Abstract
Adenosine receptors co-localize with dopamine receptors on medium spiny nucleus accumbens (NAc) neurons where they antagonize dopamine receptor activity. It remains unclear whether adenosine receptor stimulation in the NAc restores cocaine-induced enhancements in dopamine receptor sensitivity. The goal of these studies was to determine whether stimulating A1 or A2A receptors in the NAc reduces the expression of cocaine sensitization. Rats were sensitized with 7 daily treatments of cocaine (15 mg/kg, i.p.). Following one-week withdrawal, the effects of intra-NAc microinjections of the adenosine kinase inhibitor (ABT-702), the adenosine deaminase inhibitor (deoxycoformycin; DCF), the specific A1 receptor agonist (CPA) and the specific A2A receptor agonist (CGS 21680) were tested on the behavioral expression of cocaine sensitization. The results indicate that intra-NAc pretreatment of ABT-702 and DCF dose-dependently blocked the expression of cocaine sensitization while having no effects on acute cocaine sensitivity, suggesting that upregulation of endogenous adenosine in the accumbens is sufficient to non-selectively stimulate adenosine receptors and reverse the expression of cocaine sensitization. Intra-NAc treatment of CPA significantly inhibited the expression of cocaine sensitization, which was reversed by both A1 and A2A receptor antagonism. Intra-NAc treatment of CGS 21680 also significantly inhibited the expression of cocaine sensitization, which was selectively reversed by A2A, but not A1, receptor antagonism. Finally, CGS 21680 also inhibited the expression of quinpirole cross-sensitization. Together, these findings suggest that adenosine receptor stimulation in the NAc is sufficient to reverse the behavioral expression of cocaine sensitization and that A2A receptors blunt cocaine-induced sensitization of post-synaptic D2 receptors.
Keywords: A1 receptor A2A receptor, D2 receptor, psychostimulant, locomotor, dopamine receptor, adenosine kinase, adenosine deaminase, pentostatin
1. Introduction
Cocaine is a psychostimulant that can lead to dependence following continuous use, resulting in an addiction that negatively affects the individual’s physical, mental, and social health. Animals display abnormal behaviors such as locomotor hyperactivity upon acute exposure to cocaine that escalate with repeated cocaine exposure, resulting in locomotor sensitization (Robinson and Berridge, 2008, Thomas et al., 2008). The enhanced locomotor response observed in animals following withdrawal and re-exposure to the same dose of cocaine is thought to correspond to behaviors such as compulsive drug seeking and relapse seen in human cocaine addicts (Kalivas et al., 1998, Robinson and Berridge, 2008). Thus, locomotor sensitization is conceptualized as an expression of cocaine-induced neurobiological alterations that may persist in periods of withdrawal and contribute to the enhanced susceptibility to relapse. A detailed understanding of the molecular and pharmacological bases of behavioral sensitization will reveal novel molecular targets for pharmacological treatment strategies to offset cocaine-induced neural alterations.
Cocaine increases synaptic levels of the neurotransmitters dopamine, serotonin, and norepinephrine by inhibiting their respective reuptake transporters (Ritz et al., 1990). A major neural circuit involved in the development and expression of cocaine sensitization is the mesolimbic DA pathway (Di Chiara, 1995). This pathway consists of dopamine cells in the ventral tegmental area that project to medium spiny GABA neurons in the nucleus accumbens (NAc). The mesolimbic dopamine pathway plays a key role in the brain’s reward system and it is thought that dysregulation within this neural circuit occurs with repeated cocaine exposure (Koob, 2009, Self and Nestler, 1998). Five subtypes of receptors mediate cocaine-induced enhancements in dopamine neurotransmission, including the D1-like receptor family consisting of D1 and D5 receptors, and the D2-like receptor family consisting of D2, D3, and D4 receptors (Missale et al., 1998, Sibley et al., 1993). Rodent studies suggest that stimulation of the D1, D2, and D3 receptors is critical for behavioral hyperactivity and locomotor sensitization following acute and repeated cocaine exposure (Adams et al., 2001, Kita et al., 1999, Piercey et al., 1992, Ushijima et al., 1995). Additional studies have revealed that cocaine-sensitized rodents display enhanced locomotor responses to a challenge with the selective D2-receptor agonist quinpirole, suggesting that repeated cocaine exposure sensitizes D2 receptors (Bachtell et al., 2008, Collins et al., 2011, Edwards et al., 2007, Ujike et al., 1990). Thus, offsetting cocaine-induced D2 receptor hypersensitivity may represent a viable strategy to reduce the expression of locomotor sensitization.
Adenosine is an endogenous purine nucleoside that acts as a neuromodulator of dopamine signaling within the mesolimbic pathway (Ferre et al., 1992, Filip et al., 2012). Extracellular adenosine can be formed in the synaptic cleft by hydrolysis of extracellular adenosine triphosphate or by passive transport from intracellular non-vesicular stores (Cass et al., 1987, Fredholm et al., 1982, Thorn and Jarvis, 1996, White, 1977). Synaptic adenosine is removed from the extracellular space by reuptake via nucleoside transporters or degradation by two key enzymes, adenosine kinase and adenosine deaminase, which convert adenosine to adenosine monophosphate and inosine, respectively (Arch and Newsholme, 1978). Pharmacological inhibition of adenosine kinase or adenosine deaminase activity leads to elevations in local extracellular adenosine concentrations, which results in enhanced non-selective stimulation of adenosine receptors (Ballarin et al., 1991, Golembiowska and Zylewska, 2000, Huber et al., 2001, Jarvis et al., 2000, Pak et al., 1994, Sciotti and Van Wylen, 1993). Adenosine receptor signaling in the striatum is primarily mediated by the A1 and A2A receptor subtypes, which are highly expressed there (Rivkees et al., 1995, Svenningsson et al., 1999b). Within the striatum, postsynaptic adenosine receptor subtypes co-localize with specific dopamine receptor subtypes in the medium spiny neurons where they appear to antagonize postsynaptic dopamine signaling (Bertran-Gonzalez et al., 2009, Ferre et al., 1994a, Ferre et al., 1994b, Ferre et al., 1999, Gines et al., 2000, Hakansson et al., 2006, Svenningsson et al., 1998).
Emergent findings show that A1 and A2A receptors may play a critical role in regulating cocaine-induced behavioral responses (Bachtell and Self, 2009, Filip et al., 2006, Green and Schenk, 2002, Knapp et al., 2001, O'Neill et al., 2012, Poleszak and Malec, 2002b, Worley et al., 1994). Most relevant is a recent study demonstrating that systemic administration of an A2A agonist decreased, while administration of an A2A antagonist increased, cocaine-induced locomotor sensitization in rats (Filip et al., 2006). However, the specific role of striatal adenosine receptors in regulating cocaine-induced acute hyperactivity and expression of locomotor sensitization remains unclear. The current studies were designed to investigate the hypothesis that stimulation of adenosine receptors in the NAc will reverse the expression of locomotor sensitization in male Sprague Dawley rats. Specifically, we tested the effects of selective- and non-selective stimulation of adenosine receptor subtypes within the medial NAc core on the acute sensitivity to cocaine, the expression of cocaine-induced locomotor sensitization, and the expression of D2 receptor cross-sensitization in cocaine-sensitized rats. We chose to modulate adenosine receptors in the medial NAc core given structural, neurochemical and molecular changes that occur in this subregion during behavioral sensitization and the relevance of this site for other behaviors such as reinstatement to drug seeking (Brenhouse et al., 2007, Ito et al., 2004, Li et al., 2004, McFarland and Kalivas, 2001, Pierce et al., 1996). To stimulate adenosine receptors specifically in the medial NAc core, we surgically implanted rats with guide cannulae directed at the NAc core. We administered microinjections of adenosine kinase and adenosine deaminase inhibitors to assess the effects of raising endogenous extracellular adenosine and selective A1 and A2A agonists to assess the effects of selectively stimulating these receptor subtypes, on cocaine-induced locomotion. We also used subtype-selective adenosine receptor antagonists alone and in combination with the agonists to test the receptor specificity of our effects.
2. Materials and Methods
2.1 Animals and housing conditions
Male Sprague-Dawley rats (Charles River, Wilmington, MA) initially weighing 275–325 grams were individually housed with food and water available ad libitum. All experiments were conducted during the light period of a 12-h light/dark cycle in accordance with the guidelines established by the Institutional Animal Care and Use Committee at the University of Colorado at Boulder. All efforts were made to minimize animal suffering, to reduce the number of animals used, and to utilize alternatives to in vivo techniques.
2.2 Surgical procedures
Surgical implantation of intracranial cannulae was performed under halothane anesthesia (1–2.5%). Each rat was placed into a stereotaxic instrument, the scalp incised and retracted, and the head was positioned with Bregma and Lambda at the same depth coordinate. Screws were secured into the skull and holes were drilled in order to bilaterally insert guide cannulae into the NAc core (A/P: +1.7, M/L: +/−1.5, D/V: −5.7 from bregma; (Paxinos and Watson, 1998). Once inserted, guide cannulae were fixed in place with dental cement. Dummy stylets extending 1 mm beyond the tip of the cannulae were placed into the guide cannulae to maintain patency. Rats were allowed 6–7 days recovery in their home cage before experimental procedures began.
2.3 Microinjection procedure and histology
Microinjections were administered as pretreatments 5 min prior to systemic challenge injections. All microinjections occurred in the NAc at a volume of 0.5 µL. Infusions occurred over a 1 min period, and the microinjectors were removed 1 min after administration of the full volume of the infusion to ensure that the surrounding tissues took up the full volume. After all experimental procedures were complete, rats were euthanized with carbon dioxide gas and 1.0 µL/side of 0.1% cresyl violet was infused intracranially to verify cannulae tip placements. Placements were determined from coronal slices and recorded on histological maps. Data from rats with incorrect placements were excluded from these studies.
2.4 General locomotor sensitization procedure
Locomotor activity was recorded in plexiglass chambers (San Diego Instruments, San Diego, CA, USA) measuring 16x16x15 inches with 16 pairs of photobeams spaced 1 inch apart on both the×and y axes. All locomotor tests were performed in darkened chambers during the light phase of the light:dark cycle. Animals were initially habituated to the locomotor testing chambers for 2-h the day prior to start of the sensitization procedure. Saline or cocaine (15 mg/kg, i.p.) injections were performed daily for one week and horizontal locomotor activity was assessed in the activity monitoring chambers. Only animals meeting criterion for locomotor sensitization (Day 7 cocaine-induced activity > 1.4 times Day 1 cocaine-induced locomotor activity) were subsequently challenged and tested.
2.5 Effects of adenosine kinase and adenosine deaminase inhibition on expression of cocaine sensitization
After 7 daily cocaine treatments (15 mg/kg, i.p.) and home cage withdrawal (7 days), animals were habituated and given an intra-NAc pretreatment of vehicle (phosphate-buffered saline (PBS, pH 7.2), the adenosine kinase inhibitor (ABT-702; 2.5 ng/side or 5 ng/side), or the adenosine deaminase inhibitor (DCF; 10 µg/side or 20 µg/side) five minutes prior to the cocaine challenge treatment (15 mg/kg cocaine, i.p.). Locomotor activity was assessed for 2 h after the treatments. In order to assess the effects of ABT-702 and DCF on acute sensitivity, rats run in parallel were administered 7 daily saline treatments, followed by “withdrawal” (7 days) and tested with an intra-NAc pretreatment and acute cocaine challenge according to the above procedures. Forty-eight hours later, the effects of the ABT-702 and DCF alone were tested in a similar procedure without the systemic challenge treatment.
2.6 Effects of adenosine A1 and A2A receptor stimulation on expression of cocaine sensitization
After 7 daily treatments (saline or 15 mg/kg cocaine, i.p.) and home cage withdrawal (7 days), animals were habituated and given an intra-NAc pretreatment of vehicle (PBS, pH 7.2), the A1 receptor agonist (CPA: 0.75 µg/side or 1.50 µg/side), or the A2A receptor agonist (CGS 21680: 2.5 ng/side or 5 ng/side) five minutes prior to challenge treatment (15 mg/kg cocaine, i.p.). Locomotor activity was assessed for 2 h after the treatments. In order to assess the effects of CPA and CGS 21680 on acute sensitivity, rats run in parallel were administered 7 daily saline treatments, followed by “withdrawal” (7 days) and tested with an intra-NAc pretreatment and acute cocaine challenge according to the above procedures.
2.7 Effects adenosine A1 and A2A receptor blockade in cocaine-naïve and cocaine-sensitized animals
After 7 daily treatments (saline or 15 mg/kg cocaine, i.p.) and home cage withdrawal (7 days), animals were habituated and given an intra-NAc pretreatment of vehicle (50% DMSO), the A1 receptor antagonist (DPCPX): 50 µg/side), or the A2A receptor antagonist (MSX-3: 2.5 ng/side or 5 ng/side). MSX-3 is a prodrug of the selective A2A receptor antagonist MSX-2 that is rapidly converted to its active form by phosphatases in vivo (Muller et al., 1998, Sauer et al., 2000), and has been shown to be suitable for intracranial microinfusion (Hauber et al., 1998). Locomotor activity was assessed for 2 h after the treatments.
2.8 Effects of adenosine A1 and A2A receptor antagonism alone and in combination with adenosine receptor agonists on the expression of cocaine sensitization
After 7 daily treatments (15 mg/kg cocaine, i.p.) and home cage withdrawal (7 days), animals were habituated and given an intra-NAc pretreatment of vehicle (DMSO), the A1 receptor antagonist (DPCPX): 50 µg/side), or the A2A receptor antagonist (MSX-3: 2.5 ng/side or 5 ng/side) five minutes prior to a second intra-NAc treatment of vehicle (PBS, pH 7.2), the A1 receptor agonist (CPA: 1.50 µg/side), or the A2A receptor agonist (CGS 21680: 5 ng/side). Finally, a challenge treatment (15 mg/kg cocaine, i.p.) was administered and locomotor activity was assessed for 2 h after the treatments. Thus, all animals were sensitized to cocaine and challenged with cocaine on the test day following one of the 9 possible antagonist/agonist combinations.
2.9 Effects of adenosine A2A receptor stimulation on expression of quinpirole cross-sensitization
Forty-eight hours after testing the effects of adenosine A2A receptor stimulation on the expression of cocaine sensitization, animals were tested for D2 receptor cross-sensitization utilizing a 4-h within-session dose–response protocol as follows: 1-h habituation followed by hourly ascending doses of D2 receptor agonist (saline, 0.1 and 0.3 mg/kg quinpirole, s.c.). Immediately preceding the last hour of testing and treatment with 0.3 mg/kg quinpirole, animals were administered an intra-NAc treatment with either vehicle (PBS) or CGS 21680 (5 ng/side).
2.9 Drugs
The adenosine kinase inhibitor, ABT-702 (5-(3-Bromophenyl)-7-[6-(4-morpholinyl)-3-pyrido[2,3-d]byrimidin-4-amine dihydrochloride), the adenosine deaminase inhibitor, deoxycoformycin, ((8R)-3-(2-Deoxy-β-D-erythro-pentofuranosyl)-3,4,7,8-tetrahydroimidazo[4,5-d][1,3]diazepin-8-ol), the A1 receptor agonist, CPA (N6-cyclopentyladenosine) and the A2A receptor agonist, CGS 21680 [4-[2-[[6-Amino-9-(N-ethyl-b-D-ribofuranuronamidosyl)-9H-purin-2-yl]amino] ethyl]benzenepropanoic acid hydrochloride] were purchased from Tocris Bioscience (Ellisville, MO). The adenosine A1 antagonist, DPCPX (8-cyclopentyl-1,3-dipropylxanthine), the adenosine A2A receptor antagonist, MSX-3 [3,7-dihydro-8-[(1E)-2-(3-methoxyphenyl)ethenyl]-7-methyl-3-[3-(phosphonooxy)propyl-1-(2-propynyl)-1H-purine-2,6-dione disodium salt hydrate], D2-selective agonist, quinpirole [(-)-Quinpirole hydrochloride], and cocaine hydrochloride were obtained from Sigma-Aldrich (St. Louis, MO). All drugs were dissolved in sterile-filtered phosphate-buffered saline (PBS, pH 7.2), except cocaine, which was dissolved in sterile-filtered physiological saline (0.9%) and DPDPX, which was dissolved in 50% DMSO in PBS.
2.10 Statistical analyses
Locomotor data (total beam breaks) were analyzed by one-way or 2-factor ANOVA with Cocaine treatment (saline or cocaine) and challenge pre-treatment (intra-NAc pretreatment dose) as the factors. Interactive effects were followed by simple main effects analyses (one-way ANOVA or t-tests) and post hoc tests (Bonferroni’s test). Statistical significance was preset at p < 0.05.
3. Results
3.1 Inhibition of adenosine kinase or adenosine deaminase inhibits the expression of cocaine sensitization
All animals were administered 7 daily saline or cocaine (15 mg/kg, ip) injections to induce locomotor sensitization (Table 1). Following 7 days of withdrawal, animals were tested for the expression of cocaine sensitization in the presence or absence of the adenosine kinase inhibitor, ABT-702, or the adenosine deaminase inhibitor, DCF. Figure 1 illustrates that an intra-NAc pretreatment of either ABT-702 or DCF reduced the expression of cocaine sensitization. These effects were observed only in cocaine-sensitized animals. A significant cocaine X ABT-702 Dose interaction (F2,46 = 7.47; p = 0.0015) and significant main effects of cocaine (F1,46 = 7.131; p = 0.0096) and ABT-702 Dose (F2,46 = 4.18; p = 0.0215) were observed. Subsequent analysis of the interaction found that ABT-702 treatment in cocaine-naïve animals did not significantly alter acute cocaine sensitivity (F2,22 = 1.87; p = 0.1781). However, ABT-702 treatment in cocaine-sensitized animals significantly inhibited the expression of cocaine sensitization at both ABT-702 doses (F2,22 = 9.19; p < 0.0011). Significant main effects of cocaine (F1,43 = 14.73; p = 0.0004) and DCF Dose (F2,43 = 4.677; p = 0.0145) were also observed, however a significant cocaine X DCF Dose interaction (F2,43 = 2.06; p = 0.1394) was not. Subsequent analysis of the significant main effects found that only the high DCF dose (10 µg/side) significantly inhibited cocaine sensitivity (F2,46 = 3.53; p = 0.0376).
Table 1.
Development of locomotor sensitization with 7 daily saline or cocaine administrations was equivalent between groups prior to intra-NAc treatment on challenge day
| Effects of adenosine kinase and deaminase inhibition | ||
|---|---|---|
| Cocaine-naïve: Repeated Saline | ||
| Day 1 | Day 7 | Intra-NAc Challenge Treatment @ Day 14 |
| 6926 ± 496.3 | 7504 ± 2464 | Vehicle |
| 6894 ± 1014.1 | 6057 ± 1855.3 | ABT 702 (2.5 µg/side) |
| 6494 ± 924.1 | 6341 ± 1155.3 | ABT 702 (5.0 µg/side) |
| 6473 ± 660.1 | 6602 ± 714.8 | DCF (5 µg/side) |
| 6622 ± 496.3 | 6514 ± 2162 | DCF (10 µg/side) |
| Cocaine-sensitized: Repeated Cocaine | ||
| Day 1 | Day 7 | Intra-NAc Challenge Treatment @ Day 14 |
| 23871 ± 5184.2 | 34801 ± 4486.0* | Vehicle |
| 23593 ± 4234.0 | 35122 ± 4532.6* | ABT 702 (2.5 µg/side) |
| 25789 ± 3897.7 | 36662 ± 4778.2* | ABT 702 (5.0 µg/side) |
| 21809 ± 2453.3 | 32681 ± 4621.0* | DCF (5 µg/side) |
| 23699 ± 5334.0 | 33822 ± 2133.6* | DCF (10 µg/side) |
Data represent mean (± SEM) beam breaks/2 hrs.
Statistically significant difference compared to Day 1 locomotor activity, p < 0.001
Figure 1. Intra-NAc administration of adenosine kinase and adenosine deaminase inhibitors attenuates the expression of cocaine sensitization.
(a) Animals repeatedly treated with cocaine (7 X 15 mg/kg, ip) displayed significant expression of sensitization when tested with intra-NAc vehicle and 15 mg/kg cocaine (ip) following 7 days withdrawal compared with animals administered repeated saline. Intra-NAc administration of both the adenosine kinase inhibitor (ABT-702) and adenosine deaminase inhibitor (DCF) diminished the expression of cocaine sensitization. No effect of intra-NAc ABT-702 or DCF was observed on acute cocaine sensitivity since cocaine-induced locomotor activity was equivalent in cocaine-naïve animals. (b) Time-course of locomotor activity illustrating the last 30 min of the habituation period followed by the effects of 15 mg/kg cocaine (ip) with and without the intra-NAc pretreatment ABT-702 (5 µg/side) or DCF (10 µg/side) in cocaine-sensitized animals. * indicates significant from cocaine-naïve with vehicle pretreatment (p<0.0001); # indicates significant from cocaine-sensitized with vehicle pretreatment (p<0.01)
Figure 2 displays the effects of the adenosine kinase and adenosine deaminase inhibitors in the absence of a cocaine challenge in both cocaine-naïve and cocaine-sensitized animals. Intra-NAc administration of the inhibitors had no significant effect on locomotor activity in either cocaine-naïve or cocaine-sensitized animals.
Figure 2. Effects of intra-NAc administration adenosine kinase and adenosine deaminase inhibition in cocaine-sensitized and cocaine-naïve.
(a) An intra-NAc treatment with the adenosine kinase inhibitor, ABT-702, had no effect on locomotion in either cocaine-sensitized or cocaine-naive animals. (b) An intra-NAc treatment with the adenosine deaminase inhibitor, DCF, had no effect on locomotion in either cocaine-sensitized or cocaine-naive animals.
3.2 Intra-NAc stimulation of adenosine A1 and A2A receptors blocks expression of cocaine sensitization
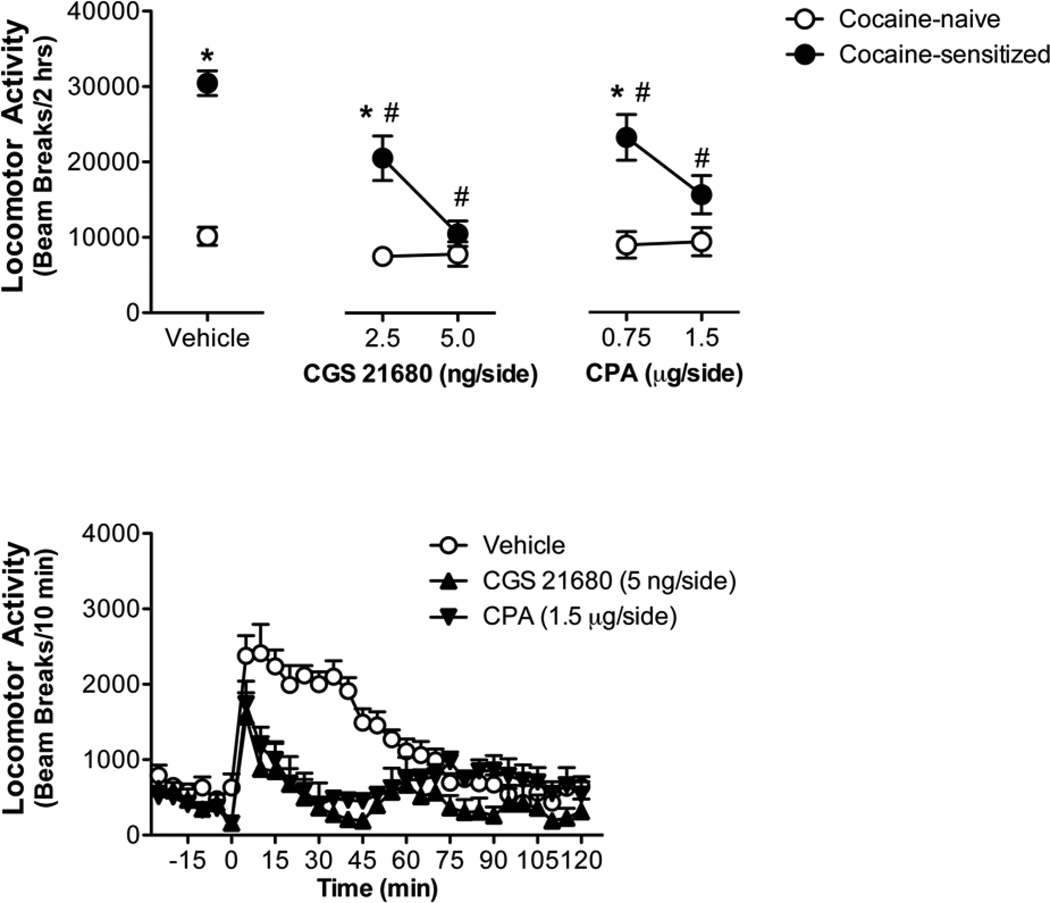
Inhibiting either adenosine kinase or adenosine deaminase is associated with accumulation of extracellular adenosine leading to non-selective stimulation of adenosine receptors (Ballarin et al., 1991, Golembiowska and Zylewska, 2000, Huber et al., 2001, Jarvis et al., 2000, Pak et al., 1994, Sciotti and Van Wylen, 1993). We next sought to determine whether selective stimulation of either the adenosine receptor A1 or A2A subtype in the NAc would recapitulate the inhibition of cocaine sensitization produced by enzyme inhibition. Similar to those studies, all animals were administered 7 daily saline or cocaine (15 mg/kg, ip) injections to induce locomotor sensitization (Table 2). Following 7 days of withdrawal, animals were tested for the expression of cocaine sensitization in the presence or absence of the adenosine A1 agonist, CPA, or the adenosine A2A agonist, CGS 21680. Figure 3 illustrates that an intra-NAc pretreatment of either CPA or CGS 21680 reduced the expression of cocaine sensitization. Like the enzyme inhibitors, these effects were observed only in cocaine-sensitized animals. A significant cocaine X CPA Dose interaction (F2,27 = 3.65; p = 0.0395) and significant main effects of cocaine (F1,27 = 42.27; p = 0.0001) and CPA Dose (F2,27 = 4.533; p = 0.0201) were observed. Subsequent analysis of the interaction found CPA treatment in cocaine-naïve animals did not significantly alter acute cocaine sensitivity (F2,9 < 1; p = 0.8771) while CPA treatment in cocaine-sensitized animals significantly inhibited the expression of cocaine sensitization at both CPA doses (F2,18 = 7.84; p = 0.0036). Similarly, a significant cocaine X CGS 21680 Dose interaction (F2,25 = 11.23; p = 0.0003) and significant main effects of cocaine (F1,25 = 52.53; p < 0.0001) and CGS 21680 Dose (F2,25 = 17.84; p < 0.0001) were observed. Subsequent analysis of the interaction shows that CGS 21680 treatment in cocaine-sensitized animals significantly inhibited the expression of cocaine sensitization at both CGS21680 doses (F2,17 = 33.06; p < 0.0001) and had no effect on cocaine sensitivity in cocaine-naïve animals (F2,14 < 1; p = 0.4498).
Table 2.
Development of locomotor sensitization with 7 daily saline or cocaine administrations was equivalent between groups prior to intra-NAc treatment on challenge day
| Effects of adenosine receptor stimulation | ||
|---|---|---|
| Cocaine-naïve: Repeated Saline | ||
| Day 1 | Day 7 | Intra-NAc Challenge Treatment @ Day 14 |
| 6921 ± 248.9 | 6585 ± 625.9 | Vehicle |
| 6712 ± 281.7 | 6184 ± 312.8 | CGS 21680 (2.5 ng/side) |
| 5765 ± 201.3 | 6500 ± 342.6 | CGS 21680 (5.0 ng/side) |
| 6176 ± 331.3 | 5680 ± 641.3 | CPA (0.75 µg/side) |
| 6528 ± 278.0 | 6277 ± 302.9 | CPA (1.5 µg/side) |
| Cocaine-sensitized: Repeated Cocaine | ||
| Day 1 | Day 7 | Intra-NAc Challenge Treatment @ Day 14 |
| 23405 ± 5154.6 | 47657 ± 3562.4* | Vehicle |
| 25105 ± 4957.7 | 48622 ± 3752.4* | CGS 21680 (2.5 ng/side) |
| 27030 ± 7532.4 | 50943 ± 3553.8* | CGS 21680 (5.0 ng/side) |
| 28193 ± 6854.1 | 51022 ± 3357.7* | CPA (0.75 µg/side) |
| 27216 ± 7212.2 | 48724 ± 4251.5* | CPA (1.5 µg/side) |
Data represent mean (± SEM) beam breaks/2 hrs.
Statistically significant difference compared to Day 1 locomotor activity, p < 0.001
Figure 3. Intra-NAc administration of adenosine receptor agonists attenuates the expression of cocaine sensitization.
(a) Animals repeatedly treated with cocaine (7 × 15 mg/kg, ip) displayed significant expression of sensitization when tested with intra-NAc vehicle and 15 mg/kg cocaine (ip) following 7 days withdrawal compared with animals administered repeated saline. Intra-NAc administration of both the adenosine A2A agonist (CGS 21680) and adenosine A1 agonist (CPA) diminished the expression of cocaine sensitization. No effect of intra-NAc CGS 21680 or CPA was observed on acute cocaine sensitivity since cocaine-induced locomotor activity was equivalent in cocaine-naïve animals. (b) Time-course of locomotor activity illustrating the last 30 min of the habituation period followed by the effects of 15 mg/kg cocaine (ip) with and without the intra-NAc pretreatment CGS 21680 (5 µg/side) or CPA (1.5 µg/side) in cocaine-sensitized animals. * indicates significant from respective cocaine-naïve group (p<0.0001); # indicates significant from cocaine-sensitized with vehicle pretreatment (p<0.001)
3.3 Intra-NAc blockade of adenosine A1 and A2A receptors reverses agonist-induced reductions of expression of cocaine sensitization
Previous work demonstrates that systemic administration of an adenosine A2A antagonist enhances the expression of cocaine sensitization (Filip et al., 2006). Therefore, we sought to determine whether adenosine receptor blockade directly in the NAc core would produce a similar effect. We first tested whether intra-NAc adenosine A1 and A2A receptor blockade would produce cross-sensitization in cocaine-sensitized animals compared to cocaine-naïve animals. Administration of the A1 and A2A receptor antagonists produced modest increases in locomotor activity in cocaine-sensitized animals compared to vehicle treatments (Figure 4). A significant main effect of cocaine (F1,26 = 5.277; p = 0.0259) and antagonist (F2,26 = 5.581; p = 0.0119) was observed, however the cocaine X antagonist interaction was not statistically significant (F2,26 = 1.256; p = 0.3015).
Figure 4. Effects of intra-NAc treatment of A1 and A2A antagonists in cocaine-naïve and cocaine-sensitized animals.
Animals sensitized to cocaine with 7 daily cocaine injections (15 mg/kg, ip) displayed modest increases in locomotor activity compared to cocaine-naïve (saline treated) animals. * indicates significant from respective cocaine-sensitized, vehicle group (p<0.05)
Given that adenosine receptor blockade produced only modest effects when administered alone, we next tested the specificity of the effects observed with intra-NAc administration of adenosine agonists. Similar to those studies, animals were administered 7 daily cocaine (15 mg/kg, ip) injections to induce locomotor sensitization. Following 7 days of withdrawal, animals were tested for the expression of cocaine sensitization with a cocaine challenge (15 mg/kg, ip). Prior to the cocaine challenge, animals received intra-NAc treatments of the adenosine A1 or A2A antagonist alone or in combination with intra-NAc treatments of the A1 or A2A agonists. Figure 5 illustrates the results of the antagonist/agonist combinations on the expression of cocaine sensitization. A significant agonist (vehicle, CPA, CGS 21680) X antagonist (vehicle, DPCPX, MSX-3) interaction (F4,37 = 4.55; p = 0.0044) and significant main effects of agonist (F2,37 = 23.81; p = 0.0001) and antagonists (F2,37 = 3.78; p = 0.0320) were observed. Subsequent analysis of the interaction found that CPA and CGS 21680 treatment alone significantly inhibited the expression of cocaine sensitization as observed above (F2,12 = 22.79; p = 0.0002). There was no effect of either antagonist pretreatment (DPCPX or MSX-3) on the expression of cocaine sensitization compared to vehicle pretreatment (F2,13 < 1; p = 0.6571). Pretreatment with DPCPX significantly reversed CPA-induced inhibition of cocaine sensitization while having no effect on CGS 21680-induced inhibition (F2,14 = 18.92; p = 0.0001). Pretreatment with MSX-3 significantly reversed CGS 21680-induced inhibition of cocaine sensitization (F2,11 = 21.95; p = 0.0001). There was a statistical trend for MSX-3 to reverse CPA-induced inhibition of cocaine sensitization, although this did not reach statistical significance (F2,13 = 3.48; p = 0.0616).
Figure 5. Effects of intra-NAc treatment of A1 and A2A on adenosine agonist-induced inhibition of cocaine sensitization.
Animals were sensitized to cocaine with 7 daily cocaine injections (15 mg/kg, ip). As reported above, CPA and CGS alone significantly reduced the expression of cocaine sensitization (left). Intra-NAc pretreatment of the A1 antagonist, DPCPX (middle), reversed CPA-induced reductions in cocaine sensitization, but not CGS 21680-induced reductions. Intra-NAc pretreatment of the A2A antagonist, MSX-3 (right), significantly reversed CGS 21680-induced and CPA-induced reductions in the expression of cocaine sensitization. * indicates significant from vehicle-vehicle group (p<0.01); # indicates significant from respective agonist-vehicle group (p<0.05)
3.4 Intra-NAc stimulation of adenosine A2A receptors inhibits quinpirole sensitivity
Cocaine sensitization is associated with a cross-sensitization in postsynaptic D2 receptors in the NAc (Bachtell et al., 2008, Collins et al., 2011, Edwards et al., 2007, Ujike et al., 1990). This study tests the hypothesis that cocaine-induced cross-sensitization in D2 receptor sensitivity will be inhibited though the stimulation of adenosine A2A receptors that are co-localized with postsynaptic D2 receptors in the NAc spiny neurons. Figure 4 illustrates that intra-NAc administration of the A2A receptor agonist, CGS 21680, suppresses quinpirole-induced locomotor activity in both cocaine-naïve and cocaine-sensitized animals. Statistical analysis reveals that cocaine-sensitized animals have a more robust locomotor response to quinpirole administration (F1,16 = 12.21; p = 0.0030) and that an intra-NAc pretreatment of CGS 21680 suppresses quinpirole-induced activity in both cocaine-naïve and cocaine sensitized animals (F1,16 = 8.65; p = 0.0096). No interactive effects were observed (F2,16 = 1.04; p = 0.3222).
4. Discussion
These studies elucidate adenosine signaling in the NAc as a key pharmacological mechanism for counteracting the expression of cocaine-induced locomotor sensitization. It is important to note that these effects do not appear to be suppressing general locomotor activity since they have no effect on the acute sensitivity of cocaine. These findings corroborate previous studies demonstrating that systemic injections of adenosine agonists counteract many cocaine-induced behavioral changes. For example, systemic administration of adenosine agonists attenuates both the development and expression of behavioral sensitization to cocaine (Filip et al., 2006, Poleszak and Malec, 2002b), impairs the acquisition of cocaine self-administration (Knapp et al., 2001), reduces the expression of cocaine conditioned place preference (Poleszak and Malec, 2002a), and attenuates cocaine seeking (Bachtell and Self, 2009). Adenosine receptors are highly expressed in the striatal structures, including the NAc, however, there is expression elsewhere in the brain. Therefore, it is important to characterize the localization of these behavioral effects. We recently demonstrated that cocaine seeking is reduced by adenosine receptor stimulation and enhanced by adenosine receptor blockade specifically in the NAc core (O'Neill et al., 2012). Together, these findings indicate that pharmacological stimulation of adenosine receptors specifically in the NAc opposes the behavioral responsiveness to cocaine.
We utilized two approaches to study the influence of adenosine signaling on cocaine sensitization. First, we infused inhibitors of the enzymes adenosine kinase and adenosine deaminase directly into the NAc prior to a cocaine challenge. These are two primary enzymes responsible for adenosine metabolism in the CNS, and they directly regulate the extracellular concentration of adenosine (Golembiowska and Zylewska, 2000, Jarvis et al., 2000). Inhibition of these enzymes produces elevations in endogenous adenosine and subsequent non-selective stimulation of adenosine receptors (Ballarin et al., 1991, Golembiowska and Zylewska, 2000, Huber et al., 2001, Jarvis et al., 2000, Pak et al., 1994, Sciotti and Van Wylen, 1993). Studies have demonstrated that adenosine kinase is the rate-limiting enzyme in ADO metabolism, while adenosine deaminase plays a less critical role in determining extracellular ADO concentration (Lloyd and Fredholm, 1995, Phillips and Newsholme, 1979, Romanowska et al., 2007). It is therefore likely that inhibiting adenosine kinase with ABT-702 leads to a more substantial increase in extracellular ADO than inhibiting adenosine deaminase with DCF. This idea may explain why ABT-702 was more efficacious and a higher dose of DCF was required to significantly block the expression of cocaine-sensitization.
As an alternative to enzyme inhibition, we also examined the effects of direct stimulation of specific ADO receptor subtypes in the NAc. ADO receptor subtypes are differentially expressed on different cellular populations within the striatum (see below). Therefore, we were exploring whether stimulation of a specific subtype localized to a unique cell population may be more or less effective in mediating cocaine-induced locomotion. Our findings suggest that both ADO A1 and A2A receptor stimulation are sufficient to reverse the expression of cocaine sensitization, however, the mechanisms of these effects may be quite different. Importantly, we show that the effects of CGS 21680 were dependent upon A2A receptor stimulation since administration of the A2A receptor antagonist, MSX-3, but not the A1 receptor antagonist, DPCPX, blocked CGS 21680-induced inhibition of cocaine sensitization. This was not true for the CPA-induced inhibition of cocaine sensitization where both A1 and A2A receptor antagonists reversed this inhibition. This suggests that A1 receptor activation may have cooperative actions with A2A receptors in the NAc akin to those observed with dopamine D1 and D2 receptors in the striatum (Bachtell et al., 2005, Hopf et al., 2003, Schmidt and Pierce, 2006, White, 1987).
The dorsal striatum and NAc are comprised primarily of medium spiny GABA neurons that include two distinct subpopulations of output neurons. These two subpopulations are differentiated by their unique expression of cellular peptides and receptor subtypes, and unique projection targets (Aubert et al., 2000, Steiner and Gerfen, 1998). For example, neurons forming the direct pathway are dynorphin/substance P-expressing and contain dopamine D1 and adenosine A1 receptors, while neurons of the indirect pathway are enkephalin-expressing and contain dopamine D2 and adenosine A2A receptors (Lu et al., 1998). There is accumulating evidence suggesting that these two populations of striatal neurons play differential roles in cocaine’s actions (Bertran-Gonzalez et al., 2008, Lee et al., 2006, Lobo et al., 2010, Lobo and Nestler, 2011). Our findings provide additional support for the unique contribution of these populations of neurons in cocaine-mediated behaviors. First, previous work indicates that systemic CPA induces c-Fos expression in the population of striatal GABA neurons containing A2A and D2 receptors (Karcz-Kubicha et al., 2006, Karcz-Kubicha et al., 2003). Similar to our effects on the expression of cocaine sensitization, administration of an A2A receptor antagonist reduces CPA-induced c-Fos expression in the striatum (Karcz-Kubicha et al., 2003). Together, these findings suggest that A1 receptor stimulation has the capacity to activate A2A/D2-containing cells through the stimulation of A2A receptors in the striatum which may reduce the expression of cocaine sensitization. Cocaine produces a robust cross-sensitization to the D2 receptor agonist, quinpirole (Bachtell et al., 2008, Collins et al., 2011, Edwards et al., 2007, Ujike et al., 1990). We show that local administration of the A2A agonist, CGS 21680, in the NAc reverses D2 receptor cross-sensitization which coincides with other recent findings showing that NAc A2A receptors inhibit D2-induced reinstatement to cocaine seeking (O'Neill et al., 2012). Thus, it appears that activation of the A2A/D2-containing cells in the striatum, either directly through A2A receptor stimulation or indirectly through A1 receptor stimulation, is important in reversing cocaine-induced enhancements in dopamine D2 receptor sensitivity that contribute to the expression of behavioral sensitization.
Generally speaking, adenosine receptor co-localization with dopamine receptors provides complementary cellular regulation where the adenosine receptors oppose cellular signaling resulting from dopamine receptor stimulation (Canals et al., 2003, Ferre, 1997, Fuxe et al., 2003, Hillion et al., 2002, Svenningsson et al., 1999a, Svenningsson et al., 1998, Svenningsson et al., 1999b). In this way, adenosine receptor stimulation can offset excessive dopamine receptor stimulation resulting from repeated psychostimulant treatments (Bailey et al., 2008, Burechailo and Martin-Iverson, 1996, Burger and Martin-Iverson, 1994, Henry and White, 1991, Ujike et al., 1990). Adenosine and dopamine receptors can alter signaling of medium spiny GABA neurons within the striatum through a variety of mechanisms. For example, these receptors form heteromeric receptor complexes through electrostatic interactions (Canals et al., 2003, Ferre et al., 1994b, Ferre et al., 1998, Franco et al., 2007, Fuxe et al., 2003, Gines et al., 2000, Hillion et al., 2002, Torvinen et al., 2002). Heteromeric formation of adenosine and dopamine receptors renders a low-affinity state of the dopamine receptor where ligand binding is inhibited and coupling of G-proteins is diminished at the dopamine receptor (Ferre et al., 1991, Ferre et al., 1998, Fuxe et al., 1998, Hillion et al., 2002, Torvinen et al., 2002, Torvinen et al., 2005). Interestingly, cocaine treatment disrupts the expression of both the A1-D1 and A2A-D2 heteromer (Marcellino et al., 2010, Toda et al., 2003), which may underlie some of the changes in behavioral responses resulting from chronic cocaine administration. Functionally, disruption of adenosine-dopamine heteromeric complexes may enable enhanced dopamine receptor activity that contributes to behavioral sensitization. Adenosine receptor stimulation may facilitate the coupling of adenosine and dopamine receptors (Vidi et al., 2008), ultimately restoring dopamine antagonism and reversing the behavioral changes following chronic cocaine administration. However, it remains unclear whether heteromeric receptor complexes or another interactive mechanism mediate our effects since receptors that are not in heteromeric complexes still play an antagonistic and reciprocal role in modulating cellular function (Ferre, 1997, Ferre et al., 1991).
Adenosine and dopamine receptors are coupled to opposing classes of G proteins (Dunwiddie and Masino, 2001, Lachowicz and Sibley, 1997). While D2 receptors are coupled to inhibitory GαI proteins, the complementary A2A receptor is coupled to stimulatory GαS proteins. Likewise, D1 receptors are coupled to stimulatory GαS proteins while its complementary A1 receptor is coupled to inhibitory GαI proteins. Thus, the complementary G-protein signaling between adenosine and dopamine receptors can have profound effects on intracellular signaling cascades and neuronal excitability (Ferre et al., 1994b, Ferre et al., 1996, Ferre et al., 1999, Schiffmann et al., 2007, Svenningsson et al., 1999a, Tozzi et al., 2007). This suggests that reciprocal regulation of downstream targets of cAMP (e.g. PKA-mediated phosphorylation targets) may play a role in the expression of cocaine sensitization. While adenosine receptors obviously play a significant role in opposing dopamine neurotransmission within the striatum, the cellular mechanisms of our effects on cocaine sensitization remain obscure and it is likely that both heteromeric receptor complexes and adenosine receptor-induced intracellular signaling contribute to the modulation of these behaviors.
It is important to recognize that adenosine receptors are expressed on other cell types in the NAc, providing other possible explanations for our results. For example, expression of A1 and A2A receptor heteromeric complexes on presynaptic glutamate terminals is involved in modulating striatal glutamate release (Ciruela et al., 2006, Ferre et al., 2008, Hettinger et al., 2001, Orru et al., 2011a, Orru et al., 2011b, Rodrigues et al., 2005). Thus, stimulation of presynaptic A2A receptors increases striatal glutamate release, while stimulation of A1 receptors produces the opposite effect (Ciruela et al., 2006, Corsi et al., 2000, Corsi et al., 1999). It seems unlikely that our findings would result from A2A-induced increases in glutamate release since stimulation of AMPA receptors in the NAc produces enhanced locomotion in cocaine sensitized animals and blockade of AMPA receptors prevents the expression of cocaine sensitization (Bachtell and Self, 2008, Bell and Kalivas, 1996, Pierce et al., 1996). Thus, we suspect that A2A receptor stimulation is primarily influencing postsynaptic A2A receptors on medium spiny neurons. Our finding that A2A stimulation inhibits quinpirole-induced locomotion in both cocaine-naïve and cocaine-sensitized animals also supports this notion.
It is plausible, however, that stimulation of A1 receptors may inhibit the ability of cocaine to enhance extracellular glutamate during the cocaine challenge that is necessary for the behavioral expression of sensitization (Bell et al., 2000, Madayag et al., 2007, Pierce et al., 1996). Adenosine A1 receptors are also thought to be expressed on a small percentage of dopamine terminals in the striatum (Alexander and Reddington, 1989, Borycz et al., 2007, Wojcik and Neff, 1983) where A1 receptor stimulation inhibits depolarization-induced dopamine release (Borycz et al., 2007, Ebstein and Daly, 1982, Michaelis et al., 1979). Thus, stimulation of A1 receptors may have a multitude of effects within the NAc that contributes to reversing the expression of cocaine sensitization including inhibition of cocaine-induced dopamine and glutamate release, inhibiting the postsynaptic signaling mediated by D1 receptors, and/or enabling A2A receptors to activate the A2A/D2-containing striatal cells.
5. Conclusions
The results of these experiments suggest an important role of adenosine and adenosine receptors within the NAc for the behavioral expression of cocaine sensitization. We demonstrate that inhibition of two enzymes responsible for degrading extracellular adenosine, adenosine kinase and adenosine deaminase, inhibit the behavioral expression of cocaine sensitization. Presumably, these effects are achieved through the non-selective stimulation of local adenosine receptors. This notion is supported by our findings that intra-NAc stimulation of either A1 or A2A receptors inhibits the behavioral expression of cocaine sensitization. Furthermore, we demonstrated that the effects of intra-NAc A2A stimulation are likely mediated through postsynaptic expression of A2A receptors since quinpirole-induced cross-sensitization was also inhibited, which is thought to be mediated by sensitization in postsynaptic D2 receptors. While the antagonistic interaction between adenosine and dopamine receptors on striatal neuronal transmission is supported by these experiments, the relative contribution of heteromeric and nonheteromeric complexes is unknown. Together, our results suggest that interactions between adenosine and dopamine receptors influence striatal signaling that mediates the expression of cocaine sensitization, but not the acute sensitivity of cocaine’s effects. Finally, the results of this study illuminate the potential for adenosine receptor stimulation as an effective strategy for reversing cocaine-induced sensitization that may underlie an addicts’ persistent susceptibility to relapse.
Figure 6. Intra-NAc treatment of an A2A receptor agonist reduces locomotor sensitivity of the D2 agonist in both cocaine-naïve and cocaine-sensitized.
Locomotor activity induced by a high dose of the D2 receptor agonist, quinpirole (0.3 mg/kg, sc) is attenuated in both cocaine-naïve and cocaine-sensitized animals. Importantly, cocaine-sensitized animals displayed crosssensitization in D2-induced locomotion, which was attenuated to levels similar to cocaine-naïve animals. * indicates significant from vehicle treated cocaine-naïve group (p<0.0001); # indicates significant from cocaine-sensitized with vehicle pretreatment (p<0.001)
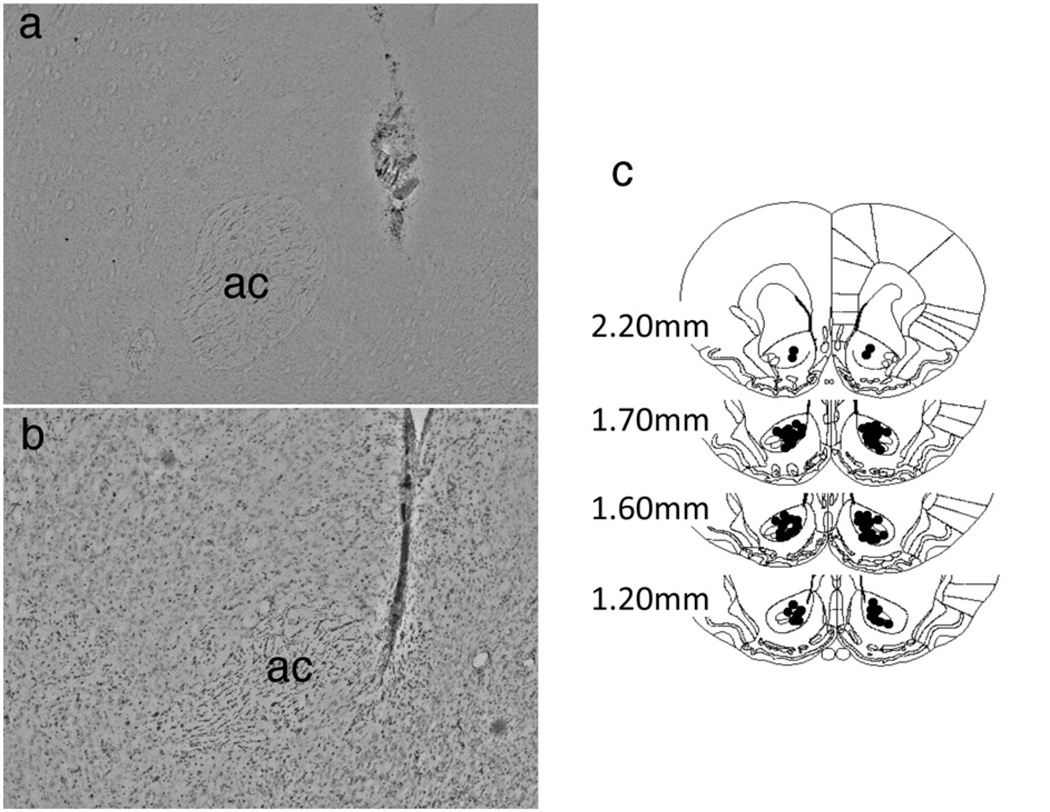
Figure 7. Localization of infusion sites in NAc core.
(a) Some infusion sites were verified by an infusion of 1.0 µL/side of 0.1% cresyl violet through the guide cannula following euthanasia and 40 µm sections were analyzed for accurate placements. (b)Infusion sites were also verified by staining non-infused 40 µm brain sections with cresyl violet. There were minimal signs of gliosis or scarring following the intra-NAc infusions. (c) Infusion sites for all animals included in the study analyses. Animals having infusion sites outside of the NAc core were eliminated from statistical analyses.
Highlights.
Elevating adenosine levels in the accumbens reverses cocaine sensitization
A1 and A2A receptor stimulation in the accumbens reverses cocaine sensitization
Accumbens adenosine receptor stimulation does not affect acute cocaine sensitivity
A1-induced reversal of sensitization involves both A1 and A2A receptors
Accumbens A2A receptor stimulation reverses D2 receptor cross-sensitivity
Acknowledgements
This work was supported by a United States Public Health Services Grant DA 029240 (R.K.B.), the Innovative Seed Grant program at the University of Colorado, and a University of Colorado CRCW Faculty Development Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams JU, Careri JM, Efferen TR, Rotrosen J. Differential effects of dopamine antagonists on locomotor activity, conditioned activity and conditioned place preference induced by cocaine in rats. Behav Pharmacol. 2001;12:603–611. doi: 10.1097/00008877-200112000-00004. [DOI] [PubMed] [Google Scholar]
- Alexander SP, Reddington M. The cellular localization of adenosine receptors in rat neostriatum. Neuroscience. 1989;28:645–651. doi: 10.1016/0306-4522(89)90011-0. [DOI] [PubMed] [Google Scholar]
- Arch JR, Newsholme EA. Activities and some properties of 5'-nucleotidase, adenosine kinase and adenosine deaminase in tissues from vertebrates and invertebrates in relation to the control of the concentration and the physiological role of adenosine. The Biochemical journal. 1978;174:965–977. doi: 10.1042/bj1740965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert I, Ghorayeb I, Normand E, Bloch B. Phenotypical characterization of the neurons expressing the D1 and D2 dopamine receptors in the monkey striatum. Journal of Comparative Neurology. 2000;418:22–32. [PubMed] [Google Scholar]
- Bachtell RK, Whisler K, Karanian D, Self DW. Effects of intra-nucleus accumbens shell administration of dopamine agonists and antagonists on cocaine-taking and cocaine-seeking behaviors in the rat. Psychopharmacology (Berl) 2005;183:41–53. doi: 10.1007/s00213-005-0133-1. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Choi KH, Simmons DL, Falcon E, Monteggia LM, Neve RL, et al. Role of GluR1 expression in nucleus accumbens neurons in cocaine sensitization and cocaine-seeking behavior. Eur J Neurosci. 2008;27:2229–2240. doi: 10.1111/j.1460-9568.2008.06199.x. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Self DW. Renewed cocaine exposure produces transient alterations in nucleus accumbens AMPA receptor-mediated behavior. J Neurosci. 2008;28:12808–12814. doi: 10.1523/JNEUROSCI.2060-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachtell RK, Self DW. Effects of adenosine A(2A) receptor stimulation on cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2009 doi: 10.1007/s00213-009-1624-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey A, Metaxas A, Yoo JH, McGee T, Kitchen I. Decrease of D2 receptor binding but increase in D2-stimulated G-protein activation, dopamine transporter binding and behavioural sensitization in brains of mice treated with a chronic escalating dose 'binge' cocaine administration paradigm. Eur J Neurosci. 2008;28:759–770. doi: 10.1111/j.1460-9568.2008.06369.x. [DOI] [PubMed] [Google Scholar]
- Ballarin M, Fredholm BB, Ambrosio S, Mahy N. Extracellular levels of adenosine and its metabolites in the striatum of awake rats: inhibition of uptake and metabolism. Acta Physiol Scand. 1991;142:97–103. doi: 10.1111/j.1748-1716.1991.tb09133.x. [DOI] [PubMed] [Google Scholar]
- Bell K, Kalivas PW. Context-specific cross-sensitization between systemic cocaine and intra-accumbens AMPA infusion in the rat. Psychopharmacology (Berl) 1996;127:377–383. doi: 10.1007/s002130050101. [DOI] [PubMed] [Google Scholar]
- Bell K, Duffy P, Kalivas PW. Context-specific enhancement of glutamate transmission by cocaine. Neuropsychopharmacology. 2000;23:335–344. doi: 10.1016/S0893-133X(00)00100-7. [DOI] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Herve D, Valjent E, et al. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci. 2008;28:5671–5685. doi: 10.1523/JNEUROSCI.1039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Hakansson K, Borgkvist A, Irinopoulou T, Brami-Cherrier K, Usiello A, et al. Histone H3 phosphorylation is under the opposite tonic control of dopamine D2 and adenosine A2A receptors in striatopallidal neurons. Neuropsychopharmacology. 2009;34:1710–1720. doi: 10.1038/npp.2008.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borycz J, Pereira MF, Melani A, Rodrigues RJ, Kofalvi A, Panlilio L, et al. Differential glutamate-dependent and glutamate-independent adenosine A1 receptor-mediated modulation of dopamine release in different striatal compartments. J Neurochem. 2007;101:355–363. doi: 10.1111/j.1471-4159.2006.04386.x. [DOI] [PubMed] [Google Scholar]
- Brenhouse HC, Howe ML, Stellar JR. Differential activation of cAMP response element binding protein in discrete nucleus accumbens subregions during early and late cocaine sensitization. Behavioral neuroscience. 2007;121:212–217. doi: 10.1037/0735-7044.121.1.212. [DOI] [PubMed] [Google Scholar]
- Burechailo L, Martin-Iverson MT. Behavioral sensitization to cocaine, but not cocaine-conditioned behavior, is associated with increased dopamine occupation of its receptors in the nucleus accumbens. Behavioral neuroscience. 1996;110:1388–1396. doi: 10.1037//0735-7044.110.6.1388. [DOI] [PubMed] [Google Scholar]
- Burger LY, Martin-Iverson MT. Increased occupation of D1 and D2 dopamine receptors accompanies cocaine-induced behavioral sensitization. Brain research. 1994;639:228–232. doi: 10.1016/0006-8993(94)91734-5. [DOI] [PubMed] [Google Scholar]
- Canals M, Marcellino D, Fanelli F, Ciruela F, de Benedetti P, Goldberg SR, et al. Adenosine A2A-dopamine D2 receptor-receptor heteromerization: qualitative and quantitative assessment by fluorescence and bioluminescence energy transfer. J Biol Chem. 2003;278:46741–46749. doi: 10.1074/jbc.M306451200. [DOI] [PubMed] [Google Scholar]
- Cass CE, Belt JA, Paterson AR. Adenosine transport in cultured cells and erythrocytes. Prog Clin Biol Res. 1987;230:13–40. [PubMed] [Google Scholar]
- Ciruela F, Casado V, Rodrigues RJ, Lujan R, Burgueno J, Canals M, et al. Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1–A2A receptor heteromers. J Neurosci. 2006;26:2080–2087. doi: 10.1523/JNEUROSCI.3574-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Truong YN, Levant B, Chen J, Wang S, Woods JH. Behavioral sensitization to cocaine in rats: evidence for temporal differences in dopamine D3 and D2 receptor sensitivity. Psychopharmacology. 2011;215:609–620. doi: 10.1007/s00213-010-2154-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsi C, Melani A, Bianchi L, Pepeu G, Pedata F. Striatal A2A adenosine receptors differentially regulate spontaneous and K+-evoked glutamate release in vivo in young and aged rats. Neuroreport. 1999;10:687–691. doi: 10.1097/00001756-199903170-00005. [DOI] [PubMed] [Google Scholar]
- Corsi C, Melani A, Bianchi L, Pedata F. Striatal A2A adenosine receptor antagonism differentially modifies striatal glutamate outflow in vivo in young and aged rats. Neuroreport. 2000;11:2591–2595. doi: 10.1097/00001756-200008030-00048. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. The role of dopamine in drug abuse viewed from the perspective of its role in motivation. Drug & Alcohol Dependence. 1995;38:95–137. doi: 10.1016/0376-8716(95)01118-i. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- Ebstein RP, Daly JW. Release of norepinephrine and dopamine from brain vesicular preparations: effects of adenosine analogues. Cellular and molecular neurobiology. 1982;2:193–204. doi: 10.1007/BF00711147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Whisler KN, Fuller DC, Orsulak PJ, Self DW. Addiction-related alterations in D1 and D2 dopamine receptor behavioral responses following chronic cocaine self-administration. Neuropsychopharmacology. 2007;32:354–366. doi: 10.1038/sj.npp.1301062. [DOI] [PubMed] [Google Scholar]
- Ferre S, Herrera-Marschitz M, Grabowska-Anden M, Ungerstedt U, Casas M, Anden NE. Postsynaptic dopamine/adenosine interaction: I. Adenosine analogues inhibit dopamine D2-mediated behaviour in short-term reserpinized mice. Eur J Pharmacol. 1991;192:25–30. doi: 10.1016/0014-2999(91)90064-w. [DOI] [PubMed] [Google Scholar]
- Ferre S, Fuxe K, von Euler G, Johansson B, Fredholm BB. Adenosine-dopamine interactions in the brain. Neuroscience. 1992;51:501–512. doi: 10.1016/0306-4522(92)90291-9. [DOI] [PubMed] [Google Scholar]
- Ferre S, O'Connor WT, Snaprud P, Ungerstedt U, Fuxe K. Antagonistic interaction between adenosine A2A receptors and dopamine D2 receptors in the ventral striopallidal system. Implications for the treatment of schizophrenia. Neuroscience. 1994a;63:765–773. doi: 10.1016/0306-4522(94)90521-5. [DOI] [PubMed] [Google Scholar]
- Ferre S, Popoli P, Gimenez-Llort L, Finnman UB, Martinez E, Scotti de Carolis A, et al. Postsynaptic antagonistic interaction between adenosine A1 and dopamine D1 receptors. Neuroreport. 1994b;6:73–76. doi: 10.1097/00001756-199412300-00020. [DOI] [PubMed] [Google Scholar]
- Ferre S, Popoli P, Tinner-Staines B, Fuxe K. Adenosine A1 receptor-dopamine D1 receptor interaction in the rat limbic system: modulation of dopamine D1 receptor antagonist binding sites. Neuroscience letters. 1996;208:109–112. doi: 10.1016/0304-3940(96)12577-5. [DOI] [PubMed] [Google Scholar]
- Ferre S. Adenosine-dopamine interactions in the ventral striatum. Implications for the treatment of schizophrenia. Psychopharmacology (Berl) 1997;133:107–120. doi: 10.1007/s002130050380. [DOI] [PubMed] [Google Scholar]
- Ferre S, Torvinen M, Antoniou K, Irenius E, Civelli O, Arenas E, et al. Adenosine A1 receptor-mediated modulation of dopamine D1 receptors in stably cotransfected fibroblast cells. The Journal of biological chemistry. 1998;273:4718–4724. doi: 10.1074/jbc.273.8.4718. [DOI] [PubMed] [Google Scholar]
- Ferre S, Rimondini R, Popoli P, Reggio R, Pezzola A, Hansson AC, et al. Stimulation of adenosine A1 receptors attenuates dopamine D1 receptor-mediated increase of NGFI-A, c-fos and jun-B mRNA levels in the dopamine-denervated striatum and dopamine D1 receptor-mediated turning behaviour. Eur J Neurosci. 1999;11:3884–3892. doi: 10.1046/j.1460-9568.1999.00810.x. [DOI] [PubMed] [Google Scholar]
- Ferre S, Ciruela F, Borycz J, Solinas M, Quarta D, Antoniou K, et al. Adenosine A1-A2A receptor heteromers: new targets for caffeine in the brain. Frontiers in bioscience: a journal and virtual library. 2008;13:2391–2399. doi: 10.2741/2852. [DOI] [PubMed] [Google Scholar]
- Filip M, Frankowska M, Zaniewska M, Przegalinski E, Muller CE, Agnati L, et al. Involvement of adenosine A2A and dopamine receptors in the locomotor and sensitizing effects of cocaine. Brain Res. 2006;1077:67–80. doi: 10.1016/j.brainres.2006.01.038. [DOI] [PubMed] [Google Scholar]
- Filip M, Zaniewska M, Frankowska M, Wydra K, Fuxe K. The Importance of the Adenosine A(2A) Receptor-Dopamine D(2) Receptor Interaction in Drug Addiction. Current medicinal chemistry. 2012;19:317–355. doi: 10.2174/092986712803414231. [DOI] [PubMed] [Google Scholar]
- Franco R, Lluis C, Canela EI, Mallol J, Agnati L, Casado V, et al. Receptor-receptor interactions involving adenosine A1 or dopamine D1 receptors and accessory proteins. Journal of neural transmission. 2007;114:93–104. doi: 10.1007/s00702-006-0566-7. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Fried G, Hedqvist P. Origin of adenosine released from rat vas deferens by nerve stimulation. Eur J Pharmacol. 1982;79:233–243. doi: 10.1016/0014-2999(82)90629-x. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Ferre S, Zoli M, Agnati LF. Integrated events in central dopamine transmission as analyzed at multiple levels. Evidence for intramembrane adenosine A2A/dopamine D2 and adenosine A1/dopamine D1 receptor interactions in the basal ganglia. Brain Res Brain Res Rev. 1998;26:258–273. doi: 10.1016/s0165-0173(97)00049-0. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Agnati LF, Jacobsen K, Hillion J, Canals M, Torvinen M, et al. Receptor heteromerization in adenosine A2A receptor signaling: relevance for striatal function and Parkinson's disease. Neurology. 2003;61:S19–S23. doi: 10.1212/01.wnl.0000095206.44418.5c. [DOI] [PubMed] [Google Scholar]
- Gines S, Hillion J, Torvinen M, Le Crom S, Casado V, Canela EI, et al. Dopamine D1 and adenosine A1 receptors form functionally interacting heteromeric complexes. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:8606–8611. doi: 10.1073/pnas.150241097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golembiowska K, Zylewska A. Effect of adenosine kinase, adenosine deaminase and transport inhibitors on striatal dopamine and stereotypy after methamphetamine administration. Neuropharmacology. 2000;39:2124–2132. doi: 10.1016/s0028-3908(00)00024-1. [DOI] [PubMed] [Google Scholar]
- Green TA, Schenk S. Dopaminergic mechanism for caffeine-produced cocaine seeking in rats. Neuropsychopharmacology. 2002;26:422–430. doi: 10.1016/S0893-133X(01)00343-8. [DOI] [PubMed] [Google Scholar]
- Hakansson K, Galdi S, Hendrick J, Snyder G, Greengard P, Fisone G. Regulation of phosphorylation of the GluR1 AMPA receptor by dopamine D2 receptors. J Neurochem. 2006;96:482–488. doi: 10.1111/j.1471-4159.2005.03558.x. [DOI] [PubMed] [Google Scholar]
- Hauber W, Nagel J, Sauer R, Muller CE. Motor effects induced by a blockade of adenosine A2A receptors in the caudate-putamen. Neuroreport. 1998;9:1803–1806. doi: 10.1097/00001756-199806010-00024. [DOI] [PubMed] [Google Scholar]
- Henry DJ, White FJ. Repeated cocaine administration causes persistent enhancement of D1 dopamine receptor sensitivity within the rat nucleus accumbens. J Pharmacol Exp Ther. 1991;258:882–890. [PubMed] [Google Scholar]
- Hettinger BD, Lee A, Linden J, Rosin DL. Ultrastructural localization of adenosine A2A receptors suggests multiple cellular sites for modulation of GABAergic neurons in rat striatum. The Journal of comparative neurology. 2001;431:331–346. doi: 10.1002/1096-9861(20010312)431:3<331::aid-cne1074>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Hillion J, Canals M, Torvinen M, Casado V, Scott R, Terasmaa A, et al. Coaggregation, cointernalization, and codesensitization of adenosine A2A receptors and dopamine D2 receptors. J Biol Chem. 2002;277:18091–18097. doi: 10.1074/jbc.M107731200. [DOI] [PubMed] [Google Scholar]
- Hopf FW, Cascini MG, Gordon AS, Diamond I, Bonci A. Cooperative activation of dopamine D1 and D2 receptors increases spike firing of nucleus accumbens neurons via G-protein betagamma subunits. Journal of Neuroscience. 2003;23:5079–5087. doi: 10.1523/JNEUROSCI.23-12-05079.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber A, Padrun V, Deglon N, Aebischer P, Mohler H, Boison D. Grafts of adenosine-releasing cells suppress seizures in kindling epilepsy. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:7611–7616. doi: 10.1073/pnas.131102898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Robbins TW, Everitt BJ. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nature Neuroscience. 2004;7:389–397. doi: 10.1038/nn1217. [DOI] [PubMed] [Google Scholar]
- Jarvis MF, Yu H, Kohlhaas K, Alexander K, Lee CH, Jiang M, et al. ABT-702 (4-amino-5-(3-bromophenyl)-7-(6-morpholinopyridin-3-yl)pyrido[2, 3-d]pyrimidine), a novel orally effective adenosine kinase inhibitor with analgesic and anti-inflammatory properties: I. In vitro characterization and acute antinociceptive effects in the mouse. J Pharmacol Exp Ther. 2000;295:1156–1164. [PubMed] [Google Scholar]
- Kalivas PW, Pierce RC, Cornish J, Sorg BA. A role for sensitization in craving and relapse in cocaine addiction. Journal of psychopharmacology. 1998;12:49–53. doi: 10.1177/026988119801200107. [DOI] [PubMed] [Google Scholar]
- Karcz-Kubicha M, Quarta D, Hope BT, Antoniou K, Muller CE, Morales M, et al. Enabling role of adenosine A1 receptors in adenosine A2A receptor-mediated striatal expression of c-fos. Eur J Neurosci. 2003;18:296–302. doi: 10.1046/j.1460-9568.2003.02747.x. [DOI] [PubMed] [Google Scholar]
- Karcz-Kubicha M, Ferre S, Diaz-Ruiz O, Quiroz-Molina C, Goldberg SR, Hope BT, et al. Stimulation of adenosine receptors selectively activates gene expression in striatal enkephalinergic neurons. Neuropsychopharmacology. 2006;31:2173–2179. doi: 10.1038/sj.npp.1301035. [DOI] [PubMed] [Google Scholar]
- Kita K, Shiratani T, Takenouchi K, Fukuzako H, Takigawa M. Effects of D1 and D2. dopamine receptor antagonists on cocaine-induced self-stimulation and locomotor activity in rats. European neuropsychopharmacology: the journal of the European College of Neuropsychopharmacology. 1999;9:1–7. doi: 10.1016/s0924-977x(97)00098-9. [DOI] [PubMed] [Google Scholar]
- Knapp CM, Foye MM, Cottam N, Ciraulo DA, Kornetsky C. Adenosine agonists CGS 21680 and NECA inhibit the initiation of cocaine self-administration. Pharmacol Biochem Behav. 2001;68:797–803. doi: 10.1016/s0091-3057(01)00486-5. [DOI] [PubMed] [Google Scholar]
- Koob GF. Dynamics of neuronal circuits in addiction: reward, antireward, and emotional memory. Pharmacopsychiatry. 2009;42(Suppl 1):S32–S41. doi: 10.1055/s-0029-1216356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachowicz JE, Sibley DR. Molecular characteristics of mammalian dopamine receptors. Pharmacology & Toxicology. 1997;81:105–113. doi: 10.1111/j.1600-0773.1997.tb00039.x. [DOI] [PubMed] [Google Scholar]
- Lee KW, Kim Y, Kim AM, Helmin K, Nairn AC, Greengard P. Cocaine-induced dendritic spine formation in D1 and D2 dopamine receptor-containing medium spiny neurons in nucleus accumbens. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:3399–3404. doi: 10.1073/pnas.0511244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Acerbo MJ, Robinson TE. The induction of behavioural sensitization is associated with cocaine-induced structural plasticity in the core (but not shell) of the nucleus accumbens. Eur J Neurosci. 2004;20:1647–1654. doi: 10.1111/j.1460-9568.2004.03612.x. [DOI] [PubMed] [Google Scholar]
- Lloyd HG, Fredholm BB. Involvement of adenosine deaminase and adenosine kinase in regulating extracellular adenosine concentration in rat hippocampal slices. Neurochem Int. 1995;26:387–395. doi: 10.1016/0197-0186(94)00144-j. [DOI] [PubMed] [Google Scholar]
- Lobo MK, Covington HE, 3rd, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–390. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, Nestler EJ. The striatal balancing act in drug addiction: distinct roles of direct and indirect pathway medium spiny neurons. Frontiers in neuroanatomy. 2011;5:41. doi: 10.3389/fnana.2011.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XY, Ghasemzadeh MB, Kalivas PW. Expression of D1 receptor, D2 receptor, substance P and enkephalin messenger RNAs in the neurons projecting from the nucleus accumbens. Neuroscience. 1998;82:767–780. doi: 10.1016/s0306-4522(97)00327-8. [DOI] [PubMed] [Google Scholar]
- Madayag A, Lobner D, Kau KS, Mantsch JR, Abdulhameed O, Hearing M, et al. Repeated N-acetylcysteine administration alters plasticity-dependent effects of cocaine. J Neurosci. 2007;27:13968–13976. doi: 10.1523/JNEUROSCI.2808-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcellino D, Navarro G, Sahlholm K, Nilsson J, Agnati LF, Canela EI, et al. Cocaine produces D2R-mediated conformational changes in the adenosine A(2A)Rdopamine D2R heteromer. Biochem Biophys Res Commun. 2010;394:988–992. doi: 10.1016/j.bbrc.2010.03.104. [DOI] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. Journal of Neuroscience. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis ML, Michaelis EK, Myers SL. Adenosine modulation of synaptosomal dopamine release. Life sciences. 1979;24:2083–2092. doi: 10.1016/0024-3205(79)90082-1. [DOI] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiological Reviews. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Muller CE, Sandoval-Ramirez J, Schobert U, Geis U, Frobenius W, Klotz KN. 8-(Sulfostyryl)xanthines: water-soluble A2A-selective adenosine receptor antagonists. Bioorganic & medicinal chemistry. 1998;6:707–719. doi: 10.1016/s0968-0896(98)00025-x. [DOI] [PubMed] [Google Scholar]
- O'Neill CE, LeTendre ML, Bachtell RK. Adenosine A2A receptors in the nucleus accumbens bi-directionally alter cocaine seeking in rats. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2012;37:1245–1256. doi: 10.1038/npp.2011.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orru M, Bakesova J, Brugarolas M, Quiroz C, Beaumont V, Goldberg SR, et al. Striatal pre-and postsynaptic profile of adenosine A(2A) receptor antagonists. PLoS ONE. 2011a;6:e16088. doi: 10.1371/journal.pone.0016088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orru M, Quiroz C, Guitart X, Ferre S. Pharmacological evidence for different populations of postsynaptic adenosine A2A receptors in the rat striatum. Neuropharmacology. 2011b;61:967–974. doi: 10.1016/j.neuropharm.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak MA, Haas HL, Decking UK, Schrader J. Inhibition of adenosine kinase increases endogenous adenosine and depresses neuronal activity in hippocampal slices. Neuropharmacology. 1994;33:1049–1053. doi: 10.1016/0028-3908(94)90142-2. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates 4th. San Diego: Academic Press; 1998. [Google Scholar]
- Phillips E, Newsholme EA. Maximum activities, properties and distribution of 5' nucleotidase, adenosine kinase and adenosine deaminase in rat and human brain. Journal of neurochemistry. 1979;33:553–558. doi: 10.1111/j.1471-4159.1979.tb05187.x. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piercey MF, Lum JT, Hoffmann WE, Carlsson A, Ljung E, Svensson K. Antagonism of cocaine's pharmacological effects by the stimulant dopaminergic antagonists, (+)-AJ76 and (+)-UH232. Brain research. 1992;588:217–222. doi: 10.1016/0006-8993(92)91578-3. [DOI] [PubMed] [Google Scholar]
- Poleszak E, Malec D. Adenosine receptor ligands and cocaine in conditioned place preference (CPP) test in rats. Pol J Pharmacol. 2002a;54:119–126. [PubMed] [Google Scholar]
- Poleszak E, Malec D. Cocaine-induced hyperactivity is more influenced by adenosine receptor agonists than amphetamine-induced hyperactivity. Pol J Pharmacol. 2002b;54:359–366. [PubMed] [Google Scholar]
- Ritz MC, Cone EJ, Kuhar MJ. Cocaine inhibition of ligand binding at dopamine, norepinephrine and serotonin transporters: a structure-activity study. Life sciences. 1990;46:635–645. doi: 10.1016/0024-3205(90)90132-b. [DOI] [PubMed] [Google Scholar]
- Rivkees SA, Price SL, Zhou FC. Immunohistochemical detection of A1 adenosine receptors in rat brain with emphasis on localization in the hippocampal formation, cerebral cortex, cerebellum, and basal ganglia. Brain research. 1995;677:193–203. doi: 10.1016/0006-8993(95)00062-u. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues RJ, Alfaro TM, Rebola N, Oliveira CR, Cunha RA. Co-localization and functional interaction between adenosine A(2A) and metabotropic group 5 receptors in glutamatergic nerve terminals of the rat striatum. J Neurochem. 2005;92:433–441. doi: 10.1111/j.1471-4159.2004.02887.x. [DOI] [PubMed] [Google Scholar]
- Romanowska M, Ostrowska M, Komoszynski MA. Adenosine ecto-deaminase (ecto-ADA) from porcine cerebral cortex synaptic membrane. Brain research. 2007;1156:1–8. doi: 10.1016/j.brainres.2007.04.037. [DOI] [PubMed] [Google Scholar]
- Sauer R, Maurinsh J, Reith U, Fulle F, Klotz KN, Muller CE. Water-soluble phosphate prodrugs of 1-propargyl-8-styrylxanthine derivatives, A(2A)-selective adenosine receptor antagonists. Journal of medicinal chemistry. 2000;43:440–448. doi: 10.1021/jm9911480. [DOI] [PubMed] [Google Scholar]
- Schiffmann SN, Fisone G, Moresco R, Cunha RA, Ferre S. Adenosine A2A receptors and basal ganglia physiology. Prog Neurobiol. 2007;83:277–292. doi: 10.1016/j.pneurobio.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HD, Pierce RC. Cooperative activation of D1-like and D2-like dopamine receptors in the nucleus accumbens shell is required for the reinstatement of cocaine-seeking behavior in the rat. Neuroscience. 2006;142:451–461. doi: 10.1016/j.neuroscience.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Sciotti VM, Van Wylen DG. Increases in interstitial adenosine and cerebral blood flow with inhibition of adenosine kinase and adenosine deaminase. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 1993;13:201–207. doi: 10.1038/jcbfm.1993.24. [DOI] [PubMed] [Google Scholar]
- Self DW, Nestler EJ. Relapse to drug-seeking: neural and molecular mechanisms. Drug Alcohol Depend. 1998;51:49–60. doi: 10.1016/s0376-8716(98)00065-9. [DOI] [PubMed] [Google Scholar]
- Sibley DR, Monsma FJ, Jr, Shen Y. Molecular neurobiology of dopaminergic receptors. International review of neurobiology. 1993;35:391–415. doi: 10.1016/s0074-7742(08)60573-5. [DOI] [PubMed] [Google Scholar]
- Steiner H, Gerfen CR. Role of dynorphin and enkephalin in the regulation of striatal output pathways and behavior. Experimental Brain Research. 1998;123:60–76. doi: 10.1007/s002210050545. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Le Moine C, Aubert I, Burbaud P, Fredholm BB, Bloch B. Cellular distribution of adenosine A2A receptor mRNA in the primate striatum. J Comp Neurol. 1998;399:229–240. doi: 10.1002/(sici)1096-9861(19980921)399:2<229::aid-cne6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Fourreau L, Bloch B, Fredholm BB, Gonon F, Le Moine C. Opposite tonic modulation of dopamine and adenosine on c-fos gene expression in striatopallidal neurons. Neuroscience. 1999a;89:827–837. doi: 10.1016/s0306-4522(98)00403-5. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Le Moine C, Fisone G, Fredholm BB. Distribution, biochemistry and function of striatal adenosine A2A receptors. Prog Neurobiol. 1999b;59:355–396. doi: 10.1016/s0301-0082(99)00011-8. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. British journal of pharmacology. 2008;154:327–342. doi: 10.1038/bjp.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn JA, Jarvis SM. Adenosine transporters. Gen Pharmacol. 1996;27:613–620. doi: 10.1016/0306-3623(95)02053-5. [DOI] [PubMed] [Google Scholar]
- Toda S, Alguacil LF, Kalivas PW. Repeated cocaine administration changes the function and subcellular distribution of adenosine A1 receptor in the rat nucleus accumbens. J Neurochem. 2003;87:1478–1484. doi: 10.1046/j.1471-4159.2003.02121.x. [DOI] [PubMed] [Google Scholar]
- Torvinen M, Gines S, Hillion J, Latini S, Canals M, Ciruela F, et al. Interactions among adenosine deaminase, adenosine A(1) receptors and dopamine D(1) receptors in stably cotransfected fibroblast cells and neurons. Neuroscience. 2002;113:709–719. doi: 10.1016/s0306-4522(02)00058-1. [DOI] [PubMed] [Google Scholar]
- Torvinen M, Torri C, Tombesi A, Marcellino D, Watson S, Lluis C, et al. Trafficking of adenosine A2A and dopamine D2 receptors. J Mol Neurosci. 2005;25:191–200. doi: 10.1385/JMN:25:2:191. [DOI] [PubMed] [Google Scholar]
- Tozzi A, Tscherter A, Belcastro V, Tantucci M, Costa C, Picconi B, et al. Interaction of A2A adenosine and D2 dopamine receptors modulates corticostriatal glutamatergic transmission. Neuropharmacology. 2007;53:783–789. doi: 10.1016/j.neuropharm.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Ujike H, Akiyama K, Otsuki S. D-2 but not D-1 dopamine agonists produce augmented behavioral response in rats after subchronic treatment with methamphetamine or cocaine. Psychopharmacology (Berl) 1990;102:459–464. doi: 10.1007/BF02247125. [DOI] [PubMed] [Google Scholar]
- Ushijima I, Carino MA, Horita A. Involvement of D1 and D2 dopamine systems in the behavioral effects of cocaine in rats. Pharmacology, biochemistry, and behavior. 1995;52:737–741. doi: 10.1016/0091-3057(95)00167-u. [DOI] [PubMed] [Google Scholar]
- Vidi PA, Chemel BR, Hu CD, Watts VJ. Ligand-dependent oligomerization of dopamine D(2) and adenosine A(2A) receptors in living neuronal cells. Molecular pharmacology. 2008;74:544–551. doi: 10.1124/mol.108.047472. [DOI] [PubMed] [Google Scholar]
- White FJ. D-1 dopamine receptor stimulation enables the inhibition of nucleus accumbens neurons by a D-2 receptor agonist. European Journal of Pharmacology. 1987;135:101–105. doi: 10.1016/0014-2999(87)90764-3. [DOI] [PubMed] [Google Scholar]
- White TD. Direct detection of depolarisation-induced release of ATP from a synaptosomal preparation. Nature. 1977;267:67–68. doi: 10.1038/267067a0. [DOI] [PubMed] [Google Scholar]
- Wojcik WJ, Neff NH. Differential location of adenosine A1 and A2 receptors in striatum. Neuroscience letters. 1983;41:55–60. doi: 10.1016/0304-3940(83)90222-7. [DOI] [PubMed] [Google Scholar]
- Worley CM, Valadez A, Schenk S. Reinstatement of extinguished cocaine-taking behavior by cocaine and caffeine. Pharmacol Biochem Behav. 1994;48:217–221. doi: 10.1016/0091-3057(94)90519-3. [DOI] [PubMed] [Google Scholar]