Abstract
Background
Sleep-disordered breathing (SDB) is an increasingly recognized risk factor for cardiovascular disease (CVD). Limited data are available from large African American cohorts.
Methods
We examined the prevalence, burden, and correlates of sleep symptoms suggestive of SDB and risk for obstructive sleep apnea (OSA) in the Jackson Heart Study (JHS), an all-African-American cohort of 5,301 adults. Data on selected daytime and nighttime sleep symptoms were collected using a modified Berlin questionnaire during the baseline examination. Risk of OSA was calculated according to published prediction model. Age and multivariable-adjusted logistic regression models were used to examine the associations between potential risk factors and measures of sleep.
Results
Sleep symptoms, burden, and risk of OSA were high among men and women in the JHS and increased with age and obesity. Being married was positively associated with sleep symptoms among women. In men, poor to fair perceived health and increased levels of stress were associated with higher odds of sleep burden, whereas prevalent hypertension and CVD were associated with higher odds of OSA risk. Similar associations were observed among women with slight variations. Sleep duration <7 hours was associated with increased odds of sleep symptoms among women and increased sleep burden among men. Moderate to severe restless sleep was consistently and positively associated with odds of adverse sleep symptoms, sleep burden, and high risk OSA.
Conclusions
Sleep symptoms in JHS had a strong positive association with features of visceral obesity, stress, and poor perceived health. With increasing obesity among younger African Americans, these findings are likely to have broad public health implications.
Keywords: African-American, epidemiology, Jackson Heart study, health status, obesity, sleep, sleep apnea syndromes, sleep disordered breathing
Introduction
Disrupted sleep is an increasingly recognized risk factor for hypertension and cardiovascular disease (CVD) [1], metabolic syndrome [2], stroke [3,4], and intracranial hypertension [5], arrhythmias [6], daytime sleepiness[7], and motor vehicle accidents [8,9]. Obstructive sleep apnea (OSA) or, more broadly, sleep-disordered breathing (SDB), is a common but underdiagnosed sleep disorder; at least 26% of the adult United States population is at risk for this condition [10] - a substantial public health burden. While prevalence appears higher among males and those who are obese, older, and African American [11], existing research has focused on elderly African Americans [12–15] and few studies have examined the extent and burden of SDB among a well-characterized cohort of African American adults [12,16]. A better understanding of the prevalence and clinical correlates of SDB among this ethnic population is essential to stem the anticipated rising tide of OSA associated with increasing obesity among younger African Americans [17,18].
Risk factors for OSA include age, male sex, obesity, upper airway congestion, increased neck size, craniofacial abnormalities, and African-American race [11]. A recent meta-analysis of ten published studies further reaffirmed the association of African-American race and both the prevalence and severity of SDB [19]. Several studies have also reported associations with socioeconomic status (SES), including annual household income, financial difficulties, and stress [16,20]; self-rated health [21]; and health-rated quality of life [13]. Epidemiologically, a diagnosis of likely OSA can be recovered from medical history utilizing the Berlin Sleep Questionnaire [22] and prediction algorithms for clinical use have been developed [23]. Successful treatment of OSA improves symptoms such as daytime sleepiness and increases quality of life [24].
We examined the prevalence and burden of symptoms suggestive of SDB in the Jackson Heart Study (JHS), a large, all-African-American cohort with wide age and body mass ranges and relatively large male proportion. We also examined the associations of sleep symptoms and risk of OSA with selected socio-demographic, anthropometric, health behaviors, and psychosocial factors, as well as chronic health conditions.
Methods
The JHS is a large, single-site, longitudinal cohort study designed to understand the etiology of CVD among African-Americans. The JHS cohort [25] includes a total of 5,301 participants partly recruited from the Jackson site of the Atherosclerosis Risk in Communities (ARIC) study, with additional randomly selected and volunteer residents of a tri-county area contained within metropolitan Jackson, MS, as well as their family members. Procedures for recruitment [26], the family study [27], and the details of the testing, anthropometric measurements, classification of CVD, hypertension, and diabetes, as well as other aspects of the study, are described in detail elsewhere [28,29]. All current data were obtained during Exam 1 (2000 – 2004). Trained interviewers asked participants questions regarding their medical history, including symptoms of SDB, socio-demographic information, health behaviors, and self-reported health status and other psychosocial characteristics.
Sleep Measures
Prevalent sleep symptoms were defined as a positive response (“Sometimes,” “Often,” or “Almost always”) to a limited set of five questions adapted from the Berlin Sleep Questionnaire [22]: “You are told that you snore loudly and bother others”; “You are told that you stop breathing (hold your breath) in sleep”; “You fall asleep during the day, particularly when not busy”; “You are tired after sleeping”; and “You feel sleepy or fall asleep while driving.” Responses of “Never”/“Seldom” to these questions were rated as a negative response for sleep symptoms. The Cronbach’s alpha for internal consistency in this cohort was 0.53. Confirmatory factor analysis, a structural equation model used to validate the structure of the sleep questions, yielded factor loadings revealing two factors corresponding to OSA and insomnia (See Table 1). Although the internal consistency is relatively low, we were interested in classifying participants in regard to the number of self-reported symptoms and not determining OSA. The number of sleep symptoms was categorized as “None” (no symptoms), “Moderate” (1–2 symptoms), or “Severe” (≥3symptoms).
Table 1.
Internal Reliability and CFA
| Scale / Individual Questions | α Coefficient | Item Total Correlation | Factor Loadings |
|---|---|---|---|
| Total Sleep Symptom Questionnaire | .53 | .24–.34 | |
| Risk of OSA | |||
| Snore | .80 | ||
| Stop Breathing | .82 | ||
| Insomnia | |||
| Fall Asleep | .68 | ||
| Tired | .60 | ||
| Feel sleepy while driving | .74 |
The burden of SDB (Sleep Burden) was quantified by first coding the responses to the sleep symptom questions (“Never,” “Seldom,” “Sometimes,” “Often,” or “Almost always”) from 0 for “Never” to 4 for “Almost Always” and then summing the individual scores, resulting in a Sleep Burden score that ranged from 0 to 20. Sleep Burden was then classified as “None” (score: 0), “Mild” (score: 1–5), “Moderate” (score: 6–10), and “Severe” (score: ≥11).
Self-reported sleep duration, defined in hours, was ascertained from the following question: “During the past month, excluding naps, how many hours of actual sleep did you get at night - or during the day, if you work at night - on average?” Perceived quality of sleep (“During the past month, how would you rate your sleep quality overall?”) was self-rated as either “Excellent,” “Very Good,” “Good,” “Fair,” or “Poor.” Restless sleep within the week prior to Exam 1 was assessed using the following question from the Centers for Epidemiologic Studies Depression Scale [30]: “During the past week, my sleep was restless,” and categorized as “None/Mild,” “Moderate,” or “Severe.”
Risk of OSA was calculated according to a clinical decision rule developed by Rodsutti et al. using 5 significant predictors of OSA: male sex, sleep complaints, including snoring and stopping breathing during sleep, categories of body mass index (BMI) and age [23]. The risk of OSA was determined by assigning the corresponding numerical value in parentheses for male sex (1.1), prevalent sleep complaints (0.9 for snoring and 0.9 for stopping breathing during sleep), BMI category: normal weight (0.0), overweight (1.0), obese (1.4) and morbidly obese (2.2), and age category: less than 30 (0.0), 30–44 (1.0), 45–59 (1.5), and 60 years and above (2.2), and then summed. The total risk of OSA score ranged from 0 to 7.3 and was categorized as low (score < 2.5), moderate (score of 2.5 to <4.2), and high (score ≥ 4.2) risk, in accord to the clinical decision rule [23].
Covariates
Socio-demographic Factors and Health Behaviors
Select socio-demographic factors included age, sex, socioeconomic status (SES), as measured by the highest level of education achieved and annual household income, and marital status. Current smoking was defined by a positive response to current cigarette use and lifetime consumption of ≥400 cigarettes. Former smoking was defined as a negative response to current cigarette use but a past consumption of ≥400 cigarettes. A total physical activity (PA) score was calculated as the sum of four individual index scores for Active Living, Work, Sport, and Home and Family Life activity using a modified version of the Baecke PA questionnaire used in the ARIC study [31].
Anthropometrics
Height was measured without shoes and recorded to the nearest centimeter. Participants stood with their feet together and head held in the Frankfurt plane. Weight was measured and recorded to the nearest kilogram using a balance scale, with participants in light clothing and not wearing shoes or constricting garments. Normal weight was defined as a measured BMI of 25 kg/m2 or less; overweight as BMI 25 – 29.99, and obesity as BMI of ≥ 30 kg/m2. Waist and neck girths were measured at the umbilicus and cricothyroid membrane, respectively, and recorded to the nearest centimeter (cm).
Psychosocial Characteristics
Perceived health status compared with others their age was classified as “Excellent,” “Good,” “Fair,” or “Poor.” The global perceived stress scale (GPSS) was developed specifically for the JHS [28] and is an eight-item questionnaire adapted from Kohn and MacDonald’s Survey of Recent Life Experiences [32], Cohen et al.’s Perceived Stress Scale [33], and Sarason et al.’s Life Events Scale [34] that measures the perception of stress experienced over a prior period of twelve months in the following areas of one’s life: employment, relationships, neighborhood of residence, caring for others, legal problems, medical problems, experiences of racism and discrimination, and meeting basic needs. Questions were primarily modified from the Sarason et al.’s Life Events Scale [34]. Participants rated stress severity on a four-point scale ranging from “not stressful” to “very stressful,” scored 0 to 3 and summed, with a total score that ranged from 0 to 24 [28]. The 20-item CES-D scale was used to measure depressive symptoms [30]. The CES-D was developed for use in large epidemiologic studies involving the general public, and it has been shown to have excellent psychometric properties in general as well as among African-Americans. Participants were asked to rate the frequency of occurrence of symptoms, and scores ranged from zero to three (depending on the question). Elevated depressive symptoms were defined as a CES-D total score ≥16 or/and treatment with antidepressants.
Clinical Parameters
Hypertension was defined as a measured blood pressure ≥ 140/90 mmHg, use of antihypertensive medications, or a self-reported physician diagnosis of hypertension in accordance to the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) criteria. In accordance to the American Diabetes Association 2004 criteria, type 2 diabetes mellitus (diabetes) was defined by a measured fasting glucose of ≥126 mg/dl, or use of insulin or oral hypoglycemic agents. Prevalent coronary heart disease (CHD) was determined by self-reported history, physician-diagnosed or ECG-determined myocardial infarction, or self-reported percutaneous coronary artery angioplasty. Prevalent cerebrovascular disease (CBD) was determined if the participant reported a physician-diagnosed stroke or a self-report of carotid endarterectomy or carotid angioplasty. CVD was defined as either prevalent CHD or prevalent CBD.
Statistical Analysis
An a priori decision was made to stratify all analyses by sex because the prevalence, extent, and burden of SDB among African-Americans is poorly understood and to determine whether heterogeneities by sex exist. Descriptive analyses and appropriate statistical tests were used to determine the prevalence of sleep symptoms and compare the distribution of socio-demographic, anthropometric, health behavior, psychosocial, and chronic disease condition variables across categories of sleep measures. Trends in the distribution of participant characteristics across sleep measures were determined by including each sleep measure as an ordinal covariate in unadjusted linear and logistic models. The sleep measures were dichotomized for regression analyses; Sleep Symptoms: “None” versus “Moderate/Severe,” Sleep Burden: “Mild” versus “Moderate/Severe,” and OSA Risk: “Low/Moderate risk” versus “High risk.” Age-adjusted logistic regression models were used to examine the bivariate associations between potential risk factors and sleep measures (Model 1). Variables that were marginally significant (p<0.10) in the bivariate associations were considered in the multivariable regression analyses. A backward elimination modeling technique, with a significance level of 0.05 for variable removal, was used to determine the most parsimonious multivariable model (Model 2). Age, BMI, waist circumference, and neck circumference were rescaled to increases of five (age, BMI, and waist circumference) and three (neck circumference) units of measurements. Odds ratios (OR) and 95% confidence intervals (CI) were computed and all statistical analyses were conducted using SAS (Version 9.1, SAS Institute, NC).
Results
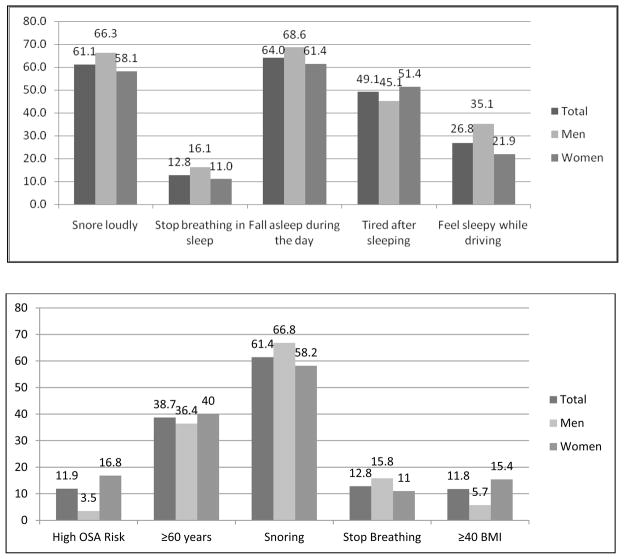
Since the prevalence of sleep symptoms in African-American populations is largely unknown, we calculated the prevalence of each sleep symptom using all available data (i.e., regardless of missing data) from 5,252 (99.1%) JHS participants who attended Exam 1 and provided responses to at least one of the sleep symptom questions. The sample size for each sleep symptom varied due to completeness in responses; however, these differences were small (<1%). Of the 5,252 participants who provided responses to the sleep symptom questionnaire, 4,690 (mean age: 54.6 ± 12.8 years; 63.2% women) had given full written consent and had complete sleep history information and data on the risk factors for impaired sleep. Affirmative answers to “Sometimes/Often/Almost always” were rated as positive responses. The cohort had high unadjusted prevalence rates of snoring (men: 66.3%; women: 58.1%), daytime somnolence (men: 68.6%; women: 61.4%), and feeling sleepy while driving (men: 35.1%; women: 21.9%) that were generally higher in men than in women (Figure 1a). However, women had a higher risk of OSA and its components than men (Figure 1b).
Figure 1.
Prevalence of sleep symptoms for the overall Jackson Heart Study cohort and stratified by sex, 2000–2004. The sample size for each sleep symptom varied due to completeness in responses; Snore loudly: N = 5250, Stop breathing in sleep: N = 5248, Fall asleep during the day: N = 5249, Tired after sleeping: N = 5248, and Feel sleepy while driving: N = 5237.
The lower part of the Figure (1.b) shows data for participants with complete respenses.
Table 2 and Table 3 summarize the sex-specific baseline characteristics across categories of sleep symptoms, sleep burden, and risk of OSA. Among women, decreasing age was associated with greater prevalence of sleep symptoms and sleep burden, but lower risk of OSA (Table 2). In addition, lower SES, being married, or being physically inactive were associated with greater prevalence of sleep symptoms and risk of OSA, but not with sleep burden. Global perceived stress and depressive symptoms had highly significant univariate associations (p<0.001) with both sleep symptoms and burden in the cohort. In general, higher sleep symptoms, sleep burden, and risk of OSA were consistently associated with higher anthropometrics and negative sociocultural characteristics and less consistently with clinical parameters. Similar associations were observed among men although trends were generally not apparent for SES and marital status (Table 3).
Table 2.
Baseline characteristics among African American women according to Sleep Symptoms, Sleep Burden, and Risk of OSA, Jackson Heart Study 2000–2004.
| Sleep Symptoms | Sleep Burden | Risk of OSA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | 0 n = 675 | 1–2 n = 1717 | 3+ n = 571 | None n = 345 | Mild n = 1772 | Moderate n = 751 | Severe n = 96 | Low n = 558 | Moderate n = 2314 | High n = 1818 |
| Age, years | 55.9±12.7 | 55.8±13.2 | 52.2±10.9 | 57.1±12.6 | 56.0±13.2 | 52.7±11.6 | 50.4±10.3c | 45.8±14.3 | 55.6±12.0 | 60.2±9.6c |
| Education, % | ||||||||||
| <High school diploma | 15.9 | 19.0 | 14.0c | 20.3 | 18.0 | 14.8 | 15.6 | 10.4 | 17.2 | 22.6c |
| High school diploma/GED | 16.7 | 21.7 | 20.8 | 20.0 | 20.4 | 20.4 | 22.9 | 16.5 | 20.4 | 23.2 |
| Some College | 26.4 | 27.4 | 35.0 | 24.1 | 27.2 | 33.6 | 34.4 | 32.3 | 28.8 | 25.7 |
| College graduate+ | 41.0 | 31.8 | 30.1 | 35.7 | 34.5 | 31.3 | 27.1 | 40.8 | 33.6 | 28.5 |
| Annual household income, % | ||||||||||
| <$25,000 | 31.9 | 38.2 | 32.1a | 35.7 | 35.9 | 35.0 | 33.3 | 31.9 | 34.1 | 41.7b |
| $25,000-$49,999 | 25.5 | 23.4 | 27.5 | 24.9 | 23.7 | 26.2 | 28.1 | 24.7 | 25.4 | 22.6 |
| $50,000-$74,999 | 14.8 | 12.8 | 14.5 | 12.5 | 13.7 | 14.1 | 11.5 | 14.5 | 14.1 | 11.8 |
| $75,000 and above | 10.8 | 9.8 | 9.1 | 10.1 | 9.9 | 10.1 | 6.3 | 12.3 | 9.9 | 8.3 |
| Unknown | 17.0 | 15.8 | 16.8 | 16.8 | 16.7 | 14.5 | 20.8 | 16.7 | 16.5 | 15.6 |
| Marital status, % | ||||||||||
| Married | 43.1 | 44.9 | 50.8a | 43.2 | 44.4 | 49.1 | 50.0 | 43.6 | 45.6 | 47.2c |
| Separated/Divorced/Widowed | 41.2 | 41.4 | 36.4 | 40.3 | 42.5 | 35.3 | 35.4 | 28.3 | 41.6 | 44.9 |
| Single/Never married | 15.7 | 13.7 | 13.8 | 16.5 | 13.1 | 15.6 | 14.6 | 28.1 | 12.8 | 7.9 |
| Smoking status, % | ||||||||||
| Current smoker | 9.6 | 9.3 | 12.8 | 9.6 | 9.4 | 10.5 | 19.8 | 12.5 | 9.5 | 9.7 |
| Former smoker | 13.0 | 16.2 | 14.4 | 13.3 | 15.5 | 15.3 | 13.5 | 10.0 | 14.8 | 19.4 |
| Total physical activity score | 8.4±2.6 | 8.1±2.6 | 8.3±2.4b | 8.2±2.6 | 8.1±2.6 | 8.4±2.5 | 7.8±2.3 | 8.8±2.5 | 8.3±2.5 | 7.5±2.5c |
| Body mass index, kg/m2 | 30.5±6.6 | 33.0±7.5 | 35.4±8.2c | 29.9±6.4 | 32.4±7.3 | 34.9±8.1 | 37.0±9.0c | 26.4±5.8 | 32.3±6.3 | 38.9±7.3c |
| Waist circumference, cm | 95.1±14.8 | 100.6±16.6 | 105.7±17.7c | 94.0±14.5 | 99.2±15.9 | 104.6±18.0 | 110.5±18.4c | 85.0±12.4 | 99.4±14.0 | 113.3±15.7c |
| Neck circumference, cm | 36.0±2.7 | 37.0±3.0 | 37.8±3.3c | 35.9±2.8 | 36.8±3.0 | 37.6±3.2 | 38.7±3.6c | 34.6±2.6 | 36.8±2.7 | 39.0 ±3.0c |
| Fair/Poor general health, % | 19.7 | 34.9 | 43.6c | 20.3 | 31.2 | 40.8 | 54.2c | 21.1 | 30.5 | 47.9c |
| Global perceived stress | 4.3±3.9 | 5.4±3.0 | 7.4±4.6c | 3.8±3.7 | 5.1±3.0 | 7.0±4.6 | 8.2±4.9c | 6.0±4.5 | 5.5±4.4 | 5.3±4.5b |
| Depressive symptoms*, % (n) | 18.6 (82) | 32.6 (358) | 48.5 (174)c | 18.4 (41) | 28.5 (322) | 44.7 (216) | 55.6 (35)c | 28.3 (94) | 31.0 (348) | 38.7 (172)b |
| Hypertension, % | 55.4 | 66.6 | 66.0c | 56.5 | 64.7 | 64.7 | 71.9a | 35.5 | 63.9 | 84.0c |
| Diabetes, % | 16.0 | 20.6 | 20.5a | 18.0 | 19.1 | 20.8 | 22.9 | 5.2 | 18.8 | 31.2c |
| Cardiovascular disease, % | 6.2 | 9.7 | 8.6a | 7.0 | 9.0 | 8.1 | 12.5 | 4.8 | 8.4 | 12.1c |
| Duration of sleep, hours | 6.7±1.4 | 6.5±1.5 | 6.0±1.5c | 6.8±1.5 | 6.5±1.5 | 6.2±1.5 | 5.7±1.6c | 6.6±1.4 | 6.5±1.5 | 6.4±1.6 |
| Perceived sleep quality, % | ||||||||||
| Excellent/Very Good | 40.0 | 30.7 | 16.1c | 41.2 | 32.8 | 20.2 | 14.6c | 32.3 | 30.0 | 28.4a |
| Good | 36.9 | 33.8 | 29.6 | 37.4 | 34.5 | 32.4 | 15.6 | 36.1 | 33.6 | 32.2 |
| Fair/Poor | 23.1 | 35.5 | 54.3 | 21.5 | 32.7 | 47.4 | 69.8 | 31.5 | 36.4 | 39.5 |
| Restless sleep**, % (n) | ||||||||||
| None | 54.2 (258) | 43.4 (501) | 30.2 (112)c | 63.9 (152) | 43.7 (524) | 36.6 (185) | 17.0 (10)c | 43.4 (154) | 44.2 (526) | 41.7 (191) |
| Some | 32.8 (156) | 32.2 (372) | 31.5 (117) | 25.6 (61) | 34.5 (414) | 29.7 (150) | 33.9 (20) | 35.5 (126) | 33.0 (392) | 27.7 (127) |
| Occasionally/All of the time | 13.0 (62) | 24.4 (282) | 38.3 (142) | 10.5 (25) | 21.8 (262) | 33.7 (170) | 49.2 (29) | 21.1 (75) | 22.8 (271) | 30.6 (140) |
Data are expressed as mean±SD or as percentages. Abbreviations: GED = Graduate equivalency degree. Sleep burden defined by the frequency of self-reported sleep symptoms (i.e., “Never,” “Seldom”, “Sometimes,” “Often,” or “Almost always”) and coded as: Low = 0; 1 ≥ Mild ≤ 5; 6 ≥ Moderate ≤ 10; Severe ≥ 11. Risk of OSA was determined by assigning the corresponding numerical value in parentheses for male sex (1.1), prevalent sleep complaints (0.9 for snoring and 0.9 for stopping breathing during sleep), BMI category: normal weight (0.0), overweight (1.0), obese (1.4) and morbidly obese (2.2), and age category: less than 30 (0.0), 30–44 (1.0), 45–59 (1.5), and 60 years and above (2.2), summed and categorized as follows: low < 2.5); 2.5 ≥ Moderate < 4.2); and High ≥ 4.2, in accord to clinical decision rule (23).
Test for trend:
p<0.05,
p<0.01,
p<0.001.
Data available for 1,903 women.
Data available for 2,003 women. The appropriate sample sizes are indicated in parentheses.
Depressive symptoms: Center of Epidemiologic Studies - Depression (CES-D) questionnaire total score ≥ 16 or treatment with antidepressants; Diabetes: Type-2 Diabetes Mellitus. OSA Risk calculated by summing up individual variables: male sex (1.1), snoring (0.9), stopping breathing during sleep (0.9), BMI category: normal weight (0.0), overweight (1.0), obese (1.4) and morbidly obese (2.2), and age category: less than 30 (0.0), 30–44 (1.0), 45–59 (1.5), and 60 years and above (2.2).
Table 3.
Baseline characteristics among African-American men according to Sleep Symptoms, Sleep Burden, and Risk of OSA, Jackson Heart Study 2000–2004.
| Sleep Symptoms | Sleep Burden | Risk of OSA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | 0 N = 335 |
1–2 N = 1009 |
3+ N = 383 |
None N =139 |
Mild N = 1006 |
Moderate N = 512 |
Severe N = 70 |
Low N = 60 |
Moderate N = 561 |
High N = 1106 |
| Age, years | 52.9±13.4 | 55.1±13.2 | 51.7±11.3c | 55.5±13.8 | 54.8±13.2 | 52.0±12.1 | 50.9±10.1c | 31.7±8.4 | 50.8±12.9 | 56.6±11.6c |
| Education, % | ||||||||||
| <High school diploma | 14.3 | 21.1 | 16.5a | 19.4 | 20.6 | 16.0 | 11.4 | 3.3 | 17.7 | 20.2 |
| High school diploma/GED | 19.1 | 18.2 | 19.8 | 27.3 | 18.1 | 17.6 | 20.0 | 21.7 | 20.0 | 18.0 |
| Some College | 29.6 | 29.1 | 30.6 | 25.9 | 28.0 | 33.0 | 32.9 | 46.7 | 31.2 | 27.8 |
| College graduate+ | 37.0 | 31.5 | 33.2 | 27.3 | 33.3 | 33.4 | 35.7 | 28.3 | 31.2 | 34.1 |
| Annual household income, % | ||||||||||
| <$25,000 | 19.7 | 23.6 | 26.1 | 26.6 | 22.6 | 24.0 | 24.3 | 28.3 | 26.2 | 21.7a |
| $25,000-$49,999 | 23.9 | 22.0 | 18.5 | 28.8 | 21.5 | 18.6 | 31.4 | 20.0 | 23.0 | 21.0 |
| $50,000-$74,999 | 15.2 | 19.6 | 20.1 | 12.2 | 18.7 | 21.3 | 17.1 | 18.3 | 18.2 | 19.3 |
| $75,000 and above | 26.3 | 28.8 | 20.6 | 20.1 | 21.1 | 20.5 | 17.1 | 11.7 | 18.5 | 22.2 |
| Unknown | 14.9 | 16.0 | 14.6 | 12.2 | 16.2 | 15.6 | 10.0 | 21.7 | 14.1 | 15.8 |
| Marital status, % | ||||||||||
| Married | 67.8 | 72.2 | 72.6 | 61.2 | 72.5 | 73.2 | 62.9 | 28.3 | 63.8 | 77.6c |
| Separated/Divorced/Widowed | 21.8 | 18.0 | 16.7 | 31.7 | 17.1 | 16.4 | 27.1 | 8.3 | 23.5 | 16.5 |
| Single/Never married | 10.5 | 9.8 | 10.7 | 7.2 | 10.4 | 10.4 | 10.0 | 63.3 | 12.7 | 6.0 |
| Smoking status, % | ||||||||||
| Current smoker | 14.9 | 18.7 | 18.3b | 17.3 | 17.4 | 19.0 | 18.6 | 15.0 | 26.0 | 13.9b |
| Former smoker | 20.6 | 28.0 | 23.2 | 18.7 | 26.1 | 27.3 | 15.7 | 15.0 | 18.5 | 29.6 |
| Total physical activity score | 9.1±2.7 | 8.6±2.7 | 8.5±2.4b | 8.8±2.7 | 8.6±2.7 | 8.7±2.5 | 8.1±2.2 | 9.9±2.2 | 9.0±2.7 | 8.4±2.6c |
| Body mass index, kg/m2 | 28.4±5.9 | 29.6±5.7 | 31.7±7.0c | 27.1±5.2 | 29.3±5.5 | 30.9±6.7 | 34.5±8.7c | 23.7±3.2 | 26.3±4.6 | 31.9±5.9c |
| Waist circumference, cm | 97.6±14.0 | 100.7±14.8 | 105.4±16.0c | 95.5±13.8 | 99.9±13.7 | 103.7±16.7 | 111.7±18.0c | 81.2±9.8 | 92.9±11.7 | 106.4±14.1c |
| Neck circumference, cm | 40.3±3.3 | 41.2±3.1 | 42.2±3.4c | 39.6±3.0 | 41.0±3.1 | 41.8±3.2 | 43.5±3.6c | 37.9±2.5 | 39.5±2.8 | 42.3±2.9c |
| Fair/Poor general health, % | 16.1 | 25.0 | 38.1c | 18.0 | 23.0 | 32.8 | 40.0c | 18.3 | 21.4 | 29.0b |
| Global perceived stress | 4.0±4.2 | 4.1±3.9 | 5.8±4.6c | 3.1±3.8 | 3.9±3.8 | 5.6±4.5 | 6.7±5.3c | 5.0±4.1 | 4.7±4.3 | 4.4±4.1 |
| Depressive symptoms*, % (n) | 12.9 (27) | 20.0 (119) | 27.6 (62)c | 14.6 (70) | 17.0 (101) | 25.1 (78) | 38.6 (17)c | 23.7 (9) | 17.1 (59) | 21.6 (140) |
| Hypertension, % | 48.7 | 62.4 | 62.1c | 50.4 | 59.8 | 60.9 | 67.1 | 11.7 | 48.3 | 68.1c |
| Diabetes, % | 11.9 | 17.6 | 19.1a | 11.5 | 16.8 | 17.8 | 21.4 | 0.0 | 8.0 | 22.2c |
| Cardiovascular disease, % | 8.7 | 12.5 | 14.1 | 10.8 | 12.1 | 12.3 | 12.9 | 3.3 | 6.8 | 15.3c |
| Duration of sleep, hours | 6.6±1.4 | 6.4±1.5 | 6.0±1.5c | 6.8±1.5 | 6.5±1.5 | 6.2±1.5 | 5.3±1.5c | 6.3±1.7 | 6.4±1.6 | 6.3±1.5 |
| Perceived sleep quality, % | ||||||||||
| Excellent/Very Good | 43.6 | 35.2 | 19.3c | 45.3 | 38.0 | 23.6 | 12.9c | 30.0 | 31.6 | 34.4 |
| Good | 34.0 | 37.3 | 33.2 | 37.4 | 36.0 | 36.3 | 24.3 | 26.7 | 36.7 | 35.7 |
| Fair/Poor | 22.4 | 27.6 | 47.5 | 17.3 | 26.0 | 40.0 | 62.9 | 43.3 | 31.7 | 29.9 |
| Restless sleep**, % (n) | ||||||||||
| None | 67.1 (143) | 54.0 (335) | 39.0 (90)c | 65.1 (54) | 59.2 (365) | 42.5 (136) | 28.9 (13)c | 54.8 (23) | 55.0 (193) | 52.4 (352) |
| Some | 24.4 (52) | 31.2 (194) | 26.4 (61) | 28.9 (24) | 28.7 (177) | 30.3 (97) | 20.0 (9) | 31.0 (13) | 29.1 (102) | 28.6 (192) |
| Occasionally/All of the time | 8.5 (18) | 14.8 (92) | 34.6 (80) | 6.0 (5) | 12.2 (75) | 27.2 (87) | 51.1 (23) | 14.3 (6) | 16.0 (56) | 19.1 (128) |
Data are expressed as mean±SD or as percentages. Abbreviations: GED = Graduate equivalency degree. Sleep burden defined by the frequency of self-reported sleep symptoms (i.e., “Never,” “Seldom,” “Sometimes,” “Often,” or “Almost always”) and coded as: Low = 0; 1 ≥ Mild ≤ 5; 6 ≥ Moderate ≤ 10; Severe ≥ 11. Risk of OSA was determined by assigning the corresponding numerical value in parentheses for male sex (1.1), prevalent sleep complaints (0.9 for snoring and 0.9 for stopping breathing during sleep), BMI category: normal weight (0.0), overweight (1.0), obese (1.4) and morbidly obese (2.2), and age category: less than 30 (0.0), 30–44 (1.0), 45–59 (1.5), and 60 years and above (2.2), summed and categorized as follows: low < 2.5); 2.5 ≥ Moderate < 4.2); and High ≥ 4.2, in accord to a clinical decision rule (23).
Test for trend:
p<0.05,
p<0.01,
p<0.001.
Data available for 1,030 men.
Data available for 1,065 men. The appropriate sample sizes are indicated in parentheses.
Depressive symptoms: Center of Epidemiologic Studies - Depression (CES-D) questionnaire total score ≥ 16 or treatment with antidepressants; Diabetes: Type-2 Diabetes Mellitus. OSA Risk calculated by summing up individual variables: male sex (1.1), snoring (0.9), stopping breathing during sleep (0.9), BMI category: normal weight (0.0), overweight (1.0), obese (1.4) and morbidly obese (2.2), and age category: less than 30 (0.0), 30–44 (1.0), 45–59 (1.5), and 60 years and above (2.2).
Model I: age-adjusted for each variable; Model II: adjusted for age and all other variables. Age and BMI are component of OSA Risk and not adjusted for OSA Risk. Significant associations are highlighted in bold.
Table 4 and Table 5 summarize the age-adjusted and multivariable regression models of the predictors of increased sleep symptoms and sleep burden and risk of OSA for women and men, respectively. In the fully-adjusted model, among women, increasing age (sleep symptoms and sleep burden) and being single/unmarried (sleep symptoms and risk of OSA) were associated with lower odds of sleep symptoms, sleep burden, and risk of OSA (Table 4). Waist and neck circumferences (but not BMI) were associated with higher odds of sleep symptoms, sleep burden and risk of OSA. In addition, poor to fair perceived general health (sleep symptoms) and higher stress (sleep symptoms and sleep burden) and depressive symptoms (sleep symptoms) were associated with higher odds of sleep symptoms and sleep burden. Prevalent hypertension was positively associated with odds of sleep symptoms, whereas prevalent diabetes was associated with lower odds of sleep symptoms. As expected, poor to fair perceived sleep quality and moderate to severe restless sleep were positively associated with odds of adverse sleep symptoms and sleep burden. Sleep durations of either <7 hours or >8 hours both were associated with higher odds of sleep burden. Similar associations were observed among men (Table 5), although a few of the associations varied slightly or lost statistical significance after multivariable adjustment. Moderate to severe restless sleep was consistently associated with odds of adverse sleep symptoms and sleep burden. In terms of sleep duration, only <7 hours was associated with higher odds for sleep symptoms in men.
Table 4.
Age-adjusted and multivariable odds of greater sleep symptoms and sleep burden and high risk of OSA among women participants in the Jackson Heart Study, 2000–2004
| Sleep Symptoms | Sleep Burden | Risk of OSA* | ||||
|---|---|---|---|---|---|---|
| Characteristic | Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 |
| Age per 5 years | 0.98 (0.94, 1.00) | 0.82 (0.87, 0.98) | 0.89 (0.87, 0.92) | 0.94 (0.90, 0.98) | ---------- | ---------- |
| Education | ||||||
| <High School | 1.66 (1.26, 2.17) | ---------- | 1.20 (0.93, 1.56) | ---------- | 1.06 (0.82, 1.38) | 0.70 (0.46, 1.06) |
| High School | 1.75 (1.36, 2.24) | ---------- | 1.24 (0.99, 1.56) | ---------- | 1.25 (0.98, 1.59) | 0.95 (0.67, 1.34) |
| Some College | 1.41 (1.14, 1.76) | ---------- | 1.33 (1.09, 1.63) | ---------- | 1.23 (0.97, 1.54) | 0.69 (0.50, 0.96) |
| ≥College Graduate | 1.0 | ---------- | 1.0 | ---------- | 1.0 | ---------- |
| Annual household income | ||||||
| <$25,000 | 1.37 (1.00, 1.86) | ---------- | 1.15 (0.86, 1.53) | ---------- | 1.15 (0.83, 1.59) | ---------- |
| $25,000-$49,999 | 1.09 (0.79, 1.49) | ---------- | 1.17 (0.86, 1.58) | ---------- | 1.05 (0.75, 1.47) | ---------- |
| $50,000-$74,999 | 1.00 (0.79, 1.42) | ---------- | 1.04 (0.74, 1.46) | ---------- | 1.05 (0.72, 1.53) | ---------- |
| ≥$75,000 | 1.0 | ---------- | 1.0 | ---------- | 1.0 | ---------- |
| Unknown | 1.11 (0.79, 1.55) | ---------- | 1.03 (0.74, 1.43) | ---------- | 0.93 (0.64, 1.34) | ---------- |
| Marital Status | ||||||
| Married | 1.0 | 1.0 | 1.0 | ---------- | 1.0 | 1.0 |
| Divorced/Widowed/Separated | 0.94 (0.78, 1.14) | 0.87 (0.68, 1.12) | 0.85 (0.71, 1.02) | ---------- | 0.87 (0.72, 1.05) | 0.95 (0.72, 1.25) |
| Single/Unmarried | 0.75 (0.57, 0.98) | 0.65 (0.46, 0.92) | 0.81 (0.63, 1.04) | ---------- | 0.69 (0.50, 0.94) | 0.42 (0.27, 0.65) |
| Cigarette Use | ||||||
| Never | 1.0 | ---------- | 1.0 | ---------- | 1.0 | ---------- |
| Former | 1.31 (1.01, 1.69) | ---------- | 1.17 (0.93, 1.47) | ---------- | 1.25 (0.99, 1.57) | ---------- |
| Current | 1.08 (0.81, 1.45) | ---------- | 1.22 (0.94, 1.58) | ---------- | 1.17 (0.87, 1.57) | ---------- |
| Physical activity score | 0.94 (0.90, 0.97) | ---------- | 0.99 (0.95, 1.02) | ---------- | 0.93 (0.90, 0.97) | 0.92 (0.87, 0.97) |
| Body mass index per 5 kg/m2 | 1.38 (1.29, 1.48) | ---------- | 1.29 (1.22, 1.36) | ---------- | ---------- | ---------- |
| Waist circumference per 5 cm | 1.15 (1.12, 1.19) | 1.10 (1.05, 1.16) | 1.14 (1.11, 1.17) | 1.09 (1.05, 1.13) | 1.44 (1.39, 1.49) | 1.30 (1.23, 1.36) |
| Neck circumference per 3 cm | 1.55 (1.42, 1.71) | 1.17 (1.01, 1.37) | 1.44 (1.33, 1.56) | 1.16 (1.01, 1.33) | 2.70 (2.43, 2.99) | 1.43 (1.23, 1.67) |
| General health | ||||||
| Excellent/Good | 1.0 | 1.0 | 1.0 | ---------- | 1.0 | ---------- |
| Fair/Poor | 2.49 (2.02, 3.07) | 1.57 (1.17, 2.10) | 1.94 (1.64, 2.30) | ---------- | 2.09 (1.75, 2.50) | ---------- |
| Global Perceived Stress | 1.10 (1.07, 1.12) | 1.05 (1.02, 1.09) | 1.10 (1.08, 1.12) | 1.09 (1.06, 1.12) | 1.03 (1.01, 1.06) | ---------- |
| Depressive symptoms | 2.52 (1.94, 3.28) | 1.49 (1.11, 2.00) | 2.32 (1.88, 2.86) | ---------- | 1.51 (1.20, 1.90) | ---------- |
| Hypertension Yes versus No | 1.95 (1.60, 2.38) | 1.46 (1.13, 1.90) | 1.58 (1.30, 1.90) | ---------- | 2.78 (2.21, 3.50) | 2.59 (1.90, 3.52) |
| Diabetes Yes versus No | 1.43 (1.13, 1.81) | 0.71 (0.52, 0.98) | 1.35 (1.10, 1.66) | ---------- | 1.96 (1.61, 2.40) | ---------- |
| Cardiovascular disease Yes versus No | 1.70 (1.20, 2.41) | ---------- | 1.26 (0.94, 1.69) | ---------- | 1.14 (0.86, 1.51) | ---------- |
| Duration of sleep | ||||||
| <7 hours | 1.69 (1.42, 2.02) | ---------- | 1.69 (1.43, 2.01) | 1.40 (1.10, 1.79) | 1.23 (1.03, 1.48) | ---------- |
| 7–8 Hours | 1.0 | ---------- | 1.0 | 1.0 | 1.0 | ---------- |
| >8 Hours | 1.35 (0.93, 1.98) | ---------- | 1.13 (0.77, 1.66) | 1.30 (0.79, 2.16) | 0.89 (0.60, 1.30) | ---------- |
| Perceived sleep quality | ||||||
| Excellent/Very Good/Good | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | ---------- |
| Fair/Poor | 2.22 (1.82, 2.71) | 1.49 (1.12, 1.98) | 2.19 (1.86, 2.58) | 1.40 (1.09, 1.80) | 1.30 (1.09, 1.56) | ---------- |
| Restless sleep | ||||||
| None/Mild | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Moderate/Severe | 2.55 (1.91, 3.41) | 1.47 (1.04, 2.06) | 2.17 (1.74, 2.69) | 1.37 (1.05, 1.79) | 1.65 (1.30, 2.10) | 1.41 (1.06, 1.88) |
Abbreviations: OSA = Obstructive sleep apnea.
Model 1 is an age-adjusted logistic regression model for bivariate associations. Model 2 is the most parsimonious multivariable model obtained using a backward elimination technique (significance level of 0.05 for variable removal).
Age and body mass index were not considered as potential risk factors because these two variables are components of the Risk of OSA score.
Model I: age-adjusted for each variable; Model II: adjusted for age and all other variables. Age and BMI are component of OSA Risk and not adjusted for OSA Risk. Significant associations are highlighted in bold.
Table 5.
Age-adjusted and multivariable odds of greater sleep symptoms and sleep burden and high risk of OSA among men participants in the Jackson Heart Study, 2000–2004
| Sleep Symptoms | Sleep Burden | Risk of OSA* | ||||
|---|---|---|---|---|---|---|
| Characteristic | Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 |
| Age per 3 years | 1.04 (0.99, 1.09) | 0.98 (0.91, 1.05) | 0.91 (0.88, 0.95) | 0.98 (0.92, 1.04) | ---------- | ---------- |
| Education | ||||||
| <High School | 1.52 (1.04, 2.24) | ---------- | 0.90 (0.65, 1.23) | 0.61 (0.37, 1.02) | 0.57 (0.41, 0.79) | 0.73 (0.42, 1.24) |
| High School | 1.13 (0.80, 1.58) | ---------- | 0.92 (0.69, 1.23) | 0.69 (0.45, 1.08) | 0.74 (0.55, 0.99) | 0.67 (0.41, 1.08) |
| Some College | 1.17 (0.87, 1.58) | ---------- | 1.09 (0.85, 1.41) | 1.15 (0.82, 1.62) | 0.89 (0.69, 1.16) | 0.91 (0.61, 1.34) |
| ≥College Graduate | 1.0 | ---------- | 1.0 | 1.0 | 1.0 | 1.0 |
| Annual household income | ||||||
| <$25,000 | 1.62 (1.13, 2.32) | ---------- | 1.19 (0.88, 1.62) | ---------- | 0.50 (0.37, 0.69) | ---------- |
| $25,000-$49,999 | 1.19 (0.84, 1.68) | ---------- | 0.95 (0.70, 1.30) | ---------- | 0.71 (0.51, 0.97) | ---------- |
| $50,000-$74,999 | 1.79 (1.22, 2.62) | ---------- | 1.17 (0.86, 1.61) | ---------- | 0.94 (0.67, 1.31) | ---------- |
| ≥$75,000 | 1.0 | ---------- | 1.0 | ---------- | 1.0 | ---------- |
| Unknown | 1.42 (0.96, 2.09) | ---------- | 1.00 (0.71, 1.40) | ---------- | 0.85 (0.60, 1.20) | ---------- |
| Marital Status | ||||||
| Married | 1.0 | ---------- | 1.0 | ---------- | 1.0 | 1.0 |
| Divorced/Widowed/Separated | 0.75 (0.56, 1.01) | ---------- | 0.96 (0.74, 1.25) | ---------- | 0.52 (0.40, 0.67) | 0.48 (0.32, 0.73) |
| Single/Unmarried | 1.02 (0.67, 1.56) | 0.81 (0.34, 1.93) | 0.75 (0.52, 1.07) | ---------- | 0.47 (0.33, 0.67) | 0.20 (0.11, 0.35) |
| Cigarette Use | ||||||
| Never | 1.0 | 1.0 | 1.0 | ---------- | 1.0 | 1.0 |
| Former | 1.47 (1.08, 2.00) | 1.57 (1.04, 2.38) | 1.29 (1.00, 1.66) | ---------- | 1.09 (0.83, 1.42) | 1.56 (1.05, 2.33) |
| Current | 1.46 (1.04, 2.05) | 1.48 (0.91, 2.41) | 1.16 (0.88, 1.52) | ---------- | 0.51 (0.39, 0.67) | 0.87 (0.54, 1.39) |
| Physical activity score | 0.93 (0.89, 0.98) | 0.93 (0.87, 0.99) | 0.96 (0.92, 1.00) | ---------- | 0.98 (0.94, 1.02) | 0.91 (0.85, 0.98) |
| Body mass index per 5 kg/m2 | 1.32 (1.18, 1.47) | ---------- | 1.33 (1.23, 1.45) | ---------- | ---------- | ---------- |
| Waist circumference per 5 cm | 1.11 (1.06, 1.16) | ---------- | 1.13 (1.09, 1.17) | 1.12 (1.07, 1.18) | 1.75 (1.64, 1.86) | 1.47 (1.32, 1.63) |
| Neck circumference per 3 cm | 1.42 (1.26, 1.59) | 1.30 (1.11, 1.52) | 1.39 (1.27, 1.54) | 3.87 (3.32, 4.51) | 1.69 (1.33, 2.15) | |
| General health | ||||||
| Excellent/Good | 1.0 | ---------- | 1.0 | 1.0 | 1.0 | ---------- |
| Fair/Poor | 2.05 (1.50, 2.81) | ---------- | 1.89 (1.51, 2.37) | 1.78 (1.26, 2.53) | 1.39 (1.09, 1.77) | ---------- |
| Global Perceived Stress | 1.05 (1.02, 1.08) | ---------- | 1.10 (1.08, 1.13) | 1.06 (1.02, 1.10) | 1.03 (1.00, 1.06) | ---------- |
| Depressive symptoms | 1.90 (1.23, 2.94) | ---------- | 1.85 (1.35, 2.53) | ---------- | 1.26 (0.90, 1.77) | ---------- |
| Hypertension Yes versus No | 1.77 (1.36, 2.29) | 1.50 (1.07, 2.12) | 1.45 (1.16, 1.82) | ---------- | 1.83 (1.47, 2.28) | 1.56 (1.13, 2.18) |
| Diabetes Yes versus No | 1.57 (1.09, 2.25) | ---------- | 1.33 (1.02, 1.75) | ---------- | 2.84 (2.02, 4.01) | ---------- |
| Cardiovascular disease Yes versus No | 1.49 (0.98, 2.27) | ---------- | 1.26 (0.92, 1.72) | ---------- | 1.75 (1.20, 2.54) | 2.37 (1.22, 4.61) |
| Duration of sleep | ||||||
| <7 Hours | 1.60 (1.25, 2.05) | 1.42 (1.02, 1.98) | 1.66 (1.34, 2.05) | ---------- | 1.25 (1.01, 1.55) | ---------- |
| 7 ± 8 Hours | 1.0 | 1.0 | 1.0 | ---------- | 1.0 | ---------- |
| >8 Hours | 1.01 (0.59, 1.74) | 0.74 (0.36, 1.50) | 0.75 (0.43, 1.31) | ---------- | 0.61 (0.37, 0.99) | ---------- |
| Perceived sleep quality | ||||||
| Excellent/Very Good/Good | 1.0 | ---------- | 1.0 | 1.0 | 1.0 | ---------- |
| Fair/Poor | 1.77 (1.33, 2.34) | ---------- | 2.15 (1.74, 2.67) | 1.80 (1.31, 2.47) | 1.01 (0.81, 1.26) | ---------- |
| Restless sleep | ||||||
| None/Mild | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | ---------- |
| Moderate/Severe | 2.78 (1.67, 4.64) | 2.48 (1.45, 4.25) | 3.31 (2.39, 4.57) | 2.35 (1.62, 3.40) | 1.38 (0.98, 1.96) | ---------- |
Abbreviations: OSA = Obstructive sleep apnea.
Model 1 is an age-adjusted logistic regression model for bivariate associations. Model 2 is the most parsimonious multivariable model obtained using a backward elimination technique (significance level of 0.05 for variable removal).
Age and body mass index were not considered as potential risk factors because these two variables are components of the Risk of OSA score.
Discussion
Sleep apnea syndromes have widespread implications on quality of life and daily functioning [7,35,36]. Given the strong association between obesity and SDB [37], a careful periodic reassessment of sleep symptoms is warranted in high-risk populations. Our study adds to the existing literature by describing the burden of sleep symptoms in this large, community-dwelling African-American population and by assessing the association with several anthropometric, lifestyle, sociocultural, and chronic disease status and features. Our study findings are significant in several respects. First, we reaffirmed the impact of body anthropometrics for sleep symptoms and an independent association of sleep symptoms with age and (depending on sex) with neck and waist circumference, but not with BMI. Second, we described relatively novel associations of increased sleep symptoms with perceived health status and global stress. These associations persisted after adjustment for multiple confounders, including demographic, anthropometric, and lifestyle variables. Third, we found persistent and independent associations between SDB symptoms with sleep quality, duration, and restlessness of sleep.
These results significantly expand our existing knowledge about African-American ethnicity and sleep apnea syndromes. African-American race has been found to be a risk factor for OSA in adults [12] and children [38] and predicts recurrence of OSA after tonsillectomy in children [39]. Additionally, the severity of respiratory disturbances was more pronounced in African-Americans, roughly twice that of Caucasians [14]. Our unadjusted prevalence rates for snoring, daytime somnolence and non-refreshing sleep were relatively high compared to the reported literature [10,36,40]. Such excess burden has been shown to persist in several studies even when adjusted for BMI, a powerful predictor of OSA [12,14,41]. On the contrary, a 2004 study of 233 participants (of these, 128 African-American) did not find a difference in severity of OSA between African-Americans and Caucasians, once adjusted for BMI and mean household income [16]. It should be noted that BMI in older studies may simply act only as a surrogate for larger waist and neck diameters, correlating with visceral obesity and upper airway narrowing. The association of sleep symptoms with marital status in our cohort was likely influenced by the availability of an external observer (bed partner), influencing both the awareness and perceived frequency of snoring or apnea, resulting in lower risk for sleep symptoms. It is interesting that this association was the strongest for predicted OSA, likely reflecting the impact of both age and BMI, as these variables were incorporated into the OSA prediction formula [23]. Similar to our results, younger, rather than old age has consistently been associated with more SDB among African-Americans[12,16].
To date, the data regarding African-American race from an unbiased population sample has been relatively limited. The largest epidemiologic survey of sleep, the Sleep Heart Study (SHS), which assessed several features of SDB, including nocturnal snoring and daytime sleepiness [42], African-American participants comprised only 648 of the 13,194 participants. SDB generally worsened with age in the SHS cohort, with a plateau effect observed around age 60 [43]. Snoring and excessive daytime sleepiness were reported less frequently in the SHS, likely reflecting, in part, different scoring [36]. Finally, a recent large meta-analysis of the existing literature to date also confirmed that African-Americans are less likely to report complaints of insomnia or sleep disturbances, compared to Caucasians[19].
SES and health-related variables accounted for the perceived racial disparity of African-Americans and confirmed association with lower exercise level, income level, and depression across races in a study with 140 elderly African-Americans and Caucasians [20]. Financial strain has also been documented to affect sleep adversely in a relatively small study involving approximately 140 African-American middle-aged female participants [44]. In our cohort, we confirmed an independent association with perceived stress and with depressive symptoms (women only), but not with SES or education. Unlike other studies, JHS measured and adjusted for multiple aspects of chronic stressors [28]. Findings with regard to perceived state of health and sleep symptoms in African-Americans have been particularly sparse. Relative to whites, elderly African-Americans are less likely to report sleep complaints and more likely to have less education, worse self-rated health, and depression [21]. In a small study of 70 elderly African-American participants pre-selected for snoring and daytime sleepiness, Stepnowsky et al. documented the significant impact of SDB on health-rated quality of life [13], in keeping with our results. The negative impact of OSA on quality of well-being was similar to that of depression or chronic obstructive pulmonary disease. The observed association of sleep symptoms with perceived state of health in our study emphasizes the importance of adequate sleep to secure a sense of well-being and deserves further investigation.
In the elderly, African-Americans had less satisfaction with sleep and had more daytime sleepiness and fatigue, despite longer sleep times when compared to Caucasians [14]. Studying elderly women, an ancillary study of 459 postmenopausal women from the Women’s Health Initiative provided suggestive data that poor quality of sleep and poor mood are associated with each other and also with African-American race and Hispanic ethnicity [45]. In our cohort, an independent association was present between sleep symptoms and restlessness of sleep, as well as perceived sleep quality. Sleep duration of <7 hours had an adverse association with sleep symptoms in both genders.
Limitations and strengths
Sleep symptoms, while they may approximate the frequency of underlying SDB, are not identical to the diagnosis of SDB or OSA obtained during multi-channel polysomnographic recording. The JHS sleep questions represented a limited version of the Berlin questionnaire and there was no gold standard test available to assess the true incidence of sleep apnea syndromes or to examine the association of these with reported sleep symptoms. Accordingly, some of the reported symptoms (snoring, apnea) may be influenced by the availability of an external observer. It also unknown whether an alternative scoring method would have arrived to identical conclusions. Various types of SDB, including OSA, central sleep apnea, obesity-hypoventilation syndrome, and Cheyne-Stokes respiration could not be differentiated based on these reported symptoms. Similarly, we could not differentiate SDB from other sleep disorders or the effect of voluntary sleep deprivation. Craniofacial and upper airway abnormalities, including allergic rhinitis, were not assessed in JHS, and the relative contribution of these abnormalities to sleep symptoms could not be discerned. Major strengths of the study included its large sample size and the representative community-derived nature of the cohort, making these observations highly applicable to African Americans from the southeastern United States. Sleep related questions were obtained early in the health history interview and answers were less likely to be compromised by interviewee fatigue. Similarly, physicians may rely on similar questions during clinical decision-making to assess the risk of SDB and triage patients for formal sleep medicine consultation.
Conclusion
Exam 1 of the JHS revealed a large burden of sleep symptoms and, likely, underlying SDB. Independent associations have been demonstrated between extent and burden of sleep symptoms and age, body anthropometrics (neck and waist circumference), married marital status, shortened sleep duration, and diagnosis of hypertension. Additionally, we confirmed global stress scores and a decreased perceived state of health as relatively novel associations. Cigarette smoking, physical activity score, depressive symptoms, or the presence of diabetes had variable and sex-dependent associations with sleep symptoms. Sleep symptoms were strongly and independently associated with other quality measures of sleep, such as self-rated quality and restlessness of sleep. Sleep quality and under-diagnosed sleep apnea syndromes may have significant public health implications; these initial results in an all-African-American cohort demonstrate the need for continued surveillance of SDB in high-risk segments of the population. The associations of SDB with adverse metabolic, cardiovascular, and psychosocial features of obesity need further exploration.
Acknowledgments
The authors would like to give a sincere thanks to the Jackson Heart Study participants, staff, and interns for their long-term commitment to the study. The Jackson Heart Study (JHS) is a collaborative study supported by the National Institutes of Health and the National Center on Minority Health and Health Disparities (study ID numbers: 5001; N01 HC95170; N01 HC95171; N01 HC95172) in partnership with Jackson State University, Tougaloo College, and the University of Mississippi Medical Center.
Dr Otis Gowdy Jr. is a graduate of the University of Mississippi, Department of Internal Medicine Renal Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Phillips B, Mannino DM. Do insomnia complaints cause hypertension or cardiovascular disease? J Clin Sleep Med. 2007;3(5):489–94. [PMC free article] [PubMed] [Google Scholar]
- 2.Troxel WM, Buysse DJ, Matthews KA, et al. Sleep symptoms predict the development of the metabolic syndrome. Sleep. 2010;33(12):1633–40. doi: 10.1093/sleep/33.12.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palomaki H. Snoring and the risk of ischemic brain infarction. Stroke. 1991;22(8):1021–5. doi: 10.1161/01.str.22.8.1021. [DOI] [PubMed] [Google Scholar]
- 4.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163(1):19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 5.Wall M, Purvin V. Idiopathic intracranial hypertension in men and the relationship to sleep apnea. Neurology. 2009;72(4):300–1. doi: 10.1212/01.wnl.0000336338.97703.fb. [DOI] [PubMed] [Google Scholar]
- 6.Gami AS, Pressman G, Caples SM, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;110(4):364–7. doi: 10.1161/01.CIR.0000136587.68725.8E. [DOI] [PubMed] [Google Scholar]
- 7.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):136–43. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horstmann S, Hess CW, Bassetti C, Gugger M, Mathis J. Sleepiness-related accidents in sleep apnea patients. Sleep. 2000;23(3):383–9. [PubMed] [Google Scholar]
- 9.George CF. Sleep apnea, alertness, and motor vehicle crashes. Am J Respir Crit Care Med. 2007;176(10):954–6. doi: 10.1164/rccm.200605-629PP. [DOI] [PubMed] [Google Scholar]
- 10.Hiestand DM, Britz P, Goldman M, Phillips B. Prevalence of symptoms and risk of sleep apnea in the US population: results from the national sleep foundation sleep in America 2005 poll. Chest. 2006;130(3):780–6. doi: 10.1378/chest.130.3.780. [DOI] [PubMed] [Google Scholar]
- 11.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 12.Redline S, Tishler PV, Hans MG, Tosteson TD, Strohl KP, Spry K. Racial differences in sleep-disordered breathing in African-Americans and Caucasians. Am J Respir Crit Care Med. 1997;155(1):186–9. doi: 10.1164/ajrccm.155.1.9001310. [DOI] [PubMed] [Google Scholar]
- 13.Stepnowsky C, Johnson S, Dimsdale J, Ancoli-Israel S. Sleep apnea and health-related quality of life in African-American elderly. Ann Behav Med. 2000;22(2):116–2. doi: 10.1007/BF02895774. [DOI] [PubMed] [Google Scholar]
- 14.Ancoli-Israel S, Klauber MR, Stepnowsky C, Estline E, Chinn A, Fell R. Sleep-disordered breathing in African-American elderly. Am J Respir Crit Care Med. 1995;152(6 Pt 1):1946–9. doi: 10.1164/ajrccm.152.6.8520760. [DOI] [PubMed] [Google Scholar]
- 15.Redline S, Kirchner HL, Quan SF, Gottlieb DJ, Kapur V, Newman A. The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Arch Int Med. 2004;164(4):406–18. doi: 10.1001/archinte.164.4.406. [DOI] [PubMed] [Google Scholar]
- 16.Scharf SM, Seiden L, DeMore J, Carter-Pokras O. Racial differences in clinical presentation of patients with sleep-disordered breathing. Sleep Breat. 2004;8(4):173–83. doi: 10.1007/s11325-004-0173-5. [DOI] [PubMed] [Google Scholar]
- 17.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288(14):1723–7. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 18.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295(13):1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 19.Ruiter ME, DeCoster J, Jacobs L, Lichstein KL. Sleep disorders in African Americans and Caucasian Americans: a meta-analysis. Behav Sleep Med. 2010;8(4):246–59. doi: 10.1080/15402002.2010.509251. [DOI] [PubMed] [Google Scholar]
- 20.Fiorentino L, Marler M, Stepnowsky C, Johnson S, Ancoli-Israel S. Sleep in older African Americans and Caucasians at risk for sleep-disordered breathing. Behav Sleep Med. 2006;4(3):164–78. doi: 10.1207/s15402010bsm0403_3. [DOI] [PubMed] [Google Scholar]
- 21.Blazer DG, Hays JC, Foley DJ. Sleep complaints in older adults: a racial comparison. J Gerontology Series A-Biological Sciences & Medical Sciences. 1995;50(5):M280–4. doi: 10.1093/gerona/50a.5.m280. [DOI] [PubMed] [Google Scholar]
- 22.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Int Med. 1999;131(7):485–91. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 23.Rodsutti J, Hensley M, Thakkinstian A, D’Este C, Attia J. A clinical decision rule to prioritize polysomnography in patients with suspected sleep apnea. Sleep. 2004;27(4):694–9. doi: 10.1093/sleep/27.4.694. [DOI] [PubMed] [Google Scholar]
- 24.Siccoli MM, Pepperell JC, Kohler M, Craig SE, Davies RJ, Stradling JR. Effects of continuous positive airway pressure on quality of life in patients with moderate to severe obstructive sleep apnea: data from a randomized controlled trial. Sleep. 2008;31(11):1551–8. doi: 10.1093/sleep/31.11.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor HA., Jr The Jackson Heart Study: an overview. Ethn Dis. 2005;15:S6–3. [PubMed] [Google Scholar]
- 26.Fuqua SR, Wyatt SB, Andrew ME, et al. Recruiting African-American research participation in the Jackson Heart Study: methods, response rates, and sample description. Ethn Dis. 2005;15:S6–29. [PubMed] [Google Scholar]
- 27.Wilson JG, Rotimi CN, Ekunwe L, et al. Study design for genetic analysis in the Jackson Heart Study. Ethn Dis. 2005;15:S6–37. [PubMed] [Google Scholar]
- 28.Payne TJ, Wyatt SB, Mosley TH, et al. Sociocultural methods in the Jackson Heart Study: conceptual and descriptive overview. Ethn Dis. 2005;15:S6–48. [PubMed] [Google Scholar]
- 29.Carpenter MA, Crow R, Steffes M, et al. Laboratory, reading center, and coordinating center data management methods in the Jackson Heart Study. Am J Med Sci. 2004;328:131–144. doi: 10.1097/00000441-200409000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Zimmerman M, Posternak MA, Chelminski I. Using a self-report depression scale to identify remission in depressed outpatients. Am J Psychiatry. 2004;161(10):1911–3. doi: 10.1176/ajp.161.10.1911. [DOI] [PubMed] [Google Scholar]
- 31.Dubbert PM, Carithers T, Ainsworth BE, Taylor HA, Jr, Wilson G, Wyatt SB. Physical activity assessment methods in the Jackson Heart Study. Ethn Dis. 2005;15(4 S6):S6-56–61. [PubMed] [Google Scholar]
- 32.Kohn PM, MacDonald JE. The Survey of Recent Life Experiences: a decontaminated hassles scale for adults. J Behav Med. 1992;15(2):221–228. doi: 10.1007/BF00848327. [DOI] [PubMed] [Google Scholar]
- 33.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 34.Sarason IG, Johnson JH, Siegel JM. Assessing the impact of life changes: development of the life experiences survey. J Consult Clin Psychol. 1978;46(5):932–946. doi: 10.1037//0022-006x.46.5.932. [DOI] [PubMed] [Google Scholar]
- 35.Cohen-Zion M, Stepnowsky C, Johnson S, Marler M, Dimsdale JE, Ancoli-Israel S. Cognitive changes and sleep disordered breathing in elderly: differences in race. J Psychosom Res. 2004;56(5):549–53. doi: 10.1016/j.jpsychores.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Baldwin CM, Ervin AM, Mays MZ, et al. Sleep disturbances, quality of life, and ethnicity: the Sleep Heart Health Study. J Clin Sleep Med. 2010;6(2):176–83. [PMC free article] [PubMed] [Google Scholar]
- 37.Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA. 2004;291(16):2013–6. doi: 10.1001/jama.291.16.2013. [DOI] [PubMed] [Google Scholar]
- 38.Redline S, Tishler PV, Schluchter M, Aylor J, Clark K, Graham G. Risk factors for sleep-disordered breathing in children: associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med. 1999;159(5 Pt 1):1527–32. doi: 10.1164/ajrccm.159.5.9809079. [DOI] [PubMed] [Google Scholar]
- 39.Amin R, Anthony L, Somers V, et al. Growth velocity predicts recurrence of sleep-disordered breathing 1 year after adenotonsillectomy. Am J Respir Crit Care Med. 2008;177(6):654–9. doi: 10.1164/rccm.200710-1610OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ram S, Seirawan H, Kumar SK, Clark GT. Prevalence and impact of sleep disorders and sleep habits in the United States. Sleep Breath. 2010;14(1):63–70. doi: 10.1007/s11325-009-0281-3. [DOI] [PubMed] [Google Scholar]
- 41.Kripke DF, Ancoli-Israel S, Klauber MR, Wingard DL, Mason WJ, Mullaney DJ. Prevalence of sleep - disordered breathing in ages 40 – 64 years: a population - based survey. Sleep. 1997;20(1):65. doi: 10.1093/sleep/20.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Connor GT, Lind BK, Lee ET, et al. Sleep Heart Health Study Investigators. Variation in symptoms of sleep-disordered breathing with race and ethnicity: the Sleep Heart Health Study. Sleep. 2003;26(1):74–9. [PubMed] [Google Scholar]
- 43.Young T, Shahar E, Nieto FJ, et al. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Sleep Heart Health Study Research Group. Arch Int Med. 2002;162(8):893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 44.Hall MH, Matthews KA, Kravitz HM, et al. Race and financial strain are independent correlates of sleep in midlife women: the SWAN sleep study. Sleep. 2009;32(1):73–82. [PMC free article] [PubMed] [Google Scholar]
- 45.Kripke DF, Jean-Louis G, Elliott JA, et al. Ethnicity, sleep, mood, and illumination in postmenopausal women. BMC Psychiatry. 2004;4:8. doi: 10.1186/1471-244X-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]