Abstract
Activation of peripheral κ opioid receptors (KOR) effectively relieves pain and hyperalgesia in preclinical and clinical models of pain. Although centrally located KOR activation results in sexually dimorphic effects, it is unclear whether peripheral KOR also produces sex dependent effects in persistent inflammatory pain conditions. In this study, we investigated whether local administration of a specific KOR agonist, U50, 488 relieve mechanical hyperalgesia induced by the injection of complete Freund’s adjuvant (CFA) in the rat hindpaw, and whether there are sex differences. The effects of U50, 488 were assessed three days after the induction of CFA-induced inflammation, a time point at which mechanical hyperalgesia was most prominent. There were no sex differences in baseline and CFA-induced changes in mechanical thresholds between male and female rats. Local treatment of U50, 488 produced moderate, but significant, anti-hyperalgesia in both male and female rats. However, U50, 488 was significantly more effective in male rats at the highest dose of U50, 488. We confirmed that the highest dose of U50, 488 used in this study did not produce systemic effects, and that the drug effect is receptor specific. On the basis of these results, we suggest that local KOR agonists are effective in mitigating mechanical hyperalgesia under a persistent inflammatory pain condition and that sex differences in anti-hyperalgesic effects become more evident at high doses.
Keywords: Sex, peripheral, kappa opioid receptor, inflammation, mechanical hyperalgesia
Targeting peripheral opioid receptors that are expressed on primary afferent terminals as effective means of treating a wide variety of pain conditions has been a topic of substantial amount of research in the past two decades [31,37]. Recently, considerable interest has been generated in developing kappa opioid receptor (KOR) ligands that are restricted to the periphery since activation of peripheral KOR does not produce many of the side effects mediated by mu or delta opioid receptors (MOR and DOR, respectively) [14,29,35,39,40].
Peripheral KORs are particularly effective in a variety of preclinical visceral pain models [6,19,32]. Consistent with the animal data, human clinical and experimental studies indicate that peripherally restricted KOR agonists produce significant analgesic effects on visceral pain [2,22]. However, peripheral KOR effects have been also demonstrated in somatic neuropathic and inflammatory pain models [4,20,24]. More recent studies showed that local KOR activation also decreases temporomandibular joint (TMJ) pain as well as inflammation and prevents the loss of alveolar bone and periodontal tissue [3,7,26,27].
It is well established that humans and animals show sex differences in KOR-mediated analgesia. While most studies that examined sex differences in KOR analgesia characterized centrally-mediated effects [10,15], few available studies that report sex differences in peripheral KORs [5,8,21] utilized persistent pain models as opposed to acute pain models that have been predominantly used to assess systemic effects of KOR treatments. Of those peripheral studies, two report a greater KOR effect in females [5,8] and one in males [21]. Thus, as with sex differences mediated by KOR in the CNS, multiple factors such as the type of stimulus modality and the intensity of a stimulus might contribute to sex differences in peripheral KOR [10,16]. However, additional studies utilizing different pain conditions are required to increase our overall understanding on neurobiological mechanisms underlying sex different responses to opioid treatments at the site of injury. In this study, we examined whether a locally applied KOR agonist attenuates complete Freund’s adjuvant (CFA)-induced mechanical hyperalgesia in a sexually dimorphic manner using a rat hindpaw model.
One hundred and two age-matched adult male and female Sprague Dawley rats (8 weeks old; 250–300 g for males and 225–260 g for females, Harlan, Indianapolis) were used in all experiments. The estrus cycle phase in female rats was not determined in this study. All animals were housed in a temperature-controlled room under a 12:12 light-dark cycle with access to food and water ad libitum. All procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and under a University of Maryland approved Institutional Animal Care and Use Committee protocol.
Mechanical sensitivity of the hindpaw was assessed with the Randall-Selitto test, an established rodent model for testing mechanical hypersensitivity of the paw. Animals were first allowed to habituate to the experimental room for 30 min for three consecutive days. The withdraw response to noxious paw pressure was assessed using a digital paw pressure Randall-Selitto applicator for rodents (IITC Life Science, Woodland Hills, CA). Each rat was placed in a cloth holder suspended in a sling, and the probe of the pressure applicator was placed under the plantar surface of the hindpaw. The probe has a spring load for easy opening and closing of the pressure applicator. The probe, which is placed under the paw, close the pressure applicator, captures and stores the pressure upon reaction. A gradually increasing pressure was applied until the rat withdraws its hindpaw. The lowest pressure necessary to elicit the withdraw response prior to inflammation was considered as the baseline mechanical threshold.
Inflammation was induced by the injection of complete Freund’s adjuvant (CFA, 50 µl; 1:1 isotonic saline) into the plantar surface of the right hindpaw with a 27-gauge needle over 5–10 s. Control animals were injected with the same volume of isotonic saline (ISO) in the same manner. Anti-hyperalgesic effects of U50, 488 (Tocris), a specific KOR agonist, was measured on day 3 after intraplantar injection of CFA or saline, during which mechanical hyperalgesia was most profound. On day 3, three different doses of U50, 488 (1, 30, or 100 µg/20 µl) were dissolved in phosphate buffer solution (PBS) or the same volume of vehicle control were administered into the plantar surface of the inflamed hindpaw of both male and female rats. A pre-injection mechanical threshold for evoking a hindpaw withdrawal response was determined 15 min prior to drug injection. Changes in mechanical sensitivity of the hindpaw were assessed 30, 60, 120 and 180 min after the administration of the drug. To rule out the possibility that local administration of U50, 488 produced systemic effects by activating KOR in the CNS, additional groups of male and female rats received the highest doses of U50, 488 (100 µg) injections into the hindpaw contralateral to the mechanical stimulation. To test the receptor specificity of the agonist, a selective antagonist for KOR, nor-BNI (Tocris; 200 µg/20 µl) was administered 10 min before U50, 488 (100 µg) treatment in male and female rats. The doses of the KOR agonist and the antagonist were adapted from previous studies [33]. All experimental and control groups consisted of 6 animals per group.
One-Way ANOVA was used to compare the differences in baseline mechanical thresholds between male and female rats. The inflammation-induced or drug-induced changes in mechanical thresholds were analyzed with a Two-Way ANOVA with repeated measures. In order to compare sex differences in the effect of individual doses of U50, 488, the mechanical thresholds were also normalized to the pre-injection threshold and the percent changes following treatment were assessed. All comparisons between multiple groups were followed by a post hoc test (Holm-Sidak method). All data are presented as mean ± standard error of the mean (SE), and differences were considered significant at p < 0.05.
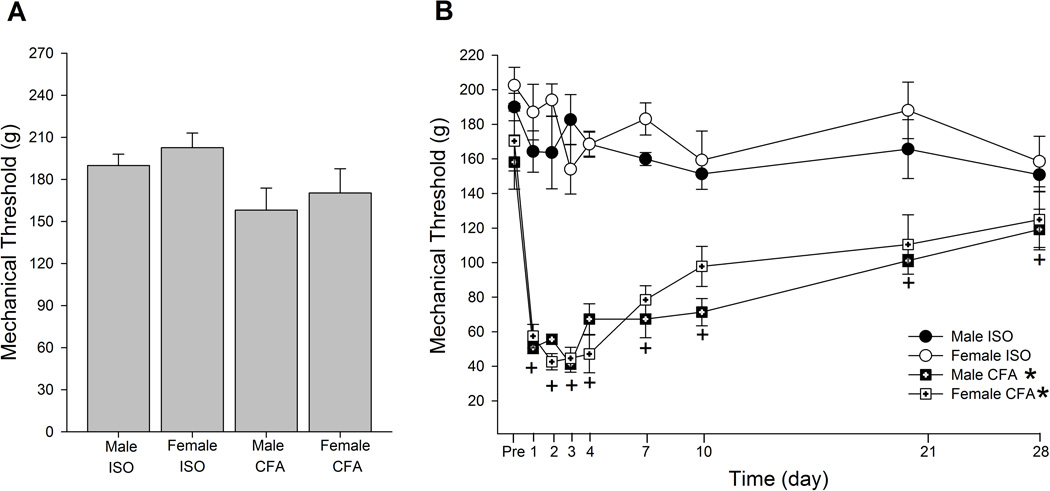
The average mechanical thresholds needed to evoke a nocifensive hindpaw withdraw response before any treatments ranged between 158 – 202 g. There was no significant difference in the baseline mechanical thresholds between male and female rats that received either CFA or ISO (Fig 1A; F=2.19, p > 0.05). Following the CFA treatment, there was a significant group effect (F=53.2, p < 0.001) and time effect (F=21.3, p < 0.001). The ISO treatment did not significantly alter the mechanical thresholds at any time point during the 28 days of observation in either male or female rats (Fig 1B). In contrast, the CFA treatment caused a significant and prolonged mechanical hyperalgesia in both male and female rats. The mean mechanical thresholds were significantly reduced from day 1, reaching the peak around day 3 (41.3 g for males and 44.7 g for females), and then showed a tendency of gradually returning to baseline over 28 days. However, a significant mechanical hyperalgesia was still present on day 28. There were no significant differences in the magnitude and the extent of CFA-induced mechanical hyperalgesia between male and female rats. A significant interaction between treatment and time was observed (F=5.89, p < 0.005).
Figure 1.
Effects of i.pl injection of CFA on hindpaw mechanical sensitivity. (A) Baseline thresholds of CFA and ISO treated male and female rats. (B) Time course of changes in mechanical thresholds of CFA and ISO treated male and female rats. * and + indicate significant group and time effect, respectively. Each group consisted of n=6.
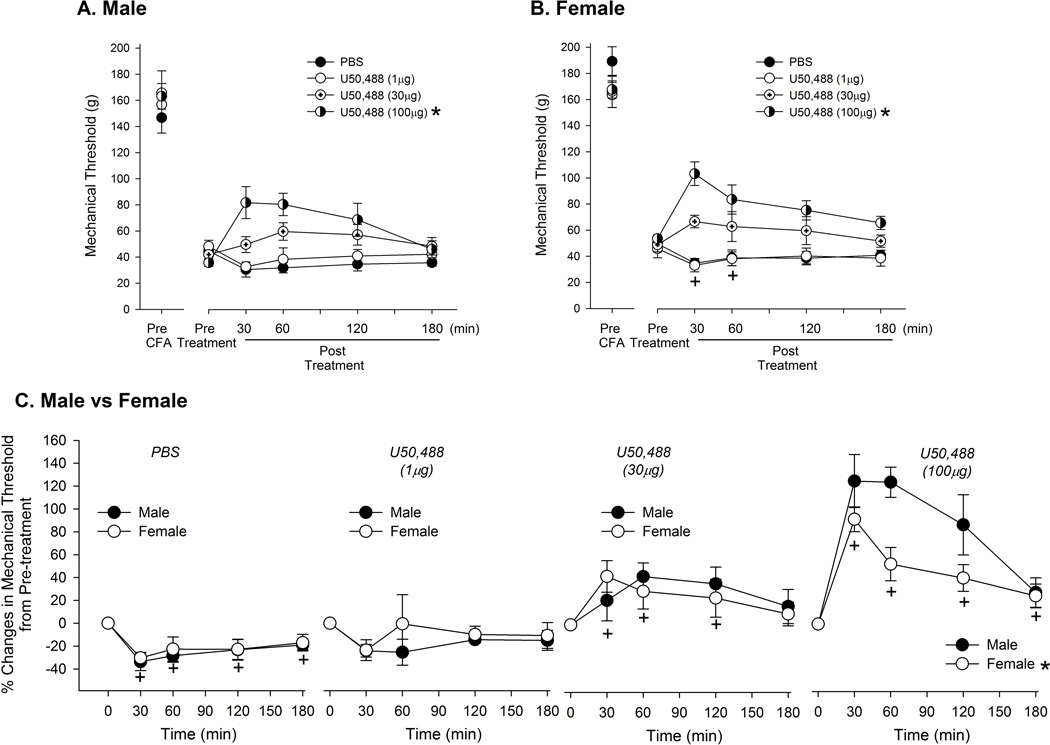
On day 3 when the CFA-induced mechanical hyperalgesia was most prominent, local injection of U50, 488 dose-dependently reversed the hyperalgesia in male rats (Fig 2A). There was a significant group effect (F=5.23, p < 0.05) without a significant time effect (F=2.4, p > 0.05). A significant interaction between dose and time was observed (F=8.1, p < 0.001). PBS injection further increased the hyperalgesia possibly due to an injection procedure in the inflamed paw (Fig 2C). U50, 488 at 1 µg did not attenuate either the injection-induced or the CFA-induced component of mechanical hyperalgesia, while U50, 488 at 30 µg slightly attenuated the CFA-induced hyperalgesia (Fig 2A). U50, 488 at 100 µg not only blocked the injection-induced exacerbation of mechanical hyperalgesia, but also significantly attenuated the CFA-induced hyperalgesia. Similarly, U50, 488 dose-dependently reversed mechanical hyperalgesia in female rats (Fig 2B; F= 9.19, p < 0.01). There was also a significant time effect at 30 and 60 min (F=5.9, p < 0.05). A significant dose and time interaction was also observed in the female group (F=4.62, p < 0.05)
Figure 2.
Effects of local treatment of U50, 488 on CFA-induced mechanical hyperalgesia. On day 3 of CFA injection, changes in mechanical thresholds after local U50, 488 treatment were measured for 180 minutes in male (A) and female (B) rats. The drug was administered 5 minutes after pre-treatment measurement of mechanical threshold. * indicates significant groups effect compared to PBS at p < 0.05. (C) Direct comparisons of percent changes in mechanical thresholds between male and female rats following PBS or drug treatment. * and + indicate significant group and time effect, respectively. Each group consisted of n=6.
In order to compare sex differences in the effectiveness of U50, 488, we normalized the data to the pre-treatment value and plotted and analyzed the responses to vehicle and each dose of U50, 488 separately (Fig 2C). PBS injection in the inflamed paw exacerbated mechanical hyperalgesia to a similar extent between male and female rats. There was a significant time effect (F=6.4, p < 0.001), but no significant sex effect (F=0.28, p > 0.05). Following PBS injection, the mechanical threshold was further reduced by approximately 30 % at 30 min and remained significantly depressed for 180 min. There was neither a significant sex effect nor time effect following the treatment with 1 µg of U50, 488 (F=0.47, p > 0.05; F=1.73, p > 0.05, respectively). The 30 µg dose of U50, 488 significantly attenuated the hyperalgesia for 120 minutes (F= 5.05, p < 0.05), but no sex differences were observed at this dose (F= 0.24, p > 0.05). More profound and prolonged anti-hyperalgesic effect was observed with U50, 488 at 100 µg. The significant anti-hyperalgesic effect was maintained for 180 min (F=25.3, p < 0.01). Interestingly, a significant sex effect was observed at this high dose with males exhibiting a greater anti-hyperalgesic effect than females (F=6.0, p < 0.05). There was no significant interaction between sex and time in any of the treatment conditions.
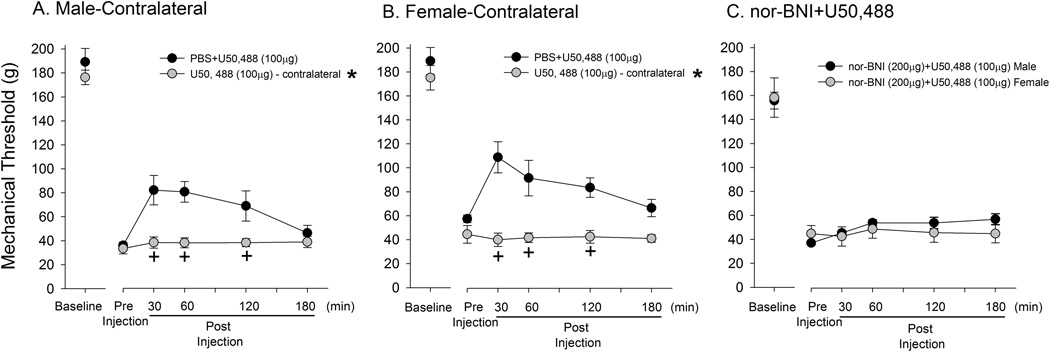
The highest dose of U50, 488 (100 µg) injected into the paw contralateral to the inflamed paw neither caused a further reduction of mechanical hyperalgesia nor attenuated the CFA-induced hyperalgesia in both male and female rats (Fig 3A,B). There was a significant drug effect (Male: F=8.06, Female: F=13, p < 0.05) and time effect (Male F=4.27, Female: F=5.3, p < 0.05) for both male and female groups. These data suggested that the anti-hyperalgesic effect produced by U50, 488, even at the highest dose utilized in this study, is mediated by local KOR. Finally, we confirmed that the anti-hyperalgesic effects produced by U50, 488 are receptor mediated by pre-treating the hindpaw with nor-BNI prior to U50, 488 administration. The nor-BNI pre-treatment completely prevented the anti-hyperalgesic effects of U50, 488 in both male and female rats (Fig 3C).
Figure 3.
Local and receptor mediated effects of U50, 488. On day 3 of CFA injection, U50, 488 injected into the hindpaw contralateral to the CFA-inflamed paw had no effect on mechanical thresholds in either males or females (A, B). On day 3 of CFA injection, changes in mechanical thresholds after local U50, 488 treatment with nor-BNI in the same paw were measured in male and female rats (C). The nor-BNI was administered 5 minutes after the pre-treatment measurement of mechanical threshold, followed by U50, 488. *, + indicates significant group and time effect, respectively. Each group consisted of n=6.
The obvious clinical advantages of avoiding the centrally mediated side effects of opioids by targeting peripheral opioid receptors continues to generate a substantive amount of data under various pain conditions. [2,12,13,14,36]. Peripheral KORs are demonstrated as a particularly important target due to their lower abuse potential and generation of fewer side effects [38]. Peripherally restricted or systemically low doses of KOR agonists reliably attenuate visceral pain in animals [6,19,32]. In humans, a peripherally restricted KOR agonist, CR665, significantly attenuates visceral pain while paradoxically enhancing somatic pain [2]. Asimadoline, another peripherally restricted KOR agonist, provides a significant relief of pain and discomfort in patients with irritable bowel syndrome [22], and ADL-10-0101 in patients with chronic pancreatitis [14]. Although preclinical studies indicate that peripheral KORs also play a role in somatic and joint pain conditions [8,11,25,27], additional studies are warranted to take full advantage of peripheral KOR agonists that are currently undergoing clinical trials [37].
To that end, we provide additional evidence that a local dose of U50, 488 can partially, but significantly, attenuate CFA-induced inflammatory mechanical hyperalgesia in somatic tissue. Previous studies that utilized inflammatory agents such as carrageenan, capsaicin, or formalin examined the anti-hyperalgesic effects of KOR agonists under pain conditions that are resolved in a relatively shorter duration and during the development phase of inflammatory pain [1,8,20,21]. Our data is particularly interesting in that U50, 488 was anti-hyperalgesic 3 days after the induction of inflammation, a time point at which the hyperalgesia was fully developed. The data is also consistent with the anti-hyperalgesic effects of asimadoline on mechanical hyperalgesia in an adjuvant-induced arthritis model [5].
In the CNS, animal studies generally report greater KOR mediated analgesia among males [9], whereas human studies with mixed action κ-agonist-antagonists have shown better analgesia in women than men in a dental pain model [16,17]. At present, there is no clear picture as to how peripheral KOR agonists produce different responses between the sexes. Peripheral KOR agonists produce a greater reduction of formalin-induced nociceptive responses in female rats [8], which is consistent with human clinical data [16,17]. In contrast, the peripheral action of KOR agonists is more potent in males whereas sex differences are not observed with mixed action opioids in capsaicin-induced hyperalgesia in rats [21]. Finally, sex differences in the effect of asimadoline are evident in attenuating only thermal, but not mechanical, hyperalgesia in a rat model of persistent arthritis [5]. Our data showed that U50, 488 dose-dependently attenuate CFA- and injection-induced mechanical hyperalgesia in both male and female rats. However, albeit small but significant sex differences can be observed at a higher dose. A small sex related difference we observed could be due to the fact that we tested the drug effect when the hyperalgesia is most prominent. It is also possible that a higher dose of U50, 488 could exaggerate sex differences. We did not test higher doses since U50, 488 may penetrate the blood brain barrier. Our data still provides important clues to studying the mechanistic basis regarding sex differences in peripheral KOR analgesia.
Although cellular mechanisms of sex differences in peripheral KOR-mediated analgesia have not been systematically investigated, it is likely that the nature of sex differences in peripheral KOR effects involve multiple mechanisms. There may exist sex differences at the level of KOR expression, KOR trafficking, and KOR signaling pathways in sensory neurons. For example, systemic activation of KORs is thought to produce sex differences via NMDA dependent mechanisms in acute pain models [18], while peripheral KOR mediated sex differences do not involve NMDA receptors [21]. Sex differences in peripheral DOR effect is in part mediated by sex differences in KATP expression, one of the downstream effectors of ORs in trigeminal sensory neurons [23,30].
Puehler et al. [28] report significant upregulation of KOR mRNA in dorsal root ganglia following the induction of paw inflammation by CFA, an effect measured only in male rats. We have shown that CFA-induced inflammation in the masseter muscle significantly upregulates MORs and KORs in trigeminal ganglia of male, but not in female, rats [41]. Male specific upregulation of KORs under inflammatory conditions could explain a lack of asimadoline effect in female patients with irritable bowel syndrome [34]. In our study, it is possible that a higher level of KOR in male rats rendered greater anti-hyperalgesic effects that became apparent with higher doses of U50, 488.
Taken together, our data bear clinical significance since the management of many types of chronic pain conditions is sexually dimorphic, and since there is increasing clinical as well as pre-clinical evidence that indicates peripheral KORs as potential therapeutic targets. Understanding the mechanical basis regarding the sex differences in KOR function will help develop sex-specific management strategies that can be directed at the periphery to ameliorate persistent types of pain.
Highlights.
CFA induced long lasting mechanical hyperalgesia in the rat hindpaw.
No sex differences in baseline or changes in mechanical sensitivity were observed.
Activation of peripheral KOR attenuated inflammatory mechanical hyperalgesia.
High dose of KOR agonist produced moderate but significant sex differences.
Acknowledgements
This study was supported by NIH grant RO1 DE19448 (J.Y.R.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no conflicts of interest associated with the present study.
References
- 1.Amarante LH, Alves DP, Duarte ID. Study of the involvement of K+ channels in the peripheral antinociception of the kappa-opioid receptor agonist bremazocine. Eur J Pharmacol. 2004 Jun 28;494(2–3):155–160. doi: 10.1016/j.ejphar.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Arendt-Nielsen L, Olesen AE, Staahl C, Menzaghi F, Kell S, Wong GY, Drewes AM. Analgesic efficacy of peripheral kappa-opioid receptor agonist CR665 compared to oxycodone in a multi-modal, multi-tissue experimental human pain model: selective effect on visceral pain. Anesthesiology. 2009 Sep;111(3):616–624. doi: 10.1097/ALN.0b013e3181af6356. [DOI] [PubMed] [Google Scholar]
- 3.Bastos JV, Queiroz-Junior CM, Caliari MV, Francischi JN, Pacheco CM, Maltos KL. Peripheral kappa opioid receptors activation reduces alveolar bone loss in rats by modulating interleukin-6 and-10. Arch Oral Biol. 2011 Jun;56(6):540–548. doi: 10.1016/j.archoralbio.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Bileviciute-Ljungar I, Spetea M. Contralateral, ipsilateral and bilateral treatments with the kappa-opioid receptor agonist U-50,488H in mononeuropathic rats. Eur J Pharmacol. 2004 Jun 28;494(2–3):139–146. doi: 10.1016/j.ejphar.2004.04.043. [DOI] [PubMed] [Google Scholar]
- 5.Binder W, Carmody J, Walker J. Effect of gender on anti-inflammatory and analgesic actions of two kappa-opioids. J Pharmacol Exp Ther. 2000 Jan;292(1):303–309. [PubMed] [Google Scholar]
- 6.Burton MB, Gebhart GF. Effects of kappa-opioid receptor agonists on responses to colorectal distension in rats with and without acute colonic inflammation. J Pharmacol Exp Ther. 1998 May;285(2):707–715. [PubMed] [Google Scholar]
- 7.Chicre-Alcântara TC, Torres-Chávez KE, Fischer L, Clemente-Napimoga JT, Melo V, Parada CA, Tambeli CH. Local kappa opioid receptor activation decreases temporomandibular joint inflammation. Inflammation. 2012 Feb;35(1):371–376. doi: 10.1007/s10753-011-9329-1. [DOI] [PubMed] [Google Scholar]
- 8.Clemente JT, Parada CA, Veiga MC, Gear RW, Tambeli CH. Sexual dimorphism in the antinociception mediated by kappa opioid receptors in the rat temporomandibular joint. Neurosci Lett. 2004 Dec 6;372(3):250–255. doi: 10.1016/j.neulet.2004.09.048. [DOI] [PubMed] [Google Scholar]
- 9.Craft RM. Sex differences in opioid analgesia: "from mouse to man". Clin J Pain. 2003 May-Jun;19(3):175–186. doi: 10.1097/00002508-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Craft RM, Mogil JS, Aloisi AM. Sex differences in pain and analgesia: the role of gonadal hormones. Eur J Pain. 2004 Oct;8(5):397–411. doi: 10.1016/j.ejpain.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Cunha TM, Souza GR, Domingues AC, Carreira EU, Lotufo CM, Funez MI, Verri WA, Jr, Cunha FQ, Ferreira SH. Stimulation of peripheral Kappa opioid receptors inhibits inflammatory hyperalgesia via activation of the PI3Kγ/AKT/nNOS/NO signaling pathway. Mol Pain. 2012 Feb 8;8:10. doi: 10.1186/1744-8069-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dionne RA, Lepinski AM, Gordon SM, Jaber L, Brahim JS, Hargreaves KM. Analgesic effects of peripherally administered opioids in clinical models of acute and chronic inflammation. Clin Pharmacol Ther. 2001 Jul;70(1):66–73. doi: 10.1067/mcp.2001.116443. [DOI] [PubMed] [Google Scholar]
- 13.Duckett JW, Cangiano T, Cubina M, Howe C, Cohen D. Intravesical morphine analgesia after bladder surgery. J Urol. 1997 Apr;157(4):1407–1409. [PubMed] [Google Scholar]
- 14.Eisenach JC, Carpenter R, Curry R. Analgesia from a peripherally active kappa-opioid receptor agonist in patients with chronic pancreatitis. Pain. 2003 Jan;101(1–2):89–95. doi: 10.1016/s0304-3959(02)00259-2. [DOI] [PubMed] [Google Scholar]
- 15.Fillingim RB, Gear RW. Sex differences in opioid analgesia: clinical and experimental findings. Eur J Pain. 2004 Oct;8(5):413–425. doi: 10.1016/j.ejpain.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Gear RW, Miaskowski C, Gordon NC, Paul SM, Heller PH, Levine JD. Kappa-opioids produce significantly greater analgesia in women than in men. Nat Med. 1996a Nov;2(11):1248–1250. doi: 10.1038/nm1196-1248. [DOI] [PubMed] [Google Scholar]
- 17.Gear RW, Gordon NC, Heller PH, Paul S, Miaskowski C, Levine JD. Gender difference in analgesic response to the kappa-opioid pentazocine. Neurosci Lett. 1996b Mar 1;205(3):207–209. doi: 10.1016/0304-3940(96)12402-2. [DOI] [PubMed] [Google Scholar]
- 18.Holtman JR, Jr, Wala EP. Characterization of antinociceptive effect of oxycodone in male and female rats. Pharmacology, Biochemistry and Behavior. 2006;83:100–108. doi: 10.1016/j.pbb.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 19.Joshi SK, Su X, Porreca F, Gebhart GF. kappa-opioid receptor agonists modulate visceral nociception at a novel, peripheral site of action. J Neurosci. 2000 Aug 1;20(15):5874–5879. doi: 10.1523/JNEUROSCI.20-15-05874.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ko MC, Johnson MD, Butelman ER, Willmont KJ, Mosberg HI, Woods JH. Intracisternal nor-binaltorphimine distinguishes central and peripheral kappa-opioid antinociception in rhesus monkeys. J Pharmacol Exp Ther. 1999 Dec;291(3):1113–1120. [PMC free article] [PubMed] [Google Scholar]
- 21.Lomas LM, Barrett AC, Terner JM, Lysle DT, Picker MJ. Sex differences in the potency of kappa opioids and mixed-action opioids administered systemically and at the site of inflammation against capsaicin-induced hyperalgesia in rats. Psychopharmacology (Berl) 2007 Apr;191(2):273–285. doi: 10.1007/s00213-006-0663-1. [DOI] [PubMed] [Google Scholar]
- 22.Mangel AW, Bornstein JD, Hamm LR, Buda J, Wang J, Irish W, Urso D. Clinical trial: asimadoline in the treatment of patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2008 Jul;28(2):239–249. doi: 10.1111/j.1365-2036.2008.03730.x. [DOI] [PubMed] [Google Scholar]
- 23.Niu K, Saloman JL, Zhang Y, Ro JY. Sex differences in the contribution of ATP-sensitive K+ channels in trigeminal ganglia under an acute muscle pain condition. Neuroscience. 2011 Apr 28;180:344–352. doi: 10.1016/j.neuroscience.2011.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Obara I, Mika J, Schafer MK, Przewlocka B. Antagonists of the kappa-opioid receptor enhance allodynia in rats and mice after sciatic nerve ligation. Br J Pharmacol. 2003 Oct;140(3):538–546. doi: 10.1038/sj.bjp.0705427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Obara I, Parkitna JR, Korostynski M, Makuch W, Kaminska D, Przewlocka B, Przewlocki R. Local peripheral opioid effects and expression of opioid genes in the spinal cord and dorsal root ganglia in neuropathic and inflammatory pain. Pain. 2009 Feb;141(3):283–291. doi: 10.1016/j.pain.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 26.Pacheco CM, Queiroz-Junior CM, Maltos KL, Caliari MV, Pacheco DF, Duarte ID, Francischi JN. Crucial role of peripheral kappa-opioid receptors in a model of periodontal disease in rats. J Periodontal Res. 2008 Dec;43(6):730–736. doi: 10.1111/j.1600-0765.2008.01102.x. [DOI] [PubMed] [Google Scholar]
- 27.Pena-dos-Santos DR, Severino FP, Pereira SA, Rodrigues DB, Cunha FQ, Vieira SM, Napimoga MH, Clemente-Napimoga JT. Activation of peripheral kappa/delta opioid receptors mediates 15-deoxy-(Delta12,14)-prostaglandin J2 induced-antinociception in rat temporomandibular joint. Neuroscience. 2009 Nov 10;163(4):1211–1219. doi: 10.1016/j.neuroscience.2009.07.052. [DOI] [PubMed] [Google Scholar]
- 28.Puehler W, Rittner HL, Mousa SA, Brack A, Krause H, Stein C, Schäfer M. Interleukin-1 beta contributes to the upregulation of kappa opioid receptor mrna in dorsal root ganglia in response to peripheral inflammation. Neuroscience. 2006 Aug 25;141(2):989–998. doi: 10.1016/j.neuroscience.2006.03.078. [DOI] [PubMed] [Google Scholar]
- 29.Rivière PJ. Peripheral kappa-opioid agonists for visceral pain. Br J Pharmacol. 2004 Apr;141(8):1331–1334. doi: 10.1038/sj.bjp.0705763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saloman JL, Niu KY, Ro JY. Activation of peripheral delta-opioid receptors leads to antihyperalgesic responses in the masseter muscle of male and female rats. Neuroscience. 2011 Sep 8;190:379–385. doi: 10.1016/j.neuroscience.2011.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sehgal N, Smith HS, Manchikanti L. Peripherally acting opioids and clinical implications for pain control. Pain Physician. 2011 May-Jun;14(3):249–258. [PubMed] [Google Scholar]
- 32.Sengupta JN, Snider A, Su X, Gebhart GF. Effects of kappa opioids in the inflamed rat colon. Pain. 1999 Feb;79(2–3):175–185. doi: 10.1016/s0304-3959(98)00175-4. [DOI] [PubMed] [Google Scholar]
- 33.Stein C, Millan MJ, Shippenberg TS, Peter K, Herz A. Peripheral opioid receptors mediating antinociception in inflammation. Evidence for involvement of mu, delta and kappa receptors. J Pharmacol Exp Ther. 1989 Mar;248(3):1269–1275. [PubMed] [Google Scholar]
- 34.Szarka LA, Camilleri M, Burton D, Fox JC, McKinzie S, Stanislav T, Simonson J, Sullivan N, Zinsmeister AR. Efficacy of on-demand asimadoline, a peripheral kappa-opioid agonist, in females with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2007 Nov;5(11):1268–1275. doi: 10.1016/j.cgh.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tao YM, Li QL, Zhang CF, Xu XJ, Chen J, Ju YW, Chi ZQ, Long YQ, Liu JG. LPK-26, a novel kappa-opioid receptor agonist with potent antinociceptive effects and low dependence potential. Eur J Pharmacol. 2008 Apr 28;584(2–3):306–311. doi: 10.1016/j.ejphar.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 36.Tegeder I, Meier S, Burian M, Schmidt H, Geisslinger G, Lötsch J. Peripheral opioid analgesia in experimental human pain models. Brain. 2003 May;126(Pt 5):1092–1102. doi: 10.1093/brain/awg115. [DOI] [PubMed] [Google Scholar]
- 37.Vadivelu N, Mitra S, Hines RL. Peripheral opioid receptor agonists for analgesia: a comprehensive review. J Opioid Manag. 2011 Jan-Feb;7(1):55–68. doi: 10.5055/jom.2011.0049. [DOI] [PubMed] [Google Scholar]
- 38.Vanderah TW. Delta and kappa opioid receptors as suitable drug targets for pain. Clin J Pain. 2010 Jan;26(Suppl 10):S10–S15. doi: 10.1097/AJP.0b013e3181c49e3a. [DOI] [PubMed] [Google Scholar]
- 39.Vanderah TW, Largent-Milnes T, Lai J, Porreca F, Houghten RA, Menzaghi F, Wisniewski K, Stalewski J, Sueiras-Diaz J, Galyean R, Schteingart C, Junien JL, Trojnar J, Rivière PJ. Novel D-amino acid tetrapeptides produce potent antinociception by selectively acting at peripheral kappa-opioid receptors. Eur J Pharmacol. 2008 Mar 31;583(1):62–72. doi: 10.1016/j.ejphar.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 40.Vanderah TW, Schteingart CD, Trojnar J, Junien JL, Lai J, Riviere PJ. FE200041 (D-Phe-DPhe-D-Nle-D-Arg-NH2): A peripheral efficacious kappa opioid agonist with unprecedented selectivity. J Pharmacol Exp Ther. 2004 Jul;310(1):326–333. doi: 10.1124/jpet.104.065391. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y, Bai G, Ro JY. Differential regulation of peripheral opioid receptor subtype expressions following inflammation in the rat masseter muscle. Washington, DC: Society for Neuroscience Meeting; 2005. [Google Scholar]