Abstract
Amblyopia, also known as lazy eye, usually occurs during early childhood and results in poor or blurred vision. Recent neuroimaging studies have found cortical structural/functional abnormalities in amblyopia. However, until now, it was still not known whether the spontaneous activity of the brain changes in amblyopia subjects. In the present study, regional homogeneity (ReHo), a measure of the homogeneity of functional magnetic resonance imaging signals, was used for the first time to investigate changes in resting-state local spontaneous brain activity in individuals with anisometropic amblyopia. Compared with age- and gender-matched subjects with normal vision, the anisometropic amblyopia subjects showed decreased ReHo of spontaneous brain activity in the right precuneus, the left medial prefrontal cortex, the left inferior frontal gyrus, and the left cerebellum, and increased ReHo of spontaneous brain activity was found in the bilateral conjunction area of the postcentral and precentral gyri, the left paracentral lobule, the left superior temporal gyrus, the left fusiform gyrus, the conjunction area of the right insula, putamen and the right middle occipital gyrus. The observed decreases in ReHo may reflect decreased visuo-motor processing ability, and the increases in ReHo in the somatosensory cortices, the motor areas and the auditory area may indicate compensatory plasticity in amblyopia.
Introduction
Amblyopia, or “lazy eye”, is a disorder of the visual system characterized by visual deficiency in an eye that is otherwise physically normal or by a deficiency that is out of proportion with the structural abnormalities of the eye [1], [2], [3]. Amblyopia is also one of the most common causes of unilateral visual deficits in adulthood [4]. In recent years, extensive neuroimaging studies have been carried out to assess cortical structural/functional abnormalities in amblyopia. Several studies have detected morphologic changes, such as reduced gray matter, in the visual cortical regions of amblyopia subjects using a voxel-based morphometry (VBM) analyses [5], [6]. These findings may indicate developmental abnormalities of the visual cortex during the critical growth period. Additionally, the advent of fMRI has produced convergent evidence of cortical dysfunction in amblyopia [7], [8], [9]. Lv et al. [10] investigated the functional dysfunction of the visual cortex that accompanies morphological changes in amblyopia and found that functional deficits may be correlate with gray matter volume in some areas, especially the occipital lobe, indicating that visual dysfunction may potentially have structural substrates in human amblyopia. Other neuroimaging tools, such as positron emission tomography and magnetoencephalography, have also provided evidence that a number of cortical areas, including the primary and secondary visual areas, regions within the parieto-occipital cortex, and the ventral temporal cortex, show reduced levels of activation in amblyopia [11].
It is generally believed that the visual cortex is the principle site of vision deficits in the visual pathway in amblyopia [7], [8], [12]. Previous studies have also observed functional deficits and morphological changes at the level of the lateral geniculate nucleus (LGN) in anisometropic amblyopia [13], [14], [15]. For example, Miki et al. [15] found that activation of the LGN is significantly affected during monocular viewing with the affected eyes. Hess et al. [14] also demonstrated that functional deficits are observable at the thalamic level using high-field fMRI in a group of human adults with amblyopia. Barnes et al. [13] found that the LGN is structurally abnormal in humans with strabismic amblyopia. Additionally, some studies have suggested that extra-striate regions are also responsible for abnormalities in special visual function in amblyopia. Barnes et al. [8] measured fMRI activation between the fixing and fellow amblyopic eyes of ten individuals with strabismic amblyopia and found that stimuli that were well within the amblyopic passband produced reduced fMRI activation in visual areas V1, V2, and V3A. Simmers and colleagues' studies suggest that, in addition to striate cortical dysfunction, both ventral and dorsal extra-striate function are affected by amblyopia, and these effects are not simply consequences of striate dysfunction [16], [17], [18].
In contrast to task-based fMRI studies, resting-state fMRI requires neither stimulation nor response and reflects the spontaneous neuronal activity or the background neurophysiological processes of the human brain [19]. Using resting-state fMRI, Biswal et al. [20] found that spontaneous low-frequency fluctuations in blood oxygen level dependent (BOLD) signals were highly synchronous within the somato-motor system and concluded that these fluctuations were physiologically meaningful. Since then, there has been increased interest in resting-state fMRI, and this method has been used in healthy subjects to investigate brain activities in various functional systems (including motor, auditory, visual, language and limbic systems), and in neurological and psychiatric diseases (including multiple sclerosis, schizophrenia and major depression) [21], [22], [23].
Zang et al. [24] proposed Regional Homogeneity (ReHo) as an index for measuring activity patterns during resting states. ReHo is a rank order statistic measure that evaluates the similarity between the time series of a given voxel and its nearest neighbors. The ReHo measure can rapidly map the level of regional activity across the whole brain of an individual [25]. Based on the hypothesis that brain activity works in clusters rather than in a single voxel, the ReHo index is robust to differences in the magnitude of the BOLD response. Regions with higher ReHo values may indicate that these brain areas have greater similarities in activity with their neighbors. In previous studies, this index has been successfully used to study a variety of neurological and psychiatric diseases (such as schizophrenia, Parkinson's disease, blindness, Alzheimer's disease etc.) and has provided new disease-related findings [26], [27], [28], [29], [30], [31], [32], [33], [34]. Regarding activity patterns of the visual cortex, Liu et al. [33] reported that blind subjects have higher ReHo values in the visual cortex compared with subjects with normal vision; this finding may be explained by abnormal cortical development and/or experience dependent plasticity in the visual cortex. The studies mentioned above indicate that the analysis of ReHo during resting states may provide a new approach to explore functional abnormalities of spontaneous brain activity. Until now, the spontaneous brain activity patterns of amblyopia subjects were still unclear. Thus, we used ReHo to address the question of where and how brain activity patterns are impaired in amblyopia.
Materials and Methods
Subjects
The study was approved by the Ethics Committee of Zhong Shan Ophthalmic Center, Sun Yat-sen University and followed the tenets of the Declaration of Helsinki. Written informed consent was obtained from all participants enrolled in the study or their legal guardians, and all participants received detailed eye examinations that included assessments of visual acuity, intraocular pressure and refraction, slit lamp examination, ophthalmoscopy, binocular alignment, ocular motility, and random-dot butterfly stereograms. In total, fourteen amblyopic patients and twenty-two healthy individuals were enrolled in the study. Two participants (1 healthy volunteer, 1 patient with amblyopia) had excessive head motion during scanning (see data preprocessing) and were excluded, leaving twenty-one healthy volunteers and thirteen patients with amblyopia who were included in the analysis. All the subjects were right-handed and had no history of strabismus, other ocular diseases, surgery, neurological disorders, or brain abnormalities based on MRI scans. The volunteers had normal or corrected to normal visual acuity in both eyes. Detailed clinical data of the subjects are shown in Table 1.
Table 1. Demographic characteristics of the participants with anisometropic amblyopia.
| Subject | Gender | Age (Y) | amblyopia eye | Acuity (20/feet) | Refraction | ||
| Right Left | Left | Right | Left | ||||
| AN01 | M | 18 | AE (L) | 20/12.5 | 20/62.55 | plano | +3.25DS/+2.25DC×80 |
| AN02 | M | 22 | AE (OU) | 20/100 | 20/100 | +10 DS/+0.5DC×180 | +11.5DS/+0.25DC×80 |
| AN03 | F | 18 | AE (R) | 20/200 | 20/12.5 | +6.5DS/+0.5DC×90 | plano |
| AN04 | F | 22 | AE (R) | 20/200 | 20/20 | −19.5DS/−1DC×65 | −6.0DS |
| AN05 | F | 18 | AE (R) | 20/80 | 20/32 | +8.0DS/+0.75DC×120 | +7.0DS/+1DC×60 |
| AN06 | F | 20 | AE (L) | 20/20 | 20/200 | −5.5DS | −20DS |
| AN07 | F | 17 | AE (R) | 20/50 | 20/20 | +2.75DS/−5.50DC×175 | +0.50DS/+0.50DC×90 |
| AN08 | F | 18 | AE (L) | 20/20 | 20/400 | −1.5DS/−0.25DC×85 | −13.0DS/−2.0DC×165 |
| AN09 | M | 17 | AE (L) | 20/20 | 20/32 | −2.5DS/−0.75DC×175 | −7.25DS/−0.50DC×180 |
| AN10 | M | 24 | AE (R) | 20/50 | 20/32 | +7.75DS | +6.0DS/+0.75DC×90 |
| AN11 | F | 30 | AE (L) | 20/16 | 20/50 | +0.50DS/+0.50DC×70 | +8.0DS/+1.0DC×120 |
| AN12 | M | 23 | AE (L) | 20/12.5 | 20/400 | +5.0DS | +7.0DS |
| AN13 | F | 43 | AE (L) | 20/20 | 20/5020/20 | −0.5DC×75 | +3.0DS |
Data acquisition
MRI data were obtained using a 3.0 Tesla MR scanner (Trio Tim system; Siemens, Erlangen, Germany). Resting-state fMRI scans were performed with an echo planar imaging (EPI) sequence with the following scan parameters: repetition time = 2000 ms, echo time = 30 ms, flip angle = 90°, matrix = 64×64, field of view = 220×220 mm2, slice thickness = 3 mm and slice gap = 1 mm. Each brain volume comprised 32 axial slices, and each functional run contained 270 volumes. During the scans, all subjects were instructed to keep their eyes closed, relax and move as little as possible. Tight, but comfortable, foam padding was used to minimize head motion, and earplugs were used to reduce scanner noise.
The structural magnetization prepared rapid gradient-echo imaging (MP-RAGE) sequence was used to acquire structural T1-weighted images in a sagittal orientation. The parameters were as follows: repetition time = 2000 ms; echo time = 2.6 ms; flip angle = 9°; acquisition matrix = 512×448; field of view = 256×224 mm2. The scanning time was approximately 5 min, and a total of 192 images with slice thicknesses of 1 mm were obtained.
Data preprocessing
All preprocessing procedures were carried out using Statistical Parametric Mapping software (SPM8, http://www.fil.ion.ucl.ac.uk/spm). To allow subjects to adapt to the scanning situation and to obtain magnetization equilibrium, the first 10 volumes of each functional time series were discarded. The remaining fMRI volumes were slice corrected and then realigned to the first volume. Subject were included if their head movement during fMRI scanning was less than 3 mm translation in any axis and less than 3° angular rotation in any axis. All realigned data were then spatially normalized to the standard Montreal Neurological Institute (MNI) EPI template and resampled to 2×2×2 mm cubic voxels. To further reduce the effects of confounding factors, six motion parameters, linear drift and the mean time series of all voxels within the white matter and the cerebrospinal fluid were removed from the data by linear regression. After that, a temporal filter (0.01–0.08 Hz) was applied to reduce the effect of low-frequency drift and high-frequency noise signals. Finally, the regressed images were smoothed with a 6 mm full width at half maximum (FWHM) to reduce spatial noise [33], [34], [35].
Structural MRI data were preprocessed in SPM8 using voxel-based morphometry (VBM) as implemented in the VBM8 toolbox with default parameters (http://dbm.neuro.uni-jena.de/vbm.html). Images were segmented into gray matter (GM), white matter and cerebrospinal fluid tissue classes. Next, the segmented GM images were bias-corrected and registered with a template image in MNI space [36]. Finally, the modulated GM volumes were resampled to 2 mm cubic voxel resolution and smoothed with a 6 mm FWHM 3D Gaussian kernel.
ReHo measurement and statistical analysis
In the present study, we investigated alterations in the spontaneous activity in anisometropic amblyopia subjects using ReHo. ReHo is a fast method for mapping regional spontaneous activity across the whole brain [24]. We do not provide the definition of ReHo here because it has been used many times, and detailed definitions can be found in Zang et al. [24].
To reduce the effect of individual variability, we normalized the ReHo value of each voxel by dividing it by the mean ReHo of the whole brain for each subject [28], [33], [34]. That is, for each voxel,
Next, a two-sample two-tailed t-test was performed to investigate the group differences in ReHo between the anisometropic amblyopia subjects and subjects with normal vision after regressing out the effects of age and gender. The statistical threshold for each voxel was set at P alpha<0.01, with a cluster size of at least 130 voxels, based on the results of a Monte Carlo simulation (http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf, AlphaSim with the following parameters: single voxel P = 0.01, FWHM = 6 mm, with AAL template in MirCroN software as a mask). Statistical comparisons of the mean fitted ReHo values between each pair of groups were performed using a two-sample two-tailed t-test at a threshold of P<0.05 (FDR corrected using groups times the number of significant brain regions). The VBM analyses were performed using the same statistical method.
Relationship between ReHo and clinical variables
To determine whether the ReHo index varied with disease progression in the patients, correlation analyses between the ReHo index in the identified regions and each of the clinical variables (visual acuity of bilateral eyes) were performed. Further, to determine if structural alteration accompanies with the altered spontanous brain activity in the patients, we also evaluated the gray matter volume in the identified regions of the amblyopia subjects. Because these analyses were exploratory in nature, we used a statistical significance level of P<0.05 (uncorrected).
Results
The demographic and psychological characteristics of the 13 patients with amblyopia (5 males, 8 females; mean age: 22.3±7.2 years) are summarized in Table 1. The 21 healthy volunteers individuals (8 males, 13 females; mean age: 23.5±2.1 years) were well matched with the amblyopia group in age (P = 0.47, two-sample two-tailed t-test) and gender (P = 0.98, Chi-squared test) distribution. Additionally, to further evaluate the influence of head motion on ReHo results, an extra evaluation of differences in movement parameters between amblyopia and subjects with normal vision was performed according the procedures described in Van Dijk et al. [37]. The mean motions of the subjects with anisometropic amblyopia and subjects with normal vision groups were 0.062±0.033 mm and 0.051±0.020 mm, respectively. No significant difference was found between the two groups (P = 0.219, two-sample, two-tailed t-test).
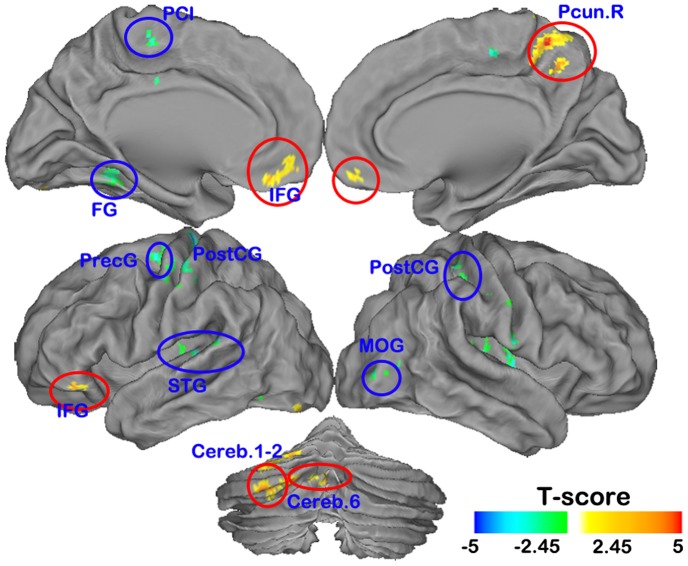
Compared with the subjects with normal vision, the anisometropic amblyopia individuals showed significantly decreased ReHo in brain regions including the right precuneus (Pcun), the left medial prefrontal cortex (MPFC), the left inferior frontal gyrus (IFG), and the left cerebellum (cerebellum area 1–2, 6) at the threshold in clusters larger than 130 voxels at P<0.01 (Alphasim corrected) (Figure 1 red, Figure 2 A and Table 2). We also found some brain areas [the bilateral conjunction area of the postcentral (PostGG) and precentral gyri (PreCG), the left paracentral lobule (PCL), the left superior temporal gyrus (STG), the left fusiform gyrus, the conjunction area of right insula and putamen and the right middle occipital gyrus (MOG)] with significantly increased ReHo at the threshold in clusters larger than 130 voxels at P<0.01 (Alphasim corrected) (Figure 1 blue, Figure 2 A and Table 3).
Figure 1. The anatomical distribution of the altered normalized ReHo in anisometropic amblyopia visualized by individuals Caret v5.61 software (P<0.01, 130 voxels, Alphasim corrected Palpha = 0.01).
Abbreviations: Pcun = precuneus, IFG = inferior frontal gyrus, MPFC = media prefrontal cortex, Cereb = cerebellum; PostCG = postcentral gyrus, PretCG = precentral gyrus, PCL = paracentral lobule, STG = superior temporal gyrus, FG = fusiform gyrus, MOG = middle occipital gyrus.
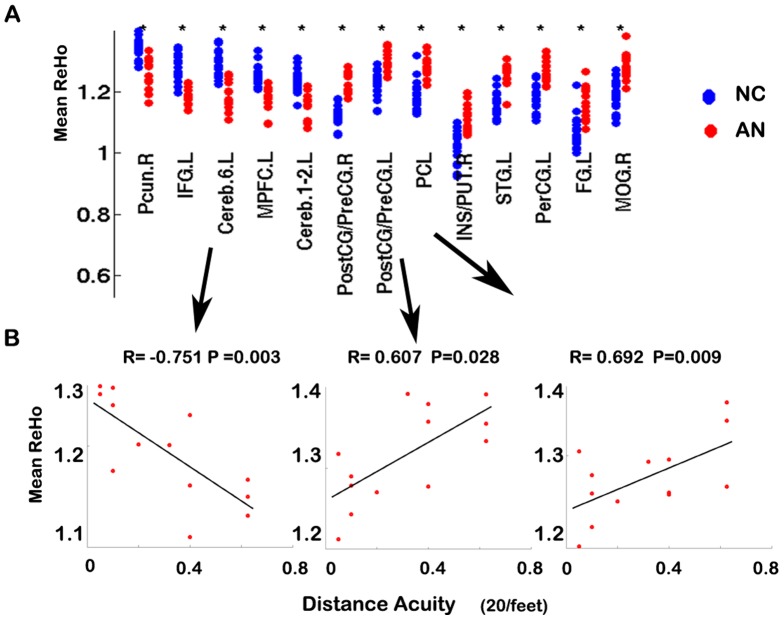
Figure 2. Plot of the normalized ReHo values in the brain areas in which ReHo values were significantly different between the subjects with normal vision and anisometropic amblyopia individuals(A).
Correlation between the mean fitted ReHo indices and visual acuities in the patients with anisometropic amblyopia (P<0.05)(B). Abbreviations: same with Figure 1.
Table 2. Brain areas with decreased ReHo in anisometropic amblyopia individuals (P<0.01, 130 voxels, Alphasim corrected Palpha = 0.01).
| Brain Region | Brodmann Region | Cluster Size | T-values | Z-scores | Coordinates in MNI (x, y, z) |
| Pcun.R | 7 | 228 | 5.81 | 4.77 | 6 −52 56 |
| 4.41 | 3.87 | 6 −56 44 | |||
| 4.01 | 3.58 | 8 −54 64 | |||
| IFG.L | 45/47 | 209 | 5.65 | 4.67 | −52 38 −4 |
| 4.36 | 3.83 | −56 26 6 | |||
| 4.33 | 3.81 | −40 34 −4 | |||
| Cereb.6.L | 132 | 4.93 | 4.22 | −16 −76 −20 | |
| 3.93 | 3.52 | −28 −82 −22 | |||
| 3.22 | 2.98 | −10 −68 −22 | |||
| MPFC | 10/11 | 191 | 4.74 | 4.10 | −2 34 −16 |
| 4.36 | 3.83 | 2 46 −10 | |||
| 3.88 | 3.49 | −10 42 −6 | |||
| Cereb.1–2.L | 184 | 4.70 | 4.06 | −32 −86 −36 | |
| 4.25 | 3.75 | −14 −90 −30 | |||
| 4.05 | 3.61 | −2 −84 −30 |
Abbreviations: Pcun = precuneus, IFG = inferior frontal gyrus, MPFC = media prefrontal cortex, Cereb = cerebellum.
L: left, R: right.
Table 3. Brain areas with increased ReHo in anisometropic amblyopia individuals (P<0.01, 130 voxels, Alphasim corrected Palpha = 0.01).
| Brain Region | Brodmann Region | Cluster Size | T-values NC-AN | Z-scores | Coordinates in MNI (x, y, z) |
| PostCG/PreCG.R | 3/4 | 522 | −5.34 | −4.48 | 24 −30 56 |
| −5.07 | −4.31 | 46 −18 40 | |||
| −4.50 | −3.93 | 58 −10 18 | |||
| PostCG/PreCG.L | 3/4 | 321 | −5.26 | −4.43 | −42 −24 54 |
| −4.58 | −3.99 | −54 −18 46 | |||
| −4.46 | −3.91 | −48 −28 48 | |||
| PCL | 4/6 | 222 | −5.02 | −4.28 | −20 −30 60 |
| −5.00 | −4.27 | −18 −30 68 | |||
| −4.34 | −3.82 | 4 −24 52 | |||
| INS/PUT.R | 152 | −4.99 | −4.26 | 36 −10 6 | |
| −4.32 | −3.81 | 28 −12 6 | |||
| −3.74 | −3.38 | 34 −14 18 | |||
| STG.L | 288 | −4.87 | −4.18 | −42 −30 8 | |
| −4.67 | −4.05 | −48 −28 20 | |||
| −4.26 | −3.76 | −32 −20 12 | |||
| PerCG.L | 6 | 224 | −4.81 | −4.14 | −32 −16 68 |
| −4.56 | −3.97 | −36 −10 56 | |||
| −3.85 | −3.47 | −26 −14 60 | |||
| FG.L | 19/37 | 133 | −4.65 | −4.03 | −38 −52 −8 |
| −3.96 | −3.55 | −28 −50 −14 | |||
| −3.37 | −3.10 | −40 −60 −12 | |||
| MOG.R | 18/19 | 157 | −4.09 | −3.64 | 28 −80 6 |
| −3.87 | −3.48 | 34 −74 2 | |||
| −3.75 | −3.39 | 44 −82 4 |
Abbreviations: PostCG = postcentral gyrus, PretCG = precentral gyrus, PCL = paracentral lobule, INS/PUT = insula and putamen, STG = superior temporal gyrus, FG = fusiform gyrus, MOG = middle occipital gyrus.
L: left, R: right.
As Figure 2 shows, ReHo in the left cerebellum (area 6), the left PostCG/PreCG and the PCL was significantly correlated with visual acuity in the anisometropic amblyopia individuals (P<0.05).
We did not find significant differences in gray matter volumes using the VBM method at our statistical threshold (P<0.01, AlphaSim corrected). Thus, our results demonstrate that gray matter volumes are not significantly altered in the identified regions in anisometropic amblyopia individuals (P<0.05, FDR corrected) (Figure S1).
Discussion
To our knowledge, this is the first study to investigate spontaneous brain activity in anisometropic amblyopia. The present study demonstrated that spontaneous activity patterns of some brain regions in the anisometropic amblyopia individuals were altered when compared to subjects with normal vision.
Decreased ReHo of spontaneous brain activity in amblyopia
The amblyopia subjects showed decreased ReHo of spontaneous brain activity in the right precuneus cortex (part of BA7) and the left cerebellum (cerebellum area 1–2, 6) (Figure 1, Table 2). The precuneus partially corresponds to the medial posterior parietal cortex, where previous studies have shown decreased glucose metabolism [38], [39] and loss of gray matter [6] in visually impaired subjects. Functionally, the medial posterior parietal cortex area is believed to play a role in visuo-motor coordination (e.g., in reaching to grasp an object) [39], [40], [41]. Similarly, the cerebellum, which functionally interacts with the frontal eye fields [42], [43], [44], [45], is also involved in the control of eye movements [46], [47], [48], [49], [50]. Neural cells in crus 1 and 2 are related to eye movements in monkeys during a visually guided eye movement tracking task [51]. In humans, the lateral cerebellar hemispheres (particularly crus 1 and 2) are involved in the executive network and play an important role in executive function [52], [53], and lesions of the lateral cerebellar hemispheres may affect smooth pursuit eye movement [54]. Previous studies have also provided evidence that the posterior lobe of the cerebellum is more closely associated with cognitive roles in motor learning, hand-eye coordination, error detection and correction, and the control of motor timing [55], [56], [57]. The roles of the oculomotor vermis (lobuli 6 and 7) in these functions are especially well documented [58]. Furthermore, recent findings suggest that visuo-motor coordination is decreased in amblyopia subjects. For example, Grant et al. [59] studied the prehension skills of subjects with amblyopia and found that patients exhibited spatio-temporal deficits in the final approach phase of reaching and grasping. Suttle et al. [60] found amblyopic children spent almost twice as much time grasping objects and made many more errors (1.5–3 times) during reach direction and grip positioning than normal sighted controls. Niechwiej-Szwedo et al. [61] found amblyopia affected both the programming and the execution of visually guided reaching. Our finding of decreased spontaneous brain activity in the precuneus (part of BA7) and the cerebellum (cerebellum area 1–2, 6) further support the role of neurophysiological deficits of visuo-motor integration processing in amblyopia.
The areas with decreased ReHo indices in the prefrontal cortices (part of BA10, 11, 45, 47) correspond partly with Broca's area and the orbitofrontal cortex. Broca's area is the region devoted to speech production and also plays a role in various cognitive and perceptual tasks [62]. The orbitofrontal cortex, which includes the medial portions of BA 9, 10, 11, and the inferior portions of BA 45, 47 in the human brain [63], is considered part of the limbic system and is involved in sensory integration and reward expectation [64]. These decreased ReHo indices may reflect decreased integration abilities in amblyopia.
Thus, we assumed that the decreases in ReHo in these regions might represent a new measure of decreases in fine visuo-motor integration processing ability in anisometropic amblyopia.
Increased ReHo of spontaneous brain activity in amblyopia
Notably, the amblyopia subjects showed increased ReHo indices in the PostCG, PreCG, PCL, and STG (BA41); these areas correspond to the primary somatosensory cortex, primary motor cortex, motor area, and primary auditory cortex, respectively. Additionally, the insula is thought to be a non-primary motor area, and it plays roles in the regulation of the body's homeostasis, motor control, self-awareness, cognitive functioning, and interpersonal experience [65]. It is generally believed that the visual areas are functionally connected to the somatosensory area [66], [67], [68], [69], [70], [71], motor area [71], [72], [73], and auditory area [71], [74]. Our finding of increased ReHo of spontaneous brain activity in these areas may reflect plasticity that compensates for amblyopia-related deficits. This potential compensatory mechanism has also been suggested in subjects with visual deficits such as blindness [71], [73], [75], [76] and neuromyelitis optica [32], [77], and these mechanisms may be general changes that enable visually impaired subjects to perform sensory-guided motor behaviors.
Increases in ReHo were also found in the parts of BA 18 and BA 19 that correspond to visual area V2, and visual area V3 (Dorsal V3 and Ventral V3) (Figure 1, Table 3). This finding is consistent with a previous, related study of the blind [33]. V2 receives strong feed-forward connections from V1 (direct and indirect via the pulvinar) and sends strong connections to V3, V4, and V5. V2 also sends strong feedback connections to V1. Dorsal V3 receives inputs from V2 and the primary visual area and projects to the posterior parietal cortex. Ventral V3 has much weaker inputs from the primary visual area and stronger connections with the inferior temporal cortex. The increased ReHo in the visual system may reflect compensatory plasticity in the feed forward system from the primary visual cortex and/or weakened feedback from the higher extra-striate visual cortex in the visual pathway. We also found increased ReHo in the left fusiform gyrus, which is consistent with some scattered reports of cortical adaptations in adults with amblyopia [78].
Limitations and Caveats
Interestingly, we did not find significant alterations of spontaneous activity in the visual cortices of amblyopia patients at the current statistical threshold. However, when we employed a relatively loose threshold, we found that the ReHo indices of visual brain regions were altered in amblyopia (Figure S2). We assume this may be because we measured spontaneous activity patterns, while previous studies mainly focused on activation patterns in response to visual stimuli in amblyopia subjects. Another possible reason might be that spontaneous activities are stronger in motor areas, and these effects overshadow any effects within the visual cortex. Further, the relatively small sample of this study may have prevented us from finding positive results in the visual cortex in the amblyopia subjects.
Additionally, in our study, we did not find statistically significance differences between the amblyopia subjects and subjects with normal vision in terms of gray matter volume with the VBM method. Furthermore we did not find significantly altered gray matter volumes in the identified ReHo regions. However, it is hard to conclude that morphologic changes do not occur in the cortex of humans with amblyopia.
We also found that the ReHo measure correlated with disease severity in the patient group (Figure 2B). However, this correlation must be considered preliminary due to the small sample size. Furthermore, our results should be interpreted carefully because we did not classify our patients based on left or right eye impairments due to the small sample size. In the future, an investigation with a large sample is required to reduce individual effects on the results.
Supporting Information
Plot of the mean gray matter volumes in which ReHo values were not significantly different between the two groups.
(TIF)
Brain areas with altered ReHo in the anisometropic amblyopia individuals (P<0.05, 30 voxels). (Red indicates ReHo indices that were higher in subjects with normal vision, and blue indicates ReHo indices that were higher in subjects with anisometropic amblyopia individuals).
(TIF)
Funding Statement
This work was supported by the Natural Science Foundation of China (Grant Numbers. 30900476, 30870694 and 60831004), and the Research Foundation of Science and Technology Plan Project, Guangdong, China (Grant Numbers: 2008B060600031, 2011B061300067). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
References
- 1. Holmes JM, Clarke MP (2006) Amblyopia. Lancet 367: 1343–1351. [DOI] [PubMed] [Google Scholar]
- 2. Information from your family doctor. Amblyopia (“lazy eye”) in your child. American family physician 75: 368. [PubMed] [Google Scholar]
- 3. McKee SP, Levi DM, Movshon JA (2003) The pattern of visual deficits in amblyopia. J Vis 3: 380–405. [DOI] [PubMed] [Google Scholar]
- 4. Kanonidou E (2011) Amblyopia: a mini review of the literature. Int Ophthalmol 31: 249–256. [DOI] [PubMed] [Google Scholar]
- 5. Xiao JX, Xie S, Ye JT, Liu HH, Gan XL, et al. (2007) Detection of abnormal visual cortex in children with amblyopia by voxel-based morphometry. Am J Ophthalmol 143: 489–493. [DOI] [PubMed] [Google Scholar]
- 6. Mendola JD, Conner IP, Roy A, Chan ST, Schwartz TL, et al. (2005) Voxel-based analysis of MRI detects abnormal visual cortex in children and adults with amblyopia. Hum Brain Mapp 25: 222–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goodyear BG, Nicolle DA, Humphrey GK, Menon RS (2000) BOLD fMRI response of early visual areas to perceived contrast in human amblyopia. J Neurophysiol 84: 1907–1913. [DOI] [PubMed] [Google Scholar]
- 8. Barnes GR, Hess RF, Dumoulin SO, Achtman RL, Pike GB (2001) The cortical deficit in humans with strabismic amblyopia. J Physiol 533: 281–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hess RF, Li X, Lu G, Thompson B, Hansen BC (2010) The contrast dependence of the cortical fMRI deficit in amblyopia; a selective loss at higher contrasts. Hum Brain Mapp 31: 1233–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lv B, He H, Li X, Zhang Z, Huang W, et al. (2008) Structural and functional deficits in human amblyopia. Neurosci Lett 437: 5–9. [DOI] [PubMed] [Google Scholar]
- 11. Anderson SJ, Swettenham JB (2006) Neuroimaging in human amblyopia. Strabismus 14: 21–35. [DOI] [PubMed] [Google Scholar]
- 12. Demer JL, Grafton S, Marg E, Mazziotta JC, Nuwer M (1997) Positron-emission tomographic study of human amblyopia with use of defined visual stimuli. J AAPOS 1: 158–171. [DOI] [PubMed] [Google Scholar]
- 13. Barnes GR, Li X, Thompson B, Singh KD, Dumoulin SO, et al. (2010) Decreased gray matter concentration in the lateral geniculate nuclei in human amblyopes. Invest Ophthalmol Vis Sci 51: 1432–1438. [DOI] [PubMed] [Google Scholar]
- 14. Hess RF, Thompson B, Gole G, Mullen KT (2009) Deficient responses from the lateral geniculate nucleus in humans with amblyopia. Eur J Neurosci 29: 1064–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miki A, Liu GT, Goldsmith ZG, Liu CS, Haselgrove JC (2003) Decreased activation of the lateral geniculate nucleus in a patient with anisometropic amblyopia demonstrated by functional magnetic resonance imaging. Ophthalmologica 217: 365–369. [DOI] [PubMed] [Google Scholar]
- 16. Simmers AJ, Ledgeway T, Mansouri B, Hutchinson CV, Hess RF (2006) The extent of the dorsal extra-striate deficit in amblyopia. Vision Res 46: 2571–2580. [DOI] [PubMed] [Google Scholar]
- 17. Simmers AJ, Ledgeway T, Hess RF (2005) The influences of visibility and anomalous integration processes on the perception of global spatial form versus motion in human amblyopia. Vision Res 45: 449–460. [DOI] [PubMed] [Google Scholar]
- 18. Simmers AJ, Ledgeway T, Hess RF, McGraw PV (2003) Deficits to global motion processing in human amblyopia. Vision Res 43: 729–738. [DOI] [PubMed] [Google Scholar]
- 19. Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, et al. (2001) A default mode of brain function. Proc Natl Acad Sci U S A 98: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Biswal B, Yetkin FZ, Haughton VM, Hyde JS (1995) Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34: 537–541. [DOI] [PubMed] [Google Scholar]
- 21. Liu Y, Wang K, Yu C, He Y, Zhou Y, et al. (2008) Regional homogeneity, functional connectivity and imaging markers of Alzheimer's disease: a review of resting-state fMRI studies. Neuropsychologia 46: 1648–1656. [DOI] [PubMed] [Google Scholar]
- 22. Jiang T, Liu Y, Shi F, Shu N, Liu B, et al. (2008) Multimodal magnetic resonance imaging for brain disorders: advances and perspectives. Brain Imaging Behav 2: 249–257. [Google Scholar]
- 23. Fox MD, Raichle ME (2007) Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8: 700–711. [DOI] [PubMed] [Google Scholar]
- 24. Zang Y, Jiang T, Lu Y, He Y, Tian L (2004) Regional homogeneity approach to fMRI data analysis. Neuroimage 22: 394–400. [DOI] [PubMed] [Google Scholar]
- 25. Kiviniemi V (2008) Endogenous brain fluctuations and diagnostic imaging. Hum Brain Mapp 29: 810–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. He Y, Chen ZJ, Evans AC (2007) Small-world anatomical networks in the human brain revealed by cortical thickness from MRI. Cereb Cortex 17: 2407–2419. [DOI] [PubMed] [Google Scholar]
- 27. Shukla DK, Keehn B, Muller RA (2010) Regional homogeneity of fMRI time series in autism spectrum disorders. Neurosci Lett 476: 46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu T, Long X, Zang Y, Wang L, Hallett M, et al. (2009) Regional homogeneity changes in patients with Parkinson's disease. Hum Brain Mapp 30: 1502–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang L, Song M, Jiang T, Zhang Y, Yu C (2011) Regional homogeneity of the resting-state brain activity correlates with individual intelligence. Neurosci Lett 488: 275–278. [DOI] [PubMed] [Google Scholar]
- 30. Yang T, Cheng Y, Li H, Jiang H, Luo C, et al. (2010) Abnormal regional homogeneity of drug-naive obsessive-compulsive patients. Neuroreport 21: 786–790. [DOI] [PubMed] [Google Scholar]
- 31. Mankinen K, Long XY, Paakki JJ, Harila M, Rytky S, et al. (2011) Alterations in regional homogeneity of baseline brain activity in pediatric temporal lobe epilepsy. Brain Res 1373: 221–229. [DOI] [PubMed] [Google Scholar]
- 32. Liang P, Liu Y, Jia X, Duan Y, Yu C, et al. (2011) Regional homogeneity changes in patients with neuromyelitis optica revealed by resting-state functional MRI. Clin Neurophysiol 122: 121–127. [DOI] [PubMed] [Google Scholar]
- 33. Liu C, Liu Y, Li W, Wang D, Jiang T, et al. (2011) Increased regional homogeneity of blood oxygen level-dependent signals in occipital cortex of early blind individuals. Neuroreport 22: 190–194. [DOI] [PubMed] [Google Scholar]
- 34. Zhang Z, Liu Y, Jiang T, Zhou B, An N, et al. (2012) Altered spontaneous activity in Alzheimer's disease and mild cognitive impairment revealed by Regional Homogeneity. Neuroimage 59: 1429–1440. [DOI] [PubMed] [Google Scholar]
- 35. Liu Y, Liang M, Zhou Y, He Y, Hao Y, et al. (2008) Disrupted small-world networks in schizophrenia. Brain 131: 945–961. [DOI] [PubMed] [Google Scholar]
- 36. Ashburner J, Friston KJ (2005) Unified segmentation. Neuroimage 26: 839–851. [DOI] [PubMed] [Google Scholar]
- 37. Van Dijk KR, Sabuncu MR, Buckner RL (2012) The influence of head motion on intrinsic functional connectivity MRI. Neuroimage 59: 431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Choi MY, Lee DS, Hwang JM, Choi DG, Lee KM, et al. (2002) Characteristics of glucose metabolism in the visual cortex of amblyopes using positron-emission tomography and statistical parametric mapping. J Pediatr Ophthalmol Strabismus 39: 11–19. [DOI] [PubMed] [Google Scholar]
- 39. Hadjidimitrakis K, Breveglieri R, Placenti G, Bosco A, Sabatini SP, et al. (2011) Fix your eyes in the space you could reach: neurons in the macaque medial parietal cortex prefer gaze positions in peripersonal space. PLoS One 6: e23335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sakata H, Taira M, Kusunoki M, Murata A, Tanaka Y (1997) The TINS Lecture. The parietal association cortex in depth perception and visual control of hand action. Trends Neurosci 20: 350–357. [DOI] [PubMed] [Google Scholar]
- 41. Milner AD, Goodale MA (2008) Two visual systems re-viewed. Neuropsychologia 46: 774–785. [DOI] [PubMed] [Google Scholar]
- 42. Junck L, Gilman S, Rothley JR, Betley AT, Koeppe RA, et al. (1988) A relationship between metabolism in frontal lobes and cerebellum in normal subjects studied with PET. J Cereb Blood Flow Metab 8: 774–782. [DOI] [PubMed] [Google Scholar]
- 43. Gamlin PD, Yoon K, Zhang H (1996) The role of cerebro-ponto-cerebellar pathways in the control of vergence eye movements. Eye (Lond) 10 Pt 2 167–171. [DOI] [PubMed] [Google Scholar]
- 44. Middleton FA, Strick PL (2001) Cerebellar projections to the prefrontal cortex of the primate. J Neurosci 21: 700–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kelly RM, Strick PL (2003) Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci 23: 8432–8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hashimoto M, Ohtsuka K (1995) Transcranial magnetic stimulation over the posterior cerebellum during visually guided saccades in man. Brain 118 Pt 5 1185–1193. [DOI] [PubMed] [Google Scholar]
- 47. Ohtsuka K, Enoki T (1998) Transcranial magnetic stimulation over the posterior cerebellum during smooth pursuit eye movements in man. Brain 121 Pt 3 429–435. [DOI] [PubMed] [Google Scholar]
- 48. Takagi M, Zee DS, Tamargo RJ (1998) Effects of lesions of the oculomotor vermis on eye movements in primate: saccades. J Neurophysiol 80: 1911–1931. [DOI] [PubMed] [Google Scholar]
- 49. Hayakawa Y, Nakajima T, Takagi M, Fukuhara N, Abe H (2002) Human cerebellar activation in relation to saccadic eye movements: a functional magnetic resonance imaging study. Ophthalmologica 216: 399–405. [DOI] [PubMed] [Google Scholar]
- 50. Nitta T, Akao T, Kurkin S, Fukushima K (2008) Involvement of the cerebellar dorsal vermis in vergence eye movements in monkeys. Cereb Cortex 18: 1042–1057. [DOI] [PubMed] [Google Scholar]
- 51. Marple-Horvat DE, Stein JF (1990) Neuronal activity in the lateral cerebellum of trained monkeys, related to visual stimuli or to eye movements. J Physiol 428: 595–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chen SH, Desmond JE (2005) Cerebrocerebellar networks during articulatory rehearsal and verbal working memory tasks. Neuroimage 24: 332–338. [DOI] [PubMed] [Google Scholar]
- 53. Monti MM, Osherson DN, Martinez MJ, Parsons LM (2007) Functional neuroanatomy of deductive inference: a language-independent distributed network. Neuroimage 37: 1005–1016. [DOI] [PubMed] [Google Scholar]
- 54. Straube A, Scheuerer W, Eggert T (1997) Unilateral cerebellar lesions affect initiation of ipsilateral smooth pursuit eye movements in humans. Ann Neurol 42: 891–898. [DOI] [PubMed] [Google Scholar]
- 55. Schmahmann JD (2010) The role of the cerebellum in cognition and emotion: personal reflections since 1982 on the dysmetria of thought hypothesis, and its historical evolution from theory to therapy. Neuropsychol Rev 20: 236–260. [DOI] [PubMed] [Google Scholar]
- 56. Schmahmann JD, Caplan D (2006) Cognition, emotion and the cerebellum. Brain 129: 290–292. [DOI] [PubMed] [Google Scholar]
- 57. Thach WT (1998) What is the role of the cerebellum in motor learning and cognition? Trends Cogn Sci 2: 331–337. [DOI] [PubMed] [Google Scholar]
- 58. Krauzlis RJ (2005) The control of voluntary eye movements: new perspectives. Neuroscientist 11: 124–137. [DOI] [PubMed] [Google Scholar]
- 59. Grant S, Melmoth DR, Morgan MJ, Finlay AL (2007) Prehension deficits in amblyopia. Invest Ophthalmol Vis Sci 48: 1139–1148. [DOI] [PubMed] [Google Scholar]
- 60. Suttle CM, Melmoth DR, Finlay AL, Sloper JJ, Grant S (2011) Eye-hand coordination skills in children with and without amblyopia. Invest Ophthalmol Vis Sci 52: 1851–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Niechwiej-Szwedo E, Goltz HC, Chandrakumar M, Hirji Z, Wong AM (2011) Effects of anisometropic amblyopia on visuomotor behavior, III: Temporal eye-hand coordination during reaching. Invest Ophthalmol Vis Sci 52: 5853–5861. [DOI] [PubMed] [Google Scholar]
- 62. Fadiga L, Craighero L (2006) Hand actions and speech representation in Broca's area. Cortex 42: 486–490. [DOI] [PubMed] [Google Scholar]
- 63. Damasio H, Grabowski TJ, Tranel D, Hichwa RD, Damasio AR (1996) A neural basis for lexical retrieval. Nature 380: 499–505. [DOI] [PubMed] [Google Scholar]
- 64. Kringelbach ML (2005) The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci 6: 691–702. [DOI] [PubMed] [Google Scholar]
- 65. Mutschler I, Schulze-Bonhage A, Glauche V, Demandt E, Speck O, et al. (2007) A rapid sound-action association effect in human insular cortex. PLoS One 2: e259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sathian K, Zangaladze A (2001) Feeling with the mind's eye: the role of visual imagery in tactile perception. Optom Vis Sci 78: 276–281. [DOI] [PubMed] [Google Scholar]
- 67. Burton H, Sinclair RJ, McLaren DG (2004) Cortical activity to vibrotactile stimulation: an fMRI study in blind and sighted individuals. Hum Brain Mapp 23: 210–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Avillac M, Deneve S, Olivier E, Pouget A, Duhamel JR (2005) Reference frames for representing visual and tactile locations in parietal cortex. Nat Neurosci 8: 941–949. [DOI] [PubMed] [Google Scholar]
- 69. Negyessy L, Nepusz T, Kocsis L, Bazso F (2006) Prediction of the main cortical areas and connections involved in the tactile function of the visual cortex by network analysis. Eur J Neurosci 23: 1919–1930. [DOI] [PubMed] [Google Scholar]
- 70. Sereno MI, Huang RS (2006) A human parietal face area contains aligned head-centered visual and tactile maps. Nat Neurosci 9: 1337–1343. [DOI] [PubMed] [Google Scholar]
- 71. Yu C, Liu Y, Li J, Zhou Y, Wang K, et al. (2008) Altered functional connectivity of primary visual cortex in early blindness. Hum Brain Mapp 29: 533–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Winstein CJ, Grafton ST, Pohl PS (1997) Motor task difficulty and brain activity: investigation of goal-directed reciprocal aiming using positron emission tomography. J Neurophysiol 77: 1581–1594. [DOI] [PubMed] [Google Scholar]
- 73. Liu Y, Yu C, Liang M, Li J, Tian L, et al. (2007) Whole brain functional connectivity in the early blind. Brain 130: 2085–2096. [DOI] [PubMed] [Google Scholar]
- 74. Beauchamp MS, Lee KE, Argall BD, Martin A (2004) Integration of auditory and visual information about objects in superior temporal sulcus. Neuron 41: 809–823. [DOI] [PubMed] [Google Scholar]
- 75. Yu C, Shu N, Li J, Qin W, Jiang T, et al. (2007) Plasticity of the corticospinal tract in early blindness revealed by quantitative analysis of fractional anisotropy based on diffusion tensor tractography. Neuroimage 36: 411–417. [DOI] [PubMed] [Google Scholar]
- 76. Shu N, Liu Y, Li J, Li Y, Yu C, et al. (2009) Altered anatomical network in early blindness revealed by diffusion tensor tractography. PLoS One 4: e7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Fiehler K, Rosler F (2010) Plasticity of multisensory dorsal stream functions: evidence from congenitally blind and sighted adults. Restor Neurol Neurosci 28: 193–205. [DOI] [PubMed] [Google Scholar]
- 78. Conner IP, Odom JV, Schwartz TL, Mendola JD (2007) Retinotopic maps and foveal suppression in the visual cortex of amblyopic adults. J Physiol 583: 159–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Plot of the mean gray matter volumes in which ReHo values were not significantly different between the two groups.
(TIF)
Brain areas with altered ReHo in the anisometropic amblyopia individuals (P<0.05, 30 voxels). (Red indicates ReHo indices that were higher in subjects with normal vision, and blue indicates ReHo indices that were higher in subjects with anisometropic amblyopia individuals).
(TIF)