Abstract
Background
Resistance of pandemic A(H1N1)2009 (H1N1pdm09) virus to neuraminidase inhibitors (NAIs) has remained limited. A new mutation I223R in the neuraminidase (NA) of H1N1pdm09 virus has been reported along with H275Y in immunocompromised patients. The aim of this study was to determine the impact of I223R on oseltamivir and zanamivir susceptibility.
Methods
The NA enzymatic characteristics and susceptibility to NAIs of viruses harbouring the mutations I223R and H275Y alone or in combination were analyzed on viruses produced by reverse genetics and on clinical isolates collected from an immunocompromised patient with sustained influenza H1N1pdm09 virus shedding and treated by oseltamivir (days 0–15) and zanamivir (days 15–25 and 70–80).
Results
Compared with the wild type, the NA of recombinant viruses and clinical isolates with H275Y or I223R mutations had about two-fold reduced affinity for the substrate. The H275Y and I223R isolates showed decreased susceptibility to oseltamivir (246-fold) and oseltamivir and zanamivir (8.9- and 4.9-fold), respectively. Reverse genetics assays confirmed these results and further showed that the double mutation H275Y and I223R conferred enhanced levels of resistance to oseltamivir and zanamivir (6195- and 15.2-fold). In the patient, six days after initiation of oseltamivir therapy, the mutation H275Y conferring oseltamivir resistance and the I223R mutation were detected in the NA. Mutations were detected concomitantly from day 6–69 but molecular cloning did not show any variant harbouring both mutations. Despite cessation of NAI treatment, the mutation I223R persisted along with additional mutations in the NA and the hemagglutinin.
Conclusions
Reduced susceptibility to both oseltamivir and zanamivir was conferred by the I223R mutation which potentiated resistance to both NAIs when associated with the H275Y mutation in the NA. Concomitant emergence of the I223R and H275Y mutations under oseltamivir treatment underlines the importance of close monitoring of treated patients especially those immunocompromised.
Introduction
Oseltamivir is considered to be the drug of choice for treatment of patients with pandemic influenza, whereas zanamivir is usually restricted to patients with suspected oseltamivir resistant strains.
Until recently, a low frequency of resistance to neuraminidase inhibitors (NAIs) was reported among seasonal as well as A(H5N1) influenza viruses, most often in drug treated and/or immunosuppressed patients [1], [2], [3]. The H275Y substitution in the neuraminidase (NA) of the N1 subtype is the most commonly observed mutation associated with oseltamivir resistance. In H1N1 viruses reported before 2007, it results in low or unstable NA activity, decreased affinity for the substrate, decreased amount of NA on the cell surface, impaired growth in cell culture and decreased viral fitness and transmission [4], [5], [6], [7]. However, natural resistance to oseltamivir in seasonal H1N1 viruses associated with the mutation H275Y in the NA emerged in 2007 in Europe and became predominant worldwide within a year [8], [9]. A permissive genetic background achieved through mutations that pre-empted the H275Y substitution and restored viral fitness of H275Y bearing viruses is likely to account for their widespread diffusion [6], [10], [11], [12]. So far, oseltamivir resistant variants were rarely reported among pandemic A(H1N1) 2009 (H1N1pdm09) influenza viruses: by October 5, 2011, a total of 605 cases have been identified worldwide (18 cases in France) with a high proportion in immunocompromised and/or oseltamivir treated patients [13]. A minority of resistant viruses were detected among patients without known exposure to oseltamivir including one in France [14].
In all cases, resistance was linked to the H275Y mutation which occurred in less than 2% of tested A(H1N1)pdm09 viruses [15] but can reach more than 13% among treated immunocompromised patients [16]. The mutation has been shown to emerge in patients infected with H1N1pdm09 virus as early as 4 days after initiation of oseltamivir treatment and to persist well after cessation of oseltamivir exposure in some immunocompromised patients [16], [17], [18], [19]. The use of zanamivir whatever the route used, inhaled (n = 8), intravenous (n = 5) or nebulised (n = 1), for treatment of patients infected with the H1N1pdm09 virus resistant to oseltamivir has been associated with reduced viral shedding or recovery in most patients (12/14) [17], [18], [20], [21], [22], [23], [24]. Recently, the emergence of an I223R mutation in the NA associated with reduced susceptibility to zanamivir was reported in two immunocompromised and one immunocompetent patients [25], [26], [27]. In immunocompromised patients, this mutation emerged subsequently to or in combination with the H275Y mutation in the NA upon failure of oseltamivir followed by zanamivir treatment.
We report here the selection of the H275Y and I223R mutations in the NA in an immunocompromised patient with sustained H1N1pdm09 virus shedding successively treated by one course of oseltamivir and two courses of zanamivir. Using reverse genetics, we demonstrate that the I223R mutation conferred reduced susceptibility to both NAIs and in the presence of the H275Y mutation potentiated resistance to both NAIs. In this patient, no viruses harboring both mutations were detected. This could be related to the impaired in vitro growth characteristics of the H275Y/I223R double mutant produced by reverse genetics.
Methods
Samples
Nasopharyngeal swabs were collected in 3 ml of Universal Transport Medium (UTM) (Copan Diagnostics Inc, Murrieta, CA). Plasma samples collected for surveillance of viral opportunistic infections were used for the detection of H1N1pdm09 RNA in blood and serology.
This study which was not part of a controlled clinical trial, was a non-interventional study with no addition to usual procedures. Biological material and clinical data were obtained only for standard viral diagnostic following physicians' prescriptions (no specific sampling, no modification of the sampling protocol). Data analyses were carried out using an anonymized database. According to the French Health Public Law (CSP Art L 1121-1.1), such protocol does not require approval of an ethics committee and is exempted from informed consent application.
Virus detection and virus isolation
Total nucleic acid was extracted from 400 µL of UTM or plasma using the EasyMag System (Biomérieux, Marcy l'Etoile, France) and eluted in 100 µl according to the manufacturer's instructions. H1N1pdm09 infection was diagnosed by real-time reverse transcription–PCR (RT-PCR) assay on a 7500 Real Time PCR System (Applied Biosystems, Foster City, CA) according to the protocol designed by the National Influenza Center Northern-France (Institut Pasteur, Paris, France) [28]. Influenza virus isolation was performed in Madin Darby Canine Kidney (MDCK) cells using standard methods. Other viral respiratory infections were investigated by a multiplex molecular assay based on the Multiplex Ligation-dependent Probe-Amplification technology (RespiFinderPlus, Pathofinder, Maastricht, The Netherlands).
Detection of mutations in the neuraminidase gene by pyrosequencing
For pyrosequencing, viral RNAs extracted from clinical specimens as well as isolates using the NucleoSpin Virus system (Macherey-Nagel, Düren, DE) were amplified with Platinum Taq (Invitrogen, Carlsbad, CA) using pyrosequencing primers (50 µM) specific for each mutation. For the H275Y mutation, primers were as described [29]. For the I223R mutation, primers GRswN1-602Fw (5′- GGGCAGTGGCTGTGTTAAAG-3′) and GRswN1-760Rv-biot (5′-biotine- TGTATGAGGCCTGTCCATCAC-3′) were designed with the Pyrosequencing Assay Design Software (version 1.0; Biotage, Uppsala, Sweden). Pyrosequencing reactions were performed on purified biotinylated amplicons using the PSQ MA 96 platform pyrosequencer (Biotage, Uppsala, Sweden) with Pyrogold reagents according to Biotage recommendations. The sequencing primers (0.40 µM) were H1N1pdm-N1-F804 [29] and GRswN1-642Fw (5′- CACTATCAAGAGTTGGAGA-3′) for detection of the H275Y and I223R mutations, respectively.
Sequencing of the neuraminidase and hemagglutinin genes
The complete NA and hemagglutinin (HA) coding sequences were determined using Big Dye v.1.1 chemistry on an ABI3730XL capillary sequencer (Applied BioSystems) and were analyzed using BioNumerics software (Applied Maths, Belgium). Accession numbers of Viral isolates are as follows: D0: A/Paris/7976/2010, NA (EPI324954), HA (EPI324955); D6: A/Paris/7975/2010, NA (EPI324951), HA (EPI324952); D11: A/Paris/7154/2009, NA (EPI324942), HA (EPI324943); D19: A/Paris/7974/2010, NA (EPI324948), HA (EPI324949); D25: A/Paris/7973/2010, NA (EPI324945), HA (EPI324946); D45: A/Paris/1156/2010, NA (EPI324957), HA (EPI324958); D69: A/Paris/1157/2010, NA (EPI324960), HA (EPI324961); D83: A/Paris/1158/2010, NA (EPI324963), HA (EPI324964); D98: A/Paris/1159/2010, NA (EPI324966), HA (EPI324967); A/Paris/2590/2009, NA (EPI180570), HA (EPI180568).
Molecular cloning and sequencing of the region of the NA spanning both 223 and 275 mutations
To characterize quasi species, the NA sequences corresponding to residues 201 to 300 were amplified by RT-PCR with primers 223SLSF (5′-GGGCAGTGGCTGTGTTAAAG-3′) and 223SLSR (5′-ATTCGAGCCATGCCAGTTAT-3′) and cloned using the TOPO TA Cloning kit for sequencing (Invitrogen). Recombinant plasmids were sequenced with 223SLSF and 223SLSR primers using an ABI 3100 Genetic Analyser (Applied Biosystems). Sequences were aligned using Sequence Navigator software version 1.0.1 (Applied Biosystems).
Construction of recombinant viruses by reverse genetics
An eight-plasmid reverse-genetics system was generated by cloning the cDNAs corresponding to the eight viral segments of the pandemic virus A/Paris/2590/2009(H1N1) at the BsmBI sites in the bidirectional pRF483 plasmid (kindly provided by R. Fouchier, Erasmus MC, Rotterdam, The Netherlands) [30]. Mutations I223R and H275Y in the NA and the N31S mutation in the M2 restoring sensitivity to amantadine were generated using the Quick change II site-directed mutagenesis kit (Stratagene). Recombinant viruses were produced essentially as previously described [31]. The HA, NA and M genes were sequenced using a Big Dye terminator sequencing kit (Applied Biosystems) and an automated sequencer (Perkin Elmer) to verify the presence of the mutations.
Titration of viruses in plaque assay
Engineered viruses were titrated in a standard plaque assay on MDCK-SIAT1 cells [32]. Briefly, the cells were incubated in the presence of serial dilutions of virus at 35°C for one hour before being overlaid with Avicel-containing medium with 1 µg/ml Tosyl-phenylalanyl-chloromethyl-ketone-treated trypsin. After 72 hours incubation at 35°C, the cells were stained with crystal violet solution containing 10% formamide and plaque numbers determined.
Replication kinetics assay
The MDCK-SIAT1 cells were incubated with the recombinant virus for 1 hour at 35°C at a multiplicity of infection (MOI) of 0.001. At the end of the incubation period, which was considered the zero time point, the cells were washed twice and fresh medium containing 1 µg/ml TPCK-treated trypsin was added. The supernatants were collected at 12, 24, 36, 48, 60 and 72 hours post infection. To quantify the levels of infectious particles in supernatants, the end-point dilution assay was performed on MDCK-SIAT1 cells using standard procedures [33].The statistical analyses were done using the Graphpad Prism (version 5) software.
NA enzymatic activity and NA inhibition assays
The enzymatic activity of the NA was measured using the 2′-(4-Methylumbelliferyl)-α-D-N-acetylneuraminic acid (MUNANA) (Sigma) as fluorogenic substrate essentially as described previously [10], [34]. For Km determination, final MUNANA concentration ranging from 10 to 500 µM and for Ki determination, final NAIs ranging from 0.01 nM to 5000 nM were used. Fluorescence was monitored using the TwinkleTM LB970T (Berthold Technologies) fluorimeter.
Results
Case Description
On November 20, 2009 (D0), a 24-year-old man was admitted to the infectious disease department with a 10-day old history of fever, coryzal symptoms, diarrhea and cough. The patient had been seen 10 days before admission for a suspicion of catheter infection and had been administrated antibiotic treatment (amoxicillin/clavulanic acid and ciprofloxacin). He was seen two days before admission for follow-up. At this time, a postexposure course of oseltamivir (75 mg×1/d) was started after influenza H1N1pdm09 had been diagnosed for his friend who had similar symptoms. The influenza-like illness syndrome of the patient that would have required a therapeutic dosage of oseltamivir was actually not considered.
The patient's medical history showed type 4 acute myelogeneous leukaemia, diagnosed in January 2008. He had undergone allogeneic cord blood stem cell transplantation in January 2009. Following graft-versus-host complications, the patient had required ongoing immunosuppression with steroids and had presented relapses of leukaemia successively in March and October 2009.
When hospitalized, the patient was being treated with prednisolone (20 mg/d), sunitinib, valaciclovir, amoxicillin, voriconazole, and cotrimoxazole.
A physical examination showed a temperature of 37.2°C. The blood pressure was 130/70 mm Hg, the pulse rate 109 beats per minute, the respiratory rate 15 breaths per minute, and the oxygen saturation 95% on room air (fraction of inspired oxygen 21%). Lung wheezes were audible, and the patient exhibited cough and rhinorrhea. Blood tests showed a neutrophil count of 1.30×109/L, a lymphocyte count of 0.40×109/L, a platelet count of 33×109/L, and a C-reactive protein level of 12 mg/L. Chest radiography showed no abnormalities. The patient was started on amoxicillin-clavulanic acid and ciprofloxacin and oseltamivir was increased to the treatment dose of 75 mg×2/d. A nasopharyngeal specimen collected on D0 tested positive for H1N1pdm09 virus. No other respiratory virus was detected. The patient improved and was discharged on D4, but nasal swabs obtained on D6 and D11 were still positive for H1N1pdm09 virus. While the symptoms did not worsen, but because of the concern for possible oseltamivir resistance, oseltamivir was discontinued and inhaled zanamivir (10 mg twice daily) was started on D15 and discontinued after a total of 10 days. The symptoms did not improve with use of inhaled zanamivir and follow-up nasal swabs taken between D19 and D69 were still positive in the context of persistent neutropenia and lymphopenia. A second 10-day course of zanamivir (10 mg twice daily) was started on D70 due to the persistence of a cough.
The patient was again admitted on D96 with a worsening fever and cough. Oxygen saturation was 95%. Chest radiography and CT-scanner remained unremarkable, but pansinusitis was diagnosed. No bacterial or fungal infections were identified. Broad spectrum antimicrobial therapy (imipenem and voriconazole) was administered without significant improvement. A nasal swab taken on D98 still contained H1N1pdm09 virus but neuraminidase inhibitor therapy was not resumed. No other specimens than nasal washes were collected to assess the presence of H1N1pdm09 throughout the respiratory tract. Blood tests confirmed the relapse of leukaemia with a leukocyte count of 170.00×109/L and 91% of blasts cells. The patient received oral chemotherapy and was discharged home for palliative care on D113. On D139, he was hospitalized after two days of fever and a vomiting episode. After a rapid deterioration of his condition in a context of impaired consciousness, the patient died on D140. The relapse of leukaemia was considered as the cause of death. No autopsy was performed.
Virus shedding
During the course of illness, nasal swabs from D0 up to D98 were found positive for H1N1pdm09 virus both by RT-PCR and virus isolation. The viral RNA level as determined by real time RT-PCR showed a slight increase shortly after the start of oseltamivir treatment, with no significant variation between D6 and D83 despite antiviral therapy, and then a significant increase (more than 2 log10) 18 days after zanamivir cessation (Fig. 1A). This viral load increase was concomitant with worsening fever and cough and relapse of the leukaemia. In accordance with the immunosuppression in this patient, no significant antibody response was detected by microneutralization (data not shown), which likely contributed to the prolonged viral shedding. To assess if this uncontrolled H1N1pdm09 infection in an immunocompromised host was associated with virus dissemination to the blood we tested for H1N1pdm09 RNA by RT-PCR in plasma samples. In agreement with previous reports, none of the ten plasma samples collected between D0 and D138 were positive for H1N1pdm09 virus.
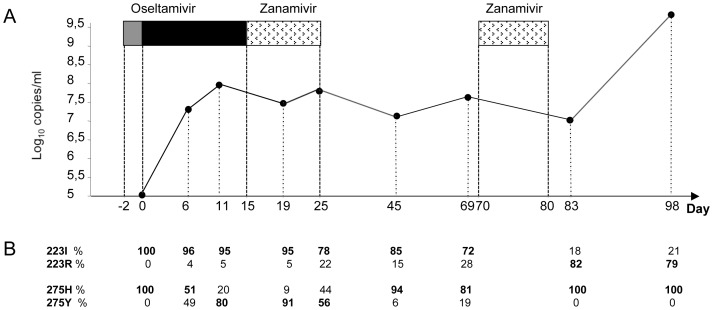
Figure 1. Treatment history and viral evolution.
A. Evolution of nasal H1N1pdm09 RNA levels and neuraminidase inhibitors treatment history. For each sequential nasopharyngeal sample the level of H1N1pdm09 RNA determined by real-time RT-PCR targeting the M gene is expressed as the log of copies per mL. Neuraminidase inhibitor treatment is represented by boxes: oseltamivir 75 mg×1/d (grey box), oseltamivir 75 mg×2/d (black box), and inhaled zanamivir 10 mg×2/d (stippled box). B. Based on the pyrosequencing quantification performed on primary specimens, the proportion of amino acids at positions 223 and 275 in the NA is indicated as a percentage below each sample. The level of the predominant residue is indicated in bold.
Mutations in the neuraminidase and hemagglutinin
Six days after initiation of oseltamivir treatment, the H275Y mutation was detected on both the primary specimen and virus isolate and remained largely predominant (>90%) at least four days after treatment interruption (Fig. 1; Table 1). Despite the absence of selective drug pressure for 54 days the mutation H275Y was still detected on D69 as a minor (19%) population. On day six, a small population of viruses with the I223R mutation in the NA was also observed. During the second course of zanamivir (D70 to D79) the proportion of these I223R mutants increased and persisted at high level (about 80%) at least 19 days after zanamivir cessation (Fig. 1). Rapidly following the detection of the H275Y and I223R mutations in the NA, additional mutations in the HA at positions 374, 415, and 418 were detected (Table 1). Further diversification of the viral sequences was observed after D45 in the HA and the NA. The functional significance of these additional mutations remains unclear at present. However, based on alignment of HA and NA sequences available in databases, mutations at positions 249 and 332 in the NA of H1N1pdm09 viruses have been observed, reflecting natural genetic variations.
Table 1. Additional mutations in neuraminidase and hemagglutinin.
| Additional mutations in NAx | Additional mutations in HAx | ||||||||||||
| A/California/7/2009 | H275 | I223 | G249 | N329 | K331 | T332 | D97 | D187 | I321 | E374 | F415 | I418 | N444 |
| A/NewYork/18/2009 | V | ||||||||||||
| D0a | |||||||||||||
| D6 | H/Y | I/R | F/L | ||||||||||
| D11 | H/Y | I/R | E*-E/K# | F/L | |||||||||
| D19 | H/Y | I/R | E*-E/K# | F*-F/L# | I#-L* | ||||||||
| D25 | H/Y | I/R | E*-E/K# | F/L | I*-I/L# | ||||||||
| D45 | H/Y | I/R | D# -D/N* | I/V | E*-E/K# | F/L | L*- I/L# | ||||||
| D69 | H/Y | I/R | G*-G/E# | N | K*-K/Q# | T*-T/A# | I/V | E*-E/K# | F/L | I*- I/L# | |||
| D83a | I/R | G* | D* | K* | T* | D* | D/N* | A/V* | E* | F* | L* | N/K* | |
| D98b | I/R | G*-G/E# | N/D | K | T*-T/A# | N* | D/N* | A/V/T/I* | E* | F/L* | L* | N/K* | |
Only amino acid mutations or mixture of wild type and mutations are indicated.
HA and NA sequence only from isolate.
HA sequence only from isolate.
: detected only on isolates.
: detected only on clinical specimens.
: relative to the sequence of A/NewYork/18/2009.
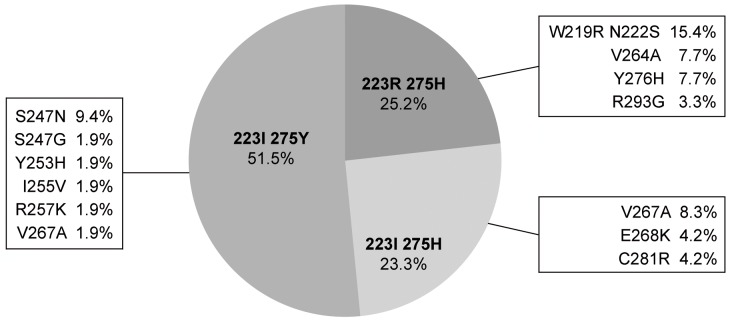
To determine if the concomitant detection of the I223R and H275Y mutations on the same specimens corresponded to viruses harbouring both mutations in their genome or to a mixture of viruses harbouring either mutation I223R or H275Y, we performed a molecular cloning on the viral RNA extracted from the specimen collected on day 25 when both mutations were readily detected and determined the sequence of the region of the NA spanning both mutations, i.e. residues 201 through 300. Of 103 clones, 53 (51.5%) harbored only 275Y, 26 (25.2%) only 223R and 24 (23.3%) were wild type (223I and 275H) (Fig. 2). None of these clones harbored both 275Y and 223R mutations simultaneously. Interestingly, 18.9% of 275Y, 34.6% of 223R and 16.7% of wild type clones showed various additional mutations. These additional mutations were not found in subsequent sequences, suggesting that they did not confer a significant selective advantage when drug pressure was withdrawn, or during the second course of zanamivir treatment.
Figure 2. Molecular cloning of the NA gene from the D25 nasal swab.
The NA region between residues 201 and 300 was amplified, cloned and sequenced. Frequencies of clones harboring various patterns of amino acids at positions 223 and 275 in the NA are represented. For each pattern, frequencies of clones with additional mutations are indicated.
Enzymatic activities of the NA of viral isolates
To evaluate the level of resistance conferred by the mutations detected in the NA that emerged upon treatment, we determined the enzymatic characteristics of the NA of viruses isolated on D0, after treatment with oseltamivir (D19) or zanamivir (D25 and 98) using a functional fluorimetric assay (see methods). The enzymatic parameters of the D0 isolate were in the same range as those of the reference strain A/California/7/2009(H1N1) (Table 2). However, for isolates harboring the H275Y (D19 isolate) or the I223R (D25, D98 isolates) mutations in major proportions (i.e. >60%; Table 2), the Km increased about two-fold indicating reduced affinity for the substrate. The Ki for oseltamivir of the D19 isolate increased 246-fold consistent with the high level of resistance to oseltamivir conferred by the H275Y mutation. For D25 and D98 isolates, in which the H275Y mutation was not detected, the Ki for oseltamivir increased about 8-fold, suggesting that the I223R mutation by itself reduced oseltamivir susceptibility. D0 and D19 isolates showed no significant change in the Ki for zanamivir in agreement with the absence or low (12%) proportion of the I223R mutation. For D25 and D98 isolates harboring the I223R mutation in 64% and 92% of the viral population respectively, the Ki increased more than 4-fold indicating reduced zanamivir susceptibility.
Table 2. Enzymatic characteristics of the NA of virus isolates.
| Virus | NA aaa | Kmb +/− SD | Ki OCb , c +/− SD | fold | Ki ZANb , d +/− SD | fold | |||
| Isolates | 223 | 275 | (µM) | (nM) | (nM) | ||||
| I% | R% | H% | Y% | ||||||
| Cal/7/09 | 100 | 0 | 100 | 0 | 44.35+/−0.25 | 0.26+/−0.05 | nae | 0.22+/−0.02 | nae |
| D 0 | 100 | 0 | 100 | 0 | 59.27+/−8.02 | 0.28+/−0.07 | nae | 0.34+/−0.01 | nae |
| D 19 | 88 | 12 | 19 | 81 | 106.00*+/−1.41 | 67.97*+/−19.95 | 246 | 0.40+/−0.04 | 1.2 |
| D 25 | 36 | 64 | 100 | 0 | 143.33*+/−11.44 | 2.34*+/−0.74 | 8.5 | 1.45*+/−0.27 | 4.3 |
| D 98 | 8 | 92 | 100 | 0 | 111.67*+/−4.50 | 2.47*+/−0.90 | 8.9 | 1.65*+/−0.12 | 4.9 |
results of pyrosequencing quantification on isolates obtained after 2 passages on MDCK cells.
mean±standard deviation of 3 independent determinations.
Ki for oseltamivir carboxylate.
Ki for zanamivir.
na: non applicable.
significantly different Km or Ki value as compared to the D0 isolate (Student t test. p<0.05).
Reverse genetics
To further determine the impact of the H275Y and I223R mutations, viruses harboring either or both mutations in the NA were produced by reverse genetics in the context of the A/Paris/2590/2009(H1N1) virus. All mutant viruses were rescued and grew to titers similar to wild-type except for the 275Y/223R mutant for which titers were reduced by 1 log (data not shown). As shown in Table 3, consistent with the enzymatic parameters determined on the patient's isolates, compared to wild-type, the Km increased about two-fold in the presence of either mutation, and more than six-fold for the double mutant. As expected, for the H275Y mutant the Ki for oseltamivir was highly increased (266-fold) with no significant effect on the Ki for zanamivir. For the I223R mutant, the Ki for both oseltamivir and zanamivir (18.4- and 8.1-fold respectively) increased. For the double mutant the Ki for oseltamivir and zanamivir further increased as compared to each single mutant (Ki = 2354.2 vs 101.08 nM, 23.3-fold increase for oseltamivir; Ki = 3.64 vs 1.94 nM, 1.9-fold increase for zanamivir). These results indicate a potentiation of the level of resistance to oseltamivir and to a lesser extent to zanamivir when both mutations are present simultaneously.
Table 3. Enzymatic characteristics of the NA of viruses produced by reverse genetics.
| NA aa | Kma+/− SD | Ki OCa , b+/− SD | fold | Ki ZANa , c+/− SD | fold | ||
| RG viruses | 223 | 275 | (µM) | (nM) | (nM) | ||
| RG-wt | I | H | 45.87±2.64 | 0.38±0.03 | nad | 0.24±0.02 | nad |
| RG-IY | I | Y | 74.55*±6.21 | 101.08*±18.27 | 266 | 0.30±0.01 | 1.3 |
| RG-RH | R | H | 113.93*±4.14 | 7.00*±0.70 | 18,4 | 1.94*±0.35 | 8.1 |
| RG-RY | R | Y | 294.78*±1,73 | 2354.20*±214.68 | 6195 | 3.64*±0.60 | 15.2 |
mean±standard deviation of 2 independent determinations.
Ki for oseltamivir carboxylate.
Ki for zanamivir.
na: non applicable.
significantly different Km or Ki value as compared to RG wild type (Student t test. p<0.05).
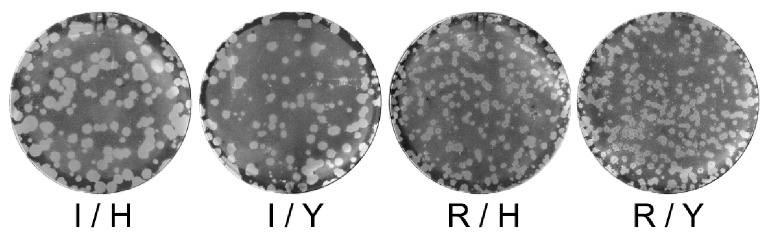
Given the impact of the mutations on the Km which indicates a reduced affinity of the NA for its substrate, we examined the virus growth on MDCK-SIAT1 cells in a standard plaque assay and measured the plaque diameters. Mean diameters ± SD were as follows: wild-type (I/H). d = 17.23±3.65; I/Y. d = 14.32±2.45; R/H. d = 8.94±2.47 and R/Y. d = 8.52±1.34. Differences were found to be significant for RG-R/H vs RG-wt(I/H), RG-R/Y vs RG-wt(I/H), RG-I/Y vs RG-R/H and RG-R/Y vs RG-I/Y in a Student t-test (p<0.0001). In addition, the virus growth kinetics were analyzed on MDCK-SIAT1 cells (Fig. 3).
Figure 3. Growth of recombinant viruses with H275Y and/or I223R mutations in MDCK-SIAT1.
Viruses produced by reverse genetics derived from the A/Paris/2590/2009(H1N1) strain and harboring the H275Y and I223R mutations alone or in combination as indicated were titrated on MDCK-SIAT-1 cells in a standard plaque assay [31]. Plaque diameters from 398 plaques (86. 87. 126. 99 plaques for RG-wt (I/H). RG-I/Y. RG-R/H and RG-R/Y. respectively) were measured.
Virus growth was slightly impaired by the H275Y mutation and even more by the I223R mutation. A cumulative effect was seen for the double mutant virus, which formed very small plaques and replicated to lower titers suggesting reduced viral fitness when both mutations were present simultaneously (Fig. 3 and 4).
Figure 4. Replication kinetics of recombinant viruses in MDCK-SIAT1.
MDCK-SIAT1 cells were inoculated at a multiplicity of infection (MOI) of 0.001 PFU with the engineered viruses. Supernatants were harvested after at 12, 24, 36, 48, 60 and 72 hours post infection and were titrated on MDCK-SIAT1 cells. The results shown are from two independent experiments. Mean titers and standard deviations are shown.
Discussion
As no vaccine was available when the influenza A(H1N1) 2009 pandemic started, NAIs, particularly oseltamivir, were the first-line strategy to combat the virus. Oseltamivir has thus been used as primary pharmacologic intervention for managing ill patients or their contacts. We showed in a severely immunocompromised patient, the selection of the H275Y mutation in the NA of the H1N1pdm09 virus 6 days after the initiation of oseltamivir treatment that was preceded by two days of oseltamivir chemoprophylaxis. While the appropriate oseltamivir dose for chemoprophylaxis in immunocompromised hosts is uncertain, the use of prophylaxis despite evidence of upper respiratory tract infection was probably a trigger for oseltamivir resistance selection. The H275Y mutation induced high-level resistance to oseltamivir which was consistent with previous reports [5], [10]. This suggests that oseltamivir monotherapy is likely not effective enough to control influenza shedding in severely immunocompromised patients and may account for resistance selection. Despite no selective drug pressure, the 275Y mutation remained detectable for 54 days.
Although H1N1pdm09 H275Y mutants remain susceptible to zanamivir, the use of this drug had no impact on viral shedding in this patient. Until recently, there have been only few reports of significant zanamivir resistance associated with mutations in the NA gene amongst human influenza viruses, in part due to a more limited use of this molecule: R152K for B virus in an immunocompromised patient [35] and Q136K/L and R371K for A or B viruses in tissue culture and in vivo [36], [37], [38]. For the H1N1pdm09 virus, two reports have shown the emergence of an I223R mutation in the NA subsequently in two immunocompromised patients upon failure of treatment with NAIs [25], [26]. In one case, the patient received oseltamivir and then intravenous zanamivir therapy following the emergence of the H275Y mutation that conferred resistance to oseltamivir [26]. The I223R mutation was detected upon relapse of virus excretion after discontinuation of zanamivir therapy at a time when the H275Y mutation could no longer be detected and persisted during the second course of zanamivir therapy. In the second case, the I223R mutation was detected along with the H275Y mutation following oseltamivir therapy and prior to the initiation of intravenous zanamivir therapy [25]. It is not known whether both mutations were simultaneously present on the same virus and the fate of the mutations after cessation of oseltamivir therapy and during zanamivir treatment was not documented. In the two cases, the occurrence of I223R was associated with an increase of the oseltamivir and zanamivir 50% inhibitory concentrations, relative to wild-type and H275Y mutant viruses, respectively.
In the case reported here, during oseltamivir treatment and before the initiation of zanamivir therapy, the I223R mutation in the NA, was detected in a minor population and became predominant when zanamivir was given. Substitutions of residue I223 for V, T or M were observed among seasonal influenza A and B or A(H5N1) viruses under oseltamivir pressure especially in immunocompromised patients [39] or after passages in vitro [40], [41]. Alone these three substitutions caused a minor decrease in oseltamivir susceptibility with no impact on zanamivir susceptibility but in combination either with H275Y or E119V they conferred major resistance to oseltamivir. In H5N1 viruses, under oseltamivir pressure, an I223M substitution in the NA was selected in vitro in combination with the H275Y mutation [41]. This dual mutation had a greater impact on the resistance to oseltamivir than H275Y alone but did not confer resistance to zanamivir and showed reduced susceptibility to peramivir. An I223V substitution was detected in the NA of H1N1pdm09 isolates in two summer campers who had received oseltamivir chemoprophylaxis and presented also with the H275Y mutation [42]. Engineered viruses harbouring the mutation I223V in their NA showed a decreased sensitivity to oseltamivir and peramivir and enhanced resistance to both NAIs in the presence of the mutation H275Y [43]. An I223K mutation was also reported to reduce susceptibility to NAIs [25]. These limited data suggest that amino acid substitutions occurring at position 223 may be selected for by oseltamivir along with the H275Y mutation. Here, the association of the I223R mutation with the reduction in zanamivir susceptibility (5 to 7-fold) was demonstrated in vitro on viral isolates and viruses produced by reverse genetics. In addition the I223R mutation conferred reduced susceptibility to oseltamivir (9 to 18-fold). These results suggest that the I223R substitution was selected for at low levels under oseltamivir treatment and eventually became predominant after zanamivir treatment. However, the modest level of resistance to zanamivir cannot explain per se the treatment failure considering that the local concentrations of zanamivir are expected to be more than 337- and 135-fold higher than the IC50s for influenza virus NAs respectively in nasal mucosa and pulmonary epithelium after a 10 mg dose given twice daily [44]. The relapse of leukaemia with profound immunodeficiency and absence of antibody response is also likely to account for lack of viral control.
In contrast to the H275Y mutation, the I223R mutation persisted at least 19 days after the last zanamivir inhalation but its disappearance thereafter cannot be excluded as viral follow-up was ceased. Potentially reduced viral fitness of 223R viruses was suggested by the reduction in plaque diameter and in virus titers achieved upon growth on MDCK-SIAT1 cells in agreement with a recent report [45] but in contrast to another study which showed that 223R viruses grew to higher titers on ST6GalI-MDCK cells [46]. In the patient studied here, persistence of 223R viruses was associated with a rebound of influenza virus shedding concomitantly with worsening fever, cough and pansinusitis. Upon prolonged virus shedding, emergence of additional mutations which persisted in the HA and in the NA was observed indicating that the viruses evolved with increasing viral population complexity. These additional mutations did not confer a higher level of resistance to zanamivir. Whether they actually improved viral fitness, and thus enabled the persistence of the I223R mutation in H1N1pdm09 viruses deserves further analysis. Viruses harboring both I223R and H275Y mutations produced by reverse genetics showed increased resistance to both zanamivir and oseltamivir compared to single mutants as also shown by Pizzorno et al. [46]. Furthermore, the simultaneous presence of both mutations resulted in further reduced plaque diameter and reduced virus growth on MDCK–SIAT1 cells, as compared to single mutants suggesting that the fitness of the double mutants was significantly impaired. This was in contrast to the observations made by Pizzorno et al. which showed similar growth for wild-type and double mutant viruses on ST6GalI-MDCK cells [46]. However, the likely reduced fitness for the viruses harboring both I223R and H275Y mutations is supported by the fact that double mutants were not detected in the clinical sample positive for both mutations.
This study clearly demonstrates that the mutation I223R alone confers reduced susceptibility to both oseltamivir and zanamivir and was primarily selected by oseltamivir. Despite zanamivir cessation, I223R mutants persisted along with additional mutations in the NA and HA. This highlights the potential for evolution of H1N1pdm09 viruses resistant to NAIs and the risk for possible emergence of sufficiently fit resistant strains able to widely spread. As prophylactic use (1×75 mg per day) has been associated with the selection of the H275Y mutation and potentially could be involved in the selection of the I223R mutation that confers resistance to both oseltamivir and zanamivir, the therapeutic dose of oseltamivir (2×75 mg per day) for prophylaxis should be carefully considered especially in immunocompromised hosts. Restricting the use of neuraminidase inhibitors only to treatment of patients that have ILI symptoms could also be an option to reduce the potential of generating resistance.
Because immunocompromised patients are at risk for sustained influenza virus shedding and for resistance selection, close monitoring and reporting of resistance to NAIs are essential. To limit the spread of any new resistant strain from this population, the promotion of prophylactic measures including influenza vaccination of relatives and healthcare providers should be encouraged as well as reinforcement of infection control practices. This study along with other reports of an I223R mutation reminds the need to develop new drugs with different viral targets.
Acknowledgments
We gratefully acknowledge the contribution of the members of the NIC (Northern-France) Mathilde Benassaya, David Briand, Frédérique Cuvelier, Sébastien Le Gal and Vanessa Roca for isolation and characterization of the viruses and excellent technical assistance. We also acknowledge the members of the Genotyping of Pathogens and Public Health platform Laure Diancourt, Anne-Sophie Delannoy-Vieillard and Jean-Michel Thiberge for sequencing of the viruses and excellent technical assistance. The bidirectional pRF483 reverse genetics plasmid was kindly provided by R. Fouchier, (Erasmus MC, Rotterdam, The Netherlands). We are indebted to Bernard Delmas and Bruno Da Costa (INRA, Jouy en Josas, France) for the pRF-HA, -M, -NS, to Nadia Naffakh, Mehdi Cherrar and Dorothée Moisy for the pRF-PB1, -PB2, -PA, NP, and to Pierre-Louis Hervé for the pRF-NA reverse genetics plasmids derived from the A/Paris/2590(H1N1)pdm isolate. Zanamivir was kindly provided by Mark von Itzstein (Institute for Glycomics, Griffith University). Oseltamivir carboxylate (GS4071), the active form of the ethyl ester prodrug oseltamivir phosphate, was kindly provided by Roche.
Funding Statement
This work was supported by the Institut Pasteur, funding from the Institut de Veille Sanitaire to the National Influenza Center (Northern-France), the Centre National de la Recherche Scientifique [URA3015], the University Paris Diderot Paris 7, Assistance Publique-Hôpitaux de Paris and the European Community's Seventh Framework Programme (FP7/2007–2013) under the project “European Management Platform for Emerging and Re-emerging Infectious disease Entities” EC [grant agreement number 223498]. GAJ was supported by a grant from the United States Department of Health and Human Services. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ison MG, Gubareva LV, Atmar RL, Treanor J, Hayden FG (2006) Recovery of drug-resistant influenza virus from immunocompromised patients: a case series. J Infect Dis 193: 760–764. [DOI] [PubMed] [Google Scholar]
- 2. Kiso M, Mitamura K, Sakai-Tagawa Y, Shiraishi K, Kawakami C, et al. (2004) Resistant influenza A viruses in children treated with oseltamivir: descriptive study. Lancet 364: 759–765. [DOI] [PubMed] [Google Scholar]
- 3. de Jong MD, Tran TT, Truong HK, Vo MH, Smith GJ, et al. (2005) Oseltamivir resistance during treatment of influenza A (H5N1) infection. N Engl J Med 353: 2667–2672. [DOI] [PubMed] [Google Scholar]
- 4. Ives JA, Carr JA, Mendel DB, Tai CY, Lambkin R, et al. (2002) The H274Y mutation in the influenza A/H1N1 neuraminidase active site following oseltamivir phosphate treatment leave virus severely compromised both in vitro and in vivo. Antiviral Res 55: 307–317. [DOI] [PubMed] [Google Scholar]
- 5. Abed Y, Goyette N, Boivin G (2004) A reverse genetics study of resistance to neuraminidase inhibitors in an influenza A/H1N1 virus. Antivir Ther 9: 577–581. [PubMed] [Google Scholar]
- 6. Bloom JD, Gong LI, Baltimore D (2010) Permissive secondary mutations enable the evolution of influenza oseltamivir resistance. Science 328: 1272–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aoki FY, Boivin G, Roberts N (2007) Influenza virus susceptibility and resistance to oseltamivir. Antivir Ther 12: 603–616. [PubMed] [Google Scholar]
- 8. Meijer A, Lackenby A, Hungnes O, Lina B, van-der-Werf S, et al. (2009) Oseltamivir-resistant influenza virus A (H1N1), Europe, 2007-08 season. Emerg Infect Dis 15: 552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moscona A (2009) Global transmission of oseltamivir-resistant influenza. N Engl J Med 360: 953–956. [DOI] [PubMed] [Google Scholar]
- 10. Rameix-Welti MA, Enouf V, Cuvelier F, Jeannin P, van der Werf S (2008) Enzymatic properties of the neuraminidase of seasonal H1N1 influenza viruses provide insights for the emergence of natural resistance to oseltamivir. PLoS Pathog 4: e1000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rameix-Welti MA, Munier S, le Gal S, Cuvelier F, Agou F, et al.. (2011) Neuraminidase of 2007–2008 influeza A(H1N1) viruses shows increased affinity for sialic acids due to the D344N substitution. Antiviral Therapy 16, in press. [DOI] [PubMed]
- 12. Holmes EC (2010) Virology. Helping the resistance. Science 328: 1243–1244. [DOI] [PubMed] [Google Scholar]
- 13. WHO (2011) Global monitoring of antiviral resistance in currently circulating human viruses, november 2011. WER 45: 497–508. [PubMed] [Google Scholar]
- 14. Chen H, Cheung CL, Tai H, Zhao P, Chan JF, et al. (2009) Oseltamivir-resistant influenza A pandemic (H1N1) 2009 virus, Hong Kong, China. Emerg Infect Dis 15: 1970–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CDC (2011) Seasonal Influenza- Weekly report : influenza summary update - 2009–2010 influenza Season Summary.
- 16. Tramontana AR, George B, Hurt AC, Doyle JS, Langan K, et al. (2010) Oseltamivir resistance in adult oncology and hematology patients infected with pandemic (H1N1) 2009 virus, Australia. Emerg Infect Dis 16: 1068–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chan PA, Connell NT, Gabonay AM, Westley B, Larkin JM, et al. (2010) Oseltamivir-resistant 2009–2010 pandemic influenza A (H1N1) in an immunocompromised patient. Clin Microbiol Infect [DOI] [PubMed] [Google Scholar]
- 18. Dulek DE, Williams JV, Creech CB, Schulert AK, Frangoul HA, et al. (2010) Use of intravenous zanamivir after development of oseltamivir resistance in a critically Ill immunosuppressed child infected with 2009 pandemic influenza A (H1N1) virus. Clin Infect Dis 50: 1493–1496. [DOI] [PubMed] [Google Scholar]
- 19. Harvala H, Gunson R, Simmonds P, Hardie A, Bennett S, et al. (2010) The emergence of oseltamivir-resistant pandemic influenza A(H1N1) 2009 virus amongst hospitalised immunocompromised patients in Scotland, November-December, 2009. Euro Surveill 15 [PubMed] [Google Scholar]
- 20. Renaud C, Boudreault AA, Kuypers J, Lofy KH, Corey L, et al. (2011) H275Y mutant pandemic (H1N1) 2009 virus in immunocompromised patients. Emerging infectious diseases 17: 653–660; quiz 765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Da Dalt L, Calistri A, Chillemi C, Cusinato R, Franchin E, et al. (2010) Oseltamivir-resistant pandemic (H1N1) 2009 treated with nebulized zanamivir. Emerging infectious diseases 16: 1813–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moore C, Galiano M, Lackenby A, Abdelrahman T, Barnes R, et al. (2011) Evidence of person-to-person transmission of oseltamivir-resistant pandemic influenza A(H1N1) 2009 virus in a hematology unit. The Journal of infectious diseases 203: 18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gaur AH, Bagga B, Barman S, Hayden R, Lamptey A, et al. (2010) Intravenous zanamivir for oseltamivir-resistant 2009 H1N1 influenza. The New England journal of medicine 362: 88–89. [DOI] [PubMed] [Google Scholar]
- 24. Esposito S, Molteni CG, Colombo C, Daleno C, Dacco V, et al. (2010) Oseltamivir-induced resistant pandemic A/H1N1 influenza virus in a child with cystic fibrosis and Pseudomonas aeruginosa infection. Journal of clinical virology: the official publication of the Pan American Society for Clinical Virology 48: 62–65. [DOI] [PubMed] [Google Scholar]
- 25. Nguyen HT, Fry AM, Loveless PA, Klimov AI, Gubareva LV (2010) Recovery of a multidrug-resistant strain of pandemic influenza A 2009 (H1N1) virus carrying a dual H275Y/I223R mutation from a child after prolonged treatment with oseltamivir. Clin Infect Dis 51: 983–984. [DOI] [PubMed] [Google Scholar]
- 26. van der Vries E, Stelma FF, Boucher CA (2010) Emergence of a multidrug-resistant pandemic influenza A (H1N1) virus. N Engl J Med 363: 1381–1382. [DOI] [PubMed] [Google Scholar]
- 27. Eshaghi A, Patel SN, Sarabia A, Higgins RR, Savchenko A, et al. (2011) Multidrug-resistant pandemic (H1N1) 2009 infection in immunocompetent child. Emerging infectious diseases 17: 1472–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHO (2009) WHO information for laboratory diagnosis of pandemic (H1N1) 2009 virus in humans - 23 november 2009.http://www.who.int/csr/resources/publications/swineflu/WHO_Diagnostic_RecommendationsH1N1_20090521.pdf. Accessed 2012 July 27.
- 29. Deyde VM, Sheu TG, Trujillo AA, Okomo-Adhiambo M, Garten R, et al. (2010) Detection of molecular markers of drug resistance in 2009 pandemic influenza A (H1N1) viruses by pyrosequencing. Antimicrob Agents Chemother 54: 1102–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. de Wit E, Spronken MI, Bestebroer TM, Rimmelzwaan GF, Osterhaus AD, et al. (2004) Efficient generation and growth of influenza virus A/PR/8/34 from eight cDNA fragments. Virus Res 103: 155–161. [DOI] [PubMed] [Google Scholar]
- 31. Rameix-Welti MA, Tomoiu A, Dos Santos Afonso E, van der Werf S, Naffakh N (2009) Avian Influenza A virus polymerase association with nucleoprotein, but not polymerase assembly, is impaired in human cells during the course of infection. J Virol 83: 1320–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Matrosovich M, Matrosovich T, Garten W, Klenk HD (2006) New low-viscosity overlay medium for viral plaque assays. Virol J 3: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reed LJ, Muench H (1938) A simple method of estimating fifty percent endpoints. Am J Hygiene 27: 493–497. [Google Scholar]
- 34. Rameix-Welti MA, Agou F, Buchy P, Mardy S, Aubin JT, et al. (2006) Natural variation can significantly alter the sensitivity of influenza A (H5N1) viruses to oseltamivir. Antimicrob Agents Chemother 50: 3809–3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gubareva LV, Matrosovich MN, Brenner MK, Bethell RC, Webster RG (1998) Evidence for zanamivir resistance in an immunocompromised child infected with influenza B virus. J Infect Dis 178: 1257–1262. [DOI] [PubMed] [Google Scholar]
- 36. Sheu TG, Deyde VM, Okomo-Adhiambo M, Garten RJ, Xu X, et al. (2008) Surveillance for neuraminidase inhibitor resistance among human influenza A and B viruses circulating worldwide from 2004 to 2008. Antimicrob Agents Chemother 52: 3284–3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hurt AC, Holien JK, Parker M, Kelso A, Barr IG (2009) Zanamivir-resistant influenza viruses with a novel neuraminidase mutation. J Virol 83: 10366–10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hurt AC, Lowther S, Middleton D, Barr IG (2010) Assessing the development of oseltamivir and zanamivir resistance in A(H5N1) influenza viruses using a ferret model. Antiviral Res [DOI] [PubMed] [Google Scholar]
- 39. Baz M, Abed Y, McDonald J, Boivin G (2006) Characterization of multidrug-resistant influenza A/H3N2 viruses shed during 1 year by an immunocompromised child. Clin Infect Dis 43: 1555–1561. [DOI] [PubMed] [Google Scholar]
- 40. Monto AS, McKimm-Breschkin JL, Macken C, Hampson AW, Hay A, et al. (2006) Detection of influenza viruses resistant to neuraminidase inhibitors in global surveillance during the first 3 years of their use. Antimicrob Agents Chemother 50: 2395–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hurt AC, Holien JK, Barr IG (2009) In vitro generation of neuraminidase inhibitor resistance in A(H5N1) influenza viruses. Antimicrob Agents Chemother 53: 4433–4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. CDC (2009) Oseltamivir-resistant 2009 pandemic influenza A (H1N1) virus infection in two summer campers receiving prophylaxis–North Carolina, 2009. MMWR Morb Mortal Wkly Rep 58: 969–972. [PubMed] [Google Scholar]
- 43. Pizzorno A, Bouhy X, Abed Y, Boivin G (2011) Generation and characterization of recombinant pandemic influenza A(H1N1) viruses resistant to neuraminidase inhibitors. The Journal of infectious diseases 203: 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Peng AW, Milleri S, Stein DS (2000) Direct measurement of the anti-influenza agent zanamivir in the respiratory tract following inhalation. Antimicrobial agents and chemotherapy 44: 1974–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van der Vries E, Veldhuis Kroeze EJ, Stittelaar KJ, Linster M, Van der Linden A, et al. (2011) Multidrug resistant 2009 A/H1N1 influenza clinical isolate with a neuraminidase I223R mutation retains its virulence and transmissibility in ferrets. PLoS pathogens 7: e1002276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pizzorno A, Abed Y, Bouhy X, Beaulieu E, Mallett C, et al. (2012) Impact of mutations at residue I223 of the neuraminidase protein on the resistance profile, replication level, and virulence of the 2009 pandemic influenza virus. Antimicrobial agents and chemotherapy 56: 1208–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]