Abstract
A novel microflow cytometer is proposed in which the particles are focused in the horizontal and vertical directions by means of the Saffman shear lift force generated within a micro-weir microchannel. The proposed device is fabricated on stress-relieved glass substrates and is characterized both numerically and experimentally using fluorescent particles with diameters of 5 μm and 10 μm, respectively. The numerical results show that the micro-weir structures confine the particle stream to the center of the microchannel without the need for a shear flow. Moreover, the experimental results show that the particles emerging from the micro-weir microchannel pass through the detection region in a one-by-one fashion. The focusing effect of the micro-weir microchannel is quantified by computing the normalized variance of the optical detection signal intensity. It is shown that the focusing performance of the micro-weir structure is equal to 99.76% and 99.57% for the 5-μm and 10-μm beads, respectively. Overall, the results presented in this study confirm that the proposed microcytometer enables the reliable sorting and counting of particles with different diameters.
INTRODUCTION
With the emergence of micro-electro-mechanical systems (MEMS) technologies in recent decades, the realization of micro-total-analysis-systems (μ-TAS) and lab-on-a-chip (LoC) devices is now an achievable reality.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 Such systems perform a complete, integrated and automated analysis of the target analyte and have many advantages compared to their macro-scale counterparts, including a reduced reagent consumption, a more rapid analysis time, and an improved potential for integration with other fluidic devices. Microflow cytometers provide a high-throughput means of sorting and counting cells/particles and are used in a wide variety of biomedical and industrial applications, including clinical hematology diagnosis, bacteria analysis, and gene diagnosis.15, 16, 17, 18, 19, 20
In a typical microflow cytometer, the cells/particles are injected into an electrolyte solution and are then focused in the horizontal plane by two sheath flows such that they pass through the detection region of the device in a single line fashion.21, 22 Fu et al.23 developed an electrokinetically driven microflow cytometer in which the sample was focused in the horizontal direction by two sheath flows and the cells/particles were then detected by means of an embedded optical fiber coupled to a gas laser and an avalanche photodiode (APD).
The literature contains many proposals for focusing cells/particles within microflow cytometers. Broadly speaking, these proposals can be classified as hydrodynamic sheath flow focusing,24, 25, 26, 27 electrokinetic sheath flow focusing,28, 29, 30 optical gradient force focusing,31, 32 dielectrophoretic force focusing,33, 34, 35, 36, 37 acoustic wave force focusing,38 and magnetic force focusing.39 However, all of these methods require the use of some form of external perturbation force. As a result, they are physically cumbersome and expensive. Consequently, the feasibility of focusing the cells/particles within a microflow cytometer using sheathless and passive techniques such as inertial lifting40 and viscoelasticity41 has attracted growing attention in recent years.
Hur et al.42 presented a sheathless parallel flow cytometer in which the cells were focused to a uniform z-position under the combined effects of a wall effect lift force and a shear gradient lift force. The validity of the proposed device was demonstrated by performing the automated counting and detection of red and white blood cells. Choi et al.43 presented a method for the sheathless 3D focusing of microbeads and blood cells by means of the transverse pressure gradients generated by a V-shaped obstacle array (VOA). Bhagat et al.44 developed a sheathless flow cytometer in which the particles were focused three-dimensionally to a single position within a spiral microchannel by means of Dean drag and inertial lift forces. The viability of the proposed cytometer was demonstrated using SH-SY5Y neuroblastoma cells. Oakey et al.45 presented a flow cytometer in which a staged channel design consisting of both curved and straight sections was used to order the particles into a single streamline by means of a Dean flow inertial focusing effect.
Zheng et al.46 examined the Saffman lift force acting on 0.2-μm particles in a microchannel with pressure-driven de-ionized (DI) water flow. The experimental results showed that the lift force was dominant in the range of 2 < z+ < 6 (where z+ = z/2r, r is the particle radius, z is the distance from the wall). Ookawara et al.47 showed that for spherical particles flowing in a microchannel, the flow near the upper wall is rapidly decelerated by viscous effects, which introduce a velocity component that moves the particle away from the wall due to the Saffman lift force in the microchannel. Candelier et al.48 showed that the time-dependent lift force acting on a particle moving in a pure shear flow at low Reynolds numbers cannot be obtained directly using the Asmolov and McLaughlin frequential results since their complicated algebraic form prevents the use of analytical inverse Fourier transformation. Consequently, a closed temporal expression for the lift force was proposed with the form of a convolution product involving an empirical kernel and the slip velocity of the particles.
The present study proposes a microflow cytometer in which the particles are focused in the horizontal and vertical directions by means of the Saffman shear lift force generated within a microchannel comprising a series arrangement of three micro-weirs with a gradually reducing height. The 3-D focusing performance of the proposed device is demonstrated by means of computational fluid dynamics (CFD) simulations. Moreover, the viability of the proposed device for practical cytometry applications is confirmed by separating and detecting a mixed sample comprising fluorescent polystyrene beads with diameters of 5 μm and 10 μm, respectively.
EXPERIMENTAL SECTION
Chip fabrication
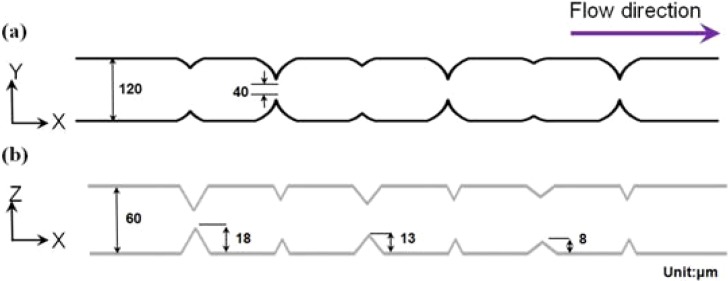
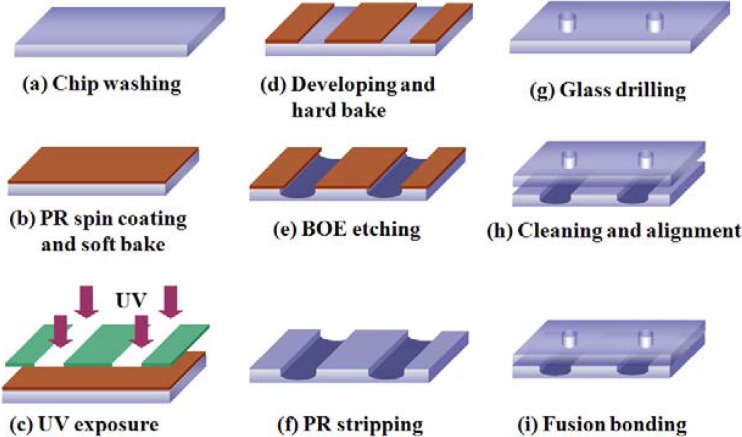
Figure 1 illustrates the configuration and geometric dimensions of the micro-weir microchannel within the proposed cytometer. As shown, the micro-weirs have a constant width (40 μm) in the horizontal plan (X-Y), but a gradually reducing height (i.e., 18 μm, 13 μm, and 8 μm) in order to focus the particle stream progressively in the vertical direction (X-Z). The cytometer was fabricated on commercial microscope slides with dimensions of 75 × 25 × 1.0 mm3 (Marienfeld, Germany). Prior to the fabrication process, the slides were annealed at a temperature of 400 °C for 4 h in order to relieve any internal residual stress. The photomasks used to pattern the lower and upper glass slides were generated using AutoCAD software and were printed with a resolution of 10 000 dpi on an EPSON 123 ink jet printer. Figure 2 shows the basic steps in the microfabrication process. The stress-relieved glass substrates were cleaned via immersion in a piranha solution (concentrated sulfuric acid mixed with concentrated hydrogen peroxide in a volume ratio of 3:1) for 10 min (see Fig. 2a). The substrates were then rinsed in DI water and blown dry with nitrogen gas. To ensure the complete removal of any residual water molecules from the surface of the substrate, the substrates were baked on a hot plate for 3 min at a temperature of 100 °C. Following the baking process, the substrates were spin-coated with a thin layer of positive photoresist (PR) (AZ4620, Clariant Corp., USA) and were then baked at 100 °C for a further 3 min (see Fig. 2b). The microchannel configuration was patterned on the PR layer using a UV lithography technique with an exposure dose of 180 mJ/cm−2 and an exposure time of 12 s (see Fig. 2c). The exposed PR layer was developed by immersing the substrate in a developer solution (AZ400K: DI water = 1:3) for 70 s. The patterned substrates were hard-baked at 150 °C for 10 min (see Fig. 2d) and were then etched for 35 min in a 6:1 ultrasonically agitated buffered oxide etchant (BOE, J. T. Baker, USA) solution to generate microchannels with a depth of 30 μm (see Fig. 2e). To remove any precipitated particles from the etched surface, the etching process was interrupted every 5 min and the substrate dipped in a 1M HCl solution for 10 s. After each dipping operation, the substrate was cleaned in DI water and then replaced in the BOE bath. Following the etching process, the remaining PR layer was stripped from the substrate using a warm potassium hydroxide solution (KOH (49%): DI water = 10:1, temperature ∼ 70 °C) (see Fig. 2f). Meanwhile, the upper substrate of the microflow cytometer was formed by drilling via holes in a plain glass slide with the same dimensions as the lower slide (see Fig. 2g). The upper and lower glass substrates were cleaned in Piranha solution and were then carefully aligned (see Fig. 2h). Finally, the two slides were thermally bonded in a sintering oven at a temperature of 670 °C for 10 min with a ramp rate of 5 °C/min to form the sealed microchip (see Fig. 2i) (Note that a more detailed explanation of the fabrication process is available in Fu et al.49).
Figure 1.
(a) Top view of micro-weir microchannel, and (b) side view of micro-weir microchannel.
Figure 2.
Major steps in microflow cytometer fabrication process.
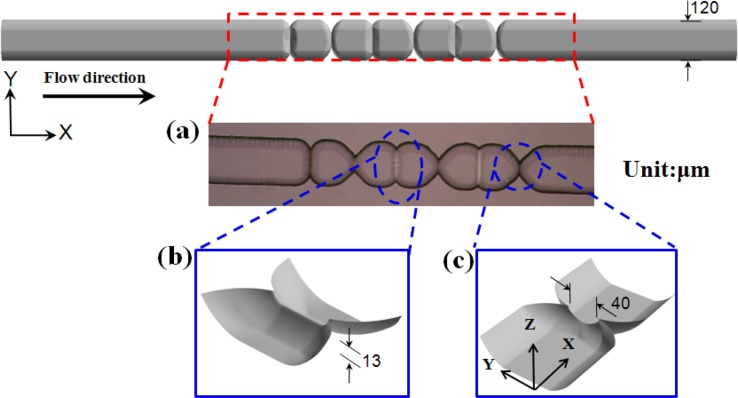
Figure 3a presents a top view optical microscope (OM) image of the fabricated micro-weir microchannel. Figures 3b, 3c present schematic illustrations showing the focusing effects of the micro-weir structures in the vertical and horizontal directions, respectively.
Figure 3.
(a) OM image of micro-weir microchannel. Schematic illustrations showing focusing effect of micro-weir structures in (b) vertical and (c) horizontal directions.
Experimental setup
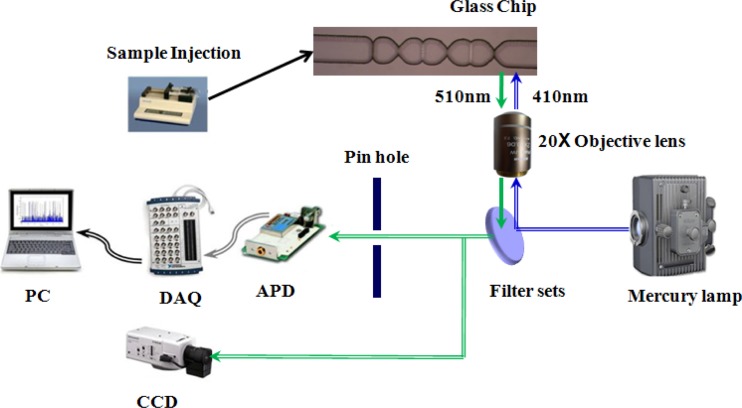
Figure 4 presents a schematic illustration of the experimental setup used to evaluate the performance of the cytometer. As shown, the sample stream was injected into the microchip by a syringe pump and was then focused in the vertical and horizontal directions as it passed through the micro-weir microchannel. The particles flowed in a single-line fashion through the detection region of the device (located 100 μm downstream of the micro-weir microchannel) and were detected at a point 100 μm downstream of the micro-weir microchannel using a laser-induced fluorescence (LIF) technique. The particles were illuminated by a mercury light source (passed through an excitation filter to produce an excitation maxima wavelength of 410 nm). The optical signals generated by the individual particles were converted into electrical signals by an APD module (C5460-01, Hamamatsu, Japan). The electrical signals were captured using a data acquisition (DAQ) card (PCI-6024E, National Instruments, USA) and interfaced to a PC for analysis purposes. The trajectories of the particles within the sample solution were observed under a microscope with a 20 × objective lens and captured by a charge-coupled device camera (CCD, model DXC-190, Sony, Japan) connected to the PC.
Figure 4.
Experimental setup used to characterize proposed microflow cytometer.
Sample preparation
The effectiveness of the focusing micro-weir microchannel was evaluated using fluorescent polystyrene beads with nominal diameters of 5 μm and 10 μm, respectively (Duke Scientific Corp. USA). The beads were suspended in a solution comprising 0.4% Tween 20 aqueous buffer solution and 14% (w/v) sodium dodecyl sulfate (SDS) in order to prevent particle sedimentation and coalescence. Prior to the experiments, the sample solutions were diluted in DI water from original concentrations of 1.4 × 108 number/ml (5-μm beads) and 1.8 × 107 number/ml (10-μm beads) to a final concentration of 1 × 104 number/ml.
NUMERICAL SIMULATION PROCEDURE AND THEORY
Governing equation and numerical methods
The trajectories of the polystyrene beads within the micro-weir microchannel were investigated given various fluid flow velocity in the range of 0.05 to 0.2 m/s by means of CFD including Saffman lift force simulations. In performing the simulations, the flow field was modeled using the 3D Navier-Stokes equations. Thus, the governing equations for mass and momentum conservation were given, respectively, as
| (1) |
| (2) |
where is the flow velocity in the direction, ρ is the fluid density, μ is the fluid dynamic viscosity, p is the static pressure, is the mass flow rate of the particles, is the time step, is the Saffman lift effect (force/unit particle mass) in the i direction per unit particle mass, and is the drag force per unit particle mass. In the present simulations, ρ and μ were assigned values of 1000 kg m−3 and 10−3 kg m−1 s−1, respectively. In addition, it was assumed that the sample flow was incompressible laminar flow with a negligible temperature variation. A uniform velocity profile was imposed at the flow inlet boundary, while atmospheric pressure conditions were imposed at the flow outlet boundary. Finally, a no-slip condition and a zero species concentration flux were imposed at all the solid wall surfaces of the microchannel.
The governing equations and corresponding boundary conditions were solved using commercial fluent CFD code. In the solution procedure, implicit equations were formed by discretizing the closed-set of governing equations using a finite volume difference method. As recommended by Hardt and Schonfeld,50 the spatial derivatives at all the interior grid points were approximated using a third-order QUICK scheme in order to minimize the effects of numerical diffusion. Finally, the resulting difference equations for the pressure-velocity coupling in the Navier-Stokes equations were solved using the Semi-Implicit method for Pressure-Linked Equation (SIMPLE) algorithm. Note that an under-relaxation technique was used to prevent divergence during the iterative solution procedure. In order to enhance the quality of the numerical solutions, the computational domain was discretized with structural elements to construct a mesh in which most of the cells had an equal side length of 5 μm (Note that a series of preliminary experiments was performed to confirm that the chosen mesh size ensured the grid independence of the particle traces.).
Particle trajectory model
The particle trajectories within the micro-weir microchannel were solved using an arbitrary Lagrangian-Eulerian method. In solving the particle trajectories, the following assumptions were made: (a) the heat and mass transfer between the fluid and the particles are sufficiently small to be ignored; (b) the individual particles do not coalesce; (c) the particles do not make contact with the solid wall surfaces; (d) the particles are solid and have a spherical form; and (e) the particles remain physically intact as they pass through the micro-weir microchannel. In accordance with the Lagrangian-Eulerian formulation, the force balance acting on each particle has the form
| (3) |
where is the particle velocity in the i direction; is the Saffman lift effect (force/unit particle mass) in the i direction per unit particle mass, and is the drag force per unit particle mass and is written as
| (4) |
where is the density of the particles, is the particle diameter, is the drag coefficient, Re is the fluid Reynolds number, and is the particle Reynolds number. Here, Re, , and are defined, respectively, as
| (5) |
| (6) |
| (7) |
where is the hydraulic diameter of the inlet microchannel and , , are constants which apply to smooth spherical particles over several Reynolds number ranges.51 The particle trajectory was finally obtained as
| (8) |
where is the particle position in the i direction.
Saffman lift effect acting on single particle in shear flow
In the present study, the particles within the cytometer are focused in the vertical and horizontal directions by the Saffman shear lift effect induced within the micro-weir microchannel. The Saffman shear lift effect including particles rotation effect can be obtained by applying a series expansion method to the original static expression given by Saffman,52 i.e.,
| (9) |
where K = 2.594 and is the deformation tensor.
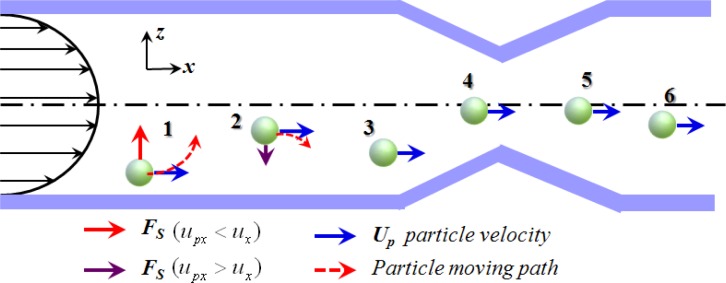
Note that Eq. 8 is valid only for low values of the Reynolds number, i.e., . As shown in Fig. 5, the lift force pushes a particle toward the center of the microchannel if the particle lags the fluid flow (i.e., ) and toward the wall if the particle leads the fluid flow (). As a result, the particles, which are originally distributed randomly within the sample flow, converge toward a specific plane in the microchannel and pass through the detection area in a single-line fashion.
Figure 5.
Schematic illustration of Saffman lift force acting on particles moving in shear flow.
RESULTS AND DISCUSSION
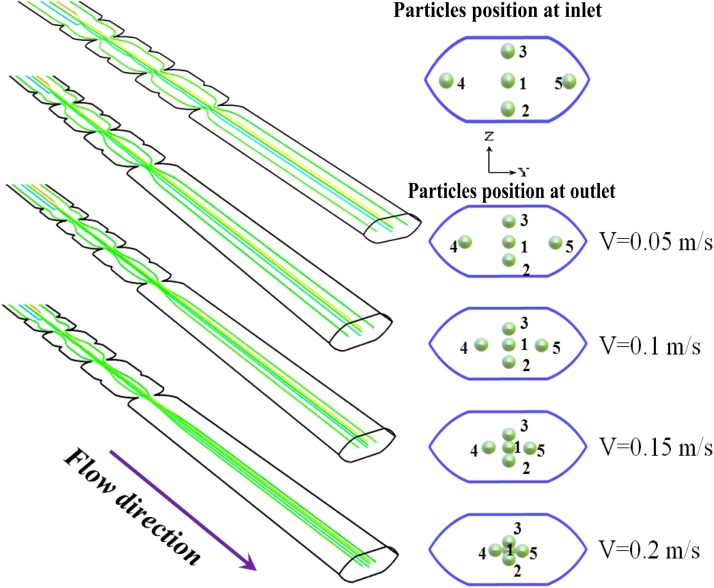
Figure 6 presents the simulation results obtained for the traces of five particles released at the inlet of the micro-weir microchannel given fluid flow velocity in the range of 0.05 to 0.2 m/s. As shown, one of the particles is released from the center of the microchannel, while the other particles are released from positions close to the microchannel walls. The results clearly show that the particle traces converge in both the vertical and the horizontal directions as the particles pass through the micro-weir microchannel. At a low fluid flow velocity (i.e., V = 0.05 m/s), the focusing effect is only very mild since the shear force induced by the micro-weir structures is very small. However, as the fluid flow velocity is increased to V = 0.2 m/s, the magnitude of the Saffman shear lift force also increases, and hence the particles are confined to the central region of the channel.
Figure 6.
Simulation results obtained for particle trace given fluid flow velocities ranging from 0.05 to 0.2 m/s.
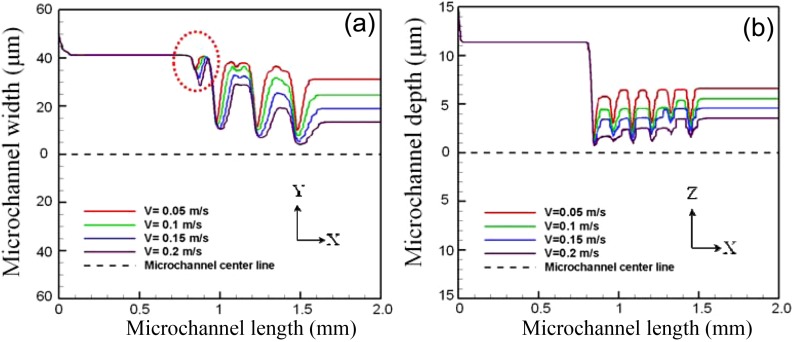
Figure 7a shows the simulation results obtained for the horizontal trajectory of particle 4 (released from a position near the microchannel wall) given fluid flow velocities ranging from 0.05 to 0.2 m/s. The results confirm that as the fluid flow velocity increases, the magnitude of the Saffman shear lift force induced by the micro-weir structures increases. It is noted that a small focusing effect is induced upstream of the first micro-weir structure (see circular dotted line). This phenomenon arises since the vertical height of the micro-weir structure (∼18 μm) is sufficient to form a focusing effect in the horizontal direction. However, it is seen that this focusing effect does not affect the subsequent convergence of the particle trajectories within the micro-weir microchannel.
Figure 7.
Simulation results for particle trajectories in (a) horizontal and (b) vertical directions given fluid flow velocities ranging from 0.05 to 0.2 m/s (Note that the particle trajectories relate to a particle released from a position close to the microchannel wall.).
Figure 7b shows the vertical trajectory of particle 3 (released from a position close to the upper surface of the microchannel) given fluid flow velocities of 0.05 to 0.2 m/s. In general, the results show that the particle trajectory converges toward the centerline of the micro-weir microchannel as the flow velocity is increased. However, as the height of the micro-weir structures reduces, the Saffman shear lift force also reduces. Thus, for all values of the fluid flow velocity, the particles diverge slightly from the centerline as they travel through the micro-weir microchannel.
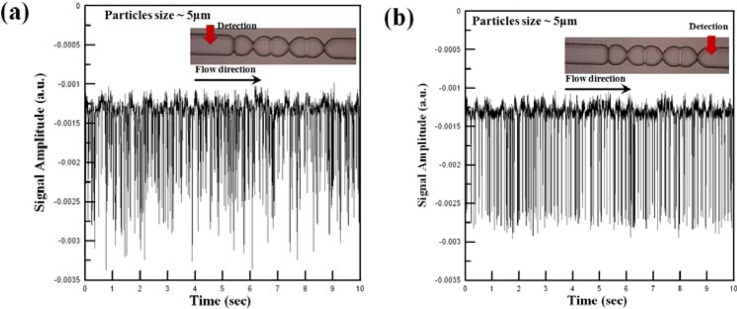
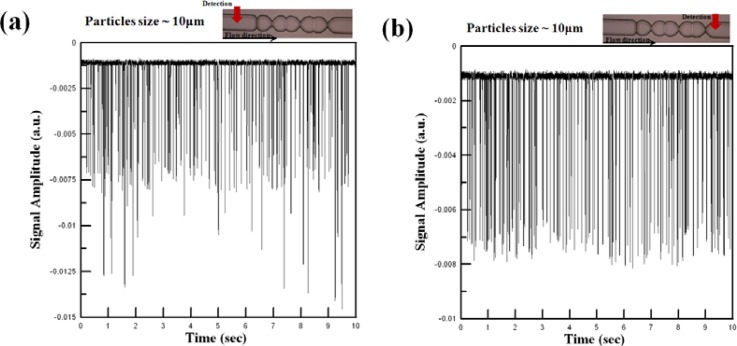
Figure 8a shows the optical signals acquired by the APD module at a position 0.5 mm upstream of the micro-weir microchannel for a fluid sample containing polystyrene beads with a diameter of 5 μm (Note that in accordance with the simulation results, the fluid flow velocity was set as 0.2 m/s.). It can be seen that the optical detection signal has both a non-uniform amplitude and a non-uniform pitch. In other words, it is inferred that the particles are overlapped in both the vertical and the horizontal directions. Figure 8b shows the detection signal obtained at a position 0.1 mm downstream of the micro-weir microchannel. In this case, the detection signal has uniform amplitude and a more regular pitch. Thus, the effectiveness of the micro-weir structures in confining the beads to the center of the microchannel such that they pass through the detection region in a single-line manner is confirmed.
Figure 8.
Optical signals collected by APD module for 5-μm beads at different positions in microflow cytometer, i.e., (a) 0.1 mm upstream of micro-weir microchannel, and (b) 0.1 mm downstream of micro-weir microchannel.
Figure 9a shows the optical signals acquired at a point 0.5 mm upstream of the micro-weir microchannel for a sample containing 10-μm particles and a fluid flow velocity of 0.2 m/s. As for the case of the 5-μm particles, the results show that the 10-μm beads are randomly arranged in the horizontal and vertical directions before entering the micro-weir microchannel. However, Fig. 9b once again confirms the effectiveness of the micro-weir structures in confining the microbeads to a single streamline along the center of the microchannel.
Figure 9.
Optical signals collected by APD module for 10-μm beads at different positions in microflow cytometer, i.e., (a) 0.1 mm upstream of micro-weir microchannel, and (b) 0.1 mm downstream of micro-weir microchannel.
As shown in Figs. 89, the amplitude of the optical signal reduces as the particles pass through the micro-weir microchannel due to the focusing effect of the micro-weir structures. In practice, the focusing effect can be quantified via the normalized variance (σ/H)2, where (σ/H)2 (referred to hereafter as the focusing performance) is defined as %, in which pi is the point intensity, is the average intensity, and n is the total number of points. From an inspection of Fig. 89, it is found that the focusing performance increases from 78.70% to 99.76% as the 5-μm particles pass through the micro-weir microchannel. Similarly, for the 10-μm particles, the focusing performance increases from 77.96% to 99.57%. In other words, the effectiveness of the micro-weir microchannel in focusing the 5-μm and 10-μm particles is confirmed.
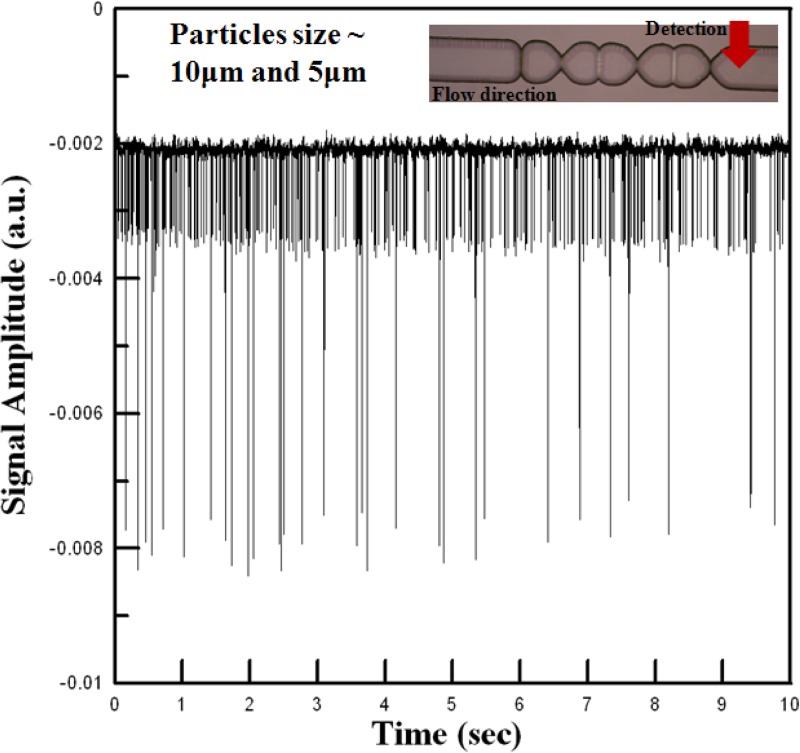
Figure 10 shows the optical signals acquired by the APD at a point 0.1 mm downstream of the micro-weir microchannel given a mixed sample comprising both 5-μm and 10 -μm particles and a fluid flow velocity of 0.2 m/s. It is seen that the signals associated with the two different particles have a clear difference in amplitude. As a result, the 5-μm and 10-μm particles can be reliably differentiated and counted. Moreover, the uniform amplitude of the two signals confirms that both particles are confined to the 3D center of the microchannel. Overall, the experimental results confirm the viability of the proposed microflow cytometer for the automatic detection and counting of cells/particles.
Figure 10.
Optical signals collected by APD module in detection region of microflow cytometer for mixed sample comprising 5-μm and 10-μm beads.
CONCLUSIONS
This study has presented a novel sheathless microflow cytometer in which the sample stream is focused three-dimensionally by means of the Saffman shear lift force developed by a series of micro-weirs of gradually reducing height. The performance of the proposed device has been characterized both numerically and experimentally. The numerical results have confirmed the effectiveness of the micro-weirs in confining the sample stream to the center of the microchannel. In addition, the experimental results have shown that the micro-weirs prompt a separation of the particles in both the horizontal and the vertical directions such that they enter the detection region of the cytometer in a single-line fashion. The separation and counting performance of the proposed cytometer has been evaluated using polystyrene beads with nominal diameters of 5 μm and 10 μm, respectively. An accurate detection performance has been observed even at fluid flow velocity as high as 0.2 m/s. The detection throughput can be approximately 80 particles/s and is potentially capable of even higher throughput. Finally, it has been shown that the micro-weir microchannel achieves a focusing performance of approximately 99.76% and 99.57% for the 5-μm and 10 -μm beads, respectively. Overall, the results presented in this study confirm that the proposed cytometer provides a simple yet extremely effective solution for the automatic separation and counting of cells/particles in a wide variety of biochemical and medical applications.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the financial support provided to this study by the National Science Council of Taiwan.
References
- Fu L. M. and Lin C. H., Biomed. Microdevices 9, 277 (2007). 10.1007/s10544-006-9036-0 [DOI] [PubMed] [Google Scholar]
- Lin C. H., Lee C. Y., Tsai C. H., and Fu L. M., Microfluid. Nanofluid. 7, 499 (2009). 10.1007/s10404-009-0403-z [DOI] [Google Scholar]
- Lim C. Y., Lam Y. C., and Yang C., Biomicrofluidics 4, 014101 (2010). 10.1063/1.3279790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong T. F., Ju W. J., Wu M. C., Tai C. H., Tsai C. H., and Fu L. M., Microfluid. Nanofluid. 9, 1125 (2010). 10.1007/s10404-010-0633-0 [DOI] [Google Scholar]
- Cho S. H., Godin J. M., Chen C. H., Qiao W., Lee H., and Lo Y. H., Biomicrofluidics 4, 043001 (2010). 10.1063/1.3511706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M. J., Johnston I. D., Tan C. K. L., and Tracey M. C., Biomicrofluidics 4, 044112 (2010). 10.1063/1.3528327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., Cao X., Chen L., Zhang J., Xia X., Fang Q., and Chen H., Electrophoresis 31, 3665 (2010). 10.1002/elps.201000258 [DOI] [PubMed] [Google Scholar]
- Hou H. H., Wang Y. N., Chang C. L., Fu L. M., and Yang R. J., Microfluid. Nanofluid. 11, 479 (2011). 10.1007/s10404-011-0813-6 [DOI] [Google Scholar]
- Paul D., Saias L., Pedinotti J., Chabert M., Magnifico S., Pallandre A., Lambert B. D., Houdayer C., Brugg B., Peyrin J., and Viovy J., Biomicrofluidics 5, 024102 (2011). 10.1063/1.3569946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movahed S. and Li D., Microfluid. Nanofluid. 10, 703 (2011). 10.1007/s10404-010-0716-y [DOI] [Google Scholar]
- Li W. K., Soong C. Y., Tzeng P. Y., and Liu C. H., Microfluid. Nanofluid. 10, 199 (2011). 10.1007/s10404-010-0663-7 [DOI] [Google Scholar]
- Ju W. J., Fu L. M., Yang R. J., and Lee C. L., Lab Chip 12, 622 (2012). 10.1039/c1lc20954j [DOI] [PubMed] [Google Scholar]
- Lin C. H., Wang Y. N., and Fu L. M., Biomicrofluidics 6, 012818 (2012). 10.1063/1.3654950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. H., Lin C. H., Fu L. M., and Chen H. C., Biomicrofluidics 6, 024108 (2012). 10.1063/1.4704504 [DOI] [Google Scholar]
- Tsai C. H., Hou H. H., and Fu L. M., Microfluid. Nanofluid. 5, 827 (2008). 10.1007/s10404-008-0284-6 [DOI] [Google Scholar]
- Hou H. H., Tsai C. H., Fu L. M., and Yang R. J., Electrophoresis 30, 2507 (2009). 10.1002/elps.200900012 [DOI] [PubMed] [Google Scholar]
- Wu H. W., Hsu R. C., Lin C. C., Hwang S. M., and Lee G. B., Biomicrofluidics 4, 024112 (2010). 10.1063/1.3454767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan X., Zhu J., and Church C., Microfluid. Nanofluid. 9, 1 (2010). 10.1007/s10404-010-0602-7 [DOI] [Google Scholar]
- Wang J., Wang C., Lin C., Lei H., and Lee G., Microfluid. Nanofluid. 10, 531 (2011). 10.1007/s10404-010-0687-z [DOI] [Google Scholar]
- Rosenauer M., Buchegger W., Finoulst I., Verhaert P., and Vellekoop M., Microfluid. Nanofluid. 10, 761 (2011). 10.1007/s10404-010-0707-z [DOI] [Google Scholar]
- Golden P., Kim J. S., Erickson J. S., Hilliard L. R., Howell P. B., Anderson G. P., Nasiar M., and Lighler F. S., Lab Chip 9, 1942 (2009). 10.1039/b822442k [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L. M., Tsai C. H., and Lin C. H., Electrophoresis 29, 1874 (2008). 10.1002/elps.200700630 [DOI] [PubMed] [Google Scholar]
- Fu L. M., Yang R. J., Lin C. H., Pan Y. J., and Lee G. B., Anal. Chim. Acta. 507, 163 (2004). 10.1016/j.aca.2003.10.028 [DOI] [Google Scholar]
- Rosenauer M. and Vellekoop M. J., Biomicrofluidics 4, 043005 (2010). 10.1063/1.3502672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummrow A., Theisen J., Frankowski M., Tuchscheerer A., Yildirim H., Brattke K., Schmidt M., and Neukammer J., Lab Chip 9, 972 (2009). 10.1039/b808336c [DOI] [PubMed] [Google Scholar]
- Lee H. C., Hou H. H., Yang R. J., Lin C. H., and Fu L. M., Microfluid. Nanofluid. 11, 469 (2011). 10.1007/s10404-011-0812-7 [DOI] [Google Scholar]
- Golden J. P., Justin G. A., Nasir M., and Ligler F. S., Anal. Bioanal. Chem. 402, 325 (2012). 10.1007/s00216-011-5415-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T. and Morgan H., Microfluid. Nanofluid. 8, 423 (2010). 10.1007/s10404-010-0580-9 [DOI] [Google Scholar]
- Zhu T., Luo C., Huang J., Xiong C., Ouyang Q., and Fang J., Biomed. Microdevices 12, 35 (2010). 10.1007/s10544-009-9355-z [DOI] [PubMed] [Google Scholar]
- Zhu J., Canter R., Keten G., Vedantam P., Tzeng T. J., and Xuan X., Microfluid. Nanofluid. 11, 743 (2011). 10.1007/s10404-011-0839-9 [DOI] [Google Scholar]
- Cho S. H., Godin J. M., Chen C. H., Qiao W., Lee H., and Lo Y. H., Biomicrofluidics 4, 043001 (2010). 10.1063/1.3511706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi N., Erickson J. S., Golden J. P., and Ligler F. S., Biomicrofluidics 5, 032009 (2011). 10.1063/1.3608136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. H., Lee G. B., Fu L. M., and Hwei B. H., J. Microelectromech. Syst. 13, 923 (2004). 10.1109/JMEMS.2004.838352 [DOI] [Google Scholar]
- Valero A., Braschler T., Demierre N., and Renaud P., Biomicrofluidics 4, 022807 (2010). 10.1063/1.3430542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piacentini N., Mernier G., Tornay R., and Renaud P., Biomicrofluidics 5, 034122 (2011). 10.1063/1.3640045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng I. F., Chung C. C., and Chang H. C., Microfluid. Nanofluid. 10, 649 (2011). 10.1007/s10404-010-0699-8 [DOI] [Google Scholar]
- Jen C. P., Weng C. H., and Huang C. T., Electrophoresis 32, 2428 (2011). 10.1002/elps.201100085 [DOI] [PubMed] [Google Scholar]
- Shi J., Yazdi S., Lin S. C. S., Ding X., Chiang I. K., Sharpd K., and Huang T. J., Lab Chip 11, 2319 (2011). 10.1039/c1lc20042a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T., Cheng R., and Mao L., Microfluid. Nanofluid. 11, 695 (2011). 10.1007/s10404-011-0835-0 [DOI] [Google Scholar]
- Carlo D. D., Lab Chip 9, 3038 (2009). 10.1039/b912547g [DOI] [PubMed] [Google Scholar]
- Sudarsan A. P. and Ugaz V. M., Lab Chip 6, 74 (2006). 10.1039/b511524h [DOI] [PubMed] [Google Scholar]
- Hur S. C., Tse H. T. K., and Carlo D. D., Lab Chip 10, 274 (2010). 10.1039/b919495a [DOI] [PubMed] [Google Scholar]
- Choi S., Song S., Choi C., and Park J. K., Small 4, 634 (2008). 10.1002/smll.200700308 [DOI] [PubMed] [Google Scholar]
- Bhagat A. A. S., Kuntaegowdanahalli S. S., Kaval N., Seliskar C. J., and Papautsky I., Biomed. Microdevices 12, 187 (2010). 10.1007/s10544-009-9374-9 [DOI] [PubMed] [Google Scholar]
- Oakey J., R. W.Applegate, Jr., Arellano E., Carlo D. D., Graves S. W., and Toner M., Anal. Chem. 82, 3862 (2010). 10.1021/ac100387b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X. and Silber-Li Z., Appl. Phys. Lett. 95, 124105 (2009). 10.1063/1.3237159 [DOI] [Google Scholar]
- Ookawara S., Agrawal M., Street D., and Ogawa K., Chem. Eng. Sci. 62, 2454 (2007). 10.1016/j.ces.2007.01.031 [DOI] [Google Scholar]
- Candelier F. and Souhar M., Phys. Rev. E 76, 067301 (2007). 10.1103/PhysRevE.76.067301 [DOI] [PubMed] [Google Scholar]
- Fu L. M., Wang J. H., Luo W. B., and Lin C. H., Microfluid. Nanofluid. 6, 499 (2009). 10.1007/s10404-008-0328-y [DOI] [Google Scholar]
- Hardt S. and Schonfeld F., AIChE J. 49, 578 (2003). 10.1002/aic.690490305 [DOI] [Google Scholar]
- Morsi S. A. and Alexander A. J., J. Fluid Mech. 55, 193 (1972). 10.1017/S0022112072001806 [DOI] [Google Scholar]
- Saffman P. G., J. Fluid Mech. 22, 385 (1965). 10.1017/S0022112065000824 [DOI] [Google Scholar]