Abstract
Background
Clinical guidelines recommend against routine prostate specific antigen (PSA) screening in older men and those with lower life expectancies. We examined providers’ decision-making regarding discontinuing PSA screening.
Methods
We administered a survey of primary providers from a large, university-affiliated primary care practice. Providers were asked about their current screening practices, factors that influence their decision to discontinue screening, and barriers to discontinuing screening. Bivariate and multivariable logistic regression analyses were used to examine whether taking age and/or life expectancy into account and barriers to discontinuing were associated with clinician characteristics and practice styles.
Results
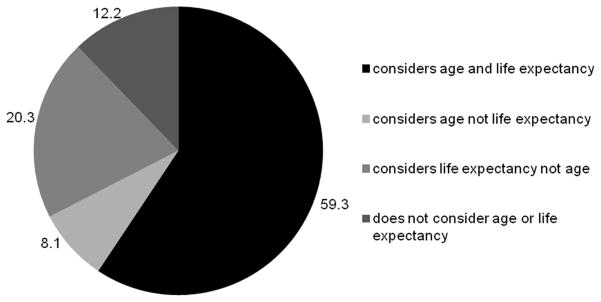
88.7% of providers participated in the survey (125 out of 141). Over half (59.3%) took both age and life expectancy into account whereas 12.2% did not consider either in their decisions to discontinue PSA screening. Providers varied with the age they typically stop screening and majority (66.4%) report difficulty in assessing life expectancy. Taking patient age and life expectancy into account was not associated with provider characteristics or practice styles. The most frequently cited barriers to discontinuing PSA screening were patient expectation (74.4%) and time constraints (66.4%). Black providers were significantly less likely than non-black providers to endorse barriers related to time constraints and clinical uncertainty, though these results are limited by the small sample size of black providers.
Conclusion
Though age and life expectancy often figure prominently in decisions to employ screening, providers face multiple barriers to discontinue PSA routine screening,
BACKGROUND
Since 2008, US Preventive Services Task Force (USPSTF) recommendations have advised against routine prostate specific antigen (PSA) screening for prostate cancer in men age 75 and over. The American Cancer Society and American Urological Association recommend incorporating life-expectancy into decisions regarding PSA screening. Draft recommendations released by the USPSTF in October 2011 discourage routine PSA screening for all men.
The recommendations suggest that the potential harms associated with PSA screening in older men and in those with limited life expectancies may outweigh the potential benefits.1,2 Patients who undergo a biopsy for an elevated PSA may experience psychological distress3 and infectious complications.4 The diagnosis and treatment of prostate cancer may lead to lower health- related quality of life;5,6 cause urinary, bowel, and erectile dysfunction;7 and result in high financial costs.8–11 A reduction in prostate cancer-specific mortality from PSA screening has not been demonstrated in older men.12
Nonetheless, screening among older men and those with limited life expectancy remains high: an estimated 57% of men aged 75 to 79 years13 and one third of men with low life expectancies received screening within the past year.14 A physician’s recommendation for PSA screening has been found to be the strongest predictor of whether a patient will undergo screening.13,15 However, little is known about what factors are important to providers in discontinuing prostate cancer screening. Previous research on colorectal cancer has reported that decisions to stop screening are complex and involve an assessment of patients’ clinical factors and functional status.16,17
We sought to examine to what extent providers report taking age and life expectancy into account when ordering PSA screening and to determine what barriers clinicians face when discontinuing PSA screening. We further tested whether taking age and life expectancy into account varied by provider clinical training, race/ethnicity, and other factors.
METHODS
Setting
Johns Hopkins Community Physicians (JHCP) is a university-affiliated practice comprised of 26 outpatient sites in 11 counties in Maryland. In 2010, approximately 40,000 patients ages 40 and over who were eligible for prostate cancer screening were seen at a JHCP site. The patient population of the clinics is diverse—27% of those eligible for PSA screening were non-white and 3 outpatient sites are located in medically underserved areas of the metropolitan area of Baltimore.
Survey design
The survey was developed in conjunction with the leadership of the Johns Hopkins Community Physicians (JHCP) and pretested by primary care providers practicing in the city of Baltimore who were not affiliated with JHCP.
Survey distribution
The survey was distributed at a yearly provider meeting. A total of 141 individuals who provide primary care for adult male patients attended the meeting and were eligible to participate. The survey was a self-administered and took approximately 10 minutes to complete. Providers were compensated $10 for their participation.
Age and PSA screening
On the survey, providers were told, “Practice guidelines vary about what age to stop PSA screening,” and were asked if there was an age at which they typically stop screening an otherwise healthy man. For those who responded affirmatively, they were asked at what age.
Role of life expectancy
Providers were asked how strongly they agreed or disagreed with the statement, “I frequently take life expectancy into account when deciding not to order PSA screening.” Providers who agreed or strongly agreed were considered to take life expectancy into account. They were then asked how easy or difficult it was for them to estimate a patient’s 10 year life expectancy in clinical practice with responses: ‘very easy’, ‘somewhat easy’, ‘neither easy nor difficult’, ‘somewhat difficult’, and ‘very difficult.’
Attitudes towards stopping PSA testing
To determine attitudes and beliefs regarding stopping PSA testing in patients who had previously been receiving routine screening, we asked providers the extent to which they agreed or disagreed with a list of statements (see Table 2). The statements were developed based on previous survey instruments,18 qualitative research16,19 and in pre-testing with providers in Baltimore. Responses were dichotomized into agree and strongly agree versus neither agree nor disagree, disagree, and strongly disagree.
Practice styles with PSA screening
Provider patterns of PSA screening were based on a single item developed by Linder and colleagues: “Over the past year, which best described your approach to prostate cancer screening with a healthy, 50-year-old man who has no other risk factors and is otherwise a candidate for screening?” with the following response options: “I generally ordered a PSA test without discussing the possible harms and benefits with the patient”, “I generally discussed the possible harms and benefits of screening with the patient, and then recommended the test”, “I generally discussed the possible harms and benefits of screening with the patient, and then let him decide whether or not to have the test”, “I generally discussed the possible harms and benefits of screening with the patient, and then recommended against the test”, and “I generally did not order the PSA test nor discussed the possible harms and benefits with the patient”.
Providers were asked how frequently, over the past year, they recommended screening in men who opted for screening with response options as “yearly”, “every other year”, or “other”. They were also asked whether they felt comfortable or uncomfortable with their ability to answer patients’ questions about PSA screening with responses ranging from very comfortable to very uncomfortable.
Demographic and clinical characteristics
The survey instrument included items on provider training (internal medicine, family medicine, internal medicine/pediatrics, and other), years since residency graduation (less than 5 years, 5 to less than 10 years, and 10 and more years) gender, and race/ethnicity.
Statistical Analysis
Descriptive analyses quantified responses on questions related to use of age and life expectancy in screening decisions and perceived barriers to screening. Bivariate analyses tested the association between (a) provider use of age and/or life expectancy with perceived barriers to screening, (b) provider use of age and/or life expectancy in screening with clinician characteristics (gender, training, race/ethnicity, and years since residency) and practice styles (patterns of PSA screening, comfort with PSA discussions, and frequency of PSA screening), and (c) perceived barriers to screening with clinician characteristics and practice styles. Multivariable logistic regression models were constructed that simultaneously adjusted for all clinician characteristics and practice styles. Separate models were built for each of the main outcomes: use of age and/or life expectancy and perceived barriers to screening. Rates of missingness for each variable were less than 3%, and observations with missing values were excluded from analyses. All analyses were conducted with Stata version 10.0 (College Station, TX). This study was approved by the Johns Hopkins University School of Medicine Institutional Review Board.
RESULTS
The response rate was 88.7% (125 out of 141). The demographic characteristics of the participants are listed in Table 1. 62.9% of the providers were female and 11.5% were black. Approximately half were internal medicine trained and the majority (67.7%) had finished residency over 10 years ago. 17.2% order PSA screening without discussing it with their patients and 33.6% discuss PSA screening and typically recommend it. When providers order PSA screening, they overwhelmingly (90%) order it on an annual basis. Providers typically felt comfortable answering patients’ questions about PSA screening (89%).
Table 1.
Demographic and practice style characteristics of Johns Hopkins Community Physicians (JHCP) survey respondents
| N (%) | 125 (100%)* |
|---|---|
| Gender | |
| Male | 47 (27.9) |
| Female | 77 (62.1) |
| Training | |
| internal medicine | 60 (48.8) |
| family practice | 48 (39.0) |
| medicine/pediatrics | 15 (12.2) |
| Years since residency | |
| <5 | 17 (13.8) |
| 5 to <10 | 23 (18.7) |
| 10+ | 83 (67.5) |
| Race/ethnicity | |
| White | 74 (60.7) |
| Black | 14 (11.5) |
| Asian | 26 (21.3) |
| Other | 8 (6.6) |
| Approach to PSA screening over the previous year | |
| order PSA without discussion | 21 (17.2) |
| discuss and recommend | 41 (33.6) |
| discuss and let patient decide | 53 (43.4) |
| discuss and recommend against | 5 (4.1) |
| do not order PSA | 2 (1.6) |
| Comfort with PSA discussions | |
| very/somewhat comfortable | 109 (89.3) |
| neutral/uncomfortable | 13 (10.7) |
| Frequency they typically recommended PSA screening | |
| on a yearly basis | 111 (90.2) |
| less than yearly | 12 (9.8) |
total numbers for individual variables may be less than 125 due to missing responses
Age to discontinue PSA screening
Nearly a third of providers (32.5%) said that they did not have an age at which they typically stop recommending PSA screening. Among the 67.5% who said they discontinued screening based on patient age, 26.8% discontinue screening at age 70 or below, 52.4% at age 75, and 20.7% age 80 or higher.
Incorporating life expectancy
The majority of providers agreed or strongly agreed that they typically take life expectancy into account when ordering PSA screening (76.8%). Providers who stated they have an age when they typically discontinued screening were also significantly more likely to take life expectancy into account (Figure 1). However, 66.4% of providers described it as very or somewhat difficult to estimate life expectancy in clinical practice. Providers who had difficulty estimating life expectancy were not significantly less likely to take life expectancy into account compared to those who do not report difficulty. In this setting, the majority of providers (69.1%) thought it would be helpful to have a clinical decision support tool that would help predict mortality.
Figure 1.
Providers who have an age at which they typically discontinue screening and those who typically take life expectancy into account (N=123 who answered both questions)
Barriers to discontinuing PSA screening
Providers endorsed multiple barriers to stopping PSA testing among men who had been previously receiving PSA testing (Table 2). The most frequently cited reason was “My patients expect me to continue getting yearly PSA tests” (74.4% of providers agreed) followed by “It takes more time to explain why I’m not screening than to just continue screening” (66.4%). Agreement with the barriers did not differ among providers who did and did not take age/life expectancy into account.
Table 2.
Number and percent of providers who agree or strongly agree with barriers to discontinuing PSA screening,* and the odds of agreement by provider race
| Total | Odds ratio for agreement with barrier, Black providers compared to non-Black providers (95% Confidence Interval)§ | |
|---|---|---|
| N (%) | 125 (100) | |
| My patients expect me to continue getting yearly PSA tests. | 64 (74.4) | 0.25 (0.07–0.92) |
| It takes more time to explain why I’m not screening than to just continue screening. | 83 (66.4) | 0.18 (0.05–0.72) |
| By not ordering a PSA, it puts me at risk for malpractice. | 67 (54.0) | 0.73 (0.20–2.61) |
| I am uncomfortable with the uncertainty if I discontinue screening. | 54 (43.6) | 0.16 (0.03–0.81) |
| Patients have been told by other doctors to get a PSA test and I’m reluctant to go against their advice. | 53 (42.4) | 0.29 (0.06–1.44) |
| If I stop PSA testing in my older patients, they will think I am trying to cut costs. | 33 (26.6) | 1.20 (0.30–4.81) |
| If I stop PSA testing, my patients will think I am ‘giving up’ on them. | 23 (18.4) | 0.29 (0.03–2.57) |
Agreement/Disagreement was on a 5-point scale; missing observations were excluded from analysis.
simultaneously adjusted for clinician characteristics (gender, training, race/ethnicity, and years since residency) and practice styles (patterns of PSA screening, comfort with PSA discussions, and frequency of PSA screening). Bivariate analyses not presented due to small cell sizes and concerns over confidentiality.
Characteristics associated with PSA screening
We examined if there was variation in using age and/or life expectancy by clinician characteristics and practice styles. In bivariate analyses, black providers were significantly less likely to have an age when they discontinued screening compared to non-black providers (percentages not reported due to small cell sizes and concerns over confidentiality, p=0.044). This result was not statistically significant after simultaneously adjusting for all clinician characteristics and practice styles. Taking life expectancy into account was not associated with provider race, other clinical characteristics, or practice styles in bivariate or multivariable logistic regression analyses.
Similarly, perceived barriers to discontinuing PSA screening varied by provider race but did not vary with other clinician characteristics or practice styles. In multivariable logistic regression analyses (Table 2), black providers were significantly less likely than non-black providers to agree with the following barriers: “It takes more time to explain why I’m not screening than to just continue screening” (odds ratio [OR] 0.18, 95% confidence interval [CI] 0.05–0.72); “I am uncomfortable with the uncertainty if I discontinue screening” (OR 0.16, 95% CI 0.03–0.81); and “My patients expect me to continue getting yearly PSA tests” (OR 0.25, 95%CI 0.07–0.92). Results of analyses remained consistent after excluding providers who generally did not order PSA screening (i.e. those who discouraged PSA screening after a discussion or those who did not discuss and did not order it; N=7).
DISCUSSION
Age and life expectancy were frequently but not universally used by primary care providers in a university-affiliated primary care network deciding whether to discontinue prostate cancer screening. The majority of these providers said they incorporate both factors into their recommendations. For these providers, it will be necessary to assist them with tools that may more accurately determine life expectancy in clinical practice. A minority of providers did not consider either age or life expectancy in their screening decisions. For these providers, it may be necessary to focus on education regarding current recommendations. Across both groups of providers, understanding and addressing barriers regarding patient expectations, time constraints, and malpractice fears are critical.
Even among JHCP providers who said they utilize life expectancy in screening decisions, many said they find it challenging to estimate it in clinical practice, and the majority of providers believed that having a tool to estimate life expectancy at the point of care would be beneficial. Provider’s difficulty with estimating life expectancy has been documented in studies on colon cancer screening,16,17 and prognostic indices for older adults require additional testing and validation.20 Providing doctors with life expectancy estimates from life tables has been shown to change screening recommendations using clinical vignettes;17 however, further research is necessary to test not only the most appropriate tool to calculate life expectancy during the clinical encounter but also whether giving providers such a tool would change decisions regarding PSA screening.
Along with difficulty estimating life expectancy, many providers reported that patient expectations were a barrier to discontinuing screening. This is consistent with studies on older patients’ expectations about discontinuing cancer screening. Interviews with older adults found that 62% of older adults believed their own life expectancy was not important in their decisions to continue cancer screening and 48% did not want to discuss their life expectancy with their clinician.21 Other studies have found that patients with limited life expectancies want to discuss this with their doctor,22 and Smith and colleagues have advocated that doctors should routinely offer to discuss prognosis with patients who have a limited life expectancy or at least by the time a patient turns 85 years old.23 Along with providing clinicians with better tools to estimate life expectancy, providers may need training in how to engage in these conversations and adequate time to do so.
The barriers that providers endorsed in discontinuing screening were similar to barriers that have been found to engaging in shared decision-making more generally. Davis and colleagues identified that competing clinical priorities (95.5%) was the most important barrier to shared decision-making followed by lack of time (80.5%) and level of patient interest (69.9%).24 To the extent that these barriers may be addressed, it may help lead to better shared decision-making and higher rates of discontinuing screening. With their emphasis on patient-centeredness, use of physician extenders, and changes in payment mechanisms (AHRQ), primary care medical homes may provide an important opportunity to mitigate these barriers.25
With the exception of provider race/ethnicity, providers who used age and life expectancy did not have different characteristics (years since residency, gender, training) compared to providers who did not use these factors. Prior research has found that black physicians were more likely to encourage PSA screening,26,27 potentially due to the higher burden of prostate cancer among black men. While no less likely to report taking age and life expectancy into account when deciding about PSA testing, black providers were significantly less likely than non-black providers to report barriers to discontinuing screening. In particular, time constraints and clinical uncertainty were seen by fewer black providers as significant barriers. The reasons for these differences are uncertain, though some of the variation may be due to underlying differences in patient panels.
This study has several potential limitations. First, despite the high response rate, non-response bias remains a concern. Responders were not significantly different from all providers who attended the retreat with respect to sex, training, and years since residency graduation. Second, questions were based on self-report which may not accurately correlate with objective measures of screening patterns and patterns of discontinuing. Third, the study was conducted shortly after the release of the USPSTF draft recommendations, which may alter providers’ perceptions of PSA screening and their response to the survey, although the recommendation against routine screening in men 75+ years old has been in place since 2008. Fourth, the providers all worked within JHCP, potentially limiting the generalizability of this study. These providers, however, represent a range of practice sizes and provide care to a diverse patient population across the state of Maryland. Their practice patterns are comparable to those found in studies of other providers,28 and the overall rates of PSA testing in 2010 (26.0% of patients over 40) are the same as the rates of PSA screening nationwide.14 Nonetheless, the results should be interpreted in the context of a single, regional health care system and it would be important to test whether similar associations are found in other health care settings and outside of Maryland. Fifth, we were unable to control for some aspects of practice and training that may affect outcomes (e.g. payer type, number of days per week in clinical practice). Last, additional factors may influence a provider’s decision to discontinue PSA screening. Though we did not ask whether factors such as prior PSA level, PSA velocity, and family history influence decisions to discontinue screening, 43% of providers stated that they often or always altered the frequency of screening based on a patient’s prior PSA level and 48% did so based on family history. These and other factors may be important when deciding to discontinue screening for an individual patient.29,30
In summary, we found that the majority of providers in a university-affiliated primary care practice consider both age and life expectancy when deciding on prostate cancer screening. However, when they do take these factors into account, there is a lack of agreement over at which age to discontinue testing and they reported difficulty in assessing life expectancy. The results suggest that, for some providers, interventions will need to teach clinicians to take age and life expectancy into account when deciding whether to initiate and continue screening. For providers that already consider these factors, the development of tools that help clinicians more accurately and quickly estimate life expectancy may be an important next step. Across both scenarios, addressing the barriers that make it difficult to stop testing—including patient expectation, lack of time, and fear of malpractice litigation—will be necessary in order to reduce the high rates of PSA screening in men who are least likely to benefit.
Acknowledgments
Funding: The Maryland Cigarette Restitution Fund at Johns Hopkins provided funding. Dr. Pollack was supported by a career development award from the National Cancer Institute and the Office of Behavioral and Social Sciences Research (K07 CA151910). Dr. Bhavsar was supported by a training grant from the Agency for Healthcare Research and Quality (T32 HS019488).
We thank Justin Bekelman for his advice on the manuscript and Erin Murphy for her assistance with the survey.
References
- 1.Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 2.Wilt TJ, Brawer MK, Barry MJ, et al. The Prostate cancer Intervention Versus Observation Trial:VA/NCI/AHRQ Cooperative Studies Program #407 (PIVOT): design and baseline results of a randomized controlled trial comparing radical prostatectomy to watchful waiting for men with clinically localized prostate cancer. Contemp Clin Trials. 2009;30:81–87. doi: 10.1016/j.cct.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Macefield RC, Metcalfe C, Lane JA, et al. Impact of prostate cancer testing: an evaluation of the emotional consequences of a negative biopsy result. Br J Cancer. 2010;102:1335–1340. doi: 10.1038/sj.bjc.6605648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loeb S, Carter HB, Berndt SI, Ricker W, Schaeffer EM. Complications After Prostate Biopsy: Data From SEER-Medicare. J Urol. 2011;186(5):1830–1834. doi: 10.1016/j.juro.2011.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balderson N, Towell T. The prevalence and predictors of pscyhological distress in men with prostate cancer who are seeking support. Br J Health Pscyhol. 2003;8:125–134. doi: 10.1348/135910703321649114. [DOI] [PubMed] [Google Scholar]
- 6.Cliff AM, MacDonagh RP. Psychosocial morbidity in prostate cancer: II. A comparison of patients and partners. BJU Int. 2000;86:834–839. doi: 10.1046/j.1464-410x.2000.00914.x. [DOI] [PubMed] [Google Scholar]
- 7.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250–1261. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 8.Snyder CF, Frick KD, Blackford AL, et al. How does initial treatment choice affect short-term and long-term costs for clinically localized prostate cancer? Cancer. 2010;116:5391–5399. doi: 10.1002/cncr.25517. [DOI] [PubMed] [Google Scholar]
- 9.Heijnsdijk EA, der Kinderen A, Wever EM, Draisma G, Roobol MJ, de Koning HJ. Overdetection, overtreatment and costs in prostate-specific antigen screening for prostate cancer. Br J Cancer. 2009;101:1833–1838. doi: 10.1038/sj.bjc.6605422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yabroff KR, Lamont EB, Mariotto A, et al. Cost of Care for Elderly Cancer Patients in the United States. J Natl Cancer Inst. 2008;100:630–641. doi: 10.1093/jnci/djn103. [DOI] [PubMed] [Google Scholar]
- 11.Mariotto AB, Robin Yabroff K, Shao Y, Feuer EJ, Brown ML. Projections of the Cost of Cancer Care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103:117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chou R, LeFevre ML. Prostate Cancer Screening—The Evidence, the Recommendations, and the Clinical Implications. JAMA. 2011;306:2721–2722. doi: 10.1001/jama.2011.1891. [DOI] [PubMed] [Google Scholar]
- 13.Bellizzi KM, Breslau ES, Burness A, Waldron W. Prevalence of Cancer Screening in Older, Racially Diverse Adults: Still Screening After All These Years. Arch Intern Med. 2011;171:2031–2037. doi: 10.1001/archinternmed.2011.570. [DOI] [PubMed] [Google Scholar]
- 14.Drazer MW, Huo D, Schonberg MA, Razmaria A, Eggener SE. Population-based patterns and predictors of prostate-specific antigen screening among older men in the United States. J Clin Oncol. 2011;29:1736–1743. doi: 10.1200/JCO.2010.31.9004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffman RM, Couper MP, Zikmund-Fisher BJ, et al. Prostate cancer screening decisions: results from the National Survey of Medical Decisions (DECISIONS study) Arch Intern Med. 2009;169:1611–1618. doi: 10.1001/archinternmed.2009.262. [DOI] [PubMed] [Google Scholar]
- 16.Lewis CL, Griffith J, Pignone MP, Golin C. Physicians’ Decisions About Continuing or Stopping Colon Cancer Screening in the Elderly: A Qualitative Study. J Gen Intern Med. 2009;24:816–821. doi: 10.1007/s11606-009-1006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis CL, Moore CG, Golin CE, Griffith J, Tytell-Brenner A, Pignone MP. Resident Physicians’ Life Expectancy Estimates and Colon Cancer Screening Recommendations in Elderly Patients. Med Decis Making. 2008;28:254–261. doi: 10.1177/0272989X07311756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunn AS, Shridharani KV, Lou W, Bernstein J, Horowitz CR. Physician-Patient Discussions of Controversial Cancer Screening Tests. Am J Prev Med. 2001;20:130–134. doi: 10.1016/s0749-3797(00)00288-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guerra CE, Jacobs SE, Holmes JH, Shea JA. Are physicians discussing prostate cancer screening with their patients and why or why not? A pilot study. J Gen Intern Med. 2007;22:901–907. doi: 10.1007/s11606-007-0142-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yourman LC, Lee SJ, Schonberg MA, Widera EW, Smith AK. Prognostic Indices for Older Adults. JAMA. 2012;307:182–192. doi: 10.1001/jama.2011.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis CL, Kistler CE, Amick HR, et al. Older adults’ attitudes about continuing cancer screeningg later in life: a pilot study interviewing residents of two continuing care communities. BMC Geriatrics. 2006;6:10. doi: 10.1186/1471-2318-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Back AL, Arnold RM. Discussing prognosis: “how much do you want to know?” Talkign to patients who do not want information or who are ambivalent. J Clin Oncol. 2006;24:4314–4217. doi: 10.1200/JCO.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 23.Smith AK, Williams BA, Lo B. Discussing Overall Prognosis with the Very Elderly. N Engl J Med. 2011;365:2149–2151. doi: 10.1056/NEJMp1109990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis K, Haisfield L, Dorfman C, Krist A, Taylor KL. Physicians’ attitudes about shared decision making for prostate cancer screening. Fam Med. 2011;43:260–266. [PMC free article] [PubMed] [Google Scholar]
- 25.Agency for Health Care Research and Quality. [Accessed February 6, 2012.];Patient Centered Medical Home Resource Center, Defining the PCMH. 2012 http://pcmh.ahrq.gov/portal/server.pt/community/pcmh__home/1483/PCMH_Defining%20the%20PCMH_v2.
- 26.Hall I, Taylor Y, Ross L, Richardson L, Richards T, Rim S. Discussions About Prostate Cancer Screening Between U.S. Primary Care Physicians and Their Patients. J Gen Intern Med. 2011;26:1098–1104. doi: 10.1007/s11606-011-1682-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stroud L, Ross LE, Rose SW. Formative evaluation of the prostate cancer screening practices of African-American physicians. J Natl Med Assoc. 2006;98:1637–1643. [PMC free article] [PubMed] [Google Scholar]
- 28.Linder SK, Hawley ST, Cooper CP, Scholl LE, Jibaja-Weiss M, Volk RJ. Primary care physicians’ reported use of pre-screening discussions for prostate cancer screening: a cross-sectional survey. BMC Fam Pract. 2009;10:19. doi: 10.1186/1471-2296-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schaeffer EM, Carter HB, Ketterman A, et al. Prostate Specific Antigen Testing Among the Elderly – When to Stop? J Urol. 2009;181:1606–14. doi: 10.1016/j.juro.2008.11.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang P, Sun L, Uhlman MA, Robertson CN, Polascik TJ, Moul JW. Prostate-specific antigen velocity based risk-adapted discontinuation of prostate cancer screening in elderly men. BJU Int. 2010;108:44–48. doi: 10.1111/j.1464-410X.2010.09812.x. [DOI] [PubMed] [Google Scholar]