Abstract
Objective
As cartilage loss and bone marrow lesions (BMLs) are associated with knee joint pain and structural worsening, this study assessed whether non-invasive estimates of articular contact stress may longitudinally predict risk for worsening of knee cartilage morphology and BMLs.
Design
This was a longitudinal cohort study of adults aged 50-79 years with risk factors for knee osteoarthritis. Baseline and follow-up measures included WORMS classification of knee cartilage morphology and BMLs. Tibiofemoral geometry was manually segmented on baseline MRI, and 3D tibiofemoral point clouds were registered into subject-specific loaded apposition using fixed-flexion knee radiographs. Discrete element analysis (DEA) was used to estimate mean and peak contact stresses for the medial and lateral compartments. The association of baseline contact stress with worsening cartilage and BMLs in the same sub-region over 30 months was assessed using conditional logistic regression.
Results
Subjects (N=38, 60.5% female) had a mean±SD age and BMI of 63.5±8.4 years and 30.5±3.7 kg/m2 respectively. Elevated mean articular contact stress at baseline was associated with worsening cartilage morphology and worsening BMLs by 30-months, with OR (95%CI) of 4.0 (2.5, 6.4) and 6.6 (2.7, 16.5) respectively. Peak contact stress also was significantly associated with worsening cartilage morphology and BMLs {1.9 (1.5, 2.3) and 2.3 (1.5, 3.6)}(all p<0.0001).
Conclusions
Detection of higher contact stress 30 months prior to structural worsening suggests an etiological role for mechanical loading. Estimation of articular contact stress with DEA is an efficient and accurate means of predicting sub-region-specific knee joint worsening and may be useful in guiding prognosis and treatment.
Keywords: joint loading, biomechanics, osteoarthritis, bone marrow lesions, cartilage loss
INTRODUCTION
The pathogenesis of knee osteoarthritis (OA) is complex and multifactorial, involving both biomechanical and biochemical processes. Focal overloading of the joint surface likely plays an important role in both initiation and progression of OA1. If the complex processes of knee OA could be obviated, a substantial cause of disability could be avoided2, 3. Many epidemiological studies have revealed population-based risk factors, but an inability to assess subject-specific focal overloading and its contribution to OA risk has until recently remained a critical barrier to assessing individual risk.
Magnetic resonance imaging (MRI) has enabled visualization of subchondral bone marrow lesions (BMLs), a feature with prognostic significance for both knee joint anatomic worsening,4-7 and symptomatic and functional decline8. BMLs are characterized by subchondral areas of high signal intensity in the distal femur or proximal tibia on T2 or proton density-weighted, fat suppressed fast spin echo or short tau inversion recovery (STIR) images7, 9. These may represent areas of osteonecrosis10, edema and bony remodeling11, ischemia and/or reperfusion injury to the overlying cartilage plate12, or impaired venous drainage leading to bone marrow congestion13, 14. It is notable that BMLs are associated with age, as well as mechanical factors such as obesity5, 8, 15, 16, malalignment6, and joint trauma8, 15, 17-22, all of which are risk factors for knee OA. In knees with symptomatic OA, more severe BMLs are predictive of progression of structural worsening5, 23, 24.
Worsening of BMLs and development of new BMLs also are associated with more rapid cartilage loss compared with when BMLs remain stable24, 25. The clinically meaningful anatomic sequellae of BMLs can guide recommendations for physical activity, in that asymptomatic older adults who participate in vigorous physical activity with BMLs have been found to be at increased risk for worsening of medial tibiofemoral cartilage defects and cartilage volume loss26. These findings suggest that BMLs have a pathogenic role in pre-clinical knee OA and may be useful as a target for prevention. Importantly, BMLs not only can increase in size, but also can resolve over time27, 28. Furthermore, correction of lower limb alignment has been shown to result in regression of BMLs29.
The ability to predict development and worsening of BMLs could inform therapies to avoid knee OA and disability later in life. In asymptomatic middle-aged adults, the development of new BMLs is associated with progressive tibiofemoral cartilage loss, while resolution of BMLs is associated with reduced cartilage loss30. In addition, resolution of BMLs has been associated with resolution of pain31. These results suggest that BMLs may be a modifiable risk factor for knee pain and knee OA.
Given the strong association between BMLs and cartilage damage and pain, in the context that most BMLs are potentially reversible9, identification of the cause as well as the mechanism by which BMLs lead to structural damage could significantly impact clinical practice to reduce disease and disability through informing the design of therapies to minimize worsening. While the exact etiology of BMLs is not known, it is likely that adverse loading plays a role32. Lower limb malalignment has been associated with a higher prevalence of tibiofemoral BMLs, in a compartment-specific fashion6. These associations, in combination with the clinical finding that BMLs occur in an injury-specific pattern following joint trauma, support the notion that increased articular contact stress may contribute to the formation of BMLs.
Measures of limb alignment33 and frontal plane moments during gait34 have provided insights into the role of altered joint loading in the development and progression of OA, but they do not directly address the issue of altered contact stress at the articular surface. Estimation of articular contact stress, using discrete element analysis (DEA), has been useful in predicting risk for incident symptomatic knee OA35. However, this technique has not been used to predict risk for structural worsening. As cartilage damage and BMLs have been cross-sectionally associated with higher compartmental loading, our purpose was to assess whether estimates of articular contact stress at baseline longitudinally predict risk for worsening of cartilage morphology and BMLs by 30-month follow-up in a community-acquired cohort. Successful prediction, using this technique could serve as a base upon which preventive and therapeutic interventions could be developed to improve public health for the growing population of adults at risk for disabling knee joint damage.
METHODS
Subjects
This study was conducted within the Multicenter Osteoarthritis (MOST) Study cohort of 3,026 adults with or at high risk for knee OA36. The MOST Study recruited 3026 community-dwelling men and women, age 50–79 years, drawn from the general population, but selected so as to be likely either to have preexisting OA or be at elevated risk based on frequent knee symptoms, history of knee injury or surgery or being overweight or obese. Exclusion criteria included bilateral knee replacement, cancer or rheumatologic disease.
For this ancillary study, a random sample of knees from the MOST cohort (1 knee per participant) were selected for this study. Eligible knees had no missing values for baseline and 30-month MRI readings of cartilage morphology or BMLs in the four central tibiofemoral subregions, and either the presence of case and control subregions for BML change (eligible for matched case-control study on BML change) or the presence of both case and control subregions for cartilage morphology change (eligible for matched case-control study on cartilage morphology change). The study protocol was approved by the institutional review boards of the participating centers.
Subject Measurements
Height in centimeters (stadiometer, Holtain, Wales, UK) and mass in kilograms (balance beam scale) were measured by trained and certified staff and body mass index (kg/m2) was calculated. Frequent knee pain was considered present if the answer to question “during the past 30 days, have you had any pain, aching, or stiffness in your left (right) knee” was “yes” on both the telephone interview and the clinic visit.35-37
Tibiofemoral Contact Stress Estimates
Tibiofemoral geometry was manually segmented from baseline knee MRIs, obtained using a coronal short T1 inversion recovery pulse sequence (ONI Medical Systems, Inc, Wilmington, MA). The segmentations were completed using an interactive pen display and the OsiriX software (The OsiriX Foundation, Geneva, Switzerland)35. The reproducibility of mean contact stress estimates obtained from multiple independent segmentations of ten knees were assessed. Bone surfaces were segmented by three raters, with two of the raters repeating the segmentations on non-consecutive days. Shrout-Fleiss Intraclass Correlation Coefficients (ICC 2,1) for the computed mean contact stresses were 0.91–0.97 for inter-rater reliability and 0.84–0.97 for dayto-day reliability.
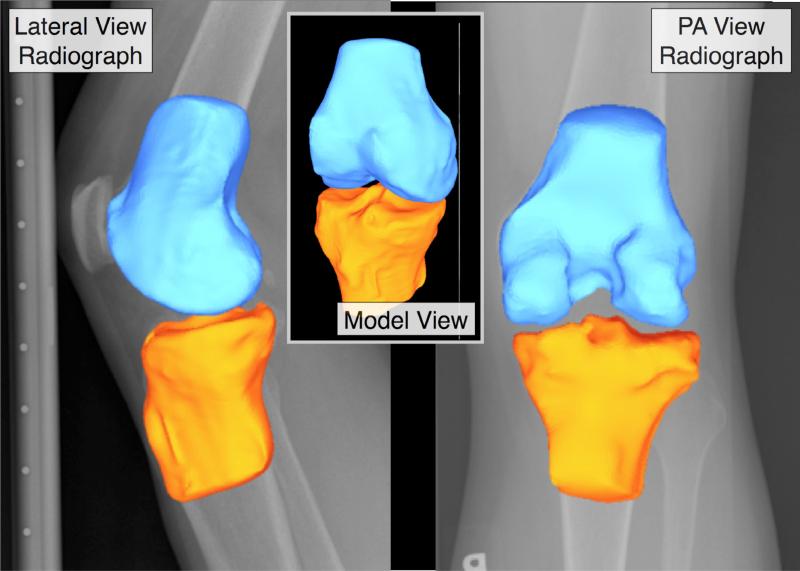
The resulting point clouds from the tibia and femur bone segmentations were wrapped as triangulated surfaces consisting of 10,000-15,000 triangles each, using Geomagic Studio software (Geomagic, Inc., Research Triangle Park, NC). Each bone surface model was smoothed, and then registered to a PA fixed-flexion radiograph of the knee using automated MATLAB algorithms (The Mathworks, Natick, MA) (Figure 1).
Figure 1.
Registration of 3-D surfaces to weight bearing configurations using radiographs.
The alignment algorithm utilized an optimization approach to minimize the difference between the silhouette of a segmented bone model obtained using a ray-casting technique and bone edges identified on PA and lateral fixed-flexion radiographs. First the radiographic scene was recreated to match the MOST clinical acquisition protocol38, and the fixed flexion radiographic image was placed into the scene to coincide with the film/detector plane. The segmented bone model was then loaded into the scene in a nominal initial position. Rays were cast from the x-ray source to the silhouette edges of the model and intersected with the film plane, creating a set of points that defined edge vertices projected onto the film. These projections were then connected using a line drawing algorithm to create a continuous contour representing the bone edge. This contour was compared to a semi-automated segmentation of bone edges from the fixed flexion radiograph. Comparison between the ray-casted contour and the segmented radiographic edge provided a basis for a cost function to align the bone model. A Monte Carlo simulation algorithm was then used to adjust the translations and rotations of the bone model to minimize the difference between the radiograph edge and the model contour. As the simulation proceeded, the algorithm was constrained to a smaller and smaller search space, until convergence to an optimal solution was achieved.
Following alignment, articular contact stresses were calculated using a validated discrete element analysis (DEA) algorithm written in MATLAB39. The stress analysis performed assumed rigid subchondral bone, with a 6 mm linear elastic cartilage layer. Nearest neighbors between facets of the apposed surfaces were computed expeditiously using a space partitioning algorithm. Each nearest neighbor pair was queried to identify and create springs between pairs, which had undergone apparent penetration. Contact stresses were then calculated using a spring model40 that relates deformation of the spring to engendered contact stress39. The overall contact force was computed from the vectorial summation of normal forces acting on each individual triangle (contact stress × triangle area). Based upon the computed contact force, the tibiofemoral apposition was adjusted in an iterative manner to obtain the translations required to achieve static equilibrium. The simulation was run in load control, utilizing a vertical loading of one-half subject body weight. The peak (maximum) and mean spatial contact stresses acting on each compartment of each knee were calculated from the DEA-computed contact stress distributions.
Measurement of BML and Cartilage Morphology
Knee MRI were performed using a 1.0 T dedicated knee system (ONI Medical Systems, OrthOne™). The protocol included axial and sagittal proton-density weighted fat-suppressed (PDFS) fast spin echo sequences (TR 4800 ms, TE 35 ms, 3.0 mm slice thickness, 0.0 mm interslice gap, FOV 14.0 cm2, matrix 288 × 192, echo train length (ETL) 8) and a STIR sequence in the coronal plane (TR 6650 ms, TE 15 ms, TI 100 ms, 3.0 mm slice thickness, 0.0 mm interslice gap, 256 × 192 matrix, FOV 14.0 cm2, ETL 8). At baseline and follow-up, two musculoskeletal radiologists assessed each tibiofemoral compartment for cartilage morphology and bone marrow lesions (BML) using the Whole-Organ Magnetic Resonance Imaging Score (WORMS) for the central medial and lateral femur, and central medial and lateral tibia. Radiologists were not blinded to order of image acquisition, but were unaware of the predictor (contact stress) measurements. The inter-reader reliability (weighted kappa) for the reading of BMLs was 0.62 and for the reading of cartilage morphology was 0.7841. Semi-quantitative assessment of BML and cartilage morphology with the WORMS scoring system can be performed accurately with the 1.0T dedicated knee scanners used for the MOST study42, and WORMS is currently believed to be the optimal method for scoring BML longitudinally43.
Statistical Analyses
A matched case-control study within subject (or knee) has been shown to provide less biased results than an unmatched study. Despite multiple adjustments, an unmatched study can still be affected by between-person differences and provide estimates of association averaged across the sample population studied44. In contrast, an M:N matched case–control study design, in which M case subregions are matched to N control subregions from the same knee, eliminates between-subject confounding and between-knee confounding (threats to internal validity in an observational study) by controlling for subject- and knee-level factors when comparing sub-regions within the same knee.
Therefore, we conducted analyses within knees, using an M:N matched case-control study design, to eliminate between-person confounding44. For cartilage worsening, each of the four central tibiofemoral sub-regions (central medial femur, central medial tibia, central lateral femur, central lateral tibia) with a baseline cartilage score less than six (ceiling), which had score increase by 30-month follow-up, was considered to have cartilage worsening. For BML worsening, sub-regions with a baseline BML score less than three (ceiling), which had a score increase by 30-month follow-up were considered to have BML worsening. Worsening included within grade worsening.45 Sub-regions not meeting these respective criteria were considered to be control regions. Only knees having at least one case and at least one control sub-region by 30-month follow-up were eligible. We used conditional logistic regression with baseline contact stress as the continuous predictor variable, and worsening of (a) cartilage morphology and (b) BMLs at 30-month follow-up as the dichotomous outcome variable. SAS 9.2 was used for statistical analyses, and an alpha level of .05 was set for statistical significance.
RESULTS
Subjects
The random sample of subjects (N=38) had a mean±SD age and BMI of 63.5±8.4 years and 30.5±3.7 kg/m2 respectively, 60.5% were female and 84.2% were Caucasian. The complete MOST cohort (N=3,026) had a mean±SD age and BMI of 62.5±8.1 years and 30.7±6.0 kg/m2 respectively, 60.2% were female and 83.3% were Caucasian. At baseline, 5.3% of subjects reported frequent knee pain35-37 in the knee being studied. Table 1 summarizes the baseline WORMS scores for the studies of worsening cartilage morphology and BMLs.
Table 1.
Percent of knees with each WORMS score at baseline
| Case-control study of cartilage worsening (N= 38 knees) | Case-control study of BML worsening (N= 38 knees) | ||||
|---|---|---|---|---|---|
| Control (N=82 subregions) | Case (N=70 subregions) | Control (N=100 subregions) | Case (N=52 subregions) | ||
| Cartilage Morphology Score | 0 | 74.4 | 27.1 | 68.0 | 23.1 |
| 2 | 9.8 | 7.1 | 9.0 | 7.7 | |
| 2.5 | 2.4 | 7.1 | 3.0 | 7.7 | |
| 3 | 9.8 | 32.9 | 12.0 | 36.5 | |
| 4 | 1.2 | 10.0 | 5.0 | 5.8 | |
| 5 | 2.4 | 12.9 | 2.0 | 17.3 | |
| 6 | 0.0 | 2.9 | 1.0 | 1.9 | |
| BML Score | 0 | 91.5 | 60.0 | 87.0 | 57.7 |
| 1 | 6.1 | 34.3 | 9.0 | 38.5 | |
| 2 | 2.4 | 5.7 | 4.0 | 3.9 | |
Contact Stresses
With medial and lateral central tibial and femoral surfaces in 38 knees, there were a total of 152 articular surfaces. The peak contact stress values averaged 5.82±1.61 MPa over the 38 knees, with a range from 2.83 to 10.45 MPa. The mean contact stress values averaged 2.47±0.54 MPa, with a range from 1.26 to 3.76 MPa.
For cartilage morphology worsening control regions, the average mean and peak contact stresses at baseline were 0.86±1.57 MPa and 2.02±3.77 MPa respectively and for case regions the average mean and peak contact stresses at baseline were 3.27±1.39 MPa and 8.02±3.68 MPa respectively. For BML worsening control regions, mean and peak contact stresses were 1.13±1.70 MPa and 2.78±4.31 MPa respectively and for case regions mean and peak contact stresses were 3.59±1.09 MPa and 8.64±2.91 MPa respectively.
Cartilage Morphology Worsening
Both higher mean articular contact stress and peak contact stress at baseline were significantly longitudinally associated with worsening cartilage morphology by 30-month follow-up (Table 2).
Table 2.
Odds ratios for the association of baseline mean and peak contact stress with (a) cartilage worsening and (b) BML worsening by 30-month follow-up. (N= 38 knees)
| Cartilage Worsening | BML Worsening | |||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Mean contact stress | 4.0 (2.5, 6.4) | <.0001 | 6.6 (2.7, 16.5) | <.0001 |
| Peak contact stress | 1.9 (1.5, 2.3) | <.0001 | 2.3 (1.5, 3.6) | <.0001 |
Bone Marrow Lesion Worsening
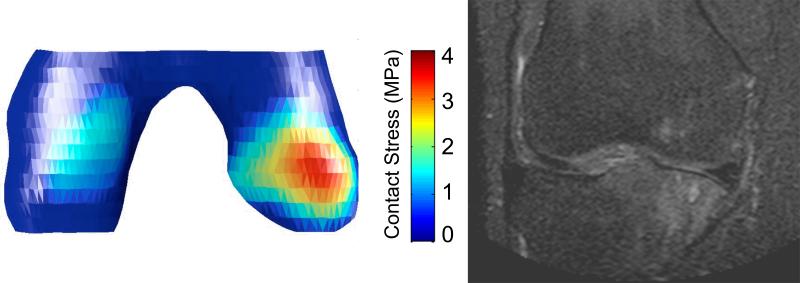
Both higher mean articular contact stress and peak contact stress at baseline were significantly longitudinally associated with worsening BMLs score by 30-month follow-up (Table 2). An illustrative case is depicted in Figure 2.
Figure 2.
Example of elevated medial contact stress at baseline and development of region-specific medial tibial and femoral BMLs on MRI by 30-month follow-up.
DISCUSSION
The results of this study indicate that higher tibiofemoral contact stress increases risk for both worsening of cartilage morphology and worsening of BMLs. These findings are consistent with the hypothesis that excessive loading within tibiofemoral joint compartments longitudinally contributes to pathology of articular cartilage and subchondral bone. Considering that BMLs predict knee symptoms7, 46, 47 and progression of structural changes including joint space narrowing6, cartilage loss5, 23, 24, 48, cartilage defects24, 49, and joint replacement50, 51, contact stress estimates derived from this implementation of DEA appear to validly predict risk for worsening of knee OA.
Prior studies have established general consensus on the range of contact stress in normal knees, with published values of peak contact stress ranging from 3 to 8 MPa, and spatial mean contact stress values ranging from 1 to 3 MPa52. The loads in these studies have varied considerably, but the results have been surprisingly consistent. The contact stress values measured in the present study were consistent with prior results, with a few knees being at the higher range of reported values. Given that the present contact stress values were obtained for a representative loading in bipedal stance, one would expect higher contact stresses for these knees during functional activities. Extrapolation to functional activities is beyond the capabilities of the current study, given expected inter-subject variability in activity frequency, intensity and type.
Use of this method of estimating contact stress has previously been shown to be an efficient and accurate means of predicting risk for development of incident symptomatic knee OA36. The results of the current study suggest that this technique may also prove useful for predicting anatomic worsening. Together, these results support utility for this technique in informing risk for both who may develop early signs of knee joint pathology and in which location in the joint.
In addition to predicting pathology, this discovery may have prognostic value for prediction of impairments and functional limitations as well. In a separate study, the MOST investigative group has found that change in BML score is strongly associated with change in knee pain53 and others have reported that resolution of BMLs is associated with resolution of pain54. The strong association between knee pain and disablement suggests that contact stress estimates may be useful for prediction of disablement as well, although further research is needed to assess this possibility.
Accurate prediction of risk for and location of BMLs may also guide therapeutic recommendations. Asymptomatic older adults with BMLs26 as well as those at risk for knee OA55 who participate in vigorous physical activity have been found to be at increased risk for worsening of medial tibiofemoral cartilage defects and cartilage volume loss. Therefore, it may be advisable to counsel those with elevated contact stress towards participation in physical activities less likely to result in structural worsening, or to assess the efficacy of interventions to attenuate risk for worsening.
Estimation of contact stress may also guide development of compartment-specific therapies to positively alter the course of knee OA. Importantly, BMLs may not only increase in size, but also may resolve over time27, 28. Correction of lower limb alignment has been shown to result in regression of BMLs29. The modeling approach used to generate estimates of compartment-specific contact stress could therefore be used to (1) guide decisions regarding which patients may benefit most from surgery, (2) guide surgical and non-surgical therapeutic planning to optimize reduction of contact stress, and (3) evaluate the efficacy of therapies for reducing damaging contact stress. Benefits could include both reduction of risk for structural worsening and disablement in later life as well as the ability to direct clinical resources to those patients at greatest risk.
Strengths of this study included the subject sampling, the availability of longitudinal highly systematic MRI readings, and the use of commonly available MRI sequences, generalizable to clinical practice. Community-dwelling adults with risk factors for knee OA, based on a history of knee injury or surgery or being overweight or obese, and in an age range in which knee OA commonly develops (50 to 79 years) were recruited. This sample was representative of those who would be most likely to benefit from development of a clinical measure to guide prevention of knee OA, making this an ideal cohort in which to test our hypotheses. The use of highly reliable longitudinal readings of MRI (WORMS) with a strict quality assurance protocol enhanced the ability of this study to assess relationships between contact stress and clinically important outcomes. Furthermore, the use of widely available MRI pulse sequences to generate a knee-specific predictive model of who will develop early OA will more easily enable translation of our findings to clinical practices regardless of magnet strength.
Implicit in the biomechanical model selection are several potential limitations, which may have affected the study findings. Neither ligaments nor menisci were included in the computational stress analysis. The rationale for this decision was based on the following. Ligaments constrain the knee primarily at extremes of motion, with notably less important contributions at the 15–20° flexion pose that was modeled. In addition, although the meniscus plays a key role in determining total area over which dynamic contact stress is distributed, our prior work supports the premise that inclusion of the meniscus is not as important when modeling a static weight-bearing pose39. In that work, in comparison with models of the same knees in which the meniscus was not included, there were only minor changes in the computed maximum contact stress values when a meniscus was included, and these changes occurred centrally on the joint surfaces.
Despite these possible limitations, contact stress estimates have been predictive of the development of incident symptomatic knee OA in prior work35, and anatomic worsening in the form of worsening cartilage morphology and BMLs in the current work. These data support utility for this approach in predicting clinically meaningful outcomes based on commonly available imaging. Further development of this approach may enable translation these initial findings to preventive strategies aimed at attenuating risk for knee OA.
Conclusion
The presence of higher estimated contact stress up to 30 months prior to development of cartilage and BML worsening suggests a role for mechanical loading in the etiology of knee joint structural worsening. Estimation of articular contact stress with discrete element analysis is an efficient and accurate means of predicting sub-region-specific knee joint worsening.
Acknowledgments
The authors thank the participants and staff of the Multicenter Knee Osteoarthritis (MOST) Study.
Role of the funding source:
This study was supported by The University of Iowa Biological Sciences Funding Program as well as NIH grants to: The University of Iowa (U01AG18832 and P50AR055533); Boston University (U01AG18820); University of Alabama (U01AG18947); University of California San Francisco (U01AG19069); and through a Paul B. Beeson Career Development Award in Aging (K23AG030945). The study sponsors (UI and NIH) were not involved in the study design; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions: Study concept and design: Segal, Niu Acquisition of data: Anderson, Kern, Lynch, Guermazi Analysis and interpretation of data: Segal, Anderson, Kern, Niu Drafting of the manuscript: Segal, Anderson Critical revision of the manuscript for important intellectual content: Segal, Anderson, Kern, Niu, Lynch, Torner, Brown, Nevitt, Guermazi Statistical Expertise: Niu Obtained funding: Torner, Nevitt Administrative, technical, or material support: Torner, Lynch, Brown, Nevitt Study supervision and take responsibility for the integrity of the work as a whole: Segal, Anderson, Niu
Competing interests:
No authors declare financial or personal relationships with other people or organizations that could potentially inappropriately influence (bias) their work and conclusions.
References
- 1.Sharma L, Song J, Felson DT, Cahue S, Shamiyeh E, Dunlop DD. The role of knee alignment in disease progression and functional decline in knee osteoarthritis. Jama. 2001;286:188–95. doi: 10.1001/jama.286.2.188. [DOI] [PubMed] [Google Scholar]
- 2.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–61. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ling SM, Fried LP, Garrett ES, Fan MY, Rantanen T, Bathon JM. Knee osteoarthritis compromises early mobility function: The Women's Health and Aging Study II. J Rheumatol. 2003;30:114–20. [PubMed] [Google Scholar]
- 4.Dieppe P, Cushnaghan J, Young P, Kirwan J. Prediction of the progression of joint space narrowing in osteoarthritis of the knee by bone scintigraphy. Ann Rheum Dis. 1993;52:557–63. doi: 10.1136/ard.52.8.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunter DJ, Zhang Y, Niu J, Goggins J, Amin S, LaValley MP, et al. Increase in bone marrow lesions associated with cartilage loss: a longitudinal magnetic resonance imaging study of knee osteoarthritis. Arthritis Rheum. 2006;54:1529–35. doi: 10.1002/art.21789. [DOI] [PubMed] [Google Scholar]
- 6.Felson DT, McLaughlin S, Goggins J, LaValley MP, Gale ME, Totterman S, et al. Bone marrow edema and its relation to progression of knee osteoarthritis. Ann Intern Med. 2003;139:330–6. doi: 10.7326/0003-4819-139-5_part_1-200309020-00008. [DOI] [PubMed] [Google Scholar]
- 7.Felson DT, Chaisson CE, Hill CL, Totterman SM, Gale ME, Skinner KM, et al. The association of bone marrow lesions with pain in knee osteoarthritis. Ann Intern Med. 2001;134:541–9. doi: 10.7326/0003-4819-134-7-200104030-00007. [DOI] [PubMed] [Google Scholar]
- 8.Guymer E, Baranyay F, Wluka AE, Hanna F, Bell RJ, Davis SR, et al. A study of the prevalence and associations of subchondral bone marrow lesions in the knees of healthy, middle-aged women. Osteoarthritis Cartilage. 2007;15:1437–42. doi: 10.1016/j.joca.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Roemer FW, Frobell R, Hunter DJ, Crema MD, Fischer W, Bohndorf K, et al. MRI-detected subchondral bone marrow signal alterations of the knee joint: terminology, imaging appearance, relevance and radiological differential diagnosis. Osteoarthritis Cartilage. 2009;17:1115–31. doi: 10.1016/j.joca.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 10.Imhof H, Breitenseher M, Kainberger F, Trattnig S. Degenerative joint disease: cartilage or vascular disease? Skeletal Radiol. 1997;26:398–403. doi: 10.1007/s002560050254. [DOI] [PubMed] [Google Scholar]
- 11.Zanetti M, Bruder E, Romero J, Hodler J. Bone marrow edema pattern in osteoarthritic knees: correlation between MR imaging and histologic findings. Radiology. 2000;215:835–40. doi: 10.1148/radiology.215.3.r00jn05835. [DOI] [PubMed] [Google Scholar]
- 12.Findlay DM. Vascular pathology and osteoarthritis. Rheumatology (Oxford) 2007;46:1763–8. doi: 10.1093/rheumatology/kem191. [DOI] [PubMed] [Google Scholar]
- 13.Arnoldi CC, Djurhuus JC, Heerfordt J, Karle A. Intraosseous phlebography, intraosseous pressure measurements and 99mTC-polyphosphate scintigraphy in patients with various painful conditions in the hip and knee. Acta Orthop Scand. 1980;51:19–28. doi: 10.3109/17453678008990764. [DOI] [PubMed] [Google Scholar]
- 14.Aaron RK, Dyke JP, Ciombor DM, Ballon D, Lee J, Jung E, et al. Perfusion abnormalities in subchondral bone associated with marrow edema, osteoarthritis, and avascular necrosis. Ann N Y Acad Sci. 2007;1117:124–37. doi: 10.1196/annals.1402.069. [DOI] [PubMed] [Google Scholar]
- 15.Baranyay FJ, Wang Y, Wluka AE, English DR, Giles GG, Sullivan RO, et al. Association of bone marrow lesions with knee structures and risk factors for bone marrow lesions in the knees of clinically healthy, community-based adults. Semin Arthritis Rheum. 2007;37:112–8. doi: 10.1016/j.semarthrit.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Kornaat PR, Kloppenburg M, Sharma R, Botha-Scheepers SA, Le Graverand MP, Coene LN, et al. Bone marrow edema-like lesions change in volume in the majority of patients with osteoarthritis; associations with clinical features. Eur Radiol. 2007;17:3073–8. doi: 10.1007/s00330-007-0711-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shelbourne KD, Jari S, Gray T. Outcome of untreated traumatic articular cartilage defects of the knee: a natural history study. J Bone Joint Surg Am. 2003;85-A(Suppl 2):8–16. doi: 10.2106/00004623-200300002-00002. [DOI] [PubMed] [Google Scholar]
- 18.Bollet AJ. Edema of the bone marrow can cause pain in osteoarthritis and other diseases of bone and joints. Ann Intern Med. 2001;134:591–3. doi: 10.7326/0003-4819-134-7-200104030-00013. [DOI] [PubMed] [Google Scholar]
- 19.Vincken PW, Ter Braak BP, van Erkel AR, Coerkamp EG, Mallens WM, Bloem JL. Clinical consequences of bone bruise around the knee. Eur Radiol. 2006;16:97–107. doi: 10.1007/s00330-005-2735-8. [DOI] [PubMed] [Google Scholar]
- 20.Costa-Paz M, Muscolo DL, Ayerza M, Makino A, Aponte-Tinao L. Magnetic resonance imaging follow-up study of bone bruises associated with anterior cruciate ligament ruptures. Arthroscopy. 2001;17:445–9. doi: 10.1053/jars.2001.23581. [DOI] [PubMed] [Google Scholar]
- 21.Palmer WE, Levine SM, Dupuy DE. Knee and shoulder fractures: association of fracture detection and marrow edema on MR images with mechanism of injury. Radiology. 1997;204:395–401. doi: 10.1148/radiology.204.2.9240526. [DOI] [PubMed] [Google Scholar]
- 22.Mink JH, Deutsch AL. Occult cartilage and bone injuries of the knee: detection, classification, and assessment with MR imaging. Radiology. 1989;170:823–9. doi: 10.1148/radiology.170.3.2916038. [DOI] [PubMed] [Google Scholar]
- 23.Phan CM, Link TM, Blumenkrantz G, Dunn TC, Ries MD, Steinbach LS, et al. MR imaging findings in the follow-up of patients with different stages of knee osteoarthritis and the correlation with clinical symptoms. Eur Radiol. 2006;16:608–18. doi: 10.1007/s00330-005-0004-5. [DOI] [PubMed] [Google Scholar]
- 24.Wluka AE, Hanna F, Davies-Tuck M, Wang Y, Bell RJ, Davis SR, et al. Bone marrow lesions predict increase in knee cartilage defects and loss of cartilage volume in middle-aged women without knee pain over 2 years. Ann Rheum Dis. 2009;68:850–5. doi: 10.1136/ard.2008.092221. [DOI] [PubMed] [Google Scholar]
- 25.Roemer FW, Guermazi A, Javaid MK, Lynch JA, Niu J, Zhang Y, et al. Change in MRI-detected subchondral bone marrow lesions is associated with cartilage loss: the MOST Study. A longitudinal multicentre study of knee osteoarthritis. Ann Rheum Dis. 2009;68:1461–5. doi: 10.1136/ard.2008.096834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teichtahl AJ, Wluka AE, Wang Y, Forbes A, Davies-Tuck ML, English DR, et al. 2009 World Congress on Osteoarthritis. Suppl 1. Vol. 17. Vol. 2009. Elsevier Ltd.; 019 The effect of long-term vigorous physical activity on knee cartilage among adults without clinical knee disease. p. S19. [Google Scholar]
- 27.Dore D, Quinn S, Ding C, Winzenberg T, Zhai G, Cicuttini F, et al. Natural history and clinical significance of MRI-detected bone marrow lesions at the knee: a prospective study in community dwelling older adults. Arthritis Res Ther. 2010;12:R223. doi: 10.1186/ar3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brem MH, Schlechtweg PM, Bhagwat J, Genovese M, Dillingham MF, Yoshioka H, et al. Longitudinal evaluation of the occurrence of MRI-detectable bone marrow edema in osteoarthritis of the knee. Acta Radiol. 2008;49:1031–7. doi: 10.1080/02841850802339413. [DOI] [PubMed] [Google Scholar]
- 29.Kroner AH, Berger CE, Kluger R, Oberhauser G, Bock P, Engel A. Influence of high tibial osteotomy on bone marrow edema in the knee. Clin Orthop Relat Res. 2007;454:155–62. doi: 10.1097/01.blo.0000238806.87411.33. [DOI] [PubMed] [Google Scholar]
- 30.Davies-Tuck ML, Wluka AE, Forbes A, Wang Y, English DR, Giles GG, et al. Development of bone marrow lesions is associated with adverse effects on knee cartilage while resolution is associated with improvement--a potential target for prevention of knee osteoarthritis: a longitudinal study. Arthritis Res Ther. 2010;12:R10. doi: 10.1186/ar2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Nevitt M, Niu J, Lewis C, Torner J, Guermazi A, et al. Fluctuation of knee pain and changes in bone marrow lesions, effusions, and synovitis on magnetic resonance imaging. Arthritis Rheum. 2011;63:691–9. doi: 10.1002/art.30148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neogi T, Nevitt M, Niu J, Sharma L, Roemer F, Guermazi A, et al. Subchondral bone attrition may be a reflection of compartment-specific mechanical load: the MOST Study. Ann Rheum Dis. 2010;69:841–4. doi: 10.1136/ard.2009.110114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma L, Song J, Dunlop D, Felson D, Lewis CE, Segal N, et al. Varus and valgus alignment and incident and progressive knee osteoarthritis. Ann Rheum Dis. 2010;69:1940–5. doi: 10.1136/ard.2010.129742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miyazaki T, Wada M, Kawahara H, Sato M, Baba H, Shimada S. Dynamic load at baseline can predict radiographic disease progression in medial compartment knee osteoarthritis. Ann Rheum Dis. 2002;61:617–22. doi: 10.1136/ard.61.7.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Segal NA, Anderson DD, Iyer KS, Baker J, Torner JC, Lynch JA, et al. Baseline articular contact stress levels predict incident symptomatic knee osteoarthritis development in the MOST cohort. J Orthop Res. 2009;27:1562–8. doi: 10.1002/jor.20936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Segal NA, Torner JC, Felson D, Niu J, Sharma L, Lewis CE, et al. Effect of thigh strength on incident radiographic and symptomatic knee osteoarthritis in a longitudinal cohort. Arthritis Rheum. 2009;61:1210–7. doi: 10.1002/art.24541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Segal NA, Torner JC, Felson DT, Niu J, Sharma L, Lewis CE, et al. Knee extensor strength does not protect against incident knee symptoms at 30 months in the multicenter knee osteoarthritis (MOST) cohort. PM R. 2009;1:459–65. doi: 10.1016/j.pmrj.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Felson DT, Nevitt MC, Yang M, Clancy M, Niu J, Torner JC, et al. A new approach yields high rates of radiographic progression in knee osteoarthritis. J Rheumatol. 2008;35:2047–54. [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson DD, Iyer KS, Segal NA, Lynch JA, Brown TD. Implementation of discrete element analysis for subject-specific, population-wide investigations of habitual contact stress exposure. J Appl Biomech. 2010;26:215–23. doi: 10.1123/jab.26.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bei Y, Fregly BJ. Multibody dynamic simulation of knee contact mechanics. Med Eng Phys. 2004;26:777–89. doi: 10.1016/j.medengphy.2004.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–90. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 42.Roemer FW, Lynch JA, Niu J, Zhang Y, Crema MD, Tolstykh I, et al. A comparison of dedicated 1.0 T extremity MRI vs large-bore 1.5 T MRI for semiquantitative whole organ assessment of osteoarthritis: the MOST study. Osteoarthritis Cartilage. 2010;18:168–74. doi: 10.1016/j.joca.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Felson DT, Lynch J, Guermazi A, Roemer FW, Niu J, McAlindon T, et al. Comparison of BLOKS and WORMS scoring systems part II. Longitudinal assessment of knee MRIs for osteoarthritis and suggested approach based on their performance: data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2010;18:1402–7. doi: 10.1016/j.joca.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neogi T, Felson D, Niu J, Lynch J, Nevitt M, Guermazi A, et al. Cartilage loss occurs in the same subregions as subchondral bone attrition: a within-knee subregion-matched approach from the Multicenter Osteoarthritis Study. Arthritis Rheum. 2009;61:1539–44. doi: 10.1002/art.24824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roemer FW, Nevitt MC, Felson DT, Niu J, Lynch JA, Crema MD, et al. MRI-based semiquantitative cartilage morphology and bone marrow lesion assessment - validity of within-grade scoring of longitudinal changes in the MOST study. Osteoarthritis Cartilage. 2011;19:s165–6. doi: 10.1016/j.joca.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Torres L, Dunlop DD, Peterfy C, Guermazi A, Prasad P, Hayes KW, et al. The relationship between specific tissue lesions and pain severity in persons with knee osteoarthritis. Osteoarthritis Cartilage. 2006;14:1033–40. doi: 10.1016/j.joca.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 47.Felson DT, Niu J, Guermazi A, Roemer F, Aliabadi P, Clancy M, et al. Correlation of the development of knee pain with enlarging bone marrow lesions on magnetic resonance imaging. Arthritis Rheum. 2007;56:2986–92. doi: 10.1002/art.22851. [DOI] [PubMed] [Google Scholar]
- 48.Tanamas S, Hanna FS, Cicuttini FM, Wluka AE, Berry P, Urquhart DM. Does knee malalignment increase the risk of development and progression of knee osteoarthritis? A systematic review. Arthritis Rheum. 2009;61:459–67. doi: 10.1002/art.24336. [DOI] [PubMed] [Google Scholar]
- 49.Kijowski R, Stanton P, Fine J, De Smet A. Subchondral bone marrow edema in patients with degeneration of the articular cartilage of the knee joint. Radiology. 2006;238:943–9. doi: 10.1148/radiol.2382050122. [DOI] [PubMed] [Google Scholar]
- 50.Tanamas SK, Wluka AE, Pelletier JP, Martel-Pelletier J, Abram F, Wang Y, et al. The association between subchondral bone cysts and tibial cartilage volume and risk of joint replacement in people with knee osteoarthritis: a longitudinal study. Arthritis Res Ther. 2010;12:R58. doi: 10.1186/ar2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scher C, Craig J, Nelson F. Bone marrow edema in the knee in osteoarthrosis and association with total knee arthroplasty within a three-year follow-up. Skeletal Radiol. 2008;37:609–17. doi: 10.1007/s00256-008-0504-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brand RA. Joint contact stress: a reasonable surrogate for biological processes? The Iowa orthopaedic journal. 2005;25:82–94. [PMC free article] [PubMed] [Google Scholar]
- 53.Felson DT, Niu J, Guermazi A, Roemer F, Aliabadi P, Clancy M, et al. The Development Of Knee Pain Correlates With Enlarging Bone Marrow Lesions On MRI. Arthritis Rheum. 2007 doi: 10.1002/art.22851. [DOI] [PubMed] [Google Scholar]
- 54.Wilson AJ, Murphy WA, Hardy DC, Totty WG. Transient osteoporosis: transient bone marrow edema? Radiology. 1988;167:757–60. doi: 10.1148/radiology.167.3.3363136. [DOI] [PubMed] [Google Scholar]
- 55.Hovis KK, Stehling C, Souza RB, Haughom BD, Baum T, Nevitt M, et al. Physical activity is associated with magnetic resonance imaging-based knee cartilage T2 measurements in asymptomatic subjects with and those without osteoarthritis risk factors. Arthritis Rheum. 2011;63:2248–56. doi: 10.1002/art.30419. [DOI] [PMC free article] [PubMed] [Google Scholar]