Abstract
Despite the fundamental roles of sialyl- and fucosyltransferases in mammalian physiology, there are few pharmacological tools to manipulate their function in a cellular setting. Although fluorinated analogs of the donor substrates are well-established transition state inhibitors of these enzymes, they are not membrane permeable. By exploiting promiscuous monosaccharide salvage pathways, we show that fluorinated analogs of sialic acid and fucose can be taken up and metabolized to the desired donor substrate-based inhibitors inside the cell. Due to the existence of metabolic feedback loops, they also act to prevent the de novo synthesis of the natural substrates, resulting in a global, family-wide shutdown of sialyl- and/or fucosyltransferases and remodeling of cell surface glycans. As an example of the functional consequences, the inhibitors drastically reduce expression of the sialylated and fucosylated ligand Sialyl Lewis X on myeloid cells, resulting in loss of binding to selectins and impaired leukocyte rolling.
Introduction
Sialylated and fucosylated glycans play key roles in development, host-pathogen interactions, cell signaling, and leukocyte trafficking1-3. Their synthesis is carried out in a non-template mediated fashion by 20 sialyltransferase (ST) and 14 fucosyltransferase (FUT) enzymes. While all members of a given family utilize the same donor substrate (CMP-NeuAc or GDP-Fucose, respectively), the unique cellular expression pattern and acceptor specificity of each enzyme allows an organism to achieve a diverse repertoire of cell-type specific glycosylation patterns, which are functionally interpreted by glycan binding proteins.
Much of the information regarding the importance of sialyl- and fucosyltransferase enzymes has come from gene ablation studies in mice4-6. These studies have illuminated the basic biology mediated by these biocatalysts, and documented the pharmacological potential of inhibiting these enzymes for the treatment of various leukocyte-mediated disorders. For instance, unique sialyltransferases have been shown to modulate B-cell receptor signaling6 and reduce the number of peripheral CD-8 T-cells7 (ST6Gal I and ST3Gal I, respectively), suggesting these enzymes as potential targets for the treatment of autoimmune diseases8. Similarly, the fucosyltransferase FUT7 has been shown to play critical roles in the biosynthesis of Sialyl Lewis X (SLeX; NeuAcα2,3Galβ1,4[Fucα1,3]GlcNAcβOR)5, a ligand for selectins, which regulates the extravasation of leukocytes from blood vessels to inflamed tissues. These mice are less susceptible to chronic inflammatory diseases such as artherosclerosis, implicating these enzymes as drug targets9,10.
Despite the fundamental importance of sialylated and fucosylated glycans, biosynthetic inhibitors to probe their function in a cellular setting are lacking. In one successful approach, cell permeable acceptor decoys, small molecule disaccharides that can compete with endogenous acceptor substrates, have been used to effectively reduce the cell surface expression of Sialyl Lewis X both in vitro11,12 and in vivo13,14. These are, however, not family-specific as they can be acted upon by multiple different enzymes, even enzymes from different families. Analogs of the nucleotide-sugar donor substrates have also been identified as potent in vitro inhibitors with selective and broad inhibition properties for both sialyl -and fucosyltransferases15,16. In one aspect of this approach, fluorinated analogs, where an electronegative fluorine atom has been placed proximal to the anomeric position, have been identified as transition state inhibitors of both of these enzyme families17,18 due to the fact that most glycosyl transfer reactions proceed through a flattened half-chair conformation with a substantial oxocarbenium-ion character19,20. Unfortunately, the high negative charge of nucleotide sugar analogs make these useful in vitro inhibitors ineffective in a cellular setting since they are not membrane penetrable.
Herein we report the development of cell-permeable, family-specific inhibitors of the sialyl- and fucosyltransferases. Taking advantage of the promiscuity of the sialic acid and fucose salvage pathways in eukaryotic cells, we show that peracetylated analogs of sialic acid and fucose bearing a fluorine atom proximal to the endocyclic oxygen are readily converted to the corresponding donor substrate analogs intracellularly. These inhibitors then act to effectively shut down the synthesis of a spectrum of sialylated and fucosylated glycan epitopes, and remodel the cell surface glycome within days. Finally, we demonstrate that these inhibitors alone or in combination dramatically inhibit the formation of the sialylated and fucosylated tetrasaccharide SLex in a human myeloid cell line (HL-60 cells), abrogating its interaction with E- and P- selectins that recruit effector cells to inflammatory sites.
Results
Strategy for the development of ST and FUT inhibitors
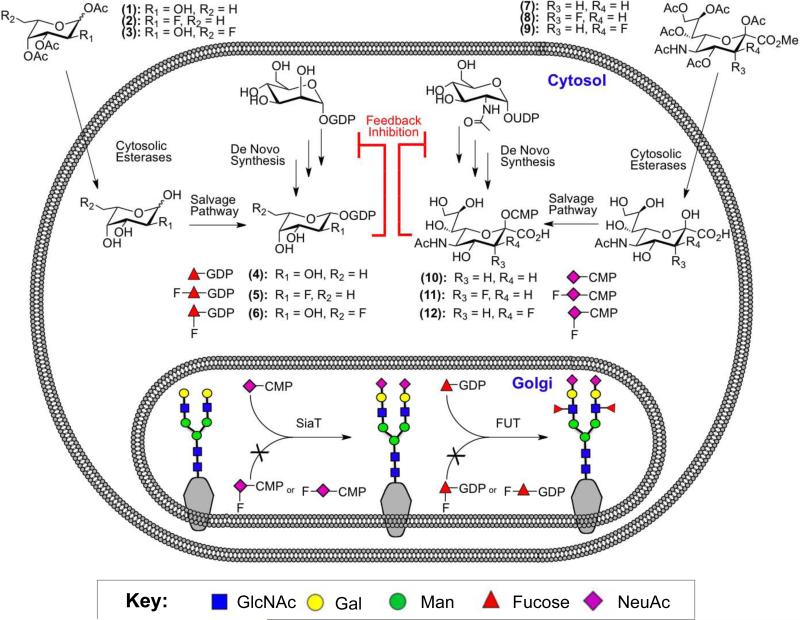
Our strategy was inspired by recent work of Vocadlo and colleagues who showed that a 5-thio-GlcNAc analog could be metabolically converted to UDP-5-thio-GlcNAc inside the cell, and selectively inhibit the activity of an O-linked GlcNAc transferase21. Since the biosynthetic pathways for synthesis of the donor substrates of fucosyl- and sialyltransferases are known to accommodate monosaccharides with artificial substituents22, we hypothesized that protected, fluorinated analogs of fucose (2-3) or sialic acid (8-9) would be readily taken into cells by passive diffusion, deacetylated, and converted into the corresponding donor substrate analog of GDP-Fucose (5-6) or CMP-NeuAc (11-12) to form the desired inhibitor inside the cell (Figure 1). Moreover, because of the structural similarity of these analogs to the natural substrates and their accumulation due to lack of turnover, we proposed that these would also act to shut down the de novo synthesis of GDP-Fucose (4) or CMP-NeuAc (10) due to existing feedback loops in these pathways (Figure 1)23,24. As a result, this dual action mechanism would make them broad, family specific inhibitors with greater potency than simple competitive inhibitors.
Figure 1. Fluorinated Monosaccharide Analogs act as Metabolic Glycosyltransferase Inhibitors.
Peracetylated Analogs of Fucose (1-3) or Sialic Acid (7-9) are taken up by cells and converted into the corresponding nucleotide sugars (4-6 and 10-12) through salvage pathways. The fluorinated analogs (5-6, 11-12) act as inhibitors of the corresponding fucosyl- or sialyltransferases and, because they are not transferred, accumulate in the cell. This accumulation then shuts down the de novo synthesis of GDP-Fucose (4) or CMP-NeuAc (10) via a feedback loop, to further decrease fucose or sialic acid expression on the cell surface.
Fluorinated analogs of fucose act as inhibitors of fucosylation in cells
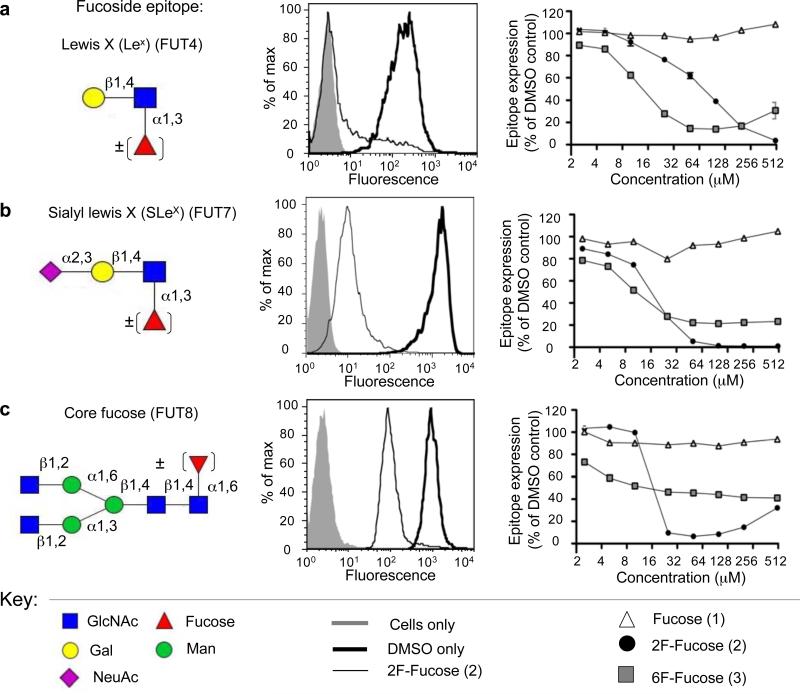
For the fucosyltransferases it has been shown that both GDP-2F-Fuc (Figure 1, (5)) and GDP-6F-Fuc (6) act as competitive inhibitors of FUTs 3, 5, 6, and 7 with Ki values in the low micromolar range17,18. Using these results as the basis for the design of metabolic inhibitors of fucosyltransferases, acetylated analogs of fucose bearing a fluorine atom at C2 (2) or C6 (3) were synthesized by modifying previously established procedures17,25 and peracetylated to improve their cell permeability (Supplementary Methods). These were then tested as inhibitors of fucosylation in cell lines using antibodies and lectins that detect biologically important fucosylated epitopes (Figure 2).
Figure 2. Fluorinated Fucose Analogs act as Fucosyltransferase Inhibitors in Cells.
HL-60 or CHO cells were treated with various concentrations of the fucose analogs (1-3). After 3 days, cells were harvested and various fucosylated epitopes were detected with anti-glycan antibodies or lectins via flow cytometry. Inhibition of the FUT4 enzyme was assessed in HL-60 cells with an anti-Lewis X antibody (a). Similarly, FUT7 inhibition was assessed in this cell line, but utilizing the anti-Sialyl Lewis X antibody (b). To analyze FUT8 inhibition, CHO cells and the lectin AAL were used (c). In all cases the data were normalized to cells treated with DMSO only as 100% fucosylated epitope expression and unstained cells as 0%. Data shown is representative of three independent experiments carried out in triplicate.
Dramatic reduction in fucosylation was obtained with the 2F-Fuc analog (2). Treatment of human HL-60 cells for 3 days led to almost complete abolition of Lewis X (LeX, Galβ1,4[α1,3Fuc]GlcNAcβOR) and Sialyl Lewis X (SLex, NeuAcα2,3Galβ1,4[α1,3Fuc]GlcNAcβOR), as assessed by flow cytometry with labeled antibodies specific for these two epitopes (Figure 2a-b), which in this cell line are products of the FUT4 and FUT7 enzymes, respectively26,27. Treatment of CHO cells, commonly used to produce bio-therapeutic proteins, resulted in dramatic reduction of core fucosylation of N-linked glycans, a product of FUT8, as probed by the AAL lectin (Figure 2c). At high concentrations of 2F-Fuc (2), however, this is slightly reversed. This is likely due to the depletion of GDP-Fucose (4) via feedback inhibition and the eventual utilization of GDP-2F-Fuc (6), a poor substrate with a high KM, at high concentrations. This is supported by experiments with the Lec13 CHO cell line, which express FUT8 but are deficient in the de novo biosynthesis of GDP-Fuc (Supplementary Figure 1), so that the only source of fucose is from the medium. Feeding cells high concentrations of 2F-Fuc (2) results in a small amount of detectable cell surface core-fucosylation in comparison to the DMSO only and Fuc (1) treated controls.
Treatment of cells with the 6F-Fuc analog (3) produced a more complex pattern of inhibition. Significant reductions of Lex, SLex, and core fucose were observed, but in all cases reduction was not complete (Figure 2a-c). For Lex, inhibiton was reversed at higher concentrations of 6F-Fuc (3), a similar phenomenon as noted above for 2F-Fuc (2) and FUT8. In contrast, the inhibition of SLex and core fucose plateaus at 20-40% of the control. To investigate if this is due to the utilization of GDP-6F-Fuc as a slow substrate with a low KM we assessed the activity of human FUT7 for its ability to utilize GDP-2F-Fuc and GDP-6F-Fuc as donor substrates. While there was no transfer of 2F-Fuc, the 6F-analog was transferred slowly as confirmed by TLC and mass spectrometry analysis (Supplementary Figure 2b and 2c). Both analogs, however, compete effectively with transfer of GDP-Fuc, with comparable IC50 values of ~2.5 μM (Supplementary Figure 2d). Thus, we suggest that the incomplete inhibition of SLex by the 6F-Fuc analog is a result of slow steady-state transfer onto glycoprotein glycans by FUT7. Using the Lec13 CHO cell line, we show that FUT8 also transfers 6F-Fuc to glycoprotein glycans (Supplementary Figure 1) and therefore the inability of (3) to fully suppress core fucosylation in CHO cells is due at least in part to the ability of hamster FUT8 to use GDP-6F-Fuc as a slow substrate.
A fluorinated analog of sialic acid acts as an inhibitor of sialylation in cells
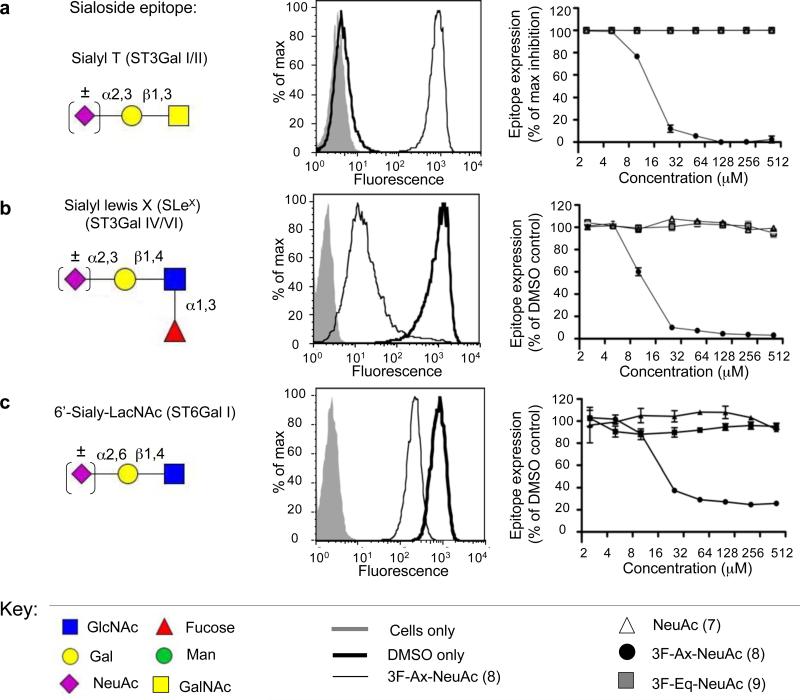
CMP-CMP-3Fax-NeuAc (11) has been shown to be a competitive inhibitor of the mammalian sialyltransferase ST6Gal I17 as well as many bacterial sialyltransferases28,29. To test applicability of the metabolic inhibitor approach to sialyltransferases, peracetylated analogs of sialic acid bearing a fluorine substitutent at C3 in either an axial (8; 3Fax-NeuAc) or equatorial (9; 3Feq-NeuAc) position were synthesized25 (and Supplementary Methods). Treatment of HL-60 cells for 3 days with (8) led to a drastic reduction in Sialyl T antigen (NeuAcα2,3Galβ1,3GalNAcαSer/Thr), the product of ST3Gal I and/or ST3Gal II30, as detected by the exposure of the underlying T antigen (Galβ1,3GalNAcαSer/Thr) with the lectin PNA (Figure 3a). Moreover, SLeX staining was completely abolished with this inhibitor, reflecting inhibition of ST3Gal IV and/or ST3Gal VI in this cell line (Figure 3b)30. Lastly, we assessed the effect on ST6Gal I, which produces high levels of NeuAcα2,6Gal in the Ramos B-cell line, using the plant lectin Sambuccus nigra agglutinin (SNA). Inhibition of this epitope was found to plateau at about ~30% of the control (Figure 3c). This is not due to utilization of (11) as a slow substrate, since no transfer is observed by ST6Gal I in vitro (Supplementary Figure 3). Instead, this may either reflect the slow turnover of glycoproteins in Ramos cells during the 3 day experiment, or that the cells can efficiently salvage sialic acid from serum and serum glycoproteins in the culture media31,32. Interestingly, in all cell lines and STs assessed, compound (9), which only differs in the orientation of the fluorine at C3, had no inhibitory effect. Since the corresponding nucleotide-sugar (12) is an inhibitor of ST6Gal I in vitro (Supplementary Figure 3), we suggest that the 3Feq-NeuAc analog 9 is not accommodated at some step in the salvage pathway.
Figure 3. A Fluorinated Sialic Acid Analogs act as a Sialyltransferase Inhibitor in Cells.
HL-60 or Ramos cells were treated with various concentrations of the sialic acid analogs (7-9). After 3 days, cells were harvested and various sialylated epitopes were detected with anti-glycan antibodies or lectins via flow cytometry. Inhibition of the ST3Gal I and/or ST3Gal II enzymes was assessed in HL-60 cells by loss of the sialylated epitope NeuAcα2,3Galβ1,3GalNAcαSer/Thr (Sialyl-T Antigen) and detection of the asialo structure Galβ1,3GalNAcαSer/Thr (T Antigen) by the lectin PNA (a). Decreases in Sialyl Lewis X formation were determined with HL-60 cells and an anti-Sialyl Lewis X antibody reflecting inhibition of ST3Gal IV and/or ST3Gal VI in this cell line (b). Inhibition of NeuAcα2,6Gal formation, the product of ST6Gal I and/or ST6Gal II, was assessed with Ramos cells and the lectin SNA (c). In each case the data represents one of three independent experiments carried out in triplicate. For (a) the expression of the T-antigen was normalized to DMSO only as 0% and the maximum inhibitor treatement as 100%. From this, Sialyl T antigen expression was expressed as 100% - (percent T-antigen expression). For (b,c) the data were normalized to cells treated with DMSO only as 100% sialylated epitope expression and unstained cells as 0%.
Mass spectrometry profiling of glycans from inhibitor treated cells
We observed that treatment with the fucosyl- and sialyltransferase inhibitors (2) and (8), respectively, did not compromise cell viability or doubling times of any of the cell lines assessed (Supplementary Figure 4 and data not shown). This is not surprising since mutant cell lines with deficiencies in sialic acid and fucose biosynthesis do not exhibit viability defects33,34.
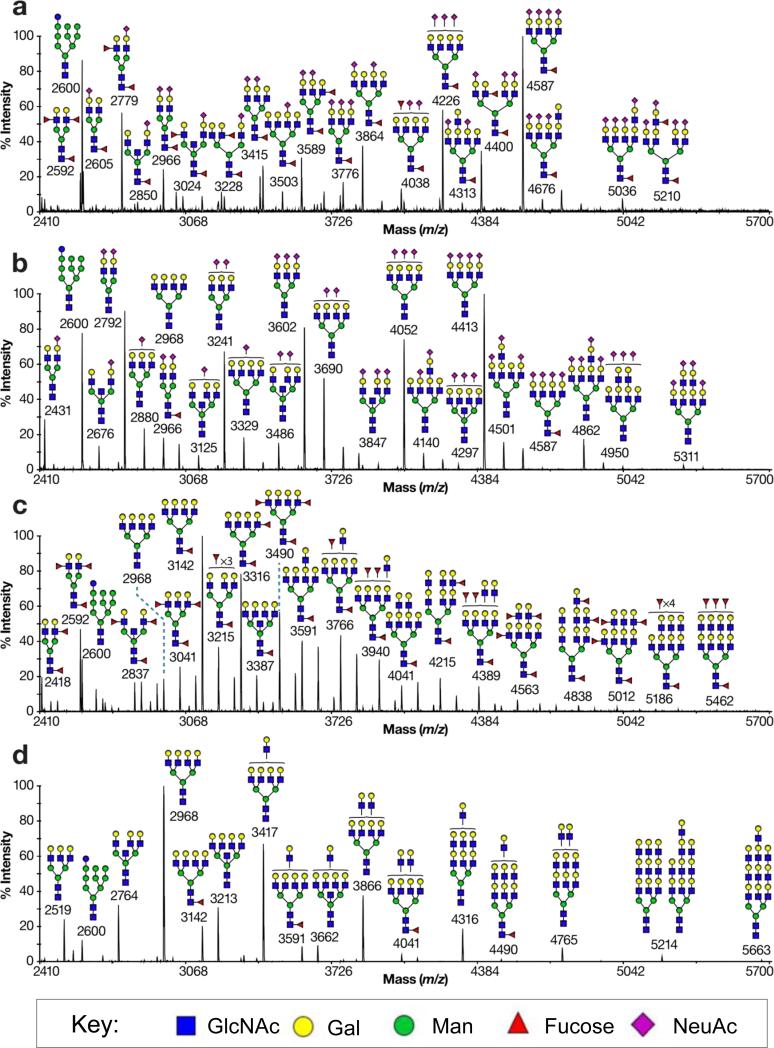
To further assess the consequences of the fucosylation inhibitor (2) and the sialylation inhibitor (8) on cell surface glycans, MALDI-TOF MS was carried out on released N- and O-glycans from HL-60 cells treated for 7 days with DMSO only, (2), (8), or both (2) and (8). As illustrated for N-linked glycans in Figure 4a, control cells show an abundance of sialylated and fucosylated epitopes in their N-glycans, reflecting the high staining of these cells with both anti-Lex and anti-SLex antibodies. Upon treatment of the cells with the fucosylation inhibitor (2), the MS data shows no loss of sialylated structures, but dramatic reduction in fucosylated structures, with only a small amount of core fucosylation remaining (Figure 4b). Flow cytometry analysis confirms a complete loss of Lex and SLex epitopes, while core fucosylation detected by AAL is reduced to ~20% of the control (data not shown). Treatment of the sialylation inhibitor (8) results in a complete loss of sialic acids and a notable increase in overall fucosylation (Figure 4c and data not shown). This is likely due to the fact that fucosyltransferases and sialyltransferases compete for the same acceptor substrates, and therefore selective inhibition of sialic acid addition allows for greater fucosylation35. Treatment with both inhibitors in concert leads to dramatic reduction of both sialic acid and fucose, with only a small amount of core-fucosylation remaining (Figure 4d). Interestingly, this dual treatment leads to elongation of the N-glycan antennae with extended poly-N-acetyllactosamine repeats, a consequence of the fact that these glycans are not ‘capped’ by sialic acid or fucose.
Figure 4. Mass spectrometry Analysis of N-glycans from Inhibitor treated cells.
HL-60 cells were treated for 7 days with DMSO only, 200 μM (2), 200 μM (8), or 200 μM each of (2) and (8). N-linked glycans from each sample were then isolated, permethylated and analyzed by MALDI-TOF MS. The four spectra represent treatment of the cells with (a) DMSO only, (b) the fucosyltransferase inhibitor (2), (c) the sialyltransferase inhibitor (8), or (d) both inhibitors in concert. All molecular ions are [M+Na]+. Putative structures are based on composition, tandem MS, and biosynthetic knowledge. Structures that show sugars outside a bracket have not been unequivocally defined.
The existence of the poly-N-acetyllactosamine chains in all samples were corroborated by endo-β-galactosidase digestion experiments on the released N-linked glycans of control HL-60 cells. This confirmed the presence of terminal sialylated and fucosylated structures in the untreated cells, while cells treated with either 2F-Fuc (2), 3F-NeuAc (8), or both inhibitors in concert, caused a corresponding dramatic reduction of fucosylated and sialylated structures (Supplementary Figure 5). Notably, there was no incorporation of 2F-Fuc or 3Fax-NeuAc detected in the mass spectrometry profiles of the HL-60 glycans. Although, slight incorporation of 2F-Fuc was seen in CHO Lec13 cells (Supplementary Figure 1), the lack of incorporation in HL-60 cells may simply be due to a difference in the human FUT8 and/or preferential use of residual GDP-Fuc present in these cells.
Analysis of the O-glycan profiles of the same inhibitor treated cells has many parallels, but some notable differences. The untreated cells show predominantly sialylated and fucosylated Core 1 and Core 2 O-glycans (Supplementary Figure 6a). Upon treatment with 2F-Fuc (2), no fucose is detected on any of these structures (Supplementary Figure 6b). However, while treatment with (8) leads to a dramatic reduction in the major disialylated branched Core 2 structure, there is a corresponding increase in a monosialylated branched Core 2 structure (m/z = 1344). Moreover, there are a series of new, elongated and fucosylated O-glycans (Supplementary Figure 6c). Treatment with both inhibitors leads to a simple pattern of structures with no fucose and some residual sialic acid (Supplementary Figure 6d). The results suggest that at least one of the sialyltransferases that transfer to branched Core 2 O-glycans is sensitive to inhibition by CMP-3Fax-NeuAc, resulting in increased extension and fucosylation, while the other is resistant resulting in the scavenge of residual CMP-NeuAc to produce the single sialylated O-linked glycans.
Taken together, the above MS data confirms the effect of the inhibitors on the biosynthesis of sialylated and fucosylated glycans, and demonstrates that some members of the respective glycosyltransferase families exhibit differential sensitivity in the context of whole cell glycosylation. It is also evident that both the fucosylation (2) and sialylation (8) inhibitors are specific for their respective families of glycosyltransferases, and have no apparent effect on the GlcNAc-, Glucosyl-, Mannosyl-, Galactosyl, and GalNAc transferases involved in the synthesis of the core N- and O-glycan structures.
Nucleotide sugar analysis of inhibitor treated cells
To definitively demonstrate the mechanism of feedback inhibition proposed for the fucosylation inhibitor 2F-Fuc (2) and the sialyltransferase inhibitor 3Fax-NeuAc (8), treated cells were subjected to nucleotide sugar analysis to quantify both the natural (4 and 10), and unnatural (5 and 11) nucleotide sugars. HL-60 cells treated for 7 days with 2F-Fuc (2) clearly produced the unnatural nucleotide sugar (5) (Table 1 and Supplementary Figure 7). Moreover, as anticipated there was a decrease in natural GDP-Fuc (4) to undetectable levels, consistent with feedback inhibition of the synthesis of GDP-Fuc. Similarly, treatment of cells with the peracetylated 3Fax -NeuAc (8) led to a dramatic accumulation of CMP-3Fax-NeuAc (11) inside the cells and a complete loss of CMP-NeuAc (10) (Table 1 and Supplementary Figure 7). Notably, CMP-NeuAc levels were not appreciably altered upon treatment with the fucosyltransferase inhibitor (2) nor were GDP-Fucose levels after treatment with the sialyltransferase inhibitor (8), which further supports the family specificity of these inhibitors.
Table 1.
Quantification of nucleotide sugars in cell extracts (2.2 × 108 cells) as determined by HPLC
| Treatment | Nucleotide Sugar | Amount (μg) |
|---|---|---|
| DMSO | GDP-Fuc (4) | 3.88 ± 0.05 |
| CMP-NeuAc (10) | 2.59 ± 0.05 | |
| 2F-Fuc (2) | GDP-Fuc (4) | Not Detected |
| 2F GDP-2F-Fuc (5) | 62.46 ± 0.49 | |
| CMP-NeuAc (10) | 2.20 ± 0.04 | |
| 3F-NeuAc (8) | GDP-Fuc (4) | 4.39 ± 0.02 |
| CMP-NeuAc (10) | Not Detected | |
| 3F CMP-NeuAc (11) | 65.18 ± 0.18 |
Metabolic fucosyl- and sialyltransferase inhibitors impair selectin binding and leukocyte rolling
To assess the functional consequences of the remodeling of HL-60 cell glycans, we investigated the impact of the reduction in the fucosylated and sialylated SLeX tetrasaccharide, which plays a critical role in the trafficking of white blood cells in innate and adaptive immunity. Its expression on various leukocyte subsets imparts on these cells the ability to roll on inflamed endothelium in blood vessels, the initial event in a cascade which eventually leads to extravasation at sites of inflammation in order to fight infection36,37. This process is mediated by the inducible expression of E-selectin on endothelium and P-selectin on endothelium and platelets in response to inflammatory stimuli. In chronic inflammatory diseases such as arthritis, atherosclerosis, and sepsis, however, an excess of leukocytes and platelets can cause tissue damage and even death38,39.
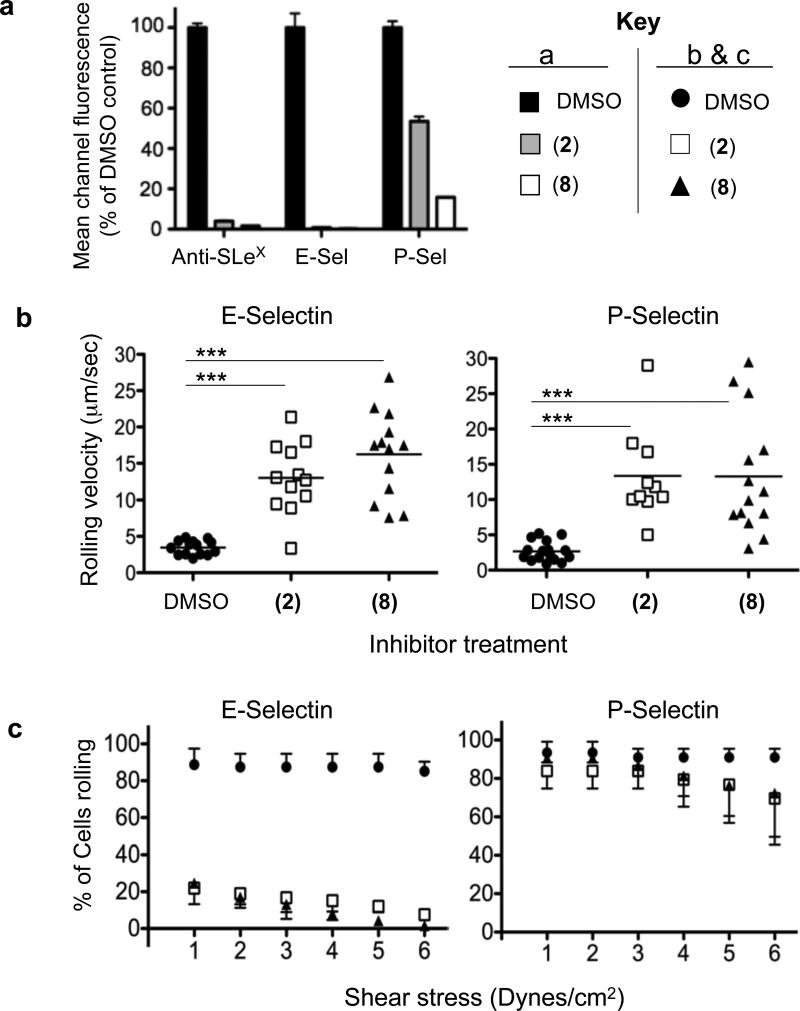
As shown in Figure 5a, treatment of cells with either (2) or (8) completely abolished staining with anti-SLex and E-Selectin-Fc protein and led to significant decreases in P-selectin binding. This residual P-selectin binding was entirely due to the known P-selectin ligand on these cells, PSGL-1 (Supplementary Figure 8a) and is not due to slow cell surface turnover (Supplementary Figure 8b-d). Since the functional epitope on PSGL-1 bearing SLex is a Core 2 O-glycan, the residual binding seen upon treatment with the ST inhibitor is consistent with the residual expression of this epitope on O-glycans as shown in the MS data (Supplementary Figure 6c). It is unclear, however, why the fucosyltransferase inhibitor (2) does not lead to a full reduction in binding since the MS data shows a complete loss of fucose on these structures (Supplementary Figure 6b) and it has been shown that this monosaccharide is important for P-selectin binding40.
Figure 5. Selectin Binding and Selectin-Mediated Leukocyte Rolling.
(a) Flow cytometry analysis of HL-60 cells treated for 5 days with 200 μM each of (2) or (8) leads to decreases in anti-Sialyl Lewis X antibody as well as recombinant E- and P-selectin binding. This leads to higher rolling velocities (at 3 dynes/cm2) of these cells on selectin coated surfaces (b) and a decreased shear resistance on E- but not P-selectin coated surfaces (c). *** corresponds to p < 0.0001.
Functionally, treated cells were found to roll substantially faster on both E-selectin and P-selectin coated surfaces (Figure 5b), due to reductions in selectin-SLex interactions, and only a fraction of the inhibitor treated cells were found rolling on E- and P-selectin when compared with the controls (Supplementary Videos 1-6). When analyzing the shear stress required to disrupt rolling interactions and cause HL-60 cells to return the bulk flow, the inhibitor treated cells were found to support E-selectin rolling only at low shear tress (Figure 5c), while those for P-selectin were nearly equivalent to the control treated cells (Figure 5c and Supplementary Videos 7-12). In summary, these results show that the metabolic ST and FUT inhibitors described herein can functionally alter leukocyte rolling properties.
Discussion
Glycosyltransferases represent the biosynthetic link between information encoded in the genome and the cellular glycome. Thus, expanding the repertoire of tools to experimentally modulate the function of the ~250 glycosyltransferases and glycan modifying enzymes should be of high priority in order to aid in the elucidation of the functions of these complex post-translational modifications. Although systematic efforts to screen for inhibitors has begun16,41-45 few glycosyltransferase inhibitors have yet emerged46,47. As an alternative to the traditional screening of small molecule inhibitors of glycosyltransferases, Vocadlo and colleagues have demonstrated the potential of using unnatural monosaccharides, which are metabolically converted to the corresponding nucleotide sugar donor analog by the cell, but inhibit glycosylation since they are bound but not used as a substrate by the enzyme21.
As documented here, exemplary peracetylated derivatives of fucose (2F-Fuc (2)) and sialic acid (3Fax-NeuAc (8)) are readily taken up by cultured cells and converted to their corresponding nucleotide sugars, GDP-2F-Fuc (5) and CMP-3Fax-NeuAc (11), by the endogenous salvage pathways. As they build up in the cell, they feedback inhibit the synthesis of the natural GDP-Fuc (4) and CMP-NeuAc (10) substrates. The choice of fluorinated monosaccharides was supported by the demonstration that the corresponding nucleotide sugars are transition state inhibitors of the sialyl- and fucosyltransferases17,18, a report that such fucose analogs could block core fucosylation of N-linked glycans of recombinant antibodies in cells48, and that there were already robust methods for their synthesis25. The fact that these inhibitors dramatically reduce sialylation of and fucosylation of cell surface glycans in a cellular setting highlights their utility as global inhibitors of sialyl- and fucosyltransferases. However, there were several notable caveats to this generalization. Core fucosylation of N-linked glycans by FUT8 in HL-60 cells was reduced, but not eliminated, and while sialylation of N-linked glycans was abolished, there was still some sialylation of O-linked glycans. In both cases the residual fucosylation and sialylation resulted from incorporation of natural sugars, suggesting that in HL-60 cells FUT8 and at least one sialyltransferase exhibits less sensitivity to these inhibitors, and/or preferentially utilizes low levels of the natural nucleotide sugar that remain in the cell.
Differences in the sensitivity of glycosyltransferases to metabolic inhibitors was also seen in the exemplary report of Gloster et al.21, who used 5-thio-GlcNAc as a precursor of UDP-5S-GlcNAc, an analog of UDP-GlcNAc, the donor substrate for GlcNAc transferases. While they demonstrated that it was a slow substrate for the cytoplasmic O-GlcNAc transferase and blocked synthesis of O-GlcNAc on cytoplasmic and nuclear proteins, no change was observed in glycan epitopes of cell surface glycans, which are products of other members of the GlcNAc transferase family. At present the mechanisms for the selectivity are not established, but even at the highest concentrations of 5-thio-GlcNAc used, there was still significant levels (~25%) of UDP-GlcNAc pools remaining for normal synthesis of glycans by enzymes that are not inhibited by the 5-thio-GlcNAc analog.
In principle, peracetylated fluorosugars used here for sialyl- and fucosyltransferases could also be used as inhibitors of other families of glycosyltransferases. Indeed, fluoro-sugar analogs of UDP-GlcNAc and UDP-Gal have been demonstrated to be inhibitors of both N-acetylglucosamine-49 and galactosyltransferases17,50, respectively. In this regard, there is an extensive literature on the use of peracetylated 4F-GlcNAc for reduction of the expression of selectin ligands, namely SLeX, both in vitro51 and in vivo52,53. While this was initially thought to be due to a chain termination effect (since 4F-GlcNAc incorporation would abolish the key nucleophile for the β1,4Galactosyltransferase)52-54, it was recently shown that this analog is not incorporated into N- or O-glycans51,55. However, it was readily made into UDP-4F-GlcNAc intracellulary, which decreased cellular levels of the natural nucleotide sugar UDP-GlcNAc51,55. This reduction is sufficient to selectively decrease the branching of N-linked glycans51, which are elaborated by enzymes with a high KM for this donor substrate56.
From the few reports to date, it is clear that metabolic inhibitors of glycosyltransferases have the potential to modulate glycosylation in cultured cells and animals, and that significant selectivity can be achieved for enzymes of a single family or even specific enzymes within a family. Since peracetylated 4F-GlcNAc administration has already demonstrated in vivo reduction of E- and P-selectin ligands52,53, it will be of interest to assess the ability of 2F-Fuc (2) and 3Fax-NeuAc (8) for their potential to reduce SLex expression in in vivo models of inflammation and cancer metastasis. Such studies will pave the way for the broader use of these inhibitors to explore the roles of sialylated and fucosylated glycans in normal biology and mechanisms of disease, and will stimulate further research into the use of monosaccharide analog precursors of nucleotide sugars as a way to modulate glycosylation pathways.
Methods
Synthesis of ST and FUT Inhibitors
Synthesis and characterization of the various inhibitors can be found in the Supporting Information. Inhibitors were dissolved in DMSO at concentrations up to 500 mM (1-3) or 400 mM (7-9).
Antibodies and Lectins
The sources of the antibodies and lectins used in this study are as follows: Anti-Sialyl Lewis X (BD Pharmingen - 551344), FITC-Anti Lewis X (Biolegend – 301904), Biotinylated-AAL (Vector Labs), FITC-PNA (Vector Labs), FITC-SNA (Vector Labs), Streptavidin Alexa Fluor-488 (Invitrogen), human E-Selectin Fc (RnD Systems), human P-Selectin Fc (RnD Systems), R-PE goat anti-human IgG Fcγ-Fragment Specific (Jackson Immunoresearch), R-PE anti-mouse IgM (Jackson Immunoresearch),
Inhibitor Titrations
For all experiments, HL-60 and Ramos cells were grown in RPMI 1640 supplemented with 10% FBS, Penicillin/Streptomycin, and L-Glutamine. CHO-K1 cells were grown in DMEM/F12 (1:1) supplemented with 10% FBS and Penicillin/Streptomycin. For titration analysis, 20,000 cells were added to each well of a 96-well plate, the media was aspirated, and 100 μl of media containing the inhibitor at the desired concentration was added (0.1% final DMSO concentration). Cells were allowed to grow for 72 hrs at 37°C, washed, and subjected to flow cytometry analysis with various lectins or anti-glycan antibodies. Each inhibitor concentration was done in triplicate and the data was normalized to the DMSO only treated control cells (100%) and unstained cells (0%).
Flow Cytometry Analysis
Cells were washed with HBSS containing 5% BSA and resuspended to 2 × 106 cells/ml. All staining is carried out in this buffer in a U-bottom 96-well plate on ice for 1hr. For directly labeled antibodies/lectins, cells are then washed two times with cold HBSS/BSA (200 μl), resuspended in this buffer (200 μl), and analyzed by flow cytometry. For indirect detections, cells are washed once with HBSS/BSA (200 μl) prior to addition of the appropriate secondary detection agent. Cells are then kept on ice for 30 minutes, washed two times with buffer, and resuspended for flow cytometry. For Sialyl Lewis X detection, 0.1 μg/ml primary antibody and 10 μg/ml R-PE anti-mouse IgM are used. For Lewis X detection, a 1:20 dilution is used. For AAL, 0.2 μg/ml AAL-biotin is used followed by 10 μg/ml Streptavidin AF-488. FITC-PNA is used at 0.5 μg/ml and FITC-SNA at 2 μg/ml. For selectin binding, HBSS/BSA containing 5 mM CaCl2 is used as the buffer. Moreover, human E-Selectin Fc (10 μg/ml) is precomplexed with R-PE goat anti-human IgG Fcγ-Fragment Specific (5 μg/ml) for 15 minutes on ice prior to diluting ten-fold and applying to cells. P-Selectin binding is done analogously.
Mass Spectrometry of Inhibitor Treated Cells
HL-60 cells were seeded at 0.2 × 106 cells/ml in media containing DMSO, 200 μM (2), 200 μM (8), or 200 μM (2) and (8). The final DMSO concentration was 0.1% in all cases. After 72 hrs, cells were pelleted and the culture media removed. These were then seeded at 0.4 × 106 cells/ml with fresh media containing the corresponding inhibitors (or DMSO only) and grown for 48 more hours. This process was then repeated and on day 7, cells were pelleted, washed three times with HBSS, counted, and ~2 × 108 cells for each condition were lyophilized. The glycomic methods used here have been described previously51,57,58. Full glycomic methods can be found in the Supplementary Methods
Nucleotide Sugar Analysis of Inhibitor Treated Cells
HL-60 cells were treated for 7 days with DMSO only, (2), or (8) as described above for the mass spectrometry analysis. On day 7, cells were pelleted, washed three times with HBSS, pelleted, and frozen. Full details for nucleotide sugar extraction and analysis can be found in the Supplementary Methods.
HL-60 Rolling Assays
Rectangular (20mm × 200mm) glass capillary flow chambers (VitroCom) were cut to 2-cm length and mounted on a glass microscope slide. Flow chambers were coated with either 40 μg/ml recombinant human P-selectin (R&D Systems) or 15 μg/ml recombinant human E-selectin (R&D Systems), and then blocked using 1% casein in PBS (Thermo Scientific). Polyethylene tubing (size PE-10) filled with saline was attached to the flow chamber outlet to control the shear stress, as previously described59. HL-60 cells treated with 200 μM of (2), (8), or DMSO control, for 5 days were suspended in HBSS containing 1mM CaCl2 and 1mM MgCl2 and added to a reservoir at the flow chamber inlet.
For HL-60 cell rolling velocity measurements, a shear stress of 3 dynes/cm2 was maintained. For HL-60 cell tethering analysis, cells were allowed to settle briefly (<10 sec) and then flow was initiated at a shear stress of 1 dyne/cm2. The shear stress was then increased in 1 dyne/cm2 increments up to 6 dyne/cm2. The number of initially settled cells that continued to roll were counted at each shear stress level. Imaging was performed using a Zeiss Axiovert 100 microscope and captured with a Sensicam QE CCD camera (Cooke).
Supplementary Material
Acknowledgements
This work was supported by NIH grants to J.C.P. (R01AI050143 and P01HL107151), C.D.R. (T32AI007606), K.L (HL111969), C.T.L. (T32AI060536), and the Complex Carbohydrate Research Center (1 P41 RR018502-01) as well as by funding from the Biotechnology and Biological Sciences Research Council (BBSRC) to A.D. and S.M.H. (BBF0083091).
Footnotes
Author Contributions
C.D.R. conceived the idea, synthesized the inhibitors, designed the experiments, and performed biochemical assays. A.A. performed the N- and O-linked glycan mass spectrometry analysis. C.T.L. performed the rolling assays. R.S. performed the nucleotide sugar analysis. J.C.P., S.M.H, A.D., K.L., and P.A. supervised the research. C.D.R and J.C.P. wrote the manuscript.
Competing Financial Interests
The authors declare no competing financial interests.
Literature Cited
- 1.Varki A, et al. Essentials of Glycobiology. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2009. [PubMed] [Google Scholar]
- 2.Becker D, Lowe J. Fucose: biosynthesis and biological function in mammals. Glycobiology. 2003;13:41R–53R. doi: 10.1093/glycob/cwg054. [DOI] [PubMed] [Google Scholar]
- 3.Varki A. Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature. 2007;446:1023–9. doi: 10.1038/nature05816. [DOI] [PubMed] [Google Scholar]
- 4.Lowe JB, Marth JD. A genetic approach to Mammalian glycan function. Annu Rev Biochem. 2003;72:643–91. doi: 10.1146/annurev.biochem.72.121801.161809. [DOI] [PubMed] [Google Scholar]
- 5.Maly P, et al. The alpha(1,3)fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell. 1996;86:643–53. doi: 10.1016/s0092-8674(00)80137-3. [DOI] [PubMed] [Google Scholar]
- 6.Hennet T, Chui D, Paulson J, Marth J. Immune regulation by the ST6Gal sialyltransferase. Proc Natl Acad Sci U S A. 1998;95:4504–9. doi: 10.1073/pnas.95.8.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Priatel J, et al. The ST3Gal-I sialyltransferase controls CD8+ T lymphocyte homeostasis by modulating O-glycan biosynthesis. Immunity. 2000;12:273–83. doi: 10.1016/s1074-7613(00)80180-6. [DOI] [PubMed] [Google Scholar]
- 8.Grewal PK, et al. ST6Gal-I restrains CD22-dependent antigen receptor endocytosis and Shp-1 recruitment in normal and pathogenic immune signaling. Mol Cell Biol. 2006;26:4970–81. doi: 10.1128/MCB.00308-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gitlin JM, et al. Disruption of tissue-specific fucosyltransferase VII, an enzyme necessary for selectin ligand synthesis, suppresses atherosclerosis in mice. Am J Pathol. 2009;174:343–50. doi: 10.2353/ajpath.2009.080036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Homeister JW, Daugherty A, Lowe JB. Alpha(1,3)fucosyltransferases FucT-IV and FucT-VII control susceptibility to atherosclerosis in apolipoprotein E-/- mice. Arterioscler Thromb Vasc Biol. 2004;24:1897–903. doi: 10.1161/01.ATV.0000141844.28073.df. [DOI] [PubMed] [Google Scholar]
- 11.Sarkar AK, Fritz TA, Taylor WH, Esko JD. Disaccharide uptake and priming in animal cells: inhibition of sialyl Lewis X by acetylated Gal beta 1-->4GlcNAc beta-O-naphthalenemethanol. Proc Natl Acad Sci U S A. 1995;92:3323–7. doi: 10.1073/pnas.92.8.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarkar AK, Rostand KS, Jain RK, Matta KL, Esko JD. Fucosylation of disaccharide precursors of sialyl LewisX inhibit selectin-mediated cell adhesion. J Biol Chem. 1997;272:25608–16. doi: 10.1074/jbc.272.41.25608. [DOI] [PubMed] [Google Scholar]
- 13.Fuster MM, Brown JR, Wang L, Esko JD. A disaccharide precursor of sialyl Lewis X inhibits metastatic potential of tumor cells. Cancer Res. 2003;63:2775–81. [PubMed] [Google Scholar]
- 14.Brown JR, et al. A disaccharide-based inhibitor of glycosylation attenuates metastatic tumor cell dissemination. Clin Cancer Res. 2006;12:2894–901. doi: 10.1158/1078-0432.CCR-05-2745. [DOI] [PubMed] [Google Scholar]
- 15.Hosoguchi K, et al. An efficient approach to the discovery of potent inhibitors against glycosyltransferases. J Med Chem. 2010;53:5607–19. doi: 10.1021/jm100612r. [DOI] [PubMed] [Google Scholar]
- 16.Lee L, et al. A potent and highly selective inhibitor of human alpha-1,3-fucosyltransferase via click chemistry. J Am Chem Soc. 2003;125:9588–9. doi: 10.1021/ja0302836. [DOI] [PubMed] [Google Scholar]
- 17.Burkart MD, et al. Chemo-enzymatic synthesis of fluorinated sugar nucleotide: useful mechanistic probes for glycosyltransferases. Bioorg Med Chem. 2000;8:1937–46. doi: 10.1016/s0968-0896(00)00139-5. [DOI] [PubMed] [Google Scholar]
- 18.Murray BW, Wittmann V, Burkart MD, Hung SC, Wong CH. Mechanism of human alpha-1,3-fucosyltransferase V: glycosidic cleavage occurs prior to nucleophilic attack. Biochemistry. 1997;36:823–31. doi: 10.1021/bi962284z. [DOI] [PubMed] [Google Scholar]
- 19.Lairson LL, Henrissat B, Davies GJ, Withers SG. Glycosyltransferases: structures, functions, and mechanisms. Annu Rev Biochem. 2008;77:521–55. doi: 10.1146/annurev.biochem.76.061005.092322. [DOI] [PubMed] [Google Scholar]
- 20.Vasella A, Davies GJ, Böhm M. Glycosidase mechanisms. Curr Opin Chem Biol. 2002;6:619–29. doi: 10.1016/s1367-5931(02)00380-0. [DOI] [PubMed] [Google Scholar]
- 21.Gloster TM, et al. Hijacking a biosynthetic pathway yields a glycosyltransferase inhibitor within cells. Nat Chem Biol. 2011;7:174–81. doi: 10.1038/nchembio.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campbell CT, Sampathkumar SG, Yarema KJ. Metabolic oligosaccharide engineering: perspectives, applications, and future directions. Mol Biosyst. 2007;3:187–94. doi: 10.1039/b614939c. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan FX, et al. Molecular cloning of human GDP-mannose 4,6-dehydratase and reconstitution of GDP-fucose biosynthesis in vitro. J Biol Chem. 1998;273:8193–202. doi: 10.1074/jbc.273.14.8193. [DOI] [PubMed] [Google Scholar]
- 24.Hinderlich S, Stäsche R, Zeitler R, Reutter W. A bifunctional enzyme catalyzes the first two steps in N-acetylneuraminic acid biosynthesis of rat liver. Purification and characterization of UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase. J Biol Chem. 1997;272:24313–8. doi: 10.1074/jbc.272.39.24313. [DOI] [PubMed] [Google Scholar]
- 25.Burkart M, Zhang Z, Hung S, Wong C. A new method for the synthesis of fluorocarbohydrates and glycosides using selectfluor. Journal of the American Chemical Society. 1997;119:11743–11746. [Google Scholar]
- 26.Weston BW, et al. A cloned CD15s-negative variant of HL60 cells is deficient in expression of FUT7 and does not adhere to cytokine-stimulated endothelial cells. Eur J Haematol. 1999;63:42–9. doi: 10.1111/j.1600-0609.1999.tb01849.x. [DOI] [PubMed] [Google Scholar]
- 27.Nakayama F, et al. CD15 expression in mature granulocytes is determined by alpha 1,3-fucosyltransferase IX, but in promyelocytes and monocytes by alpha 1,3-fucosyltransferase IV. J Biol Chem. 2001;276:16100–6. doi: 10.1074/jbc.M007272200. [DOI] [PubMed] [Google Scholar]
- 28.Chiu CP, et al. Structural analysis of the sialyltransferase CstII from Campylobacter jejuni in complex with a substrate analog. Nat Struct Mol Biol. 2004;11:163–70. doi: 10.1038/nsmb720. [DOI] [PubMed] [Google Scholar]
- 29.Ni L, et al. Crystal structures of Pasteurella multocida sialyltransferase complexes with acceptor and donor analogues reveal substrate binding sites and catalytic mechanism. Biochemistry. 2007;46:6288–98. doi: 10.1021/bi700346w. [DOI] [PubMed] [Google Scholar]
- 30.Marathe DD, Chandrasekaran EV, Lau JT, Matta KL, Neelamegham S. Systems-level studies of glycosyltransferase gene expression and enzyme activity that are associated with the selectin binding function of human leukocytes. FASEB J. 2008;22:4154–67. doi: 10.1096/fj.07-104257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oetke C, et al. Evidence for efficient uptake and incorporation of sialic acid by eukaryotic cells. Eur J Biochem. 2001;268:4553–61. doi: 10.1046/j.1432-1327.2001.02379.x. [DOI] [PubMed] [Google Scholar]
- 32.Mendla K, Baumkötter J, Rosenau C, Ulrich-Bott B, Cantz M. Defective lysosomal release of glycoprotein-derived sialic acid in fibroblasts from patients with sialic acid storage disease. Biochem J. 1988;250:261–7. doi: 10.1042/bj2500261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keppler OT, et al. UDP-GlcNAc 2-epimerase: a regulator of cell surface sialylation. Science. 1999;284:1372–6. doi: 10.1126/science.284.5418.1372. [DOI] [PubMed] [Google Scholar]
- 34.Ripka J, Adamany A, Stanley P. Two Chinese hamster ovary glycosylation mutants affected in the conversion of GDP-mannose to GDP-fucose. Arch Biochem Biophys. 1986;249:533–45. doi: 10.1016/0003-9861(86)90031-7. [DOI] [PubMed] [Google Scholar]
- 35.Beyer TA, et al. Biosynthesis of mammalian glycoproteins. Glycosylation pathways in the synthesis of the nonreducing terminal sequences. J Biol Chem. 1979;254:12531–4. [PubMed] [Google Scholar]
- 36.Sperandio M, Gleissner CA, Ley K. Glycosylation in immune cell trafficking. Immunol Rev. 2009;230:97–113. doi: 10.1111/j.1600-065X.2009.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lowe JB. Glycan-dependent leukocyte adhesion and recruitment in inflammation. Curr Opin Cell Biol. 2003;15:531–8. doi: 10.1016/j.ceb.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 38.Mestas J, Ley K. Monocyte-endothelial cell interactions in the development of atherosclerosis. Trends Cardiovasc Med. 2008;18:228–32. doi: 10.1016/j.tcm.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chandra A, Enkhbaatar P, Nakano Y, Traber LD, Traber DL. Sepsis: emerging role of nitric oxide and selectins. Clinics (Sao Paulo) 2006;61:71–6. doi: 10.1590/s1807-59322006000100012. [DOI] [PubMed] [Google Scholar]
- 40.Leppänen A, White SP, Helin J, McEver RP, Cummings RD. Binding of glycosulfopeptides to P-selectin requires stereospecific contributions of individual tyrosine sulfate and sugar residues. J Biol Chem. 2000;275:39569–78. doi: 10.1074/jbc.M005005200. [DOI] [PubMed] [Google Scholar]
- 41.Rillahan CD, Brown SJ, Register AC, Rosen H, Paulson JC. High-throughput screening for inhibitors of sialyl- and fucosyltransferases. Angew Chem Int Ed Engl. 2011;50:12534–7. doi: 10.1002/anie.201105065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gross B, Kraybill B, Walker S. Discovery of O-GlcNAc transferase inhibitors. J Am Chem Soc. 2005;127:14588–9. doi: 10.1021/ja0555217. [DOI] [PubMed] [Google Scholar]
- 43.Gross BJ, Swoboda JG, Walker S. A strategy to discover inhibitors of O-linked glycosylation. J Am Chem Soc. 2008;130:440–1. doi: 10.1021/ja078125s. [DOI] [PubMed] [Google Scholar]
- 44.Ahsen O, et al. A miniaturized high-throughput screening assay for fucosyltransferase VII. Anal Biochem. 2008;372:96–105. doi: 10.1016/j.ab.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 45.Hang HC, et al. Small molecule inhibitors of mucin-type O-linked glycosylation from a uridine-based library. Chem Biol. 2004;11:337–45. doi: 10.1016/j.chembiol.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 46.Brown J, Crawford B, Esko J. Glycan antagonists and inhibitors: a fount for drug discovery. Crit Rev Biochem Mol Biol. 2007;42:481–515. doi: 10.1080/10409230701751611. [DOI] [PubMed] [Google Scholar]
- 47.Kiessling LL, Splain RA. Chemical approaches to glycobiology. Annu Rev Biochem. 2010;79:619–53. doi: 10.1146/annurev.biochem.77.070606.100917. [DOI] [PubMed] [Google Scholar]
- 48.Alley SCJ, Scott C, Sussman Django, Benjamin Dennis R., Toki Brian, Burke Patrick J. In: Methods and Compositions for Making Antibodies and Anitbody Derivatives with Reduced Core Fucosylation. Office, U.S.P., editor. Seattle Genetics, Inc.; United States: 2009. [Google Scholar]
- 49.Frantom PA, Coward JK, Blanchard JS. UDP-(5F)-GlcNAc acts as a slow-binding inhibitor of MshA, a retaining glycosyltransferase. J Am Chem Soc. 2010;132:6626–7. doi: 10.1021/ja101231a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hayashi T, Murray BW, Wang R, Wong CH. A chemoenzymatic synthesis of UDP-(2-deoxy-2-fluoro)-galactose and evaluation of its interaction with galactosyltransferase. Bioorg Med Chem. 1997;5:497–500. doi: 10.1016/s0968-0896(96)00263-5. [DOI] [PubMed] [Google Scholar]
- 51.Barthel SR, et al. Peracetylated 4-fluoro-glucosamine reduces the content and repertoire of N- and O-glycans without direct incorporation. J Biol Chem. 2011;286:21717–31. doi: 10.1074/jbc.M110.194597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dimitroff CJ, Kupper TS, Sackstein R. Prevention of leukocyte migration to inflamed skin with a novel fluorosugar modifier of cutaneous lymphocyte-associated antigen. J Clin Invest. 2003;112:1008–18. doi: 10.1172/JCI19220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gainers ME, et al. Skin-homing receptors on effector leukocytes are differentially sensitive to glyco-metabolic antagonism in allergic contact dermatitis. J Immunol. 2007;179:8509–18. doi: 10.4049/jimmunol.179.12.8509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Descheny L, Gainers ME, Walcheck B, Dimitroff CJ. Ameliorating skin-homing receptors on malignant T cells with a fluorosugar analog of N-acetylglucosamine: P-selectin ligand is a more sensitive target than E-selectin ligand. J Invest Dermatol. 2006;126:2065–73. doi: 10.1038/sj.jid.5700364. [DOI] [PubMed] [Google Scholar]
- 55.Nishimura S, Hato M, Hyugaji S, Feng F, Amano M. Glycomics for drug discovery: Metabolic perturbation in androgen-independent prostate cancer cells induced by unnatural hexosamine mimics. Angewante Chemie. 2012;51 doi: 10.1002/anie.201108742. in press. [DOI] [PubMed] [Google Scholar]
- 56.Grigorian A, et al. Control of T Cell-mediated autoimmunity by metabolite flux to N-glycan biosynthesis. J Biol Chem. 2007;282:20027–35. doi: 10.1074/jbc.M701890200. [DOI] [PubMed] [Google Scholar]
- 57.Jang-Lee J, et al. Glycomic profiling of cells and tissues by mass spectrometry: fingerprinting and sequencing methodologies. Methods Enzymol. 2006;415:59–86. doi: 10.1016/S0076-6879(06)15005-3. [DOI] [PubMed] [Google Scholar]
- 58.Ceroni A, et al. GlycoWorkbench: a tool for the computer-assisted annotation of mass spectra of glycans. J Proteome Res. 2008;7:1650–9. doi: 10.1021/pr7008252. [DOI] [PubMed] [Google Scholar]
- 59.Chesnutt BC, et al. Induction of LFA-1-dependent neutrophil rolling on ICAM-1 by engagement of E-selectin. Microcirculation. 2006;13:99–109. doi: 10.1080/10739680500466376. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.