chd1 RNAi ES cells, indicating that chromatin is less breathable. This data suggests that formation of open chromatin, required for pluripotency, is dependent on CHD1 levels. Additional evidence to support this notion comes from the reduced capacity of Chd1 knockdown fibroblasts to undergo induced pluripotency. Since CHD1 is viewed as an adaptor protein for targeting many other specialized complexes, the full spectrum of CHD1 action in ES cells has yet to be discovered.
Non-Coding RNAs in Stem Cell Regulation
A new, integrated regulatory network is currently emerging based on the dynamic interplay of chromatin modifying, chromatin remodeling and DNA methylation components with non-protein coding RNAs (ncRNAs). These mechanisms synergize to choreograph cellular stemness and to generate cellular diversity. New evidence indicates the existence of an extensive regulatory network involving RNA signaling.133 This is based on the notion that although only 1.2% of the human genome encodes protein, a large fraction of it is transcribed. Indeed, as much as 98% of the transcriptional output in humans and other mammals has been proposed to consist of non-protein coding RNAs.134 The number of known functional ncRNA genes has risen dramatically in recent years, and many of these appear to be expressed and function in a developmentally specific manner. Such ncRNAs include microRNAs (miRNAs), long non-coding RNAs (lnRNAs), PIWI RNAs (piRNAs), short heterochromatic RNAs (shRNAs), endogenous short interfering RNAs (endo-siRNAs) and transcripts originating from retrotransposon repeats and pseudogenes.135,136 There is also considerable evidence that ncRNAs regulate chromosome dynamics, chromatin modification and epigenetic memory, including imprinting, DNA methylation and transcriptional gene silencing (reviewed in ref. 137). We will now discuss several examples of ncRNAs and how their functions pertain to ES cell maintenance and differentiation.
microRNAs
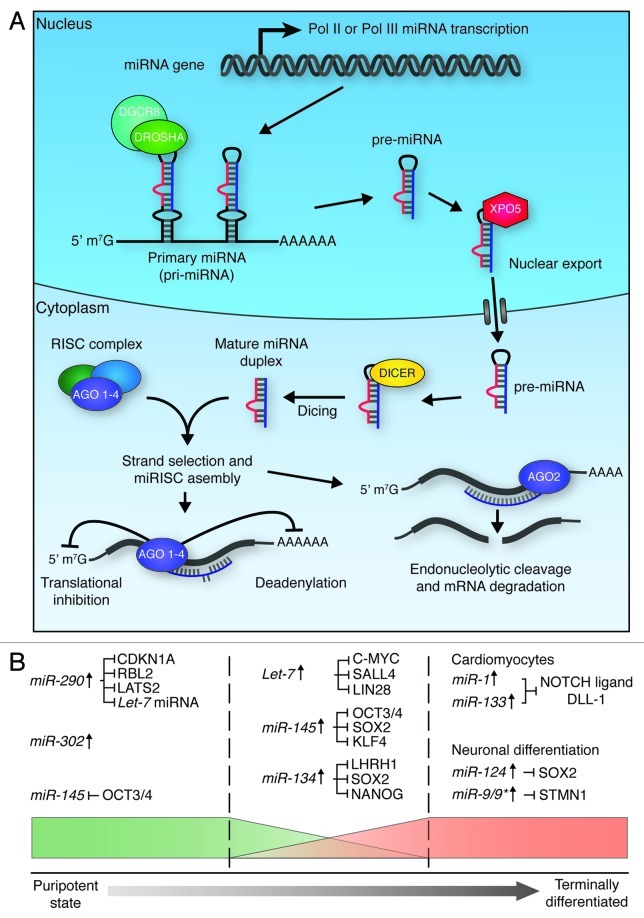
miRNAs are a class of small, ~21 nt, non-coding RNAs that play an important role in post-transcriptional gene expression through the regulation of mRNA stability and, consequently, protein abundance. miRNAs recognize and bind their targets through short 2–8 nt seed sequences.138 As each miRNA can recognize many hundreds of targets, and multiple miRNAs may target individual mRNAs, these gene regulatory networks can become rather complex.138 For a review of miRNA biogenesis, see reference 139 and Figure 5A.
Figure 5. miRNA biogenesis and function in ES cell differentiation. (A) Micro RNA biogenesis. Primary transcripts (pri-miRNAs) are generated through RNA pol II or pol III transcription, which then undergo RNase III cleavage, mediated by the DROSHA/DGCR8 complex, to generate ~70 nt pre-miRNAs. XPO5 exports these pre-miRNAs to the cytoplasm, where they are further cleaved by DICER to generate mature double-stranded RNA duplexes. One strand of these duplexes is then bound by one of four Argonaute proteins (AGO 1–4) to form active RISC complexes, which can modulate gene expression through translational inhibition, or mRNA deadenylation. If the miRNA is perfectly matched to the target sequence, endonucleolytic cleavage of the mRNA transcript can occur through the ‘slicer’ activity of AGO2.139,145 (B) miRNAs play roles in the maintenance of pluripotency (left section), the onset of differentiation (middle section), and the maintenance of terminal differentiation (right section). In order to maintain pluripotency, miRNAs act to promote maintenance of cell cycle progression (CDKN1A, RBL2 and LATS2 inhibition) and de novo DNA methylation (RBL2 inhibition), and suppress factors that promote differentiation (Let-7 inhibition). In order to promote differentiation, miRNAs act to block self-renewal and core pluripotency factor production. miRNAs maintain terminal differentiation by suppressing gene expression associated with other lineages (miR-1 and miR-133), block self-renewal (miR-124) and maintain the state of the specific lineage (miR-9/9*).
miRNAs are appealing candidates to control ES cell pluripotency and drive cellular differentiation. There are two main reasons for this argument. First, key regulators of pluripotency such as OCT3/4, SOX2 and NANOG have been found to occupy the promoters of a host of miRNAs, and act to either activate or repress their expression depending on the miRNA species.140 Furthermore, histone marks associated with active transcription (H3K4me3 and H3K36me3) and silenced transcription (H3K27me3) are associated with active and silent miRNAs, respectively, almost mimicking the epigenetic patterns of protein coding genes.140 Second, knockouts of the miRNA processing enzymes Dicer and Dgcr8 abolish the ability of ES cells to silence their self-renewal program and cause severe defects in their ability to differentiate.141,142
Several miRNAs have been linked to cellular stemness, including those from miR-290 and miR-302 clusters. Expression of mature ES cell-specific miR-290 family members can rescue the Dgcr8 null proliferation defects in ES cells by controlling the expression of negative regulators of ES cell cycle, such as CDKN1A, RBL2 and LATS2 (Fig. 5B).143 miR-290 family miRNAs also maintain de novo DNA methylation through the control of RBL2, which transcriptionally represses DNA methyltransferases.144 Furthermore, miR-290 family members antagonize the activity of differentiation-related miRNAs such as the let-7 family.145
Let-7 family members are a highly conserved group of miRNAs that repress C-MYC, SALL4 and LIN28 protein production, leading to a loss of ES cell self-renewing capacity and subsequent ES cell differentiation (Fig. 5B).146 LIN28 plays an important role in ES cell maintenance by inhibiting pre-let-7 miRNA cleavage and destabilization of pre-let-7 miRNA.147 Inhibition of let-7 substantially enhances somatic cell reprogramming into iPS cells.146 miR-145 is another miRNA associated with ES cell differentiation, acting through inhibition of the master regulators of pluripotency OCT3/4, SOX2 and KLF4.148 In self-renewing ES cells, however, OCT3/4 transcriptionally represses miR-145 expression to maintain a pluripotent state.149 Along this line, it has been shown that retinoic acid-inducible miR-134 promotes differentiation of mouse ES cells to the ectodermal lineage, with probable targeting of Sox2, LHRH1 and Nanog transcripts.150,151
Finally, a number of lineage-specific miRNAs are responsible for maintaining ES cell differentiation (Fig. 5B). For example, miR-1 and miR-133 are muscle-specific miRNAs that are activated upon ES cell differentiation to cardiomyocytes.152 These miRNAs promote mesoderm differentiation by repressing non-muscle gene expression through downregulation of the Notch pathway ligand, DLL-1.152 Another miRNA, miR-9/9*, has recently been linked to the molecular pathways of neural differentiation, and provides a good example in which several mechanisms of epigenetic regulation feedback on one another to mediate a specific function. In particular, as a part of neural differentiation circuitry, downregulation of the gene Rest/Nrsf, responsible for the repression of neuronal-specific genes, lifts repression on miR9/9* and miR-124 promoters, resulting in elevated expression of these miRNAs. This in turn leads to a switch in mSWI/SNF subunit composition, due to miRNA mediated BAF53A repression, exit from cell cycle, and concomitant BAF53B activation.153 As discussed above, this leads to transitioning of neural precursors to terminally differentiated neurons.
There is ever-growing evidence of the increased and complex network of miRNA function in ES cells. Although not all of the players have been identified to date, evidence points to three distinct mechanisms of their actions: (1) Participation in maintenance of stem cell self-renewal and pluripotency through the inhibition of negative factors controlling these events; (2) Initiation of stem cell differentiation through the inhibition of master pluripotency factors; (3) Maintenance of lineage definition through restricting the expression of genes from other lineages. There may be other, as yet undiscovered functions.
Polycomb-associated non-coding RNAs
A number of recent publications indicate that the Polycomb group complex PRC2 (discussed above) utilizes non-coding RNA co-factors as sequence specific guides to direct Polycomb group complexes to their cognate binding sites within the genome. In mouse ES cells, RNA immunoprecipitation (RIP), combined with RNA-sequencing (RIP-seq), has uncovered novel Polycomb group-interacting RNA genome-wide.154 This study has identified at least 9000 distinct ncRNA transcripts that bind PRC2, revealing a highly complex and abundant population of long non-coding RNAs, which may direct PRC2 to its target loci throughout the genome. Previous reports also indicate that large intergenic non-coding RNA (lincRNA) associated with members of the Polycomb group were cataloged from a number of human stem and somatic cells.155 Although less than 2% of the PRC2 non-coding transcriptome identified by Zhao et al. intersects with lincRNAs, the jury is still out as to whether or not PRC2 associated non-coding RNA interactions have cell-type specificity, allowing PRC2 to employ a variety of ncRNAs to differentially suppress genomic loci in a cell-type specific manner. Similarly, the ncRNA ANRIL (also known as CDKN2B-AS1) reportedly interacts with the chromodomain of CBX7 (a component of the PRC1 complex), and modulates its binding to H3K27me3 in vitro (Fig. 6).156 Relevance of ANRIL ncRNA in ES cell biology has yet to be investigated, but detailed investigations of the functional significance of individual ncRNAs that interact with PRC2, such as Hotair, Xist RNA, Tsix and RepA, demonstrate that long ncRNAs may contribute to the cellular epigenome through modulation of DNA methylation,157,158 changes in chromatin modifications,154,155,159,160 or interception with RNAi and miRNA pathways.161-164 These observations suggest that long ncRNAs might represent a “flexible scaffold,” mediating interactions between DNA and protein complexes.165,166
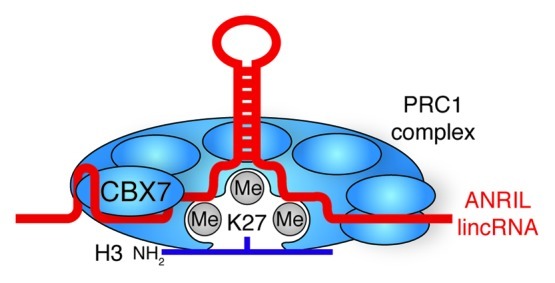
Figure 6. lincRNA stabilization of Polycomb Group complexes. ANRIL lincRNA bind and stabilizes the interaction of PRC1 with H3K27me3 through the CBX7 subunit.
OCT3/4 and NANOG-associated ncRNAs
Of note, several lines of evidence indicate that master pluripotency regulators such as OCT3/4 and NANOG might be involved in the regulation of transcriptional activity of ES cell-specific non-coding RNAs.70 The functional relevance of two of these RNAs, Rncr2/Ak028326/Gomafu/Miat (OCT3/4-activated) and Ak141205 (NANOG-repressed), was recently investigated in the context of mES stemness and differentiation.167 In particular, knockdown of these ncRNAs altered Oct3/4 and/or Nanog transcript levels, and modulated mESC differentiation toward specific lineages in the presence of LIF. Overexpression of either long ncRNA led to enhancement of mesodermal, endodermal, and ectodermal differentiation in the presence of LIF. This data suggests that non-coding RNA can be integral part of transcriptional factor circuitry in ES cells.
Conclusions
The cumulative research data only briefly discussed in this review suggests an ever-expanding realm of epigenetic players intimately involved in a multilayered but interconnected network of epigenetic regulation within stem cells. This staggering complexity holds the key not only to the puzzle of mammalian development, but also tissue and organ regeneration and ultimately the emerging paradigms of human aging and age-related diseases. Epigenetic memory operates on combinatorial read-outs of histone modifications, DNA methylation, alterations of chromatin structure due to chromatin remodeling and non-coding RNAs. These represent another crucial mechanism, besides just a network of transcriptional factors, that governs the fine-tuning and precision of gene expression programs. The elucidation of epigenetic mechanisms promises to have important implications for novel advances in stem cell research and nuclear reprogramming, and may offer novel targets for combating human diseases, potentially leading to new diagnostic and therapeutic avenues.
Acknowledgments
We apologize to our colleagues for the omission of many important research contributions due to space constraints in this review. We thank members of Lunyak lab, Benjamin Blackwell and Regina Brunauer, for their critical reading and suggestions. J.R.T and V.V.L are supported by Buck Institute Start-up Fund to V.V.L.
Footnotes
Previously published online: www.landesbioscience.com/journals/epigenetics/article/21141
References
- 1.Reik W. Stability and flexibility of epigenetic gene regulation in mammalian development. Nature. 2007;447:425–32. doi: 10.1038/nature05918. [DOI] [PubMed] [Google Scholar]
- 2.Schaniel C, Ang YS, Ratnakumar K, Cormier C, James T, Bernstein E, et al. Smarcc1/Baf155 couples self-renewal gene repression with changes in chromatin structure in mouse embryonic stem cells. Stem Cells. 2009;27:2979–91. doi: 10.1002/stem.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pasini D, Bracken AP, Hansen JB, Capillo M, Helin K. The polycomb group protein Suz12 is required for embryonic stem cell differentiation. Mol Cell Biol. 2007;27:3769–79. doi: 10.1128/MCB.01432-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loh YH, Zhang W, Chen X, George J, Ng HH. Jmjd1a and Jmjd2c histone H3 Lys 9 demethylases regulate self-renewal in embryonic stem cells. Genes Dev. 2007;21:2545–57. doi: 10.1101/gad.1588207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaspar-Maia A, Alajem A, Polesso F, Sridharan R, Mason MJ, Heidersbach A, et al. Chd1 regulates open chromatin and pluripotency of embryonic stem cells. Nature. 2009;460:863–8. doi: 10.1038/nature08212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armstrong L, Hughes O, Yung S, Hyslop L, Stewart R, Wappler I, et al. The role of PI3K/AKT, MAPK/ERK and NFkappabeta signalling in the maintenance of human embryonic stem cell pluripotency and viability highlighted by transcriptional profiling and functional analysis. Hum Mol Genet. 2006;15:1894–913. doi: 10.1093/hmg/ddl112. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong L, Lako M, Dean W, Stojkovic M. Epigenetic modification is central to genome reprogramming in somatic cell nuclear transfer. Stem Cells. 2006;24:805–14. doi: 10.1634/stemcells.2005-0350. [DOI] [PubMed] [Google Scholar]
- 8.Ang YS, Tsai SY, Lee DF, Monk J, Su J, Ratnakumar K, et al. Wdr5 mediates self-renewal and reprogramming via the embryonic stem cell core transcriptional network. Cell. 2011;145:183–97. doi: 10.1016/j.cell.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–26. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 10.Boyer LA, Plath K, Zeitlinger J, Brambrink T, Medeiros LA, Lee TI, et al. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature. 2006;441:349–53. doi: 10.1038/nature04733. [DOI] [PubMed] [Google Scholar]
- 11.Mikkelsen TS, Ku M, Jaffe DB, Issac B, Lieberman E, Giannoukos G, et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature. 2007;448:553–60. doi: 10.1038/nature06008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takahashi K, Okita K, Nakagawa M, Yamanaka S. Induction of pluripotent stem cells from fibroblast cultures. Nat Protoc. 2007;2:3081–9. doi: 10.1038/nprot.2007.418. [DOI] [PubMed] [Google Scholar]
- 13.Li E. Chromatin modification and epigenetic reprogramming in mammalian development. Nat Rev Genet. 2002;3:662–73. doi: 10.1038/nrg887. [DOI] [PubMed] [Google Scholar]
- 14.Williams RR, Azuara V, Perry P, Sauer S, Dvorkina M, Jørgensen H, et al. Neural induction promotes large-scale chromatin reorganisation of the Mash1 locus. J Cell Sci. 2006;119:132–40. doi: 10.1242/jcs.02727. [DOI] [PubMed] [Google Scholar]
- 15.Lessard JA, Crabtree GR. Chromatin regulatory mechanisms in pluripotency. Annu Rev Cell Dev Biol. 2010;26:503–32. doi: 10.1146/annurev-cellbio-051809-102012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patterton D, Wolffe AP. Developmental roles for chromatin and chromosomal structure. Dev Biol. 1996;173:2–13. doi: 10.1006/dbio.1996.0002. [DOI] [PubMed] [Google Scholar]
- 17.Constantinescu D, Gray HL, Sammak PJ, Schatten GP, Csoka AB. Lamin A/C expression is a marker of mouse and human embryonic stem cell differentiation. Stem Cells. 2006;24:177–85. doi: 10.1634/stemcells.2004-0159. [DOI] [PubMed] [Google Scholar]
- 18.Butler JT, Hall LL, Smith KP, Lawrence JB. Changing nuclear landscape and unique PML structures during early epigenetic transitions of human embryonic stem cells. J Cell Biochem. 2009;107:609–21. doi: 10.1002/jcb.22183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meshorer E, Yellajoshula D, George E, Scambler PJ, Brown DT, Misteli T. Hyperdynamic plasticity of chromatin proteins in pluripotent embryonic stem cells. Dev Cell. 2006;10:105–16. doi: 10.1016/j.devcel.2005.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiblin AE, Cui W, Clark AJ, Bickmore WA. Distinctive nuclear organisation of centromeres and regions involved in pluripotency in human embryonic stem cells. J Cell Sci. 2005;118:3861–8. doi: 10.1242/jcs.02500. [DOI] [PubMed] [Google Scholar]
- 21.Rasmussen TP. Embryonic stem cell differentiation: a chromatin perspective. Reprod Biol Endocrinol. 2003;1:100. doi: 10.1186/1477-7827-1-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Surani MA. Reprogramming of genome function through epigenetic inheritance. Nature. 2001;414:122–8. doi: 10.1038/35102186. [DOI] [PubMed] [Google Scholar]
- 23.Bassett A, Cooper S, Wu C, Travers A. The folding and unfolding of eukaryotic chromatin. Curr Opin Genet Dev. 2009;19:159–65. doi: 10.1016/j.gde.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 24.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 25.Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–5. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 26.Lee JS, Smith E, Shilatifard A. The language of histone crosstalk. Cell. 2010;142:682–5. doi: 10.1016/j.cell.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamakaka RT, Biggins S. Histone variants: deviants? Genes Dev. 2005;19:295–310. doi: 10.1101/gad.1272805. [DOI] [PubMed] [Google Scholar]
- 28.Old RW, Woodland HR. Histone genes: not so simple after all. Cell. 1984;38:624–6. doi: 10.1016/0092-8674(84)90256-3. [DOI] [PubMed] [Google Scholar]
- 29.Meshorer E, Misteli T. Chromatin in pluripotent embryonic stem cells and differentiation. Nat Rev Mol Cell Biol. 2006;7:540–6. doi: 10.1038/nrm1938. [DOI] [PubMed] [Google Scholar]
- 30.Ahmad K, Henikoff S. The histone variant H3.3 marks active chromatin by replication-independent nucleosome assembly. Mol Cell. 2002;9:1191–200. doi: 10.1016/S1097-2765(02)00542-7. [DOI] [PubMed] [Google Scholar]
- 31.Ng RK, Gurdon JB. Epigenetic memory of an active gene state depends on histone H3.3 incorporation into chromatin in the absence of transcription. Nat Cell Biol. 2008;10:102–9. doi: 10.1038/ncb1674. [DOI] [PubMed] [Google Scholar]
- 32.Nan X, Ng HH, Johnson CA, Laherty CD, Turner BM, Eisenman RN, et al. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998;393:386–9. doi: 10.1038/30764. [DOI] [PubMed] [Google Scholar]
- 33.Lunyak VV, Burgess R, Prefontaine GG, Nelson C, Sze SH, Chenoweth J, et al. Corepressor-dependent silencing of chromosomal regions encoding neuronal genes. Science. 2002;298:1747–52. doi: 10.1126/science.1076469. [DOI] [PubMed] [Google Scholar]
- 34.Klose RJ, Bird AP. Genomic DNA methylation: the mark and its mediators. Trends Biochem Sci. 2006;31:89–97. doi: 10.1016/j.tibs.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 35.Ooi SK, Bestor TH. The colorful history of active DNA demethylation. Cell. 2008;133:1145–8. doi: 10.1016/j.cell.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 36.Cui H, Fedoroff NV. Inducible DNA demethylation mediated by the maize Suppressor-mutator transposon-encoded TnpA protein. Plant Cell. 2002;14:2883–99. doi: 10.1105/tpc.006163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reik W, Dean W. DNA methylation and mammalian epigenetics. Electrophoresis. 2001;22:2838–43. doi: 10.1002/1522-2683(200108)22:14<2838::AID-ELPS2838>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 38.Gong Z, Morales-Ruiz T, Ariza RR, Roldán-Arjona T, David L, Zhu JK. ROS1, a repressor of transcriptional gene silencing in Arabidopsis, encodes a DNA glycosylase/lyase. Cell. 2002;111:803–14. doi: 10.1016/S0092-8674(02)01133-9. [DOI] [PubMed] [Google Scholar]
- 39.Morales-Ruiz T, Ortega-Galisteo AP, Ponferrada-Marín MI, Martínez-Macías MI, Ariza RR, Roldán-Arjona T. DEMETER and REPRESSOR OF SILENCING 1 encode 5-methylcytosine DNA glycosylases. Proc Natl Acad Sci U S A. 2006;103:6853–8. doi: 10.1073/pnas.0601109103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rai K, Huggins IJ, James SR, Karpf AR, Jones DA, Cairns BR. DNA demethylation in zebrafish involves the coupling of a deaminase, a glycosylase, and gadd45. Cell. 2008;135:1201–12. doi: 10.1016/j.cell.2008.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Popp C, Dean W, Feng S, Cokus SJ, Andrews S, Pellegrini M, et al. Genome-wide erasure of DNA methylation in mouse primordial germ cells is affected by AID deficiency. Nature. 2010;463:1101–5. doi: 10.1038/nature08829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science. 2001;293:1089–93. doi: 10.1126/science.1063443. [DOI] [PubMed] [Google Scholar]
- 43.Mayer W, Niveleau A, Walter J, Fundele R, Haaf T. Demethylation of the zygotic paternal genome. Nature. 2000;403:501–2. doi: 10.1038/35000656. [DOI] [PubMed] [Google Scholar]
- 44.Yamanaka S. A fresh look at iPS cells. Cell. 2009;137:13–7. doi: 10.1016/j.cell.2009.03.034. [DOI] [PubMed] [Google Scholar]
- 45.Farthing CR, Ficz G, Ng RK, Chan CF, Andrews S, Dean W, et al. Global mapping of DNA methylation in mouse promoters reveals epigenetic reprogramming of pluripotency genes. PLoS Genet. 2008;4:e1000116. doi: 10.1371/journal.pgen.1000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lister R, Pelizzola M, Dowen RH, Hawkins RD, Hon G, Tonti-Filippini J, et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature. 2009;462:315–22. doi: 10.1038/nature08514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lister R, Ecker JR. Finding the fifth base: genome-wide sequencing of cytosine methylation. Genome Res. 2009;19:959–66. doi: 10.1101/gr.083451.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kriaucionis S, Heintz N. The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science. 2009;324:929–30. doi: 10.1126/science.1169786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–5. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ficz G, Branco MR, Seisenberger S, Santos F, Krueger F, Hore TA, et al. Dynamic regulation of 5-hydroxymethylcytosine in mouse ES cells and during differentiation. Nature. 2011;473:398–402. doi: 10.1038/nature10008. [DOI] [PubMed] [Google Scholar]
- 51.Williams K, Christensen J, Pedersen MT, Johansen JV, Cloos PA, Rappsilber J, et al. TET1 and hydroxymethylcytosine in transcription and DNA methylation fidelity. Nature. 2011;473:343–8. doi: 10.1038/nature10066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Valinluck V, Sowers LC. Endogenous cytosine damage products alter the site selectivity of human DNA maintenance methyltransferase DNMT1. Cancer Res. 2007;67:946–50. doi: 10.1158/0008-5472.CAN-06-3123. [DOI] [PubMed] [Google Scholar]
- 53.Bhutani N, Burns DM, Blau HM. DNA demethylation dynamics. Cell. 2011;146:866–72. doi: 10.1016/j.cell.2011.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.He YF, Li BZ, Li Z, Liu P, Wang Y, Tang Q, et al. Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA. Science. 2011;333:1303–7. doi: 10.1126/science.1210944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang L, Lu X, Lu J, Liang H, Dai Q, Xu GL, et al. Thymine DNA glycosylase specifically recognizes 5-carboxylcytosine-modified DNA. Nat Chem Biol. 2012;8:328–30. doi: 10.1038/nchembio.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ito S, D’Alessio AC, Taranova OV, Hong K, Sowers LC, Zhang Y. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature. 2010;466:1129–33. doi: 10.1038/nature09303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guo JU, Su Y, Zhong C, Ming GL, Song H. Hydroxylation of 5-methylcytosine by TET1 promotes active DNA demethylation in the adult brain. Cell. 2011;145:423–34. doi: 10.1016/j.cell.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pastor WA, Pape UJ, Huang Y, Henderson HR, Lister R, Ko M, et al. Genome-wide mapping of 5-hydroxymethylcytosine in embryonic stem cells. Nature. 2011;473:394–7. doi: 10.1038/nature10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu H, D’Alessio AC, Ito S, Wang Z, Cui K, Zhao K, et al. Genome-wide analysis of 5-hydroxymethylcytosine distribution reveals its dual function in transcriptional regulation in mouse embryonic stem cells. Genes Dev. 2011;25:679–84. doi: 10.1101/gad.2036011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koh KP, Yabuuchi A, Rao S, Huang Y, Cunniff K, Nardone J, et al. Tet1 and Tet2 regulate 5-hydroxymethylcytosine production and cell lineage specification in mouse embryonic stem cells. Cell Stem Cell. 2011;8:200–13. doi: 10.1016/j.stem.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dawlaty MM, Ganz K, Powell BE, Hu YC, Markoulaki S, Cheng AW, et al. Tet1 is dispensable for maintaining pluripotency and its loss is compatible with embryonic and postnatal development. Cell Stem Cell. 2011;9:166–75. doi: 10.1016/j.stem.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oguro H, Yuan J, Ichikawa H, Ikawa T, Yamazaki S, Kawamoto H, et al. Poised lineage specification in multipotential hematopoietic stem and progenitor cells by the polycomb protein Bmi1. Cell Stem Cell. 2010;6:279–86. doi: 10.1016/j.stem.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 63.Mohn F, Weber M, Rebhan M, Roloff TC, Richter J, Stadler MB, et al. Lineage-specific polycomb targets and de novo DNA methylation define restriction and potential of neuronal progenitors. Mol Cell. 2008;30:755–66. doi: 10.1016/j.molcel.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 64.Rodriguez J, Muñoz M, Vives L, Frangou CG, Groudine M, Peinado MA. Bivalent domains enforce transcriptional memory of DNA methylated genes in cancer cells. Proc Natl Acad Sci U S A. 2008;105:19809–14. doi: 10.1073/pnas.0810133105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Szutorisz H, Canzonetta C, Georgiou A, Chow CM, Tora L, Dillon N. Formation of an active tissue-specific chromatin domain initiated by epigenetic marking at the embryonic stem cell stage. Mol Cell Biol. 2005;25:1804–20. doi: 10.1128/MCB.25.5.1804-1820.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee KK, Workman JL. Histone acetyltransferase complexes: one size doesn’t fit all. Nat Rev Mol Cell Biol. 2007;8:284–95. doi: 10.1038/nrm2145. [DOI] [PubMed] [Google Scholar]
- 67.Katsumoto T, Aikawa Y, Iwama A, Ueda S, Ichikawa H, Ochiya T, et al. MOZ is essential for maintenance of hematopoietic stem cells. Genes Dev. 2006;20:1321–30. doi: 10.1101/gad.1393106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee JH, Hart SR, Skalnik DG. Histone deacetylase activity is required for embryonic stem cell differentiation. Genesis. 2004;38:32–8. doi: 10.1002/gene.10250. [DOI] [PubMed] [Google Scholar]
- 69.Zhong X, Jin Y. Critical roles of coactivator p300 in mouse embryonic stem cell differentiation and Nanog expression. J Biol Chem. 2009;284:9168–75. doi: 10.1074/jbc.M805562200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Loh YH, Wu Q, Chew JL, Vega VB, Zhang W, Chen X, et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38:431–40. doi: 10.1038/ng1760. [DOI] [PubMed] [Google Scholar]
- 71.Schuettengruber B, Chourrout D, Vervoort M, Leblanc B, Cavalli G. Genome regulation by polycomb and trithorax proteins. Cell. 2007;128:735–45. doi: 10.1016/j.cell.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 72.Wang J, Rao S, Chu J, Shen X, Levasseur DN, Theunissen TW, et al. A protein interaction network for pluripotency of embryonic stem cells. Nature. 2006;444:364–8. doi: 10.1038/nature05284. [DOI] [PubMed] [Google Scholar]
- 73.Lund AH, van Lohuizen M. Polycomb complexes and silencing mechanisms. Curr Opin Cell Biol. 2004;16:239–46. doi: 10.1016/j.ceb.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 74.Lee TI, Jenner RG, Boyer LA, Guenther MG, Levine SS, Kumar RM, et al. Control of developmental regulators by Polycomb in human embryonic stem cells. Cell. 2006;125:301–13. doi: 10.1016/j.cell.2006.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang L, Brown JL, Cao R, Zhang Y, Kassis JA, Jones RS. Hierarchical recruitment of polycomb group silencing complexes. Mol Cell. 2004;14:637–46. doi: 10.1016/j.molcel.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 76.Jørgensen HF, Giadrossi S, Casanova M, Endoh M, Koseki H, Brockdorff N, et al. Stem cells primed for action: polycomb repressive complexes restrain the expression of lineage-specific regulators in embryonic stem cells. Cell Cycle. 2006;5:1411–4. doi: 10.4161/cc.5.13.2927. [DOI] [PubMed] [Google Scholar]
- 77.Ringrose L, Paro R. Epigenetic regulation of cellular memory by the Polycomb and Trithorax group proteins. Annu Rev Genet. 2004;38:413–43. doi: 10.1146/annurev.genet.38.072902.091907. [DOI] [PubMed] [Google Scholar]
- 78.Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, et al. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science. 2002;298:1039–43. doi: 10.1126/science.1076997. [DOI] [PubMed] [Google Scholar]
- 79.Cao R, Zhang Y. SUZ12 is required for both the histone methyltransferase activity and the silencing function of the EED-EZH2 complex. Mol Cell. 2004;15:57–67. doi: 10.1016/j.molcel.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 80.Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–56. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Erhardt S, Su IH, Schneider R, Barton S, Bannister AJ, Perez-Burgos L, et al. Consequences of the depletion of zygotic and embryonic enhancer of zeste 2 during preimplantation mouse development. Development. 2003;130:4235–48. doi: 10.1242/dev.00625. [DOI] [PubMed] [Google Scholar]
- 82.O’Carroll D, Erhardt S, Pagani M, Barton SC, Surani MA, Jenuwein T. The polycomb-group gene Ezh2 is required for early mouse development. Mol Cell Biol. 2001;21:4330–6. doi: 10.1128/MCB.21.13.4330-4336.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Valk-Lingbeek ME, Bruggeman SW, van Lohuizen M. Stem cells and cancer; the polycomb connection. Cell. 2004;118:409–18. doi: 10.1016/j.cell.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 84.Wang H, Wang L, Erdjument-Bromage H, Vidal M, Tempst P, Jones RS, et al. Role of histone H2A ubiquitination in Polycomb silencing. Nature. 2004;431:873–8. doi: 10.1038/nature02985. [DOI] [PubMed] [Google Scholar]
- 85.Azuara V, Perry P, Sauer S, Spivakov M, Jørgensen HF, John RM, et al. Chromatin signatures of pluripotent cell lines. Nat Cell Biol. 2006;8:532–8. doi: 10.1038/ncb1403. [DOI] [PubMed] [Google Scholar]
- 86.Montgomery ND, Yee D, Chen A, Kalantry S, Chamberlain SJ, Otte AP, et al. The murine polycomb group protein Eed is required for global histone H3 lysine-27 methylation. Curr Biol. 2005;15:942–7. doi: 10.1016/j.cub.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 87.Zhou Q, Chipperfield H, Melton DA, Wong WH. A gene regulatory network in mouse embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104:16438–43. doi: 10.1073/pnas.0701014104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bibikova M, Chudin E, Wu B, Zhou L, Garcia EW, Liu Y, et al. Human embryonic stem cells have a unique epigenetic signature. Genome Res. 2006;16:1075–83. doi: 10.1101/gr.5319906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liang G, Chan MF, Tomigahara Y, Tsai YC, Gonzales FA, Li E, et al. Cooperativity between DNA methyltransferases in the maintenance methylation of repetitive elements. Mol Cell Biol. 2002;22:480–91. doi: 10.1128/MCB.22.2.480-491.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shiota K, Kogo Y, Ohgane J, Imamura T, Urano A, Nishino K, et al. Epigenetic marks by DNA methylation specific to stem, germ and somatic cells in mice. Genes Cells. 2002;7:961–9. doi: 10.1046/j.1365-2443.2002.00574.x. [DOI] [PubMed] [Google Scholar]
- 91.Viré E, Brenner C, Deplus R, Blanchon L, Fraga M, Didelot C, et al. The Polycomb group protein EZH2 directly controls DNA methylation. Nature. 2006;439:871–4. doi: 10.1038/nature04431. [DOI] [PubMed] [Google Scholar]
- 92.Cao F, Chen Y, Cierpicki T, Liu Y, Basrur V, Lei M, et al. An Ash2L/RbBP5 heterodimer stimulates the MLL1 methyltransferase activity through coordinated substrate interactions with the MLL1 SET domain. PLoS One. 2010;5:e14102. doi: 10.1371/journal.pone.0014102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dou Y, Milne TA, Ruthenburg AJ, Lee S, Lee JW, Verdine GL, et al. Regulation of MLL1 H3K4 methyltransferase activity by its core components. Nat Struct Mol Biol. 2006;13:713–9. doi: 10.1038/nsmb1128. [DOI] [PubMed] [Google Scholar]
- 94.Wysocka J, Swigut T, Milne TA, Dou Y, Zhang X, Burlingame AL, et al. WDR5 associates with histone H3 methylated at K4 and is essential for H3 K4 methylation and vertebrate development. Cell. 2005;121:859–72. doi: 10.1016/j.cell.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 95.Gan Q, Thiébaud P, Thézé N, Jin L, Xu G, Grant P, et al. WD repeat-containing protein 5, a ubiquitously expressed histone methyltransferase adaptor protein, regulates smooth muscle cell-selective gene activation through interaction with pituitary homeobox 2. J Biol Chem. 2011;286:21853–64. doi: 10.1074/jbc.M111.233098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Thompson BA, Tremblay V, Lin G, Bochar DA. CHD8 is an ATP-dependent chromatin remodeling factor that regulates beta-catenin target genes. Mol Cell Biol. 2008;28:3894–904. doi: 10.1128/MCB.00322-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bhaumik SR, Smith E, Shilatifard A. Covalent modifications of histones during development and disease pathogenesis. Nat Struct Mol Biol. 2007;14:1008–16. doi: 10.1038/nsmb1337. [DOI] [PubMed] [Google Scholar]
- 98.Seward DJ, Cubberley G, Kim S, Schonewald M, Zhang L, Tripet B, et al. Demethylation of trimethylated histone H3 Lys4 in vivo by JARID1 JmjC proteins. Nat Struct Mol Biol. 2007;14:240–2. doi: 10.1038/nsmb1200. [DOI] [PubMed] [Google Scholar]
- 99.Shi Y, Whetstine JR. Dynamic regulation of histone lysine methylation by demethylases. Mol Cell. 2007;25:1–14. doi: 10.1016/j.molcel.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 100.Trojer P, Reinberg D. Histone lysine demethylases and their impact on epigenetics. Cell. 2006;125:213–7. doi: 10.1016/j.cell.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 101.Whetstine JR, Nottke A, Lan F, Huarte M, Smolikov S, Chen Z, et al. Reversal of histone lysine trimethylation by the JMJD2 family of histone demethylases. Cell. 2006;125:467–81. doi: 10.1016/j.cell.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 102.Agger K, Cloos PA, Christensen J, Pasini D, Rose S, Rappsilber J, et al. UTX and JMJD3 are histone H3K27 demethylases involved in HOX gene regulation and development. Nature. 2007;449:731–4. doi: 10.1038/nature06145. [DOI] [PubMed] [Google Scholar]
- 103.De Santa F, Totaro MG, Prosperini E, Notarbartolo S, Testa G, Natoli G. The histone H3 lysine-27 demethylase Jmjd3 links inflammation to inhibition of polycomb-mediated gene silencing. Cell. 2007;130:1083–94. doi: 10.1016/j.cell.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 104.Lee MG, Villa R, Trojer P, Norman J, Yan K-P, Reinberg D, et al. Demethylation of H3K27 Regulates Polycomb Recruitment and H2A Ubiquitination. Science (New York, NY 2007:1149042. [DOI] [PubMed] [Google Scholar]
- 105.Jepsen K, Solum D, Zhou T, McEvilly RJ, Kim HJ, Glass CK, et al. SMRT-mediated repression of an H3K27 demethylase in progression from neural stem cell to neuron. Nature. 2007;450:415–9. doi: 10.1038/nature06270. [DOI] [PubMed] [Google Scholar]
- 106.Lan F, Bayliss PE, Rinn JL, Whetstine JR, Wang JK, Chen S, et al. A histone H3 lysine 27 demethylase regulates animal posterior development. Nature. 2007;449:689–94. doi: 10.1038/nature06192. [DOI] [PubMed] [Google Scholar]
- 107.Saleque S, Kim J, Rooke HM, Orkin SH. Epigenetic regulation of hematopoietic differentiation by Gfi-1 and Gfi-1b is mediated by the cofactors CoREST and LSD1. Mol Cell. 2007;27:562–72. doi: 10.1016/j.molcel.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 108.Tsukada Y, Fang J, Erdjument-Bromage H, Warren ME, Borchers CH, Tempst P, et al. Histone demethylation by a family of JmjC domain-containing proteins. Nature. 2006;439:811–6. doi: 10.1038/nature04433. [DOI] [PubMed] [Google Scholar]
- 109.Takeuchi T, Yamazaki Y, Katoh-Fukui Y, Tsuchiya R, Kondo S, Motoyama J, et al. Gene trap capture of a novel mouse gene, jumonji, required for neural tube formation. Genes Dev. 1995;9:1211–22. doi: 10.1101/gad.9.10.1211. [DOI] [PubMed] [Google Scholar]
- 110.Wu H, D’Alessio AC, Ito S, Xia K, Wang Z, Cui K, et al. Dual functions of Tet1 in transcriptional regulation in mouse embryonic stem cells. Nature. 2011;473:389–93. doi: 10.1038/nature09934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xiao T, Hall H, Kizer KO, Shibata Y, Hall MC, Borchers CH, et al. Phosphorylation of RNA polymerase II CTD regulates H3 methylation in yeast. Genes Dev. 2003;17:654–63. doi: 10.1101/gad.1055503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shafa M, Krawetz R, Rancourt DE. Returning to the stem state: epigenetics of recapitulating pre-differentiation chromatin structure. Bioessays. 2010;32:791–9. doi: 10.1002/bies.201000033. [DOI] [PubMed] [Google Scholar]
- 113.Hargreaves DC, Crabtree GR. ATP-dependent chromatin remodeling: genetics, genomics and mechanisms. Cell Res. 2011;21:396–420. doi: 10.1038/cr.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang W, Côté J, Xue Y, Zhou S, Khavari PA, Biggar SR, et al. Purification and biochemical heterogeneity of the mammalian SWI-SNF complex. EMBO J. 1996;15:5370–82. [PMC free article] [PubMed] [Google Scholar]
- 115.Phelan ML, Sif S, Narlikar GJ, Kingston RE. Reconstitution of a core chromatin remodeling complex from SWI/SNF subunits. Mol Cell. 1999;3:247–53. doi: 10.1016/S1097-2765(00)80315-9. [DOI] [PubMed] [Google Scholar]
- 116.Kaeser MD, Aslanian A, Dong MQ, Yates JR, 3rd, Emerson BM. BRD7, a novel PBAF-specific SWI/SNF subunit, is required for target gene activation and repression in embryonic stem cells. J Biol Chem. 2008;283:32254–63. doi: 10.1074/jbc.M806061200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kidder BL, Palmer S, Knott JG. SWI/SNF-Brg1 regulates self-renewal and occupies core pluripotency-related genes in embryonic stem cells. Stem Cells. 2009;27:317–28. doi: 10.1634/stemcells.2008-0710. [DOI] [PubMed] [Google Scholar]
- 118.Ho L, Ronan JL, Wu J, Staahl BT, Chen L, Kuo A, et al. An embryonic stem cell chromatin remodeling complex, esBAF, is essential for embryonic stem cell self-renewal and pluripotency. Proc Natl Acad Sci U S A. 2009;106:5181–6. doi: 10.1073/pnas.0812889106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gao X, Tate P, Hu P, Tjian R, Skarnes WC, Wang Z. ES cell pluripotency and germ-layer formation require the SWI/SNF chromatin remodeling component BAF250a. Proc Natl Acad Sci U S A. 2008;105:6656–61. doi: 10.1073/pnas.0801802105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lessard J, Wu JI, Ranish JA, Wan M, Winslow MM, Staahl BT, et al. An essential switch in subunit composition of a chromatin remodeling complex during neural development. Neuron. 2007;55:201–15. doi: 10.1016/j.neuron.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Roberts CW, Galusha SA, McMenamin ME, Fletcher CD, Orkin SH. Haploinsufficiency of Snf5 (integrase interactor 1) predisposes to malignant rhabdoid tumors in mice. Proc Natl Acad Sci U S A. 2000;97:13796–800. doi: 10.1073/pnas.250492697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lickert H, Takeuchi JK, Von Both I, Walls JR, McAuliffe F, Adamson SL, et al. Baf60c is essential for function of BAF chromatin remodelling complexes in heart development. Nature. 2004;432:107–12. doi: 10.1038/nature03071. [DOI] [PubMed] [Google Scholar]
- 123.Kim JK, Huh SO, Choi H, Lee KS, Shin D, Lee C, et al. Srg3, a mouse homolog of yeast SWI3, is essential for early embryogenesis and involved in brain development. Mol Cell Biol. 2001;21:7787–95. doi: 10.1128/MCB.21.22.7787-7795.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Guidi CJ, Sands AT, Zambrowicz BP, Turner TK, Demers DA, Webster W, et al. Disruption of Ini1 leads to peri-implantation lethality and tumorigenesis in mice. Mol Cell Biol. 2001;21:3598–603. doi: 10.1128/MCB.21.10.3598-3603.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bultman SJ, Gebuhr TC, Pan H, Svoboda P, Schultz RM, Magnuson T. Maternal BRG1 regulates zygotic genome activation in the mouse. Genes Dev. 2006;20:1744–54. doi: 10.1101/gad.1435106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Klochendler-Yeivin A, Fiette L, Barra J, Muchardt C, Babinet C, Yaniv M. The murine SNF5/INI1 chromatin remodeling factor is essential for embryonic development and tumor suppression. EMBO Rep. 2000;1:500–6. doi: 10.1093/embo-reports/kvd129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ho L, Jothi R, Ronan JL, Cui K, Zhao K, Crabtree GR. An embryonic stem cell chromatin remodeling complex, esBAF, is an essential component of the core pluripotency transcriptional network. Proc Natl Acad Sci U S A. 2009;106:5187–91. doi: 10.1073/pnas.0812888106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Singhal N, Graumann J, Wu G, Araúzo-Bravo MJ, Han DW, Greber B, et al. Chromatin-Remodeling Components of the BAF Complex Facilitate Reprogramming. Cell. 2010;141:943–55. doi: 10.1016/j.cell.2010.04.037. [DOI] [PubMed] [Google Scholar]
- 129.Lorch Y, Maier-Davis B, Kornberg RD. Mechanism of chromatin remodeling. Proc Natl Acad Sci U S A. 2010;107:3458–62. doi: 10.1073/pnas.1000398107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang ZK, Davies KP, Allen J, Zhu L, Pestell RG, Zagzag D, et al. Cell cycle arrest and repression of cyclin D1 transcription by INI1/hSNF5. Mol Cell Biol. 2002;22:5975–88. doi: 10.1128/MCB.22.16.5975-5988.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sims RJ, 3rd, Chen CF, Santos-Rosa H, Kouzarides T, Patel SS, Reinberg D. Human but not yeast CHD1 binds directly and selectively to histone H3 methylated at lysine 4 via its tandem chromodomains. J Biol Chem. 2005;280:41789–92. doi: 10.1074/jbc.C500395200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chen X, Xu H, Yuan P, Fang F, Huss M, Vega VB, et al. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell. 2008;133:1106–17. doi: 10.1016/j.cell.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 133.Mattick JS, Amaral PP, Dinger ME, Mercer TR, Mehler MF. RNA regulation of epigenetic processes. Bioessays. 2009;31:51–9. doi: 10.1002/bies.080099. [DOI] [PubMed] [Google Scholar]
- 134.Mattick JS. Challenging the dogma: the hidden layer of non-protein-coding RNAs in complex organisms. Bioessays. 2003;25:930–9. doi: 10.1002/bies.10332. [DOI] [PubMed] [Google Scholar]
- 135.Dinger ME, Amaral PP, Mercer TR, Pang KC, Bruce SJ, Gardiner BB, et al. Long noncoding RNAs in mouse embryonic stem cell pluripotency and differentiation. Genome Res. 2008;18:1433–45. doi: 10.1101/gr.078378.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mattick JS. Linc-ing Long noncoding RNAs and enhancer function. Dev Cell. 2010;19:485–6. doi: 10.1016/j.devcel.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 137.Mattick JS, Makunin IV. Small regulatory RNAs in mammals. Hum Mol Genet. 2005;14(Spec No 1):R121–32. doi: 10.1093/hmg/ddi101. [DOI] [PubMed] [Google Scholar]
- 138.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11:597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 140.Marson A, Levine SS, Cole MF, Frampton GM, Brambrink T, Johnstone S, et al. Connecting microRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells. Cell. 2008;134:521–33. doi: 10.1016/j.cell.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Murchison EP, Partridge JF, Tam OH, Cheloufi S, Hannon GJ. Characterization of Dicer-deficient murine embryonic stem cells. Proc Natl Acad Sci U S A. 2005;102:12135–40. doi: 10.1073/pnas.0505479102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang Y, Medvid R, Melton C, Jaenisch R, Blelloch R. DGCR8 is essential for microRNA biogenesis and silencing of embryonic stem cell self-renewal. Nat Genet. 2007;39:380–5. doi: 10.1038/ng1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang Y, Baskerville S, Shenoy A, Babiarz JE, Baehner L, Blelloch R. Embryonic stem cell-specific microRNAs regulate the G1-S transition and promote rapid proliferation. Nat Genet. 2008;40:1478–83. doi: 10.1038/ng.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Benetti R, Gonzalo S, Jaco I, Muñoz P, Gonzalez S, Schoeftner S, et al. A mammalian microRNA cluster controls DNA methylation and telomere recombination via Rbl2-dependent regulation of DNA methyltransferases. Nat Struct Mol Biol. 2008;15:268–79. doi: 10.1038/nsmb.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yi R, Fuchs E. MicroRNAs and their roles in mammalian stem cells. J Cell Sci. 2011;124:1775–83. doi: 10.1242/jcs.069104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Melton C, Judson RL, Blelloch R. Opposing microRNA families regulate self-renewal in mouse embryonic stem cells. Nature. 2010;463:621–6. doi: 10.1038/nature08725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Viswanathan SR, Daley GQ, Gregory RI. Selective blockade of microRNA processing by Lin28. Science. 2008;320:97–100. doi: 10.1126/science.1154040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xu N, Papagiannakopoulos T, Pan G, Thomson JA, Kosik KS. MicroRNA-145 regulates OCT4, SOX2, and KLF4 and represses pluripotency in human embryonic stem cells. Cell. 2009;137:647–58. doi: 10.1016/j.cell.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 149.Martinez NJ, Gregory RI. MicroRNA gene regulatory pathways in the establishment and maintenance of ESC identity. Cell Stem Cell. 2010;7:31–5. doi: 10.1016/j.stem.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tay YM, Tam WL, Ang YS, Gaughwin PM, Yang H, Wang W, et al. MicroRNA-134 modulates the differentiation of mouse embryonic stem cells, where it causes post-transcriptional attenuation of Nanog and LRH1. Stem Cells. 2008;26:17–29. doi: 10.1634/stemcells.2007-0295. [DOI] [PubMed] [Google Scholar]
- 151.Tay Y, Zhang J, Thomson AM, Lim B, Rigoutsos I. MicroRNAs to Nanog, Oct4 and Sox2 coding regions modulate embryonic stem cell differentiation. Nature. 2008;455:1124–8. doi: 10.1038/nature07299. [DOI] [PubMed] [Google Scholar]
- 152.Ivey KN, Muth A, Arnold J, King FW, Yeh RF, Fish JE, et al. MicroRNA regulation of cell lineages in mouse and human embryonic stem cells. Cell Stem Cell. 2008;2:219–29. doi: 10.1016/j.stem.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yoo AS, Staahl BT, Chen L, Crabtree GR. MicroRNA-mediated switching of chromatin-remodelling complexes in neural development. Nature. 2009;460:642–6. doi: 10.1038/nature08139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhao J, Ohsumi TK, Kung JT, Ogawa Y, Grau DJ, Sarma K, et al. Genome-wide identification of polycomb-associated RNAs by RIP-seq. Mol Cell. 2010;40:939–53. doi: 10.1016/j.molcel.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Khalil AM, Guttman M, Huarte M, Garber M, Raj A, Rivea Morales D, et al. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci U S A. 2009;106:11667–72. doi: 10.1073/pnas.0904715106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yap KL, Li S, Muñoz-Cabello AM, Raguz S, Zeng L, Mujtaba S, et al. Molecular interplay of the noncoding RNA ANRIL and methylated histone H3 lysine 27 by polycomb CBX7 in transcriptional silencing of INK4a. Mol Cell. 2010;38:662–74. doi: 10.1016/j.molcel.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Boumil RM, Ogawa Y, Sun BK, Huynh KD, Lee JT. Differential methylation of Xite and CTCF sites in Tsix mirrors the pattern of X-inactivation choice in mice. Mol Cell Biol. 2006;26:2109–17. doi: 10.1128/MCB.26.6.2109-2117.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sado T, Okano M, Li E, Sasaki H. De novo DNA methylation is dispensable for the initiation and propagation of X chromosome inactivation. Development. 2004;131:975–82. doi: 10.1242/dev.00995. [DOI] [PubMed] [Google Scholar]
- 159.Kim DH, Jeon Y, Anguera MC, Lee JT. X-chromosome epigenetic reprogramming in pluripotent stem cells via noncoding genes. Semin Cell Dev Biol. 2011;22:336–42. doi: 10.1016/j.semcdb.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Payer B, Lee JT, Namekawa SH. X-inactivation and X-reactivation: epigenetic hallmarks of mammalian reproduction and pluripotent stem cells. Hum Genet. 2011;130:265–80. doi: 10.1007/s00439-011-1024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ogawa Y, Sun BK, Lee JT. Intersection of the RNA interference and X-inactivation pathways. Science. 2008;320:1336–41. doi: 10.1126/science.1157676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chow JC, Ciaudo C, Fazzari MJ, Mise N, Servant N, Glass JL, et al. LINE-1 activity in facultative heterochromatin formation during X chromosome inactivation. Cell. 2010;141:956–69. doi: 10.1016/j.cell.2010.04.042. [DOI] [PubMed] [Google Scholar]
- 163.Navarro P, Oldfield A, Legoupi J, Festuccia N, Dubois A, Attia M, et al. Molecular coupling of Tsix regulation and pluripotency. Nature. 2010;468:457–60. doi: 10.1038/nature09496. [DOI] [PubMed] [Google Scholar]
- 164.Navarro P, Chambers I, Karwacki-Neisius V, Chureau C, Morey C, Rougeulle C, et al. Molecular coupling of Xist regulation and pluripotency. Science (New York, NY 2008; 321:1693-5. [DOI] [PubMed]
- 165.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10:155–9. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 166.Mercer TR, Qureshi IA, Gokhan S, Dinger ME, Li G, Mattick JS, et al. Long noncoding RNAs in neuronal-glial fate specification and oligodendrocyte lineage maturation. BMC Neurosci. 2010;11:14. doi: 10.1186/1471-2202-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sheik Mohamed J, Gaughwin PM, Lim B, Robson P, Lipovich L. Conserved long noncoding RNAs transcriptionally regulated by Oct4 and Nanog modulate pluripotency in mouse embryonic stem cells. RNA. 2010;16:324–37. doi: 10.1261/rna.1441510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chi TH, Wan M, Lee PP, Akashi K, Metzger D, Chambon P, et al. Sequential roles of Brg, the ATPase subunit of BAF chromatin remodeling complexes, in thymocyte development. Immunity. 2003;19:169–82. doi: 10.1016/S1074-7613(03)00199-7. [DOI] [PubMed] [Google Scholar]
- 169.Pedersen TA, Kowenz-Leutz E, Leutz A, Nerlov C. Cooperation between C/EBPalpha TBP/TFIIB and SWI/SNF recruiting domains is required for adipocyte differentiation. Genes Dev. 2001;15:3208–16. doi: 10.1101/gad.209901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bultman SJ, Gebuhr TC, Magnuson TA. A Brg1 mutation that uncouples ATPase activity from chromatin remodeling reveals an essential role for SWI/SNF-related complexes in beta-globin expression and erythroid development. Genes Dev. 2005;19:2849–61. doi: 10.1101/gad.1364105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wu JI, Lessard J, Olave IA, Qiu Z, Ghosh A, Graef IA, et al. Regulation of dendritic development by neuron-specific chromatin remodeling complexes. Neuron. 2007;56:94–108. doi: 10.1016/j.neuron.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 172.Huang X, Gao X, Diaz-Trelles R, Ruiz-Lozano P, Wang Z. Coronary development is regulated by ATP-dependent SWI/SNF chromatin remodeling component BAF180. Dev Biol. 2008;319:258–66. doi: 10.1016/j.ydbio.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 173.Reyes JC, Barra J, Muchardt C, Camus A, Babinet C, Yaniv M. Altered control of cellular proliferation in the absence of mammalian brahma (SNF2alpha) EMBO J. 1998;17:6979–91. doi: 10.1093/emboj/17.23.6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Takeuchi JK, Lickert H, Bisgrove BW, Sun X, Yamamoto M, Chawengsaksophak K, et al. Baf60c is a nuclear Notch signaling component required for the establishment of left-right asymmetry. Proc Natl Acad Sci U S A. 2007;104:846–51. doi: 10.1073/pnas.0608118104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chi TH, Wan M, Zhao K, Taniuchi I, Chen L, Littman DR, et al. Reciprocal regulation of CD4/CD8 expression by SWI/SNF-like BAF complexes. Nature. 2002;418:195–9. doi: 10.1038/nature00876. [DOI] [PubMed] [Google Scholar]