Abstract
Early life experiences, including those in utero, have been linked to increased risk for adult-onset chronic disease. The underlying assumption is that there is a critical period of developmental plasticity in utero when selection of the fetal phenotype that is best adapted to the intrauterine environment occurs. The current study is the first to test the idea that extreme maternal psychosocial stressors, as observed in the Democratic Republic of Congo, may modify locus-specific epigenetic marks in the newborn resulting in altered health outcomes. Here we show a significant correlation between culturally relevant measures of maternal prenatal stress, newborn birth weight and newborn methylation in the promoter of the glucocorticoid receptor NR3C1. Increased methylation may constrain plasticity in subsequent gene expression and restrict the range of stress adaptation responses possible in affected individuals, thus increasing their risk for adult-onset diseases.
Keywords: DNA Methylation, developmental origins of health and disease, epigenetics, maternal stress
Introduction
According to the developmental origins of health and disease (DOHaD) hypothesis, events in early development are directly related to disease risk in later life.1,2 The rationale is that fetal tissues are especially sensitive to the intrauterine environment, which results in selection of an optimal fetal phenotype. If the intrauterine environment is unusually limiting or unrepresentative of the environment in later life, the selected phenotype may be maladaptive in the adult and result in increased risk for a number of diseases. In recent years, the role of epigenetically determined changes in gene expression during early development has emerged as a possible mechanism by which the most adaptive phenotype is expressed.3,4
Early life stress is associated with poor stress adaptation, elevated disease risk and mortality in later life.5-7 Glucocorticoids (GCs) are associated with stress responses and have been proposed as a primary candidate for fetal programming in part because elevated GC levels during pregnancy are correlated with low birth weight.8-10 Specifically, experimental and clinical data in humans and animal models demonstrate that both prenatal stress and prenatal overexposure to GCs are associated with reduced birth weight, possibly via related actions in a shared pathway such as feeding behavior.8 NR3C1 is the GC receptor and is involved in cell proliferation and differentiation and specifically implicated in newborn birth weight, thus providing a biological mechanism by which NR3C1 expression may influence birth weight. The current study tests the hypothesis that maternal prenatal stress may result in epigenetic changes at NR3C1 in newborns that are related to birth outcomes (Fig. 1). We measure the effects of maternal stress on newborn birth weight and investigate if any observed correlation is associated with epigenetic modifications at the newborn NR3C1. Specifically, we assay methylation status at 39 CpG sites in the upstream promoter of NR3C1, the same region that has been associated with changes in methylation and/or gene expression correlated with childhood abuse-related suicide,11 prenatal exposure to intimate partner violence12 and post-traumatic stress disorder.13
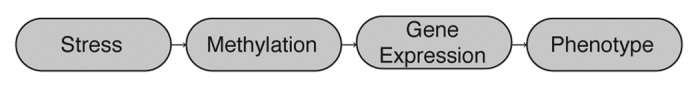
Figure 1. Proposed relationship of environmental stress influencing a phenotype via an epigenetic mechanism that alters gene expression.
Results and Discussion
In the current study, we developed three models of maternal prenatal stress; specifically, material deprivation intended to reflect availability of financial resources, mundane stressors to reflect daily psychosocial stress, and war stress to measure the influence of war-related events (see Table S1 for a list of questions associated with each maternal stressor). We find a strong correlation between each maternal stressor and our birth outcome phenotype of newborn birth weight (Fig. 2). War stress has the largest effect and accounts for a notable 35% of the variance in birth weight (Fig. 2C, Pearson’s correlation = -0.62, p = 0.0009). Within the war stress variables, personal experience of rape (past rape, rape resulting in pregnancy, rape during pregnancy) accounts for 31% of birth weight variance and eclipses the effect of other war stressors (refugee status, family member killed, past kidnapping, parents or self a result of rape), which can then account for only an additional 4% of the variance in birth weight. This association between maternal stress and newborn birth weight provides the foundation upon which to test a possible epigenetic mechanism that mediates the stressful effects of the intrauterine environment to the newborn (Fig. 1).
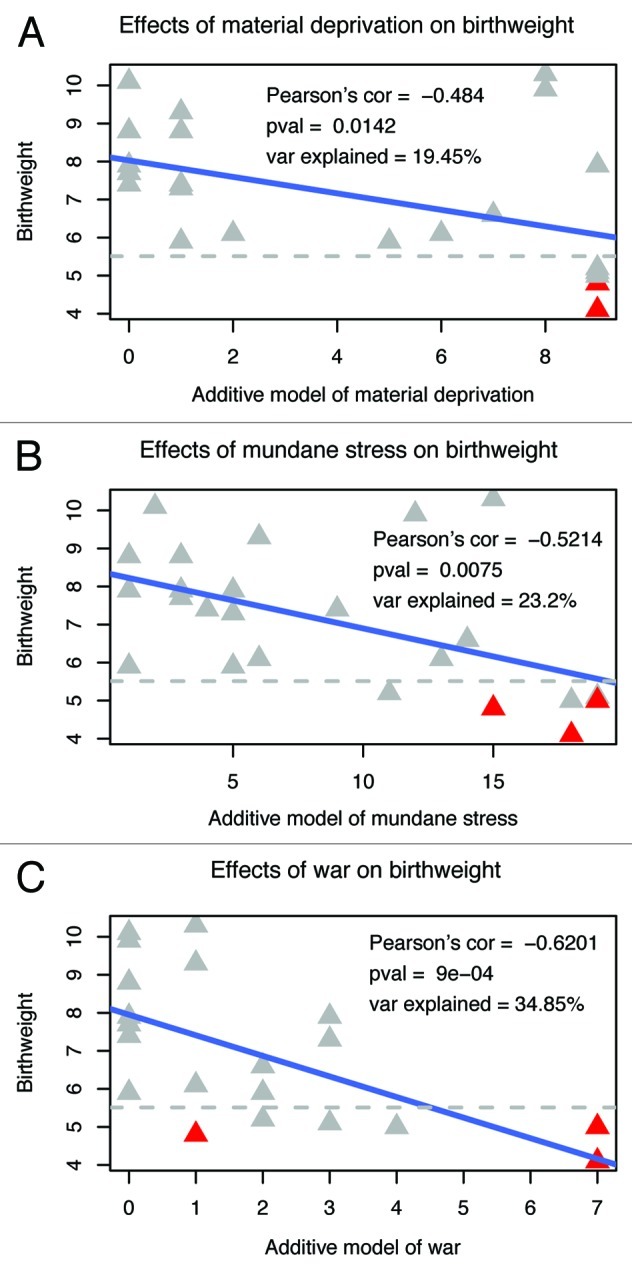
Figure 2. Correlation of maternal stressors with newborn birth weight. Pearson’s correlation, associated p value and percent explained variation in birth weight are reported in each figure for each maternal stressor. Preterm births are indicated in red. Preterm birth is associated with both the predictor maternal stressor variables and the outcome birth weight variable. Since our data include only three preterm births and since preterm birth is involved as both predictor and outcome, we focus on maternal stressors and birth weight.
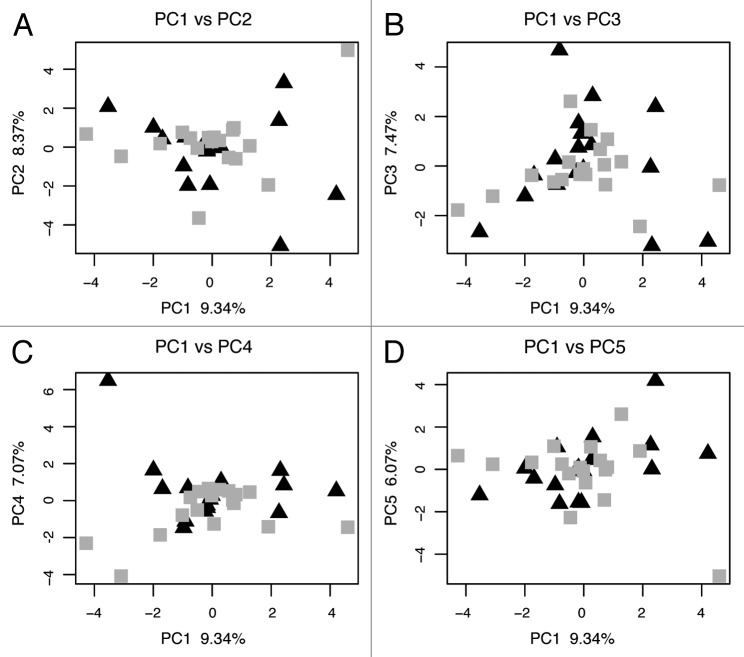
For the methylation data, we performed a principal component analysis (PCA) of the percent methylation at all 39 CpG sites. This method allows the identification of independent components that explain observed, complex variation from across the entire data set and may reflect different environmental, genetic or biological influences on methylation. Each principle component is driven by variation at a subset of the data allowing the creation of a data driven summary statistic as opposed to testing each site separately or devising a simple summary statistic. Our methylation results reveal several important aspects of methylation profiles and mother-to-newborn transmission of epigenetic information. First, we find no identically methylated sequences between mothers and their newborns, implying that all maternal methylation marks at the NR3C1 promoter have been removed from the newborn’s genomes and new marks generated. Lack of identical methylation marks between mother and newborn suggests that methylation marks are not strongly genetically determined at this locus and, thus, may be more susceptible to environmental influences, perhaps providing the NR3C1 gene with an increased capacity for rapid change and adaptation. Second, previous studies have shown a high degree of tissue specificity in methylation profiles, e.g., between blood and soft tissue samples.14 However, our PCA of methylation profiles reveals no separation of mother and newborn samples (Fig. 3), suggesting that maternal venous blood and umbilical cord blood do not display tissue-specific methylation marks at NR3C1. Thus, differences between maternal and newborn samples are unlikely to be due to tissue specificity.
Figure 3. Principal component analysis of maternal venous blood and newborn cord blood methylation profiles. The first principal component (PC1) of maternal and newborn samples is plotted with PC2, 3, 4 and 5 in four graphs. Mothers are indicated by triangles and newborns are indicated by squares.
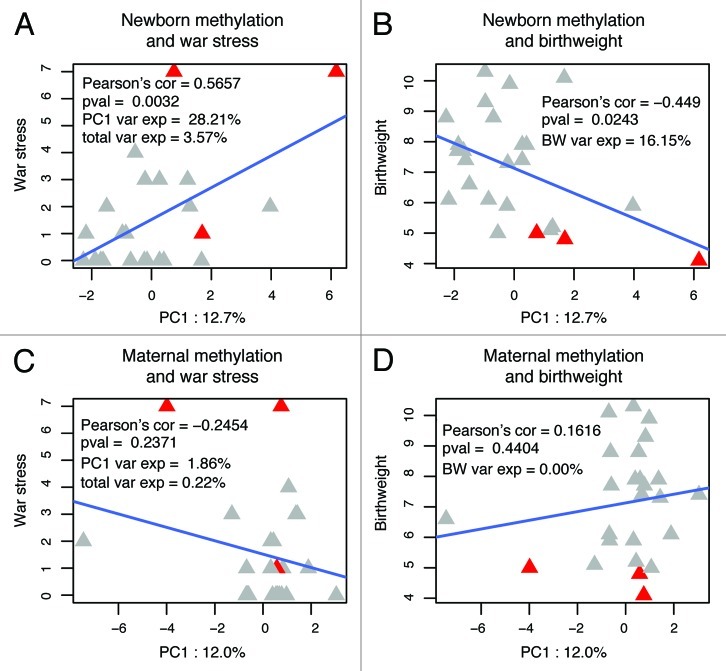
With respect to testing the effect of stress on methylation patterns, we see differences in correlation of the NR3C1 methylation data and maternal stressor, but only when mothers and newborns are analyzed separately (Fig. 4). We see the strongest correlation between war stress and newborn methylation (when analyzed as the first principal component, i.e., PC1; Figure 4A, p = 0.0032). Furthermore, newborn methylation-PC1 is strongly correlated with newborn birth weight (Fig. 4B, p = 0.024), thus completing the cycle from stress to methylation to phenotype/birth weight outlined in Figure 1. All three maternal stressors are significantly correlated with each other (Table S2); consequently, material deprivation and mundane stress are also correlated with newborn methylation-PC1 (Fig. S1; significant correlation of newborn methylation-PC1 with war stress and material deprivation). An exploratory factor analysis was performed to determine which CpG sites are correlated with PC1 and CpG sites 25, 20, 23, 10, 21 and 24 were each found to have an additive effect (with loadings of 0.998, 0.652, 0.560, 0.344, 0.292 and 0.192, respectively).
Figure 4. Correlation of newborn and maternal methylation with war stress and newborn birth weight. Newborn (A and B) and maternal (C and D) methylation were analyzed in a principal components analysis (PCA) and correlation of the first principal component (PC1) with maternal war stress (A and C) and newborn birth weight (B and D) is shown here. Only newborn methylation-PC1 shows significant correlation with war stress and newborn birth weight. Plot abbreviations are as follows: PC1 Var Exp = amount of variance in methylation-PC1 explained by war stress; Total Var Exp = total amount of methylation variance explained by war stress; BW Var Exp = amount of variance in birth weight explained by methylation-PC1.
In contrast to the results with newborn methylation, no correlation is seen between maternal methylation and stress or newborn birth weight (Figs. 4C and D). A correlation with newborn methylation, but not maternal methylation, is consistent with the tenets of the DOHaD hypothesis in which maternal stress modifies offspring biology. Moreover, methylation marks are determined by a combination of environmental, genetic and biological factors, thus a PCA may be necessary to separate the various factors that influence methylation. We see no correlation of newborn methylation with maternal stress or newborn birth weight when total methylation is analyzed, consistent with the idea that multiple factors determine methylation and these factors must be separated in order to evaluate their individual effects. We also see no correlation of newborn methylation PC2, 3 or 4 with maternal stress or newborn birth weight suggesting that PC1 may be particularly influenced by, and mediating the effects of, maternal stress.
Increased methylation that occurs in response to in utero stress may constrain plasticity in expression at stress-related genes in later life and thus restrict the range of stress adaptation responses possible in the affected individuals. Limited stress adaptations may, in turn, increase an individual’s risk of developing chronic complex disorders later in life. Kinnally et al.4 found that increased methylation (genome-wide and gene-specific) interacted with early life stress in macaques to predict higher stress reactivity. Specifically, at-risk individuals showed enhanced reactivity to higher intensity stressors, which may be the most comparable factor to stress-related disorders in humans. It has been shown that glucocorticoid receptors (GRs) and mineralocorticoid receptors (MRs) provide complementary functions in mediating the stress response in humans and that a GR:MR imbalance increases vulnerability to stress-related psychiatric disorders.15 Increased methylation of the GR, as seen in our study (Fig. S2, increased infant methylation PC1 is correlated with increased mean methylation) could, potentially, perturb the GR:MR balance if it were not matched by a similar change in MR methylation and gene expression, thus setting the stage for increased stress reactivity. The fact that our results are robust to different types of stressors is important given the chronic nature of stress exposure and our ability to deal with stress. Animal models have found that dietary supplements during pregnancy cause a permanent shift in coat color associated with increased methylation upstream of the Agouti coat color gene.16,17 These studies suggest we may be able to devise treatments to manipulate environmentally induced epigenetic alterations, which has profound implications for our ability to deal with the increasing prevalence of stress-related disorders in the United States.
Materials and Methods
Maternal venous blood and umbilical cord blood samples were collected from 25 mother-newborn dyads in July–August, 2010 in eastern Democratic Republic of Congo (DRC), a region plagued by ongoing war and extreme violence to women. Detailed ethnographic interviews18 and perinatal trauma surveys19 were administered to all mothers including questions designed to develop emic, i.e., culturally relevant, measures of stress. An additive model was built to capture stress levels in which each stressor was unweighted and/or weighted based on factor and association analyses. Genomic DNA was extracted from all 50 samples and treated with sodium bisulfite. A 321 bp region of the NR3C1 promoter was amplified,11 agarose gel-purified and cloned. An average of 21 clones per individual was sequenced. Methylation was analyzed using a principal component analysis (PCA) of percent methylation across all 39 assayed sites. In PCA, the first principal component (PC1) accounts for the largest portion of variation in the data set, followed by the second and subsequent PCs. In our case, PC1 accounted for 12.7% and 12.0% of variation in newborn and maternal methylation, respectively. All statistical analyses including regressions, analyses of variance (ANOVAs) and PCAs were completed in R.20 See Supplemental Materials for more information.
Supplementary Material
Acknowledgments
We thank the women of the Democratic Republic of Congo for their participation in this study. This work was supported by grants from the University of Florida (UF) Clinical and Translational Science Institute and UF College of Liberal Arts and Science, and a UF Research Opportunity Seed Fund award.
Disclosure of Potential Conflicts of Interest
The authors declare no competing financial interests.
Author Contributions
C.J.M. designed the study, generated sequence data, performed analyses and wrote the paper; N.C.D. collected the ethnographic and survey data and saliva samples and performed initial DNA extractions; J.S. generated sequence data; D.A.H. performed statistical analyses and helped write the paper.
Footnotes
Previously published online: www.landesbioscience.com/journals/epigenetics/article/21180
References
- 1.Barker DJ. The fetal and infant origins of adult disease. BMJ. 1990;301:1111. doi: 10.1136/bmj.301.6761.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gluckman PD, Hanson MA. The developmental origins of health and disease: The breadth and importance of the concept. In: Wintour EM, Owens JA, eds. Early Life Origins of Health and Disease. 573: Springer, 2006: 1-17. [Google Scholar]
- 3.Cole DA, Cai L, Martin NC, Findling RL, Youngstrom EA, Garber J, et al. Structure and measurement of depression in youths: applying item response theory to clinical data. Psychol Assess. 2011;23:819–33. doi: 10.1037/a0023518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kinnally EL, Feinberg C, Kim D, Ferguson K, Leibel R, Coplan JD, et al. DNA methylation as a risk factor in the effects of early life stress. Brain Behav Immun. 2011;25:1548–53. doi: 10.1016/j.bbi.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Entringer S, Epel ES, Kumsta R, Lin J, Hellhammer DH, Blackburn EH, et al. Stress exposure in intrauterine life is associated with shorter telomere length in young adulthood. Proc Natl Acad Sci U S A. 2011;108:E513–8. doi: 10.1073/pnas.1107759108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kajantie E. Fetal origins of stress-related adult disease. Ann N Y Acad Sci. 2006;1083:11–27. doi: 10.1196/annals.1367.026. [DOI] [PubMed] [Google Scholar]
- 7.Monaghan P, Heidinger BJ, D’Alba L, Evans NP, Spencer KA. For better or worse: reduced adult lifespan following early-life stress is transmitted to breeding partners. Proc Biol Sci. 2012;279:709–14. doi: 10.1098/rspb.2011.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cottrell EC, Seckl J. Prenatal stress, glucocorticoids and the programming of adult disease. Frontiers in Behavioral Neuroscience 2009;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis EP, Sandman CA. The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Dev. 2010;81:131–48. doi: 10.1111/j.1467-8624.2009.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seckl JR, Meaney MJ. Glucocorticoid “programming” and PTSD risk. Ann N Y Acad Sci. 2006;1071:351–78. doi: 10.1196/annals.1364.027. [DOI] [PubMed] [Google Scholar]
- 11.McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonté B, Szyf M, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12:342–8. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Radtke KM, Ruf M, Gunter HM, Dohrmann K, Schauer M, Meyer A, et al. Transgenerational impact of intimate partner violence on methylation in the promoter of the glucocorticoid receptor. Transcult Psychiatry. 2011;1:e21. doi: 10.1038/tp.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vukojevic V, Papassotiropoulos A, de Quervain D. Epigenetic regulation at the NR3C1 gene promoter and PTSD. International Congress of Human Genetics. Montreal, Canada; 2011. [Google Scholar]
- 14.Yang HH, Hu N, Wang C, Ding T, Dunn BK, Goldstein AM, et al. Influence of genetic background and tissue types on global DNA methylation patterns. PLoS One. 2010;5:e9355. doi: 10.1371/journal.pone.0009355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Kloet ER, Derijk RH, Meijer OC. Therapy Insight: is there an imbalanced response of mineralocorticoid and glucocorticoid receptors in depression? Nat Clin Pract Endocrinol Metab. 2007;3:168–79. doi: 10.1038/ncpendmet0403. [DOI] [PubMed] [Google Scholar]
- 16.Dolinoy DC, Weidman JR, Waterland RA, Jirtle RL. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ Health Perspect. 2006;114:567–72. doi: 10.1289/ehp.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003;23:5293–300. doi: 10.1128/MCB.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spradley J. The Ethnographic Interview. Belmont: Wadsworth; 1979. [Google Scholar]
- 19.Brunet A, Weiss DS, Metzler TJ, Best SR, Neylan TC, Rogers C, et al. The Peritraumatic Distress Inventory: a proposed measure of PTSD criterion A2. Am J Psychiatry. 2001;158:1480–5. doi: 10.1176/appi.ajp.158.9.1480. [DOI] [PubMed] [Google Scholar]
- 20.Team RDCR. A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria; 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.