Abstract
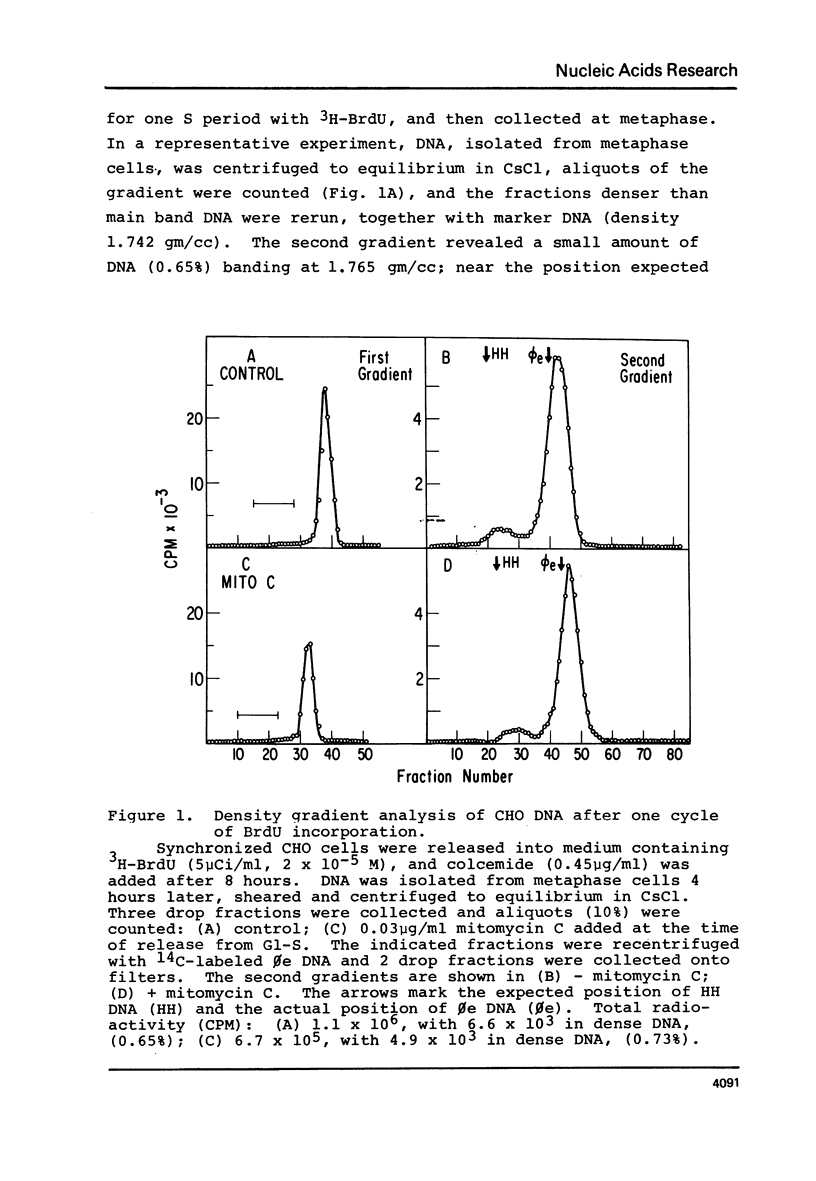
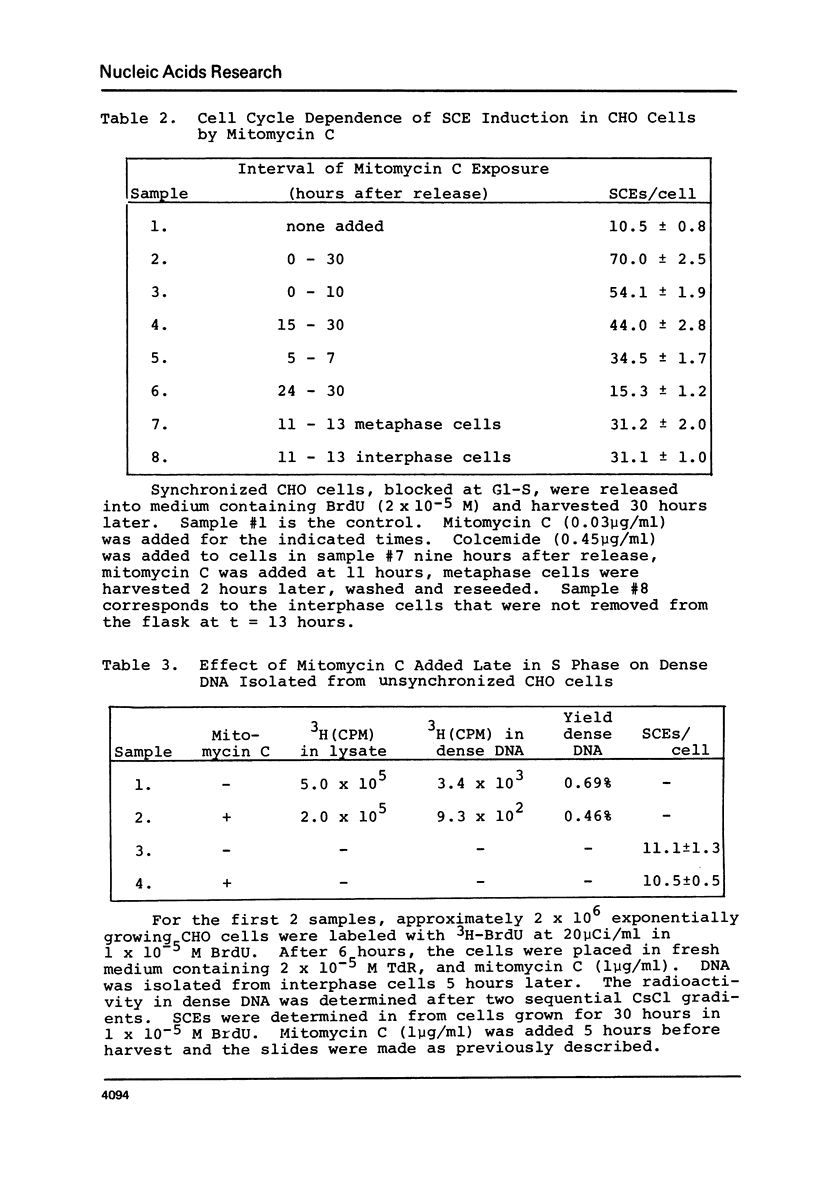
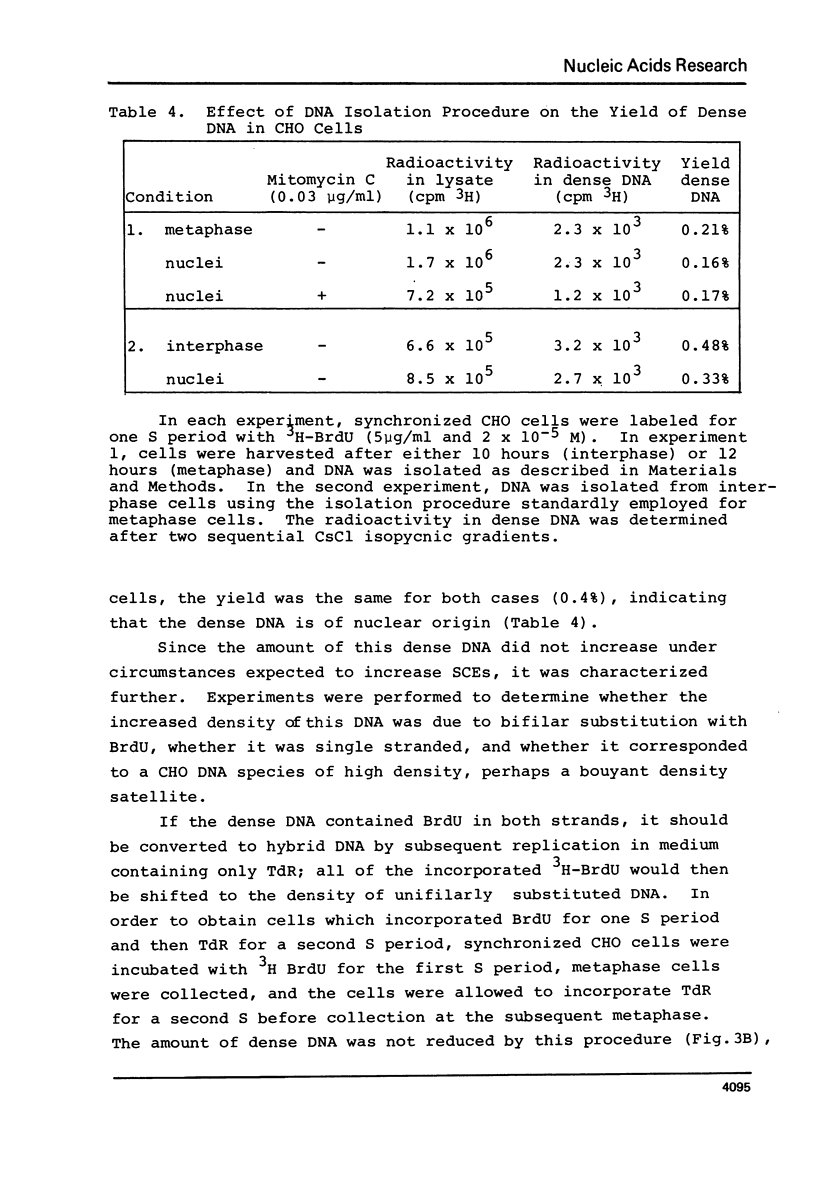
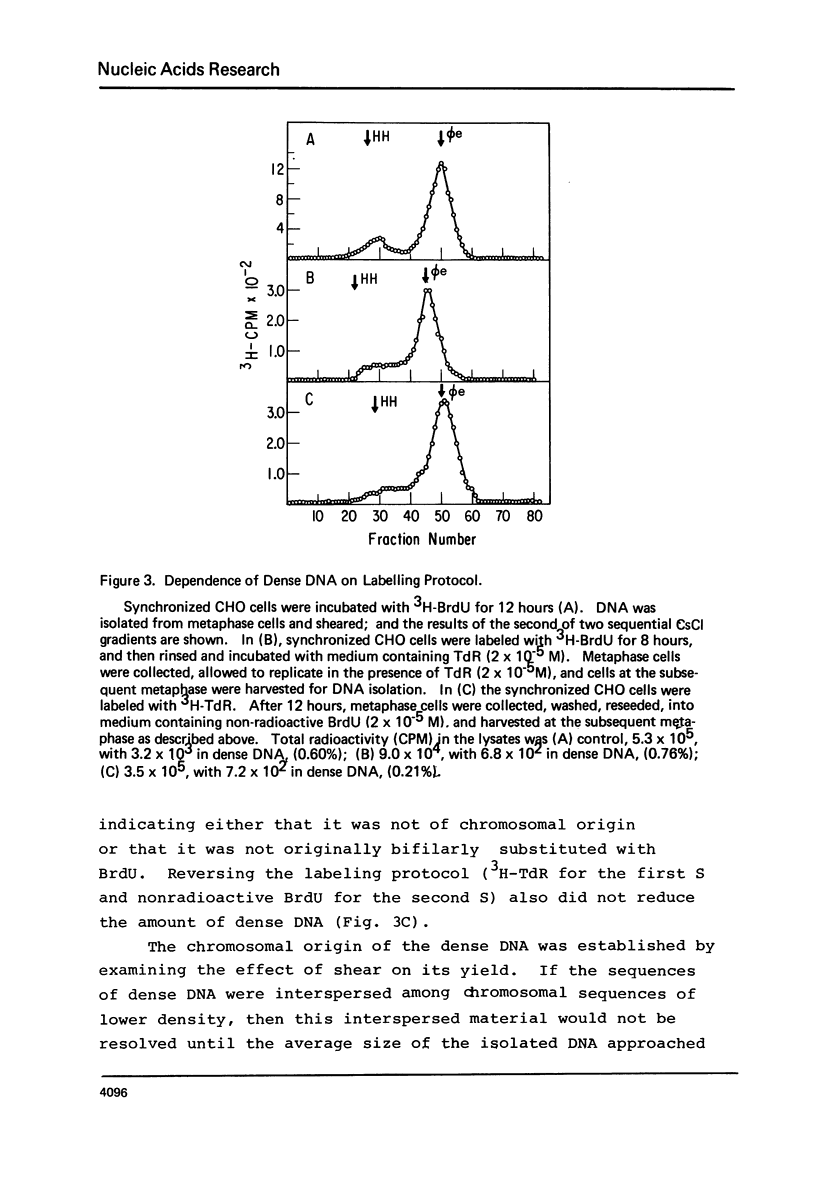
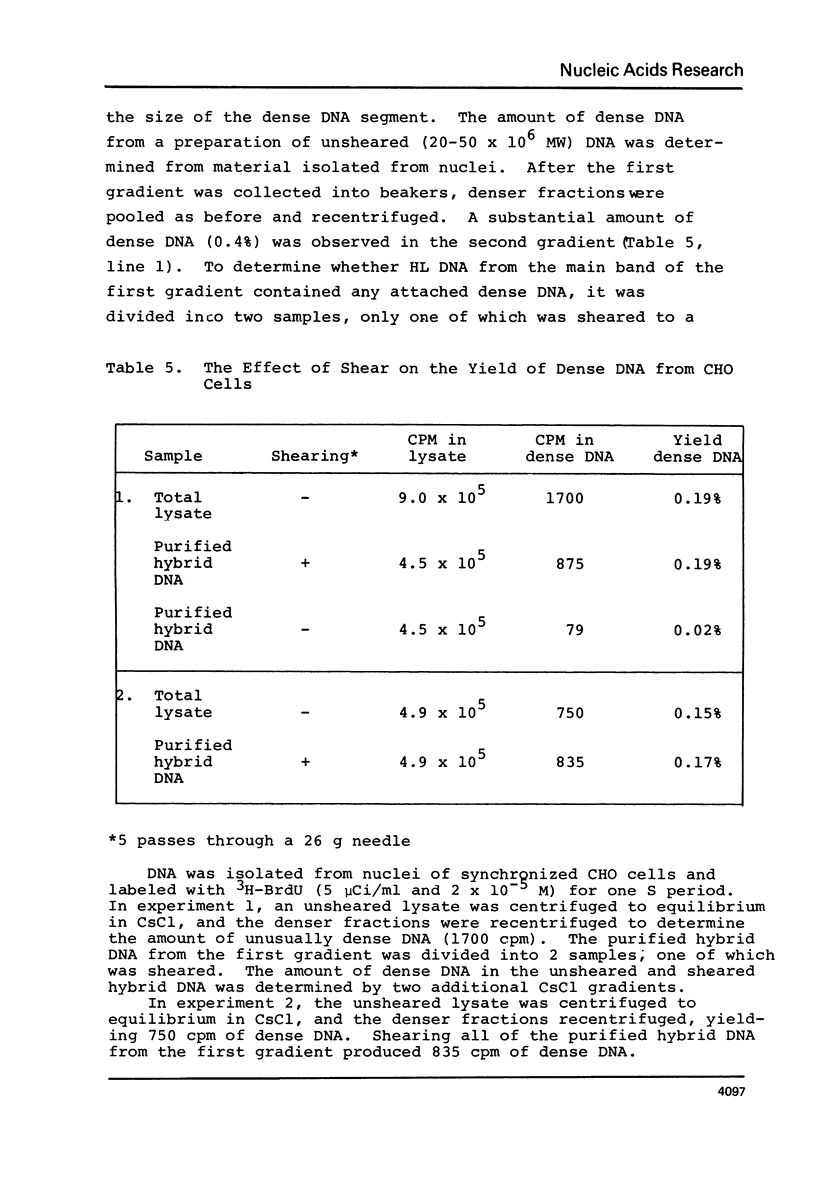
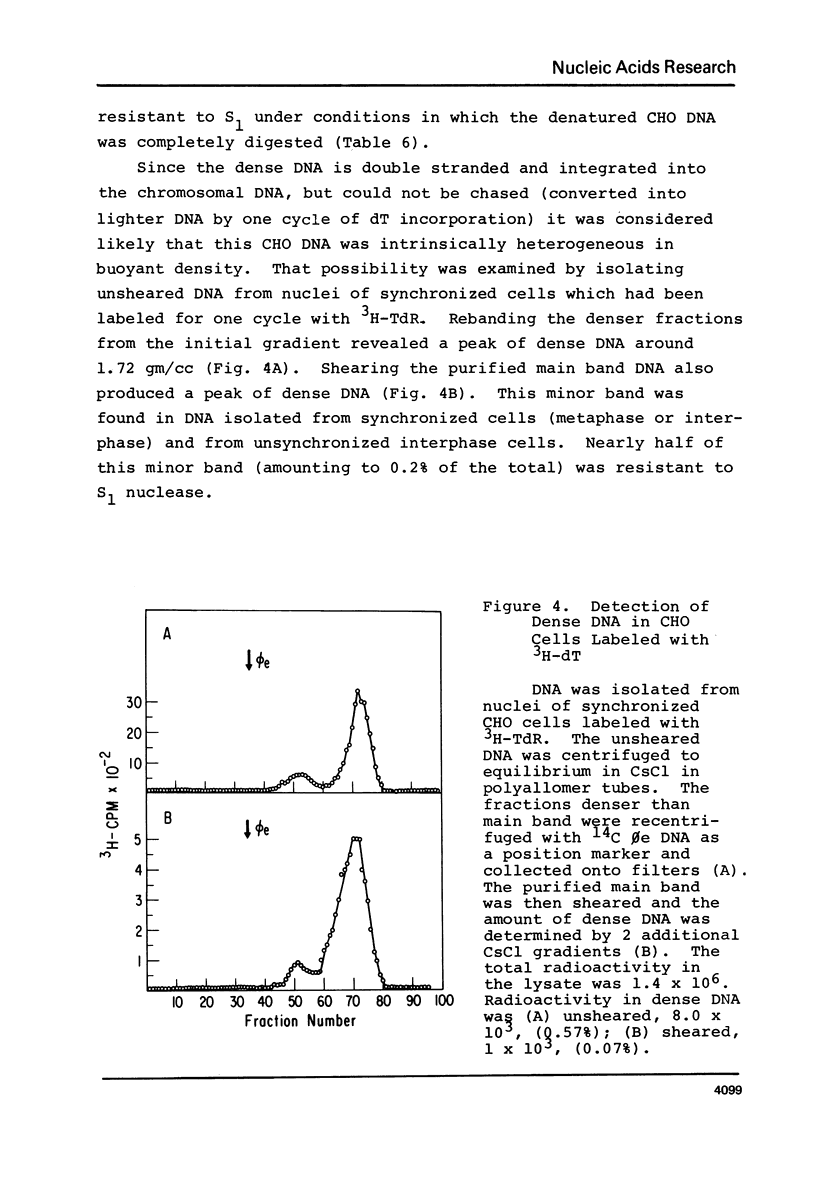
Chinese hamster ovary cells (CHO) grown for one cycle in bromodeoxyuridine (BrdU) contain a small amount (0.5%) of unusually dense double stranded DNA. This dense DNA has been previously interpreted as being bifilarly substituted with BrdU and hence evidence that sister chromatid exchange (SCE) formation proceeds via the Holliday model of recombination. However, the amount of this dense DNA is 100 times greater than that expected based on the SCE frequency in similarly cultured CHO cells, and it is not increased by treating the cells with mitomycin C. Moreover, contrary to expectations for bifilary substituted DNA, the amount of this dense DNA is not reduced by growing BrdU-labeled cells for a second cycle in TdR. Finally, DNA isolated from CHO cells contains a minor band (0.5%) with a density 0.025 gm/cc greater than that of the main band, whether or not BrdU has been incorporated. These results call into question the identification of this unusually dense DNA as bifilarly substituted and hence its previously postulated relationship to SCE formation.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. W., Latt S. A. Analysis of sister chromatid exchange formation in vivo in mouse spermatogonia as a new test system for environmental mutagens. Nature. 1976 Apr 1;260(5550):449–451. doi: 10.1038/260449a0. [DOI] [PubMed] [Google Scholar]
- Chaganti R. S., Schonberg S., German J. A manyfold increase in sister chromatid exchanges in Bloom's syndrome lymphocytes. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4508–4512. doi: 10.1073/pnas.71.11.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway S. M., Evans H. J. Sister chromatid exchange in human chromosomes from normal individuals and patients with ataxia telangiectasia. Cytogenet Cell Genet. 1975;15(1):17–29. doi: 10.1159/000130495. [DOI] [PubMed] [Google Scholar]
- Hamlin J. L., Pardee A. B. S phase synchrony in monolayer CHO cultures. Exp Cell Res. 1976 Jul;100(2):265–275. doi: 10.1016/0014-4827(76)90147-6. [DOI] [PubMed] [Google Scholar]
- Hanania N., Caneva R., Tapiero H., Harel J. Distribution of repetitious DNA in randomly growing and synchronized Chinese hamster cells. Exp Cell Res. 1975 Jan;90(1):79–86. doi: 10.1016/0014-4827(75)90359-6. [DOI] [PubMed] [Google Scholar]
- Higgins N. P., Kato K., Strauss B. A model for replication repair in mammalian cells. J Mol Biol. 1976 Mar 5;101(3):417–425. doi: 10.1016/0022-2836(76)90156-x. [DOI] [PubMed] [Google Scholar]
- Kato H. Spontaneous sister chromatid exchanges detected by a BUdR-labelling method. Nature. 1974 Sep 6;251(5470):70–72. doi: 10.1038/251070a0. [DOI] [PubMed] [Google Scholar]
- Korenberg J. R., Freedlender E. F. Giemsa technique for the detection of sister chromatid exchanges. Chromosoma. 1974;48(4):355–360. doi: 10.1007/BF00290992. [DOI] [PubMed] [Google Scholar]
- Krishan A. Rapid flow cytofluorometric analysis of mammalian cell cycle by propidium iodide staining. J Cell Biol. 1975 Jul;66(1):188–193. doi: 10.1083/jcb.66.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latt S. A., Loveday K. S. Characterization of sister chromatid exchange induction by 8-methoxypsoralen plus near UV light. Cytogenet Cell Genet. 1978;21(4):184–200. doi: 10.1159/000130896. [DOI] [PubMed] [Google Scholar]
- Latt S. A. Sister chromatid exchanges, indices of human chromosome damage and repair: detection by fluorescence and induction by mitomycin C. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3162–3166. doi: 10.1073/pnas.71.8.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latt S. A., Stetten G., Juergens L. A., Buchanan G. R., Gerald P. S. Induction by alkylating agents of sister chromatid exchanges and chromatid breaks in Fanconi's anemia. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4066–4070. doi: 10.1073/pnas.72.10.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loveday K. S., Fox M. S. The fate of bacteriophage phie transfecting DNA. Virology. 1978 Apr;85(2):387–403. doi: 10.1016/0042-6822(78)90447-6. [DOI] [PubMed] [Google Scholar]
- Meselson M. S., Radding C. M. A general model for genetic recombination. Proc Natl Acad Sci U S A. 1975 Jan;72(1):358–361. doi: 10.1073/pnas.72.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P. D., Holliday R. Evidence for the formation of hybrid DNA during mitotic recombination in Chinese hamster cells. Cell. 1976 Aug;8(4):573–579. doi: 10.1016/0092-8674(76)90225-7. [DOI] [PubMed] [Google Scholar]
- Munroe S. H., Latt S. A. Comparison of the subunit organization of early and late replicating chromatin. Exp Cell Res. 1977 Dec;110(2):299–313. doi: 10.1016/0014-4827(77)90296-8. [DOI] [PubMed] [Google Scholar]
- Perry P., Evans H. J. Cytological detection of mutagen-carcinogen exposure by sister chromatid exchange. Nature. 1975 Nov 13;258(5531):121–125. doi: 10.1038/258121a0. [DOI] [PubMed] [Google Scholar]
- Perry P., Wolff S. New Giemsa method for the differential staining of sister chromatids. Nature. 1974 Sep 13;251(5471):156–158. doi: 10.1038/251156a0. [DOI] [PubMed] [Google Scholar]
- Rommelaere J., Miller-Faurès A. Detection by density equilibrium centrifugation of recombinant-like DNA molecules in somatic mammalian cells. J Mol Biol. 1975 Oct 15;98(1):195–218. doi: 10.1016/s0022-2836(75)80109-4. [DOI] [PubMed] [Google Scholar]
- Solomon E., Bobrow M. Sister chromatid exchanges--a sensitive assay of agents damaging human chromosomes. Mutat Res. 1975 Nov;30(2):273–278. [PubMed] [Google Scholar]
- Stetka D. G., Wolff S. Sister chromatid exchange as an assay for genetic damage induced by mutagen-carcinogens. II. In vitro test for compounds requiring metabolic activation. Mutat Res. 1976 Dec;41(2-3):343–350. doi: 10.1016/0027-5107(76)90107-x. [DOI] [PubMed] [Google Scholar]
- Tatsumi K., Strauss B. Production of DNA bifilarly substituted with bromodeoxyuridine in the first round of synthesis: branch migration during isolation of cellular DNA. Nucleic Acids Res. 1978 Feb;5(2):331–347. doi: 10.1093/nar/5.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J H. Sister Chromatid Exchanges in Tritium-Labeled Chromosomes. Genetics. 1958 May;43(3):515–529. doi: 10.1093/genetics/43.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. H., Woods P. S., Hughes W. L. THE ORGANIZATION AND DUPLICATION OF CHROMOSOMES AS REVEALED BY AUTORADIOGRAPHIC STUDIES USING TRITIUM-LABELED THYMIDINEE. Proc Natl Acad Sci U S A. 1957 Jan 15;43(1):122–128. doi: 10.1073/pnas.43.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tice R., Chaillet J., Schneider E. L. Evidence derived from sister chromatid exchanges of restricted rejoining of chromatid subunits. Nature. 1975 Aug 21;256(5519):642–644. doi: 10.1038/256642a0. [DOI] [PubMed] [Google Scholar]
- Vogel W., Bauknecht T. Differential chromatid staining by in vivo treatment as a mutagenicity test system. Nature. 1976 Apr 1;260(5550):448–449. doi: 10.1038/260448a0. [DOI] [PubMed] [Google Scholar]
- Wolff S., Bodycote J., Thomas G. H., Cleaver J. E. Sister chromatid exchange in xeroderma pigmentosum cells that are defective in DNA excision repair or post-replication repair. Genetics. 1975 Oct;81(2):349–355. doi: 10.1093/genetics/81.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]




