Abstract
Adrenergic-receptor beta2 (ADRB2) and beta3 (ADRB3) are obesity genes that play a key role in the regulation of energy balance by increasing lipolysis and thermogenesis. The Glu27 allele in ADRB2 and the Arg64 allele in ADRB3 are associated with abdominal obesity and early onset of non-insulin-dependent diabetes mellitus (NIDDM) in many ethnic groups. Peroxisome proliferator-activated receptor γ (PPARG) is required for adipocyte differentiation. Pro12Ala mutation decreases PPARG activity and resistance to NIDDM. In humans, energy-expense alleles, Gln27 in ADRB2 and Trp64 in ADRB3, are at higher frequencies than Glu27 and Arg64, respectively, but Ala12 in PPARG is at lower frequency than Pro12. Adaptation of humans for lipolysis, thermogenesis, and reduction of fat accumulation could be considered by examining which alleles in these genes are dominant in non-human primates (NHP). All NHP (P. troglodytes, G. gorilla, P. pygmaeus, H. agilis and macaques) had energy-thrifty alleles, Gly16 and Glu27 in ADRB2, and Arg64 in ADRB3, but did not have energy-expense alleles, Arg16, Gln27 and Trp64 alleles. In PPARG gene, all NHP had large adipocyte accumulating type, the Pro12 allele.
Conclusions
These results indicate that a tendency to produce much more heat through the energy-expense alleles developed only in humans, who left tropical rainforests for savanna and developed new features in their heat-regulation systems, such as reduction of body hair and increased evaporation of water, and might have helped the protection of entrails from cold at night, especially in glacial periods.
Introduction
The polymorphisms of adrenergic receptor genes have been focused on because these receptors have important roles in lipolysis and thermogenesis and cause differences in energy expenditure. Adrenergic-receptor beta3 (ADRB3) is located mainly on the surface of visceral and brown adipose cells and promotes lipolysis and thermogenesis by noradrenaline release from the sympathetic nerves stimulated by cold temperature or food consumption [1], [2]. Trp64Arg mutation of ADRB3 is associated with lower resting metabolic rate [3], abdominal obesity [4], [5], weight gain [6], and difficulty losing weight [7]. Adipose cells with ADRB3 of Trp64/Arg64 or Arg64/Arg64 showed 2/3-fold reduced ability to produce intracellular cAMP [8] and lipolytic glycerol [9] compared with those with Trp64/Trp64. The frequency of Trp64Arg variant was examined in several ethnic groups [3], [10]–[17].
It is known that ADRB2 stimulates lipolysis in adipose cells as well as ADRB3 [18]. Three polymorphisms of ADRB2, Arg16Gly, Gln27Glu, and Thr164Ile, were studied in humans [19]. The Glu27 allele has a tendency to increase BMI, body fat mass, fat cell volume, and waist∶hip ratio [20]–[23], as well as type II diabetes [24], and to suppress lipid oxidation [25]. Male African-Americans and Caucasians with Gly16 of ADRB2 were found to gain weight more from childhood to young adulthood than those with Arg16 [26]. However, the contribution of Gly16 to obesity is controversial. From the analysis of nucleotide sequences of a chimpanzee and haplotypes of ADRB2 and ADRB3 in humans, it was proved that Gly16, Glu27, and Thr164 in ADRB2 and Arg64 in ADRB3 are ancient types. In ADRB2, substitution of the energy-expense type, Gly16∶Gln27 (GC haplotype), for Gly16∶Glu27 (GG haplotype) occurred first 1.9 million years ago (Ma), then substitution Arg16∶Gln27 (AC haplotype) occurred, and then there was recombination between some GG and AC haplotypes [27], [28].
Little information has been accumulated about alleles of ADRB2 or ADRB3 in NHP, except for the determination of the Arg64 type in fifteen obese M. mulatta [29].
Peroxisome proliferator-activated receptor γ (PPARG) is required for adipocyte differentiation from precursor cells. PPARG forms a heterodimer with retinoid receptor, binds to peroxisome proliferation response element of the target genes in the precursor cells, and activates differentiation into small size adipocytes. These small size adipocytes secrete leptin and/or adiponectin, a factor that increases insulin sensitivity. However, upon consumption of a high-fat diet, PPARG induces adipocytes to transform from small size to large size by accumulating fat, which secrete insulin-resistance factors, such as TNFα, resistin, and free fatty acids [30], [31]. Pro12Ala mutation was found in PPARG and the Ala12 allele frequency was found to be high in Caucasians (0.12) and low in Asians (0.01) [32]. The transcriptional activity of PPARG with Ala12 was lower than that with Pro12 when these were stimulated by the ligand of PPARG, thiazolidinedione [33]. Pro12Ala substitution was found to be associated with lower body mass index. In an obese group, subjects with Ala12 were more insulin-sensitive than those with Pro12 [33]. The frequency of Ala12 allele in type 2 diabetic Japanese subjects (0.018) was significantly lower than that in a healthy group (0.043) [34].
To determine whether the appearance of energy-expense-type alleles in these genes was specific for humans, we examined SNP for the 16th and 27th amino acids of ADRB2, the 64th amino acid of ADRB3, and the 12th amino acid of PPARG in NHP (30 Pan troglodytes, 8 Gorilla gorilla, 17 Pongo pygmaeus, 15 Hylobates agilis and 108 macaques).
Results
The nucleotide sequences, the predicted restriction map, and obtained restriction fragment length polymorphism (RFLP) patterns including codon 16 of ADRB2 are shown in Figures 1, 2A and 2B. All non-human hominoids showed a 108 bp fragment instead of a 130 bp fragment, indicating homozygosity for the Gly16 allele, as shown in Table 1. Since the nucleotide was substituted from T to C, restriction site with BsrDI was not retained in the five macaques (Fig. 1), and we did not perform the RFLP method for these monkeys. However, it was confirmed by nucleotide sequence analysis that the target 16th amino acid was Gly in each individual of five species of macaques.
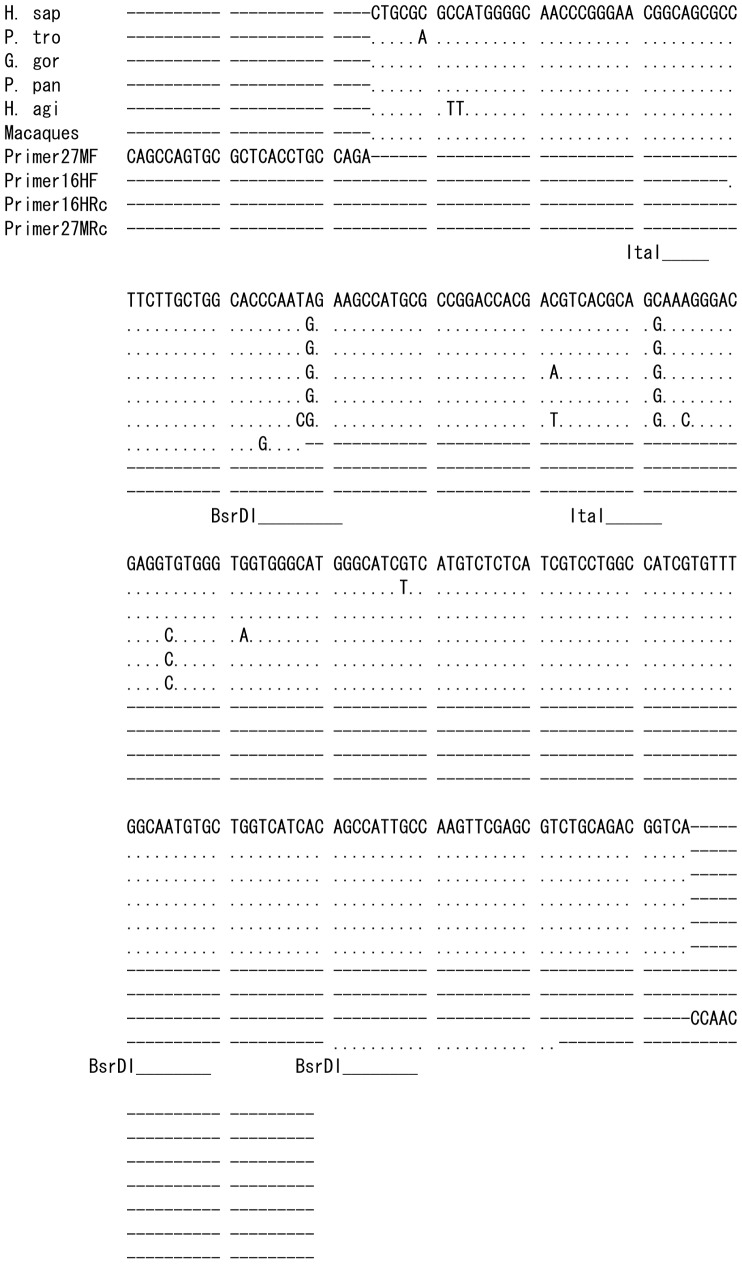
Figure 1. Nucleotide sequences in ADRB2 of humans and NHPs.
Primers 16HF and 16HRc (Rc means the complementary sequence of reverse primer) indicate a primer set used for the PCR to amplify the region for the 16th amino acid in hominoids. One nucleotide of primer 16HF was changed to create the restriction site of BsrDI. Primers 27MF and 27MRc indicate a primer set used for the PCR to amplify the region for the 27th amino acid in macaques. The underlines show the restriction site (GCAATGNN) with BsrDI for the 16th amino acid and the restriction site (GCNGC) with ItaI for the 27th amino acid. The nucleotide sequences for hominoids were determined from G. gorilla (DDBJ Accession No. AB669098), P. pygmaeus (AB669099) and H. agilis (AB669100), and obtained from the Ensembl database for ADRB2 of H. sapiens (ENSG00000169252) and P. troglodytes (ENSPTRG00000017391). All macaques, M. fascicularis, M. fuscata, M. nemestrina, M. radiata (AB669101∼AB669104, respectively) and M. mulatta (ENSMMUG 00000002214), show the same sequence.
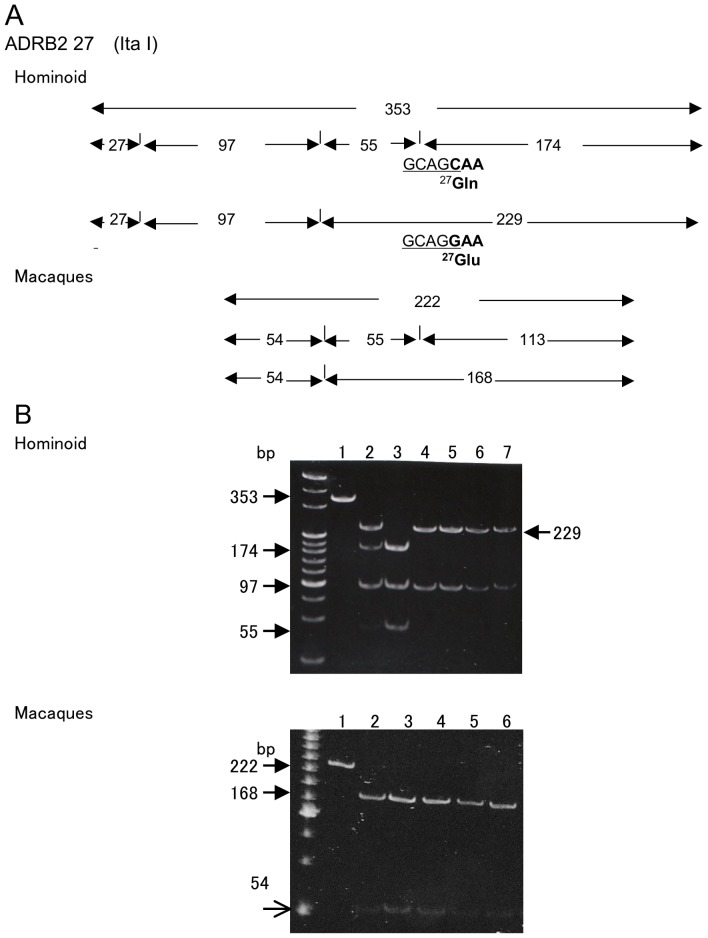
Figure 2. All hominoids had Gly16 allele in ADRB2.
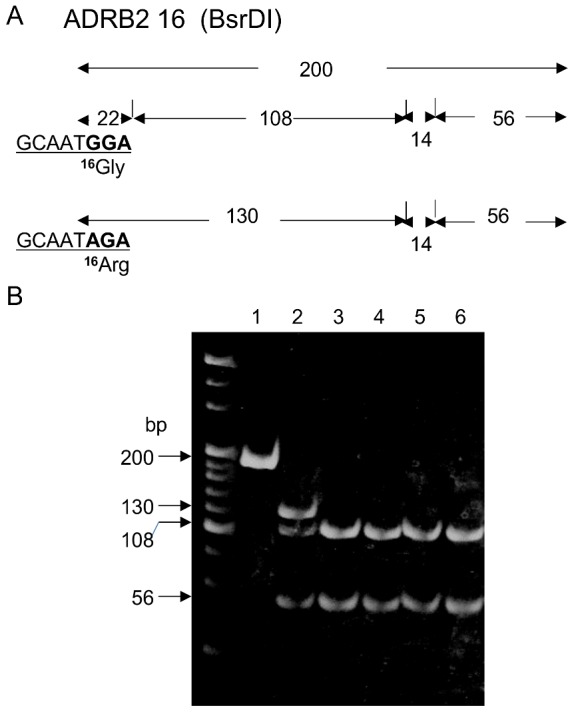
A) Restriction map of ADRB2 for the 16th amino acid digested with BsrDI (GCAATGNN). This restriction map was predicted from the nucleotide sequences of hominoids (Fig. 1). B) RFLP patterns of PCR products of ADRB2 for the 16th amino acid digested with BsrDI in hominoids. Lane 1: PCR product of a human; not digested (200 bp). Lane 2: Fragments of human Arg16/Gly16 (130,108 and 56 bp (22 and 14 bp fragments were undetectable)). Lane 3 to lane 6: Fragments from P. troglodytes, G. gorilla, P. pygmaeus, and H. agilis, respectively (108 and 22 bp instead of 130 bp).
Table 1. Frequencies of the thrifty type amino acids in ADRB2, ADRB3 and PPARG of non-human primates.
| Species | ADRB2 16 | ADRB2 27 | ADRB3 64 | PPARG 12 | ||||
| n | Frequency of Gly16 | n | Frequency of Glu27 | n | Frequency of Arg64 | n | Frequency of Pro12 | |
| P. troglodytes | 30 | 1.0 | 30 | 1.0 | 30 | 1.0 | 30 | 1.0 |
| G. gorilla | 8 | 1.0 | 8 | 1.0 | 8 | 1.0 | 8 | 1.0 |
| P. pygmaeus | 11 | 1.0 | 17 | 1.0 | 17 | 1.0 | 14 | 1.0 |
| H. agilis | 15 | 1.0 | 15 | 1.0 | 15 | 1.0 | ND | |
| Macaques | 5 | 1.0 | 108 | 1.0 | 108 | 1.0 | 93* | 1.0 |
| H. sap (African) | 226 | 0.50[94] | 120 | 0.18[95] | 49 | 0.12[3] | 53 | 0.97[32] |
| H. sap (Europian) | 140 | 0.64[20] | 140 | 0.40[20] | 48 | 0.08[3] | 26 | 0.88[32] |
| H. sap (Asian) | 508 | 0.49[24] | 508 | 0.08[24] | 642 | 0.31[3] ** | 50 | 0.99[32] |
Macaques are M. mulatta, M. fuscata, M. fascicularis, M. nemestrina, and M. radiata.
(M. mulatta, M. fuscata and M. fascicularis). ND:not detected.
Pima Indians.
The upper panel of Figure 3A shows the predicted restriction map of ADRB2 digested by ItaI (GCAGC) for hominoids from the DNA sequences. The upper panel of Figure 3B shows the obtained RFLP pattern of ItaI-digested PCR product of ADRB2 containing the Gln27Glu site of hominoids. All apes, P. troglodytes, G. gorilla, P. pygmaeus, and H. agilis were homozygous for Glu27 because a 229 bp fragment was obtained instead of 174 and 55 bp fragments (lanes 4–7). This region of the macaques could not be amplified with the primer set for hominoids but could be amplified with the new primer set to produce a 222 bp amplicon (Fig. 1 from primer 27MF to 27MRc). The predicted restriction map digested by ItaI for macaques is shown in the lower panel of Figure 3A. The ItaI-digested macaque amplicon yielded fragments of 168 bp for the homozygous Glu allele instead of 55 and 113 bp fragments (lower panel of Fig. 3B). All hominoids and macaques were homozygous for Glu27 in ADRB2 (Table 1).
Figure 3. All NHP had Glu27 allele in ADRB2.
A) Restriction map of ADRB2 for the 27th amino acid digested with ItaI. This restriction map was predicted from the nucleotide sequences of humans (Ensembl database ENSG00000169252) and P. troglodytes (Ensembl database ENSPTRG00000017391) and from obtained macaque nucleotide sequences (Fig. 1). B) RFLP patterns of PCR products of ADRB2 for the 27th amino acid digested with ItaI. Upper panel: for hominoids. Lane 1: PCR products of a human; not digested (353 bp). Lane 2: fragments of human Gln27/Glu27 (229, 174, 97, 55 and 27 bp which was undetectable at this concentration). Lane 3: fragments of human Gln27/Gln27 (174, 97, 55 and 27 bp). Lane 4 to lane 7: fragments from P. troglodytes, G. gorilla, P. pygmaeus, and H. agilis, respectively (229 bp instead of 174 and 55 bp). Lower panel: for macaques. Lane 1: PCR products of M. mulatta; not digested (222 bp). From lane 2 to lane 6: fragments from M. mulatta, M. fuscata, M. fascicularis, M. nemestrina, and M. radiata, respectively (168 bp instead of 113 and 55 bp).
The nucleotide sequences of 70 bp including codon 64 of ADRB3 in G. gorilla (AB669105), P. pygmaeus (AB669106), H. agilis (AB669107), M. fascicularis (AB669108), M. fuscata (AB669109), M. nemestrina (AB669110), and M. radiata (AB669111) were determined. All of them confirmed that the restriction site with MvaI was CCCGG not CCTGG together with P. troglodytes (ENSPTRG00000024086) and M. mulatta (ENSMMUG00000005876). The restriction map digested by MvaI (CC (T/A) GG) of this region was predicted from these sequences among humans and NHP, as shown in Figure 4A. Since all digested ADRB3 amplicons from NHP gave a 95 bp fragment but no 61 and 34 bp fragments, as shown in lanes 4 to 12 in Figure 4B, they had a homozygous form of Arg64, as shown in Table 1.
Figure 4. All NHP had Arg64 allele in ADRB3.
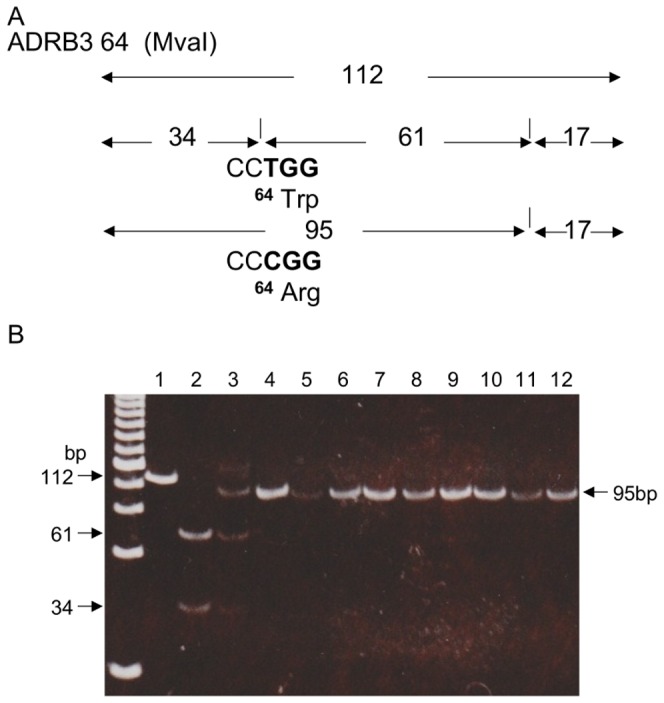
A) Restriction map of ADRB3 digested with MvaI for humans and NHP. B) RFLP patterns of PCR product of ADRB3 digested with MvaI for humans and NHP. Lane 1: PCR products of a human; not digested (112 bp). Lane 2: fragments of the human Trp64/Trp64 (61, 34 and 17 bp which was undetectable at this concentration.) Lane3: fragments of human Arg64/Trp64 (95, 61, 34 and 17 bp). Lane 4 to lane 12: fragments from P. troglodytes, G. gorilla, P. pygmaeus, H. agilis, M. mulatta, M. fuscata, M. fascicularis, M. nemestrina, and M. radiata, respectively (95 bp instead of 61 and 34 bp).
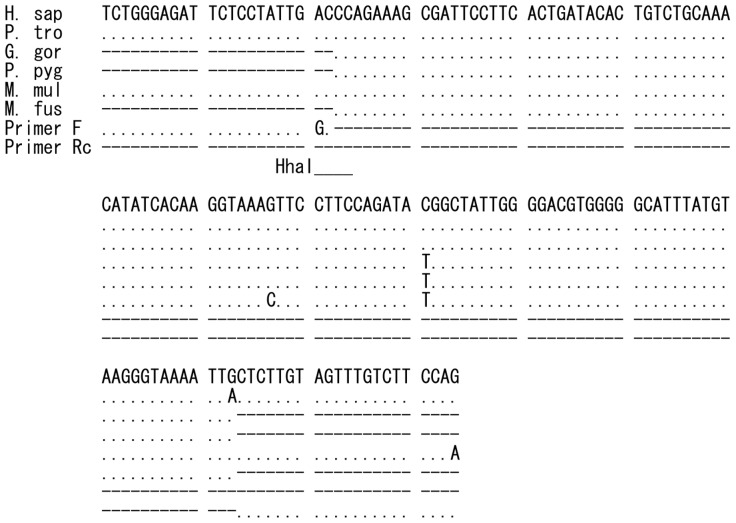
Figure 5 shows the nucleotide sequences of PPARG between primers. RFLP method by digestion with HhaI (GCGC) was carried out according to Hara et al. [34]. All examined NHPs had 154 bp instead of 23 and 131 bp fragments, indicating the thrifty type, Pro12 allele (Table 1).
Figure 5. Nucleotide sequences in PPARG of primates.
The determined sequences of G. gorilla (AB669114), P. pygmaeus (AB669115), and M. fuscata (AB669116) together with the Ensembl database of human (ENSG00000132170), P. troglodytes (ENSPTRG00000014632) and M. mulatta (ENSMMUG00000007191). Underline shows the restriction site (GCGC) with HhaI. The restriction site region of M. fascicularis (AY048695) from the GenBank database was the same as in other macaques. One nucleotide of primer F was changed to create the restriction site of HhaI [34].
Consequently, previously unknown features were identified that all NHPs had thrifty-type alleles, Gly16 and Glu27 in ADRB2, Arg64 in ADRB3, and Pro12 in PPARG.
Discussion
The adrenergic receptors, ADRB2 and ADRB3, have an important role in lipolysis and thermogenesis, and the polymorphism of these genes causes differences in energy expenditure. Alleles of both Glu27 in ADBR2 and Arg64 in ADRB3 are associated with obesity and/or non-insulin-dependent diabetes mellitus (NIDDM) in humans.
Neel [35] proposed the concept of a thrifty gene in humans, which nowadays causes diabetes mellitus given the high availability of food, but was beneficial in the periods of feast or famine experienced in hunter-gatherer cultures. Our present study shows that all examined NHP including apes had the thrifty type in the functional hot spots, Glu27 in ADRB2 and Arg64 in ADRB3. This means that these thrifty-type alleles did not develop at the time of hunter-gathering in humans, but rather that NHP had them already.
Although it is not clear whether the Gly16 in ADRB2 is related to obesity, polymorphism of this site was not found in the non-human hominoids; all of them had Gly16. Since the restriction site of BsrDI was not retained in the macaques, we did not analyze them. However, all macaque species for which the nucleotide sequence was determined had the Gly16 allele (see Fig. 1).
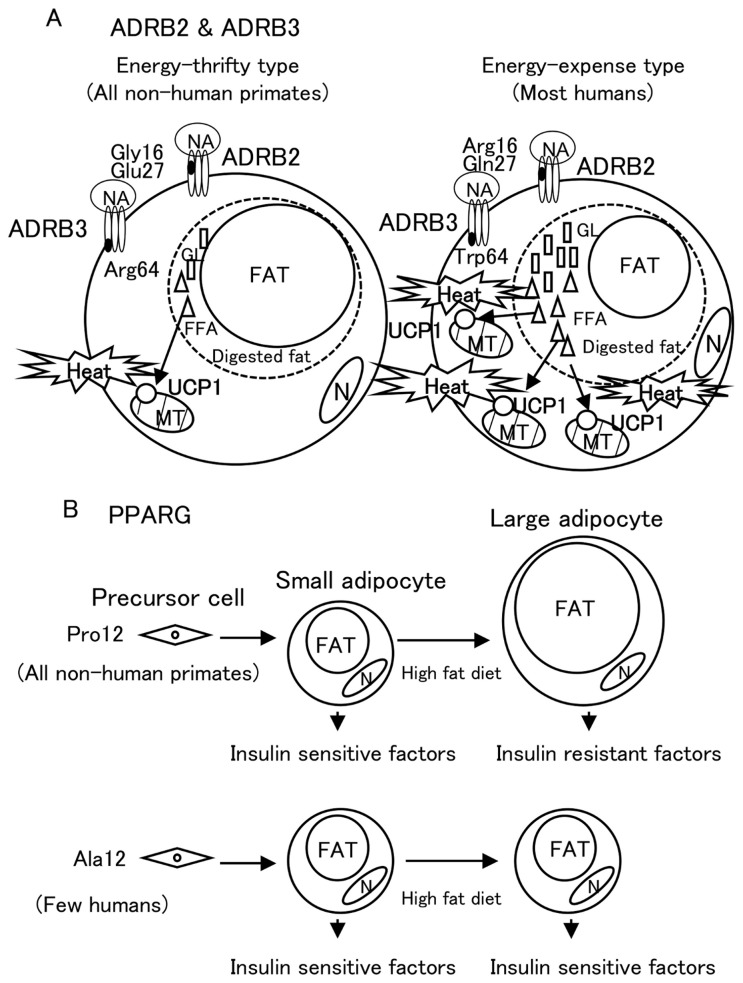
PPARG activates differentiation of precursor cells of adipocytes to the small-sized adipocytes, which secrete factors that prevent diabetes mellitus. If rodents that possess PPARG with Pro12 consume a high-fat diet, the small-sized adipocytes accumulate more fat to change to hypertrophic adipocytes, which secrete factors that promote diabetes mellitus, such as TNFα, resistin and free fatty acids. However, heterozygous PPARG-deficient mice showed protection from insulin resistance induced by a high-fat diet and an increased number of small-sized adipocytes [31]. The number of small-sized adipocytes is thought to increase with a high-fat diet in the case of PPARG with Ala 12 allele, the same as in heterozygous PPARG deficiency, because the transcriptional activity of PPARG with Ala12 was shown to be lower [33]. Actually, human subjects with Ala12 showed insulin sensitivity and the frequency of Ala12 allele was lower in diabetics than in healthy humans [34]. Thus, PPARG with Pro12 allele is thought to be a thrifty type. All NHP were found to have the thrifty type Pro12 allele in PPARG. The differences of these functions between alleles and results are summarized in Figure 6. These thrifty-type alleles, Glu27 in ADRB2, Arg64 in ADRB3 and Pro12 in PPARG, are preserved in some present-day humans and cause NIDDM in food-abundant conditions, as presumed by Neel. Antagonism of insulin, as mentioned by Neel, might suggest insulin-resistant substances secreted from hypertrophic adipocytes, such as TNFα, resistin and free fatty acids.
Figure 6. Summaries of effects of SNPs in ADRBs and PPARG.
A) ADRBs with energy expense-type allele found only in humans stimulate the digestion of accumulated fat in adipocytes at a higher level. A lot of generated free fatty acids (FFA) stimulate both transcription of the uncoupling protein (UCP1) gene and the activity of UCP1 proteins, which generate heat in mitochondria. GL: glycerol. B) PPARG activates differentiation from precursor cells into small-sized adipocytes, which secrete insulin-sensitive factors. PPARG with Ala12 found only in humans causes reduction of transcriptional activity of PPARG and leads to protection from high-fat-diet-induced hypertrophy of adipocytes, which secrete insulin-resistant factors [30], [34].
Why do non-human primates retain thrifty genes?
All the examined NHP showed the thrifty type at these four hot spots. However, it might be necessary to confirm the current findings using much larger sample sizes as, in this study, the sample sizes, especially those for the great apes, were limited, because these DNA samples were nonrelated.
The food abundance and/or food intake of NHP changes seasonally not only in temperate regions [36] but also in tropical regions [37]–[43]. Accordingly, body weight [44], fat deposition [45], and energy-consuming activities, such as traveling [46], [47], are reduced during periods when food is scarce. Therefore, if an individual with the energy-loss types, Gln27 in ADRB2 or Trp64 in ADRB3, happened to appear, they might be at a fitness disadvantage because they would lose energy by higher lypolysis and thermogenesis and could not accumulate enough fat in the adipose tissues to survive during food shortages. Pro12 allele in PPARG might help in the accumulation of more fat to produce large adipocytes during the higher-fat-diet season, which could be utilized in subsequent seasons with a food shortage.
Why did humans evolve energy-loss genes?
As the Gln27 allele of ADRB2 and the Trp64 allele of ADRB3 are found all over the world in human populations, these alleles might have appeared before the migration out of Africa and now be present in many populations. Cagliani et al. [27], [28] and Wilson et al. [28] speculated that the divergence occurred around 1.9 MYA and that, from Fisher's exact test of ADRB2, this gene may be associated with balancing selection or relaxed constraint. The lipolysis activity of ADRB3 with the Trp64 allele is higher than that with Arg64. The production of second messenger, cAMP [8], [48], and glycerol by lipolysis was 1.5-fold higher in those homozygous for Trp64 than in those homozygous for Arg64 [9]. Blood glycerol level and hydroxybutyrate level were higher, that is, higher lipolysis, in humans with Gln27 in ADRB2 [49]. Free fatty acids produced by lipolysis by stimulation of ADRBs accelerate transcription of the UCP1 gene and increase the thermogenesis activity of the UCP1 protein.
The human lineage is thought to have diverged from the chimpanzee lineage 6 MYA. It took a long time for the ancestors of humans to arrive at the savanna from the tropical forests and to live as hunter-gatherers. Fossils of Ardipithecus dated to 5.6 MYA [50] and 4.4 MYA [51], [52] indicated that they combined arboreal palmigrade clambering with terrestrial primitive bipedality. Although the footprints of bipedals dated 3.6 MYA were found at Laetoli, Tanzania [53], Australopithecus from 3.2 MYA might have exhibited a mixture of terrestrial and arboreal locomotion [54]. The oldest stone tools have been dated to between 2.6 and 2.5 MYA [55]. Homo habilis, which emerged around 2.3 MYA [56], had a bigger brain (600–800 ml) [57] and could make unifacial core stone tools [58], but they were thought to be able to eat meat only by scavenging or hunting small animals [59] because their bipedal locomotion was still incomplete [60]–[62].
Homo erectus, which emerged 1.8 MYA, was the first Homo that could walk completely bipedally [63]–[65]. They could hunt large animals in the savanna after chasing them over long distances since their brain size had increased dramatically compared with that of habilis, from 800 to 1200 ml [57]. They could also make hand axes (stone tools in Acheulean culture) [66] and might have had sufficiently high intelligence to enable advanced cooperation. They migrated out of Africa to Asia and Europe 1.8 -1.0 MYA [67], [68], but they were not the origin of modern humans.
Mitochondrial DNA analysis of the present-day world population has suggested that a small proportion of the descendants of Homo erectus migrated from Africa 0.15 MYA and spread throughout the world 60–70,000 years ago [69]. This theory about the single origin of modern humans is supported by nuclear DNA analysis [70], [71].
The human ancestors who left tropical rainforests for savanna and developed new features in their heat-regulation systems owing to stronger solar radiation, such as reduction of body hair and increased evaporation of water through an increased number of eccrine glands, might have been Homo erectus who were fully bipedal and had sufficiently big brains for making tools and cooperating at an advanced level. Apocrine glands are located deep in the dermis and their ducts lead to hair follicles associated with sebaceous glands. Eccrine glands, which lie in a shallow portion of the dermis between hair follicles and have ducts that open to the surface of the skin directly, are not associated with hair follicles, and can secrete water including sodium chloride. The proportion of eccrine glands to apocrine glands was found to be around 100% in humans but 70% in G. gorilla and P. troglodytes [72]. Baboons and P. troglodytes increase the activity of sweat glands and the respiratory rate upon an increase of environmental temperature to 40°C. Humans, however, do not increase respiratory frequency but instead increase the activity of sweat glands [73], [74]. The hair density of NHPs decreases systematically with increasing body surface area, that is, massive primates have fewer hairs to lose metabolically generated heat owing to a relatively low ratio of surface/mass; however, Homo erectus, unique among primates, had hairless skin except in small areas because the evaporation of water from the developed eccrine glands might have occurred efficiently owing to a loss of hair for cooling during overheating among those who lived under the high radiant heat loads of the savanna [75], [76].
It is known that a glacial period started around 2.4 MYA, which was identified by carbonate content and oxygen isotope analysis of cores from the North Atlantic [77]. The global temperature changed periodically. A deep (more than 150 m) and wide (several 100 m2) freshwater lake appeared twice in the South Kenyan and Tanzanian Rift Area (1.9-1.7 MYA and 1.0 -0.7 MYA) owing to the precessional forcing and progressive rifting of East Africa [78]. Extreme aridity occurred and C4 plants grew in the absence of these wet phases [79]–[81]. Almost the same changes of oxygen isotope as occurred during the last glacial period also occurred at the beginning of the glacial periods 2.4-2.3 MYA and 2.1-2.0 MYA; slightly smaller changes also continued periodically until the last glacial period [77]. The lowest global average temperature in the glacial period was speculated to be as much as 8°C lower than the present value, as determined from the relationship between temperature and oxygen isotope, 1.4°K/‰ [82], in the ice cores from several sites (Greenland [83], Antarctica [84], and Mt. Kilimanjaro [85]) and North Atlantic deep sea drilled cores [77]. As the highest and lowest temperatures at Arusha, 150 km from Olduvai Gorge in Tanzania, are 23°C and 13°C in July, the winter and dry season in the present day, Homo erectus might have experienced temperatures of around 5°C at night.
Consequently, Homo erectus might have felt cold with large temperature differences at night, especially in the glacial period. It might have been hard to maintain their body temperature at night, especially for children. Present-day children, however, have four times more brown adipose tissue than adults until the age of ten years old, even though adults keep half of their brown adipose tissue in the abdominal area [86]. The brown adipose tissue expresses UCP1 and might be able to produce heat. Fortunately, there was a mutation of ADRB2 from a thrifty type to an energy expense type 1.9 MYA [27], [28]. Noradrenaline from the sympathetic nervous system is secreted by cold stimulation and produces much more heat through the Gln27 allele of ADRB2 and the Trp64 allele of ADRB3, which helps maintenance of body temperature of Homo erectus more than the Glu27 allele or the Arg64 allele.
However, the increase of thermogenesis might have caused increased total energy expense, which might have required increased energy production. Homo erectus could hunt large animals with high nutritional value using hand axes and narrow slivers of rock, as well as by utilizing full bipedal locomotion. Wrangham [87] proposed that Homo erectus might have cooked using fire on the basis of several findings: large anatomical differences from Homo habilis, especially smaller tooth size, less flared rib cage, and narrower pelvis indicating a smaller gut [64], [88], which could digest cooked food easier than raw food and absorb more energy. If this is true, we can state that Homo erectus could survive by hunting animals using tools and by using fire to obtain more energy in spite of the single nucleotide changes in ADRB2 and ADRB3 genes that conveyed a greater energy loss but high thermogenesis to protect entrails from cold in the glacial period. In addition, fire might have warmed their bodies during the night.
During the period when Homo erectus remained in Africa from 1.8 to 0.15 MYA, these energy expense-type alleles spread among them and, when they migrated out of Africa, every subsequently formed population might have had both thrifty-type and expense-type alleles.
In conclusion, the energy expense-type alleles, Gln27 of ADRB2, Trp64 of ADRB3, and Ala12 of PPARG, which induce small-sized adipocytes, are specific SNP that have only accumulated in humans, and convey abilities of both high energy loss and high thermogenesis, and less accumulation of fat in adipocytes. The advantage of energy expense-type alleles of ADRB2 and ADRB3 for increasing body temperature of Homo erectus and the ancestors of modern humans in the glacial period may explain the higher frequencies of energy expense-type alleles than thrifty-type alleles.
Materials and Methods
This study was carried out within the ethical guidelines and framework of Kyoto University and was approved by the Primate Research Institute, Kyoto University, and Kumamoto Sanctuary. Macaques in the Primate Research Institute, Kyoto University, were bred following the third edition of The Guide for the Care and Use of Laboratory Primates (Primate Research Institute, Kyoto University) in accordance with the National Research Council Guidelines “Guide for the care and use of laboratory animals 1996”. Individual cages for macaques with a body weight (BW) of 3–10 Kg measured 0.39 m2 floor area ×76.2 cm height, and those for macaque with BW of 10–15 Kg were 0.54 m2×81.3 cm. The temperature was set at 27°C in summer and 23°C in winter for the long-tailed macaques, and for other macaques at 20°C in winter. Humidity was set at 40–70%.
Macaques in an open-air corral were bred at less than 30 heads per 500 m2. They were provided with a jungle gym made of wooden logs with two small rooms to avoid rain or low temperature, a stream and a pond with small fish. Feeding was as follows: Japanese macaques received 48–55 kcal/kg/day monkey chow and rhesus macaques received 40–50 kcal/kg/day, with feeding twice a day and some sweet potatoes every two days. Wheat or dried soybeans were fed to macaques in the open-air corral twice a week. Some blood samples of the macaques bred at the PRI, Kyoto University, were taken before 1996 when all procedures were conducted according to the second edition of the Guide for Care and Use of Laboratory Primates following the guidelines issued by the NIH in 1985. The cages were wider and higher than those prescribed by the third edition from 1996.
All procedures for chimpanzees were conducted according to the third edition of the Guide for the Care and Use of Laboratory Primates (Primate Research Institute, Kyoto University) and the Guidelines for Care of Chimpanzees (Kumamoto Sanctuary). All chimpanzees lived in social groups. They could spend time in an outdoor playground where a jungle gym with fire hoses and ropes to climb had been set up. Trees and grass had also been planted. The ground area was 15–60 m2 per head. Their basic food per day was monkey chow (PS by Oriental Yeast Co., Ltd., and Monkey Bit by Nosan Corporation) at 300–500 g depending on the BW, one banana, two oranges (around 500 g), sweet potatoes at 500 g, carrots at 150 g, cabbage at 500 g, and seasonal vegetables or fruit at 500 g. Wild grass and twigs with leaves were given every day. Soybeans, sunflower seeds, peanuts with shells, honey, fruit juice and sugar cane were set in puzzle feeders to be eaten freely. Chimpanzees were fed more than five times per day.
Blood samples of non-human primates were obtained from 30 chimpanzees (one Pan troglodytes schweinfurthii, 29 Pan troglodytes verus) from The Kumamoto Sanctuary, Kyoto University (previously named The Chimpanzee Sanctuary Uto) (permission numbers P1988-08 [89]). Blood samples of sixteen orangutans (Pongo pygmaeus) were collected for a biochemical health check, testing for hepatitis virus and evolutionary study with permission from the Sepilok Rehabilitation Center in West Malaysia in 1988 [90]. Three agile gibbons (Hylobates agilis) were from Ragunan Zoo and 12 pet monkeys were from a field survey at Pangkalan Bun in Kalimantan, Indonesia, with the permission of Bogor Agricultural University and supported by Competitive Research Grant from the Ministry of Education and Culture of Indonesia awarded to Dr. Bambang Suryobroto (No. 03/P2IPT/DPPM/96/PHB I/5/1996) and by Grant-in-Aid for Scientific Research (Overseas Scientific Survey) No. 08041147 from the Ministry of Education, Culture, Sports, Science and Technology of Japan in 1996 [91]. 108 macaques including rhesus macaques (Macaca mulatta) that originated in China and India, Japanese macaques (Macaca fuscata fuscata) that originated in Wakasa, Arashiyama, Awajishima, and Koshima, Japan, long-tailed macaques (Macaca fascicularis) that originated in Indonesia, bonnet macaques (Macaca radiata) from India and pig-tailed macaques (Macaca nemestrina) were from the Primate Research Institute, Kyoto University.
Muscle samples of 8 Western lowland gorillas (Gorilla gorilla gorilla) were imported (Import permission No. JP9129795, June 25, 1992) from the Gorilla Orphanage in Brazzaville, the Republic of Congo, for clinical pathological study and evolutionary study of great apes in Africa (Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (No. 04044153)) in 1992 by the late Professor S. Hayama and his team. Many baby gorillas had died and been kept in a stock freezer in the Gorilla Orphanage in Brazzaville. Prof. Hayama and his team went to the Republic of Congo in 1991 and 1992 at the request of the Government of the Republic of Congo and the Gorilla Rescue Center (Dr. Mark Attwater) to investigate the causes of death. They carried out anatomical study in Brazzaville and brought back several organs including muscles for more precise clinical pathological studies and evolutionary studies in Japan. It was found that the gorillas had suffered from helminthiasis, dehydration, anorexia nervosa, influenza viral pneumonia and acute anterior poliomyelitis [92], [93].
The blood samples were not originally collected for the present study, but as part of routine health examinations and field survey by the late Professor Osamu Takenaka. During these examinations, chimpanzees were sedated with oral midazolam (1 mg/kg) or droperidol (0.2 mg/kg), and their blood was collected while they were anesthetized with ketamine hydrochloride (7 mg/kg) or a combination of ketamine hydrochloride (3.5 mg/kg) and medetomidine hydrochloride (0.035 mg/kg). The blood samples of other primates were collected under general anesthesia with ketamine hydrochloride (5–10 mg/Kg BW)+atropine (0.02–0.05 mg/Kg BW) by intramuscular injection.
PCR was performed using 10 ng of DNA and Ampli TaqR Gold DNA polymerase (Applied Biosystems Co. Ltd.) in a total volume of 25 µl under the following conditions: ADRB2 fragment with the Arg16Gly variant site was amplified in accordance with Large et al. [20] with modification of the forward primer to create the restriction site for BsrDI (GCAATGNN). ADRB2 amplicon containing Gln27Glu substitution site was amplified by the method of Large et al. [20] using a primer set of 5′-GAATGAGGCTTCAGGCGTC-3′ (forward) and 5′-GGCCCATGACC AGATCAGCA-3′ (reverse) in the presence of dimethylsulfoxide for hominoids, and other primers of 5′-CAGACAGTGCGCTCACCTGCCAGA-3′ (forward) and 5′-ACGCTCGAACTTGGCAATGGCT-3′ (reverse) for macaques because of a lack of amplification with the primers for hominoids. ADRB3 PCR product containing the Trp64Arg polymorphism site was amplified at 95°C for 9 min, then 32 cycles of 95°C for 1 min, 55°C for 1 min, and 72°C for 2 min, followed by 72°C at 5 min using primers of 5′-TGGGAGGCAACCTGCTGGTCAT-3′ (forward) and 5′-AGGAGTCCCATCACCAGGTC-3′ (reverse). PCR product of PPARG containing Pro12Ala polymorphism site was amplified by the method of Hara et al. [34] using the same forward primer, which creates the restriction site of HhaI. However, the reverse primer region includes one more T in the sequence of H. sapiens, P. troglodytes, and M. mulatta from the Ensembl database, so the reverse primer including one more complementary nucleotide A was used (Fig. 5).
ADRB2 fragment with the Arg16Gly variant site with 5 U of BsrDI (New England Bio Labs Co. Ltd.) at 65°C for 1.5 hrs. ADRB2 amplicon for the Gln27Glu substitution site (5 µl) was digested with 5 U of ItaI (Roche Applied Science Co. Ltd.) at 37°C for 1 hr. ADRB3 PCR product containing the Trp64Arg polymorphism site (5 µl) was digested with 5 U of MvaI (CC(A/T)GG) (TAKARA Co. Ltd.) at 37°C for 1 hr and PPARG amplicon containing Pro12 Ala variant site was digested with 5 U of HhaI (GCGC) (TAKARA Co. Ltd.) at 37°C for 3 hrs. The digested fragments were separated by 12% polyacrylamide gel electrophoresis and visualized using SYBR Green I (Cambrex Co. Ltd.) under a UV illuminator.
DNA sequence analysis was performed to confirm the restriction site of PCR products of ADRB2, ADRB3, and PPARG using an ABI PRISM™ 310-20 Genetic Analyzer.
Acknowledgments
We thank the late Professor Osamu Takenaka and Mr. Nobukatsu Miwa of Primate Research Institute, Kyoto University and the late Professor Sugio Hayama of Kansai Medical University for great efforts to collect primate samples. We would also like to thank Associate Professor Naofumi Nakagawa of Kyoto University for giving us very helpful suggestions and Ms. M. Uehara, M. Inagaki, S. Watanabe, A. Otsuka, M. Yamamoto, S. Watanabe, S Higuchi, K. Itoh, R. Mukai, Mr. Y. Kabuto, and I. Yamakami for assisting with the experiments.
Funding Statement
This study was financially supported by the Cooperation Research Program of Primate Research Institute, Kyoto University. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Girardier L, Seydoux J (1981) Is there a sympathetic regulation of the efficiency of energy utilization? Diabetologia 20: 362–365. [PubMed] [Google Scholar]
- 2. Tappy L (1996) Thermic effect of food and sympathetic nervous system activity in humans. Reprod Nutr Dev 36: 391–397. [DOI] [PubMed] [Google Scholar]
- 3. Walston J, Silver K, Bogardus C, Knowler WC, Celi FS, et al. (1995) Time of onset of non-insulin- dependent diabetes mellitus and genetic variation in the β3-adrenergic receptor gene. N Engl J Med 333: 343–347. [DOI] [PubMed] [Google Scholar]
- 4. Widén E, Lehto M, Kanninen T, Walston J, Shuldiner AR, et al. (1995) Association of a polymorphism in the β3-adrenergic receptor gene with features of the insulin resistance syndrome in Finns. N Engl J Med 333: 348–351. [DOI] [PubMed] [Google Scholar]
- 5. Kim-Motoyama H, Yasuda K, Yamaguchi T, Yamada N, Katakura T, et al. (1997) A mutation of the beta-3-adrenergic receptor is associated with visceral obesity but decreased serum triglyceride. Diabetologia 40: 469–472. [DOI] [PubMed] [Google Scholar]
- 6. Clément K, Vaisse C, Manning BSJ, Basdevant A, Guy-Grand B, et al. (1995) Genetic variation in the β3-adrenergic receptor and an increased capacity to gain weight in patients with morbid obesity. N Engl J Med 333: 352–354. [DOI] [PubMed] [Google Scholar]
- 7. Yoshida T, Sakane N, Umekawa T, Sakai M, Takahashi T, et al. (1995) Mutation of β3-adrenergic-receptor gene and response to treatment of obesity. Lancet 346: 1433–1434. [DOI] [PubMed] [Google Scholar]
- 8. Piétri-Rouxel F, St John Manning B, Gros J, Strosberg AD (1997) The biochemical effect of the naturally occurring Trp64→Arg mutation on human β3-adrenoceptor activity. Eur J Biochem 247: 1174–1179. [DOI] [PubMed] [Google Scholar]
- 9. Umekawa T, Yoshida T, Sakane N, Kogure A, Kondo M, et al. (1999) Trp64Arg mutation of β3-adrenoceptor gene deteriorates lipolysis induced by β3-adrenoceptor agonist in human omental adipocytes. Diabetes 48: 117–120. [DOI] [PubMed] [Google Scholar]
- 10. Kadowaki H, Yasuda K, Iwamoto K, Otabe S, Shimokawa K, et al. (1995) A mutation in the β3-adrenergic receptor gene is associated with obesity and hyperinsulinemia in Japanese subjects. Biochem Biophys Res Commun 215: 555–560. [DOI] [PubMed] [Google Scholar]
- 11. Biery AJ, Ebbesson SOE, Shuldiner AR, Boyer BB (1997) The β3-adrenergic- receptor TRP64ARG polymorphism and obesity in Alaskan Eskimos. Int J Obesity 21: 1176–1179. [DOI] [PubMed] [Google Scholar]
- 12. Sakane N, Yoshida T, Umekawa T, Kondo M, Sakai Y, et al. (1997) β3-adrenergic-receptor polymorphism: a genetic maker for visceral fat obesity and the insulin resistance syndrome. Diabetologia 40: 200–204. [DOI] [PubMed] [Google Scholar]
- 13. Sun L, Yoko I, Shun I (2000) Investigation and comparison of the beta3-adrenergic receptor gene Trp64Arg mutation in the Chinese and Japanese. Zhonghua Yi Xue Za Zhi 80: 107–110. [PubMed] [Google Scholar]
- 14. Silver K, Walston J, Wang Y, Dowse G, Zimmet P, et al. (1996) Molecular scanning for mutations in the β3-adrenergic receptor gene in Nauruans with obesity and noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 81: 4155–4158. [DOI] [PubMed] [Google Scholar]
- 15. Silver K, Mitchell BD, Walston J, Sorkin JD, Stern MP, et al. (1997) Trp64Arg β3-adrenergic receptor and obesity in Mexican Americans. Hum Genet 101: 306–311. [DOI] [PubMed] [Google Scholar]
- 16. Büettner R, Schäffler A, Arndt H, Rogler G, Nusser J, et al. (1998) The Trp64Arg polymorphism of the β3-adrenergic receptor gene is not associated with obesity or type 2 diabetes mellitus in a large population-based Caucasian cohort. J Clin Endocrinol Metab 83: 2892–2897. [DOI] [PubMed] [Google Scholar]
- 17. Corella D, Guillén M, Portolés O, Sorlí JV, Alonso V, et al. (2001) Gender specific associations of the Trp64Arg mutation in theβ3-adregergic receptor gene with obesity-related phenotypes in a Mediterranean population: interaction with a common lipoprotein lipase gene variation. J Int Med 250: 348–360. [DOI] [PubMed] [Google Scholar]
- 18. Barbe P, Millet L, Galitzky J, Lafontan M, Berlan M (1996) In situ assessment of the role of the β1-, β2- and β3-adrenoceptors in the control of lipolysis and nutritive blood flow in human subcutaneous adipose tissue. Br J Pharmacol 117: 907–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Green SA, Turki J, Hall IP, Liggett SB (1995) Implication of genetic valiability of human β2-adrenergic receptor structure. Pulm Phamacol 8: 1–10. [DOI] [PubMed] [Google Scholar]
- 20. Large V, Hellström L, Reynisdottir S, Lönnqvist F, Eriksson P, et al. (1997) Human beta-2 adrenoceptor gene polymorphisms are highly frequent in obesity and associate with altered adipocyte beta-2 adrenoceptor function. J Clin Invest 100: 3005–3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. González Sánchez JL, Proenza AM, Martínez Larrad MT, Ramis JM, Fernández Pérez C, et al. (2003) The glutamine 27 glutamic acid polymorphism of the β2-adrenoceptor gene is associated with abdominal obesity and greater risk of impaired glucose tolerance in men but not in women: a population-based study in Spain. Clin Endocrinol 59: 476–481. [DOI] [PubMed] [Google Scholar]
- 22. Masuo K, Katsuya T, Kawaguchi H, Fu Y, Rakugi H, et al. (2006) β2-adrenoceptor polymorphisms relate to obesity through blunted leptin-mediated sympathetic activation. Am J Hypertens 19: 1084–1091. [DOI] [PubMed] [Google Scholar]
- 23. Hellström L, Large V, Reynisdottir S, Wahrenberg H, Arner P (1999) The different effects of a Gln27Glu β2-adrenoceptor gene polymorphism on obesity in males and in females. J Int Med 245: 253–259. [DOI] [PubMed] [Google Scholar]
- 24. Ishiyama-Shigemoto S, Yamada K, Yuan X, Ichikawa F, Nonaka K (1999) Association of polymorphisms in the β2-adrenergic receptor gene with obesity, hypertriglyceridaemia, and diabetes mellitus. Diabetologia 42: 98–101. [DOI] [PubMed] [Google Scholar]
- 25. Macho-Azcarate T, Marti A, Calabuig J, Martinez JA (2003) Basal far oxidation and after a peak oxygen consumption test in obese women with a β2 adrenoceptor gene polymorphism. J Nutr Biochem 14: 275–279. [DOI] [PubMed] [Google Scholar]
- 26. Ellsworth DL, Coady SA, Chen W, Srinivasan SR, Elkasabany A, et al. (2002) Influence of the β2-adrenergic receptor Arg16Gly polymorphism on longitudinal changes in obesity from childhood through young adulthood in a biracial cohort: the Bogalusa Heart Study. Int J Obesity 26: 928–937. [DOI] [PubMed] [Google Scholar]
- 27. Cagliani R, Fumagalli M, Pozzoli U, Riva S, Comi GP, et al. (2009) Diverse evolutionary histories for β-adrenoreceptor genes in humans. Am J Hum Genet 85: 64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wilson RH, Moran CN, Cole JJ, Pitsiladis YP, Bailey MES (2010) Evolutionary history of the ADRB2 gene in humans. Am J Hum Genet 86: 490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Walston J, Lowe A, Silver K, Yang Y, Bodkin NL, et al. (1997) The β3-adrenergic receptor in the obesity and diabetes prone rhesus monkey is very similar to human and contains arginine at codon 64. Gene 188: 207–213. [DOI] [PubMed] [Google Scholar]
- 30. Kadowaki T (2000) Insights into insulin resistance and type2 diabetes from knockout mouse models. J Clin Invest 106: 459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kubota N, Terauchi Y, Miki H, Tamemoto H, Yamauchi T, et al. (1999) PPARγ mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol Cell 4: 597–609. [DOI] [PubMed] [Google Scholar]
- 32. Yen CJ, Beamer BA, Negri C, Silver K, Brown KA, et al. (1997) Molecular scanning of the human peroxisome proliferator activated receptor γ (hPPARγ) gene in diabetic Caucasians: Identification of a Pro12Ala PPAR γ2 missense mutation. Biochem Biophys Res Commun 241: 270–274. [DOI] [PubMed] [Google Scholar]
- 33. Deeb SS, Fajas L, Nemoto M, Pihlajamäki J, Mykkänen L, et al. (1998) A Pro12Ala substitution in PPARγ2 associated with decreased receptor activity, lower body mass index and improved insulin sensitivity. Nat Genet 20: 284–287. [DOI] [PubMed] [Google Scholar]
- 34. Hara K, Okada T, Tobe K, Yasuda K, Mori Y, et al. (2000) The Pro12Ala polymorphism in PPARγ2 may confer resistance to type 2 diabetes. Biochem Biophys Res Commun 271: 212–216. [DOI] [PubMed] [Google Scholar]
- 35. Neel JV (1962) Diabetes mellitus: A “thrifty” genotype rendered detrimental by “progress”? Am J Hum Genet 14: 353–362. [PMC free article] [PubMed] [Google Scholar]
- 36. Nakagawa N (1997) Determinants of the dramatic seasonal changes in the intake of energy and protein by Japanese monkeys in a cool temperature forest. Am J Primatol 41: 267–288. [DOI] [PubMed] [Google Scholar]
- 37. Symington MM (1990) Fission-fusion social organization in Ateles and Pan . Int J Primatol 11: 47–61. [Google Scholar]
- 38. Tutin CEG, Ham RM, White LJT, Harrison MJS (1997) The primate community of the Lopé Reserve, Gabon: diets, responses to fruit scarcity, and effects on biomass. Am J Primatol 42: 1–24. [DOI] [PubMed] [Google Scholar]
- 39. Nakagawa N (2000) Foraging energetics in patas monkeys (Erythrocebus patas) and tantalus monkeys (Cercopithecus aethiops tantalus): Implications for reproductive seasonality. Am J Primatol 52: 169–185. [DOI] [PubMed] [Google Scholar]
- 40. Hashimoto C, Suzuki S, Takenoshita Y, Yamagiwa J, Basabbose AK, et al. (2003) How fruit abundance affects the chimpanzee party size: a comparison between four study sites. Primates 44: 77–81. [DOI] [PubMed] [Google Scholar]
- 41. Takemoto H (2004) Seasonal change in terrestriality of chimpanzees in relation to microclimate in the tropical forest. Am J Phys Anthropol 124: 81–92. [DOI] [PubMed] [Google Scholar]
- 42. Yamagiwa J, Basabose AK (2006) Diet and seasonal changes in sympatric gorillas and chimpanzees at Kahuzi-Biega National Park. Primates 47: 74–90. [DOI] [PubMed] [Google Scholar]
- 43. Thompson ME, Knott CD (2008) Urinary C-peptide of insulin as a non-invasive marker of energy balance in wild orangutans. Horm Behav 53: 526–535. [DOI] [PubMed] [Google Scholar]
- 44. Uehara S, Nishida T (1987) Body weights of wild chimpanzees (Pan troglodytes schweinfurthii) of the Mahale Mountains National Park, Tanzania. Am J Phys Anthropol 72: 315–321. [DOI] [PubMed] [Google Scholar]
- 45. Muroyama Y, Kanamori H, Kitahara E (2006) Seasonal variation and sex differences in the nutritional status in two local populations of wild Japanese macaques. Primates 47: 355–364. [DOI] [PubMed] [Google Scholar]
- 46. Harrison MJS (1985) Time budget of the green monkey, Cercopithecus sabaeus: some optimal strategies. Int J Primatol 6: 351–376. [Google Scholar]
- 47. Agetsuma N, Nakagawa N (1998) Effects of habitat differences on feeding behaviors of Japanese monkeys: comparison between Yakushima and Kinkazan. Primates 39: 275–289. [Google Scholar]
- 48. Kimura K, Sasaki N, Asano A, Mizukami J, Kayahashi S, et al. (2000) Mutated human beta-3-adrenergic receptor (Trp64Arg) lowers the response to beta3-adrenergic agonists in transfected 3T3-L1 predipocytes. Horm Metab Res 32: 91–96. [DOI] [PubMed] [Google Scholar]
- 49. Macho-Azcarate T, Marti A, González A, Martinez JA, Ibañez J (2002) Gln27Glu polymorphism in the beta2 adrenergic receptor gene and lipid metabolism during exercise in obese women. Int J Obesity 26: 1434–1441. [DOI] [PubMed] [Google Scholar]
- 50. Haile-Selassie Y, Suwa G, White TD (2004) Late Miocene teeth from middle Awash, Ethiopia, and early hominid dental evolution. Science 303: 1503–1505. [DOI] [PubMed] [Google Scholar]
- 51. White TD, Suwa G, Asfaw B (1994) Australopithecus ramidus, a new species of early hominid from Aramis, Ethiopia. Nature 371: 306–312. [DOI] [PubMed] [Google Scholar]
- 52. White TD, Asfaw B, Beyene Y, Haile-Selassie Y, Lovejoy CO, et al. (2009) Ardipithecus ramidus and the paleobiology of early hominids. Science 326: 75–86. [PubMed] [Google Scholar]
- 53. Leakey MD, Hay RL (1979) Pliocene footprints in the Laetolil Beds at Laetoli, northern Tanzania. Nature 278: 317 323. [Google Scholar]
- 54. McHenry HM, Berger LR (1998) Body proportions in Australopithecus afarensis and A. africanus and the origin of the genus Homo . J Human Evol 35: 1–22. [DOI] [PubMed] [Google Scholar]
- 55. Semaw S, Renne P, Harris JWK, Feibel CS, Bernor RL, et al. (1997) 2.5-million-year-old stone tools from Gona, Ethiopia. Nature 385: 333–336. [DOI] [PubMed] [Google Scholar]
- 56. Kimbel WH, Johanson DC, Rak Y (1997) Systematic assessment of a maxilla of Homo from Hadar, Ethiopia. Am J Phys Anthropol 103: 235–262. [DOI] [PubMed] [Google Scholar]
- 57. McHenry HM (1994) Tempo and mode in human evolution. Proc Natl Acad Sci USA 91: 6780–6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leakey MD (1976) A summary and discussion of the archaeological evidence from bed I and bed II, Olduvai Gorge, Tanzania. In: G. L. Isaac & E. McCown (eds.), Human origins: Louis Leakey and the East African evidence (pp. 431–59). Menlo Park: Staples Press.
- 59. Bunn HT (1981) Archaeological evidence for meat-eating by Plio-Pleistocene hominids from Koobi Fora and Olduvai Gorge. Nature 291: 574–577. [Google Scholar]
- 60. Wood B, Collard M (1999) The human genus. Science 284: 65–71. [DOI] [PubMed] [Google Scholar]
- 61. Susman RL, Stern JT (1982) Functional morphology of Homo habilis . Science 217: 931–934. [DOI] [PubMed] [Google Scholar]
- 62. Harcourt-Smith WEH, Aiello LC (2004) Fossila, feet and the evolution of human bipedal locomotion. J Anat 204: 403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ruff CB, Walker AC (1993) Body size and body shape. In: Walker AC, Leakey RE, editors. The nariokotome Homo erectus skeleton. Cambridge, MA: Harvard Press pp.234–265
- 64. Antón SC (2003) Natural history of Homo erectus . Yrbk Phys Anthropol 46: 126–169. [DOI] [PubMed] [Google Scholar]
- 65. Aiello L, Wells JCK (2002) Energetics and the evolution of the genus Homo . Ann Rev Anthropol 31: 323–338. [Google Scholar]
- 66. Lepre CJ, Roche H, Kent DV, Harmand S, Quinn RL, et al. (2011) An earlier origin for the Acheulian. Nature 477: 82–85. [DOI] [PubMed] [Google Scholar]
- 67. Swisher CC III, Curtis GH, Jacob T, Getty AG, Suprijo A, et al. (1994) Age of the earliest known hominids in Java, Indonesia. Science 263: 1118–1121. [DOI] [PubMed] [Google Scholar]
- 68. Gabunia L, Antón SC, Lordkipanidze D, Vekua A, Justus A, et al. (2001) Dmanisi and dispersal. Evol Anthropol 10: 158–170. [Google Scholar]
- 69. Cann RL, Stoneking M, Wilson AC (1987) Mitochondrial DNA and human evolution. Nature 325: 31–36. [DOI] [PubMed] [Google Scholar]
- 70. Tishkoff SA, Dietzsch E, Speed W, Pakstis AJ, Kidd JR, et al. (1996) Global patterns of linkage disequilibrium at the CD4 locus and modern human origins. Science 271: 1380–1387. [DOI] [PubMed] [Google Scholar]
- 71. Li JZ, Absher DM, Tang H, Southwick AM, Casto AM, et al. (2008) Worldwide human relationships inferred from genome-wide patterns of variation. Science 319: 1100–1104. [DOI] [PubMed] [Google Scholar]
- 72. Folk GE Jr, Semken HA Jr (1991) The evolution of sweat glands. Int J Biometeorol 35: 180–186. [DOI] [PubMed] [Google Scholar]
- 73. Hiley PG (1976) The thermoregulatory responses of the galago (Galago crassicaudatus), the baboon (Papio cynocephalus) and the chimpanzee (Pan satyrus) to heat stress. J Physiol 254: 657–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Robertshaw D (1975) Catecholamines and the control of sweat glands. In Handbook of Physiology, pp.591–603. Bethesda, Md.: American Physiological Society.
- 75. Schwartz GG, Rosenblum LA (1981) Allometry of primate hair density and the evolution of human hairlessness. Am J Phys Anthropol 55: 9–12. [DOI] [PubMed] [Google Scholar]
- 76. Wheeler PE (1992) The influence of the loss of functional body hair on the water budgets of early hominids. J Hum Evol 23: 379–388. [Google Scholar]
- 77. Shackleton NJ, Backman J, Zimmerman H, Kent DV, Hall MA, et al. (1984) Oxygen isotope calibration of the onset of ice-rafting and history of glaciation in the North Atlantic region. Nature 307: 620–623. [Google Scholar]
- 78. Trauth MH, Maslin MA, Deino AL, Strecker MR, Bergner AGN, et al. (2007) High– and low-latitude forcing of Plio-Pleistocene east African climate and human evolution. J Hum Evol 53: 475–486. [DOI] [PubMed] [Google Scholar]
- 79. Levin NE, Quade J, Simpson SW, Semaw S, Rogers M (2004) Isotopic evidence for Plio-Pleistocene environmental change at Gona, Ethiopia. Earth Planet Sci Lett 219: 93–110. [Google Scholar]
- 80. Wynn JG (2004) Influence of Plio-Pleistocene aridification on human evolution: Evidence from paleosols of theTurkana Basin, Kenya. Am J Phys Anthropol 123: 106–118. [DOI] [PubMed] [Google Scholar]
- 81. deMenocal PB (2004) African climate change and faunal evolution during the Pliocene-Pleistocene. Earth Planet Sci Lett 220: 3–24. [Google Scholar]
- 82.Dansgaard W (2004) Frozen annals: Greenland ice cap research. Narayana Press.
- 83. Grootes PM, Stuiver M, White JWC, Johnsen S, Jouzel J (1993) Comparison of oxygen isotope records from the GISP2 and GRIP Greenland ice cores. Nature 366: 552–554. [Google Scholar]
- 84. Jouzel J, Lorius C, Petit JR, Genthon C, Barkov NI, et al. (1987) Vostok ice core: a continuous isotope temperature record over the last climate cycle (160,000 years). Nature 329: 403–408. [Google Scholar]
- 85. Thompson LG, Mosley-Thompson E, Davis ME, Henderson KA, Brecher HH, et al. (2002) Kilimanjaro ice core records: Evidence of Holocene climate change in tropical Africa. Science 298: 589–593. [DOI] [PubMed] [Google Scholar]
- 86. Heaton JM (1972) The distribution of brown adipose tissue in the human. J Anat 112: 35–39. [PMC free article] [PubMed] [Google Scholar]
- 87.Wrangham R (2009) How cooking made us human. New York, Basic Press.
- 88. McHenry HM, Coffing K (2000) Australopithecs to Homo: Transformations in body and mind. Annu Rev Anthropol 29: 125–146. [Google Scholar]
- 89. Hong KW, Weiss A, Morimura N, Udono T, Hayasaka I, et al. (2011) Polymorphism of the tryptophan hydroxylase 2 (TPH2) gene is associated with chimpanzee neuroticism. PLoS ONE 6 7:e22144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Takenaka A, Udono T, Miwa N, Varavudhi P, Takenaka O (1993) High frequency of triplicated α-globin genes in tropical primates, crab-eating macaques (Macaca fascicularis), chimpanzees (Pan troglodytes), and orangutans (Pongo pygmaeus). Primates 34: 55–60. [Google Scholar]
- 91. Hirai H, Mootnick AR, Takenaka O, Suryobroto B, Mouri T, et al. (2003) Genetic mechanism and property of a whole-arm translocation (WAT) between chromosomes 8 and 9 of agile gibbons (Hylobates agilis). Chromosome Res 11: 37–50. [DOI] [PubMed] [Google Scholar]
- 92.Hayama S, Suzuki K, Yanai T, Masegi T, Kuroda S, et al.. (1993) Constations Clonicopathologiques sur les Gorilles Orphelins du Congo, Rapport Annuel 1992–1993 par les quipes Japanaises et Congolaises: République du et Centre des Etudes Africaines, Université de Kyoto Japan.
- 93. Hayama S (1994) The clinical pathological studies and comparable anatomical studies of African great apes in Republic of the Congo. Africa Research 44: 97–107 (in Japanese). [Google Scholar]
- 94.NCBI website. Available: http://www.ncbi.nlm.nih.gov/projects/SNP/snp_ref.cgi?rs=1042713. Accessed 2012 Jan 30.
- 95.NCBI website. Available: http://www.ncbi.nlm.nih.gov/projects/SNP/snp_ref.cgi?rs=1042714. Accessed 2012 Jan 30.