Abstract
Aims
Hypoxia is known to influence cardiovascular (CV) function, in part, through adenosine receptor activation. We have shown in a mouse model that during primary cardiac morphogenesis, acute maternal hypoxia negatively affects fetal heart rate, and recurrent maternal caffeine exposure reduces fetal cardiac output (CO) and down-regulates fetal adenosine A2A receptor gene expression. In the present study, we investigated whether maternal caffeine dosing exacerbates the fetal CV response to acute maternal hypoxia during the primary morphogenesis period.
Methods
Gestational day 11.5 pregnant mice were exposed to hypoxia (45 second duration followed by 10 minutes of recovery and repeated 3 times) while simultaneously monitoring maternal and fetal CO using high-resolution echocardiography.
Results
Following maternal hypoxia exposure, maternal CO transiently decreased and then returned to pre-hypoxia baseline values. In contrast to a uniform maternal cardiac response to each exposure to hypoxia, the fetal CO recovery time to the baseline decreased, and CO rebounded above baseline following the 2nd and 3rd episodes of maternal hypoxia. Maternal caffeine treatment inhibited the fetal CO recovery to maternal hypoxia by lengthening the time to CO recovery and eliminating the CO rebound post-recovery. Selective treatment with an adenosine A2A receptor antagonist, but not an adenosine A1 receptor antagonist, reproduced the altered fetal CO response to maternal hypoxia created by caffeine exposure.
Conclusions
Results suggest an additive negative effect of maternal caffeine on the fetal CV response to acute maternal hypoxia, potentially mediated via adenosine A2A receptor inhibition during primary cardiovascular morphogenesis.
Keywords: adenosine receptor, caffeine, fetus, hemodynamics, hypoxia
Introduction
The period of primary cardiovascular (CV) morphogenesis is also time of greatest vulnerability to epigenetic events (1). During this critical developmental window the fetal CV system is the first functioning organ to transport oxygen and nutrients between the utero-placental and feto-placental circulations (2). A broad range of developmental errors can occur during this time and the investigation of CV morphogenesis and adaptation reveals significant epigenetic plasticity, supporting the important role of epigenetic events on developmental fate (3). Maladaptation in response to these epigenetic events results in deviations from the normal trajectory of fetal development.
Maternal hypoxia is a well-known factor that affects developing fetal CV function, major organ development including the brain, heart, liver, and kidney, and can cause intrauterine growth restriction (IUGR) (4–6). We previously reported in a mouse model that acute maternal hypoxia affects fetal heart rate during primary CV morphogenesis (7). We have also reported that modest maternal caffeine exposure in a mouse model acutely impairs fetal CV function during this critical developmental window and recurrent maternal caffeine exposure produces intrauterine growth restriction. The negative effect of caffeine exposure on fetal CV function is mediated by caffeine inhibition of the adenosine A2A receptor (8).
Adenosine and adenosine receptors play a crucial role in cellular and tissue response to hypoxia (9). For example, adenosine mediates local blood flow regulation during ischemia and hypoxia. Plasma adenosine concentration is usually higher in the fetus than in adults (10, 11). Recent studies have shown that fetal adenosine levels increase under various stress conditions, such as hypoxia or placental dysfunction (12, 13). The elevation of umbilical venous plasma adenosine levels has also been demonstrated in newborns after vaginal delivery (10) and in fetuses with intrauterine growth restriction (11). These findings suggest that adenosine and adenosine receptor function play an integral role in the developing fetal stress response to hypoxia. If adenosine modulates the fetal hemodynamic response to hypoxia then adenosine antagonists, including caffeine, may have a negative adaptive effect during the response to hypoxic conditions. The cellular effects of adenosine are mediated via G protein-coupled membrane-bound receptors. At present, four distinct adenosine receptors subtypes, A1, A2A, A2B, and A3, have been cloned and characterized in several species (14–17). Of these subtypes, the A1 and A2A receptors are activated at low adenosine concentrations and A1 adenosine receptors mediate the negative dromotropic, chronotropic, and inotropic adenosine effects (14, 15). Activation of adenosine A2A and perhaps A2B receptors triggers vasodilatation (14, 16, 17). Many studies suggested that adenosine is potentially beneficial for salvaging ischemic and/or reperfused myocardium. These properties include vasodilatation, β-adrenergic antagonism (18, 19), and glycolytic stimulation (20).
The plasma concentration of adenosine in the fetus is usually higher than in adults (10, 11, 21) and adenosine concentration in the placenta increases 100-fold during delivery (22). Intrauterine hypoxia and acidosis increase the plasma level of adenosine in the fetus and the placenta (10–12). Kubonoya et al. reported that plasma adenosine level increased cyclically with intermittent umbilical cord occlusion in fetal sheep and they speculated that adenosine may have a protective effect against hypoxia (23). Adenosine is a vasodilator in most vascular beds, but is a vasoconstrictor of renal glomerular afferent arteriole and placental circulation (16). Read et al. speculated that release of adenosine by local ischemia in the placenta contributes to the ensuing vasoconstrictor response, directing blood to the placental villi that have higher oxygen tensions where its vasodilator effect could help sustain adequate blood flow (21).
Previous reports regarding the action of adenosine on the fetal CV system during the period of primary morphogenesis are limited to those of studies performed in vitro. Xu et al. showed that the affinity of the A2A receptor was 60-fold higher than that of the A2B receptor in ventricular cells cultured from 14-day chick embryos and that adenosine and N-ethyladenosine-5'-uronic acid (NECA), capable of activating both the adenosine A2A and A2B receptors, caused a greater increase in the contractile amplitude (24). Previous studies showed that A1 receptor agonist reduced heart rate in a dose-dependent manner and A1 receptor antagonist increased heart rate in a murine fetuses from GD 8 and older (25–27). Revkees et al. suggested that adenosine may be a regulator of cardiac cell division during early development (26).
In a recent large prospective cohort study, it has been shown that caffeine consumption during pregnancy was associated with an increased risk of fetal growth restriction and this association continued throughout pregnancy (28). Furthermore, Weng et al. demonstrated an elevated risk of miscarriage associated with caffeine consumption during pregnancy in their prospective cohort study (29).
These studies, including our own, suggest that negative fetal CV function by acute maternal hypoxia, which could be induced by anaerobic exercises, smoking, and/or even emotional events, may be exacerbated by additive caffeine exposure (ingestion of caffeinated foods and drinks). Therefore, in the present study, we investigated whether the embryo/fetal CV response to the acute maternal hypoxia is negatively impacted by additive maternal caffeine exposure or by exposure to adenosine receptor inhibitors that may recapitulate the caffeine effect.
Materials and Methods
Animals
Eight- to 12-week-old virgin female CD-1 mice were mated with CD-1 males (2:1) overnight and were examined for copulatory plugs the next morning. We defined gestational day (GD) 0.5 as noon of that day. Animal care and experimental protocols were approved by the Institutional Animal Care and Use Committee, University of Pittsburgh.
Experimental Protocol
Saline (control group), caffeine (10 mg/kg per dose, caffeine group), the adenosine A1 receptor antagonist 8-cyclopentyl-1, 3-dimethylxanthine (4.8 mg/kg per dose, CPT group), or the adenosine A2A receptor antagonist 3-(3-hydroxypropyl)-8-(m-methoxystyryl)-7-methyl-1-propargylxanthine phosphate disodium salt (3.0 mg/kg per dose, MSX-3 group) was administered to CD-1 pregnant mice on GD 11.5 by subcutaneous injection. Caffeine dose was determined based on our previous study of maternal caffeine exposure mimicking regular daily human caffeine ingestion (8). Selective adenosine receptor antagonist doses are the minimal effective doses required to inhibit adenosine receptor (8). Each group consisted of seven dams. Thirty minutes after drug administration, dams were subjected to hypoxia using 100% nitrogen for 45 seconds and the changes in maternal and fetal hemodynamics were measured simultaneously using two echocardiography systems. The maternal hypoxia stress was repeated three times at intervals of 10 minutes, and maternal and fetal hemodynamics were continuously monitored for a total of 40 minutes.
Drugs
All drugs were dissolved in sterile saline (with a few drops of 0.1 N NaOH for CPT and MSX-3; final pH adjusted to 7.4) and administered subcutaneously in a volume of 0.01 ml/g of maternal body weight. The dose of the adenosine receptor antagonists was chosen according to the previous report by Schindler et al.(30) which is the same dosage as in our previous study (8). Maternal serum caffeine concentrations measured in our previous study at 30 and 60 min after administration of 10 mg/kg of caffeine were 1.1±0.27 (n=4) and 1.1±0.25 (n=5) µg/ml, respectively (8).
Echocardiography
Standard transthoracic (dam) and maternal transabdominal (fetus) echocardiography were performed simultaneously using two ultrasound imaging systems. We used a high frequency ultrasound biomicroscope (Vevo 660; VisualSonics, Toronto, ON, Canada) with a 40-MHz mechanical sector transducer to measure fetal dorsal aortic flow, and an Acuson Sequoia C256 system with a 13-MHz linear ultrasonic transducer (15L8; Acuson Corporation, Mountain View, CA) to measure maternal pulmonary arterial flow. Pregnant mice were anesthetized with isoflurane gas (2% isoflurane for 1 minute induction and 1.5 to 1.7% for maintenance with 100% oxygen gas) and restrained in the supine position. Maternal blood pressure was monitored using a tail cuff blood pressure system (XBP 1000; Kent Scientific, Torrington, CT). The ambient temperature was maintained at approximately 37°C with a temperature controller (THM 1000; Visual Sonics) and a heating lamp (8). We measured the maternal heart rate (HR), the velocity-time integral (VTI, cm) of pulmonary flow from pulsed-Doppler velocity tracings, and main pulmonary artery diameter (D, cm) from B-mode images. We calculated maternal cardiac output (CO, ml/min) as CO = π(D/2)2 × VTI × HR. We chose a single fetus in order to visualize the fetus in the longitudinal axis of the body (sagittal view), which enables us to monitor both ventricle and developing dorsal aorta within an acceptable angle (less than 15 degrees between the longitudinal axis of the dorsal aorta and Doppler ultrasound beam) to estimate blood flow. The fetal CO (µl/minute) was calculated from pulsed-Doppler velocity waveforms and M-mode images of the dorsal aorta applying the same formula for maternal CO calculation.
Statistical Analysis
Data are expressed as means ± SE. Maternal and fetal hemodynamic data from different treatments were analyzed using two-way repeated analysis of variance (ANOVA) with the Bonferroni post hoc test.
Results
Baseline maternal and fetal heart rate (HR), stroke volume (SV), and cardiac output (CO)
The baseline maternal and fetal HR, SV, and CO were not different among control, caffeine, adenosine A1 receptor antagonist (CPT), and adenosine A2A antagonist (MXS-3) treated groups before hypoxia exposure (Table 1).
Table 1.
Baseline hemodynamics
| Control | Caffeine | A1 antagonist (CPT) |
A2A antagonist (MSX-3) |
p-value | ||
|---|---|---|---|---|---|---|
| Mother | HR (/min) | 517 ± 12.4 | 533 ± 19.6 | 500 ± 64.1 | 506 ± 13.8 | n.s. |
| CO (ml/min) | 27.7 ± 1.0 | 27.3 ± 1.4 | 25.2 ± 3.1 | 28.4 ± 1.5 | n.s. | |
| Fetus | HR (/min) | 183 ± 22.6 | 181 ± 7.5 | 209 ± 38.2 | 176 ± 11.0 | n.s. |
| CO (µl/min) | 83.1 ± 7.9 | 73.9 ± 5.9 | 81.7 ± 10.7 | 62.9 ± 7.6 | n.s. | |
| SV (µl | 0.47 ± 0.031 | 0.41 ± 0.031 | 0.41 ± 0.36 | 0.36 ± 0.035 | n.s. | |
HR: heart rate, CO: cardiac output, SV: stroke volume
Maternal hemodynamics
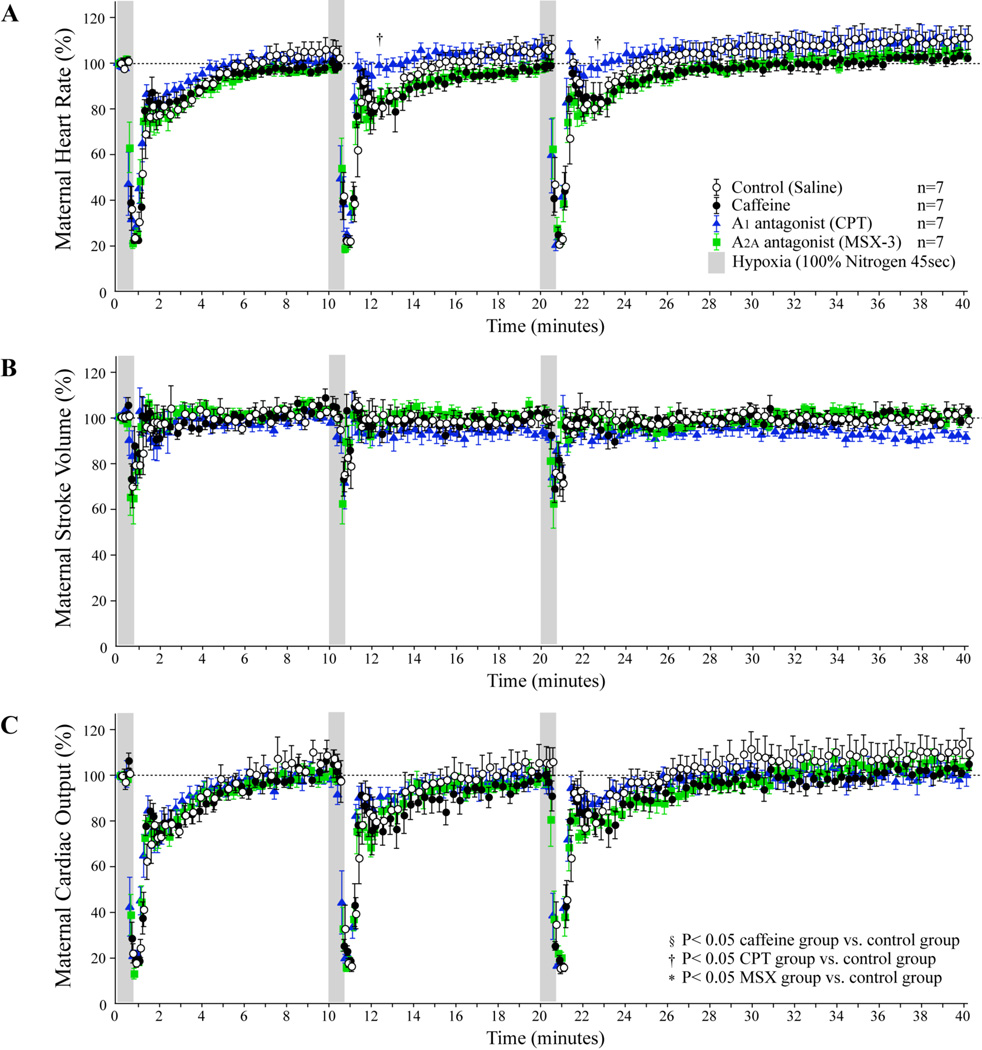
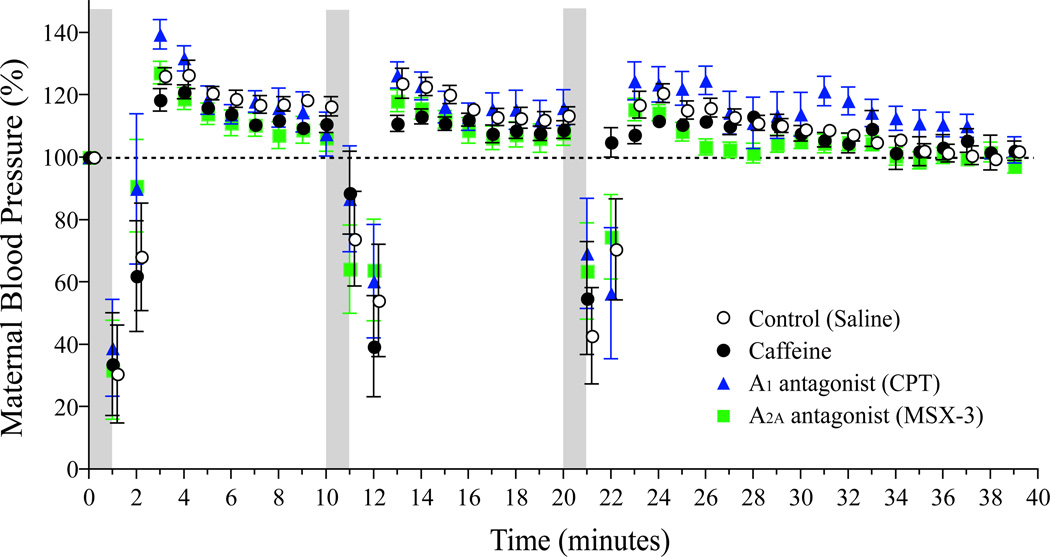
In the control group, maternal HR, SV, and CO decreased suddenly approximately 30 seconds (s) after the beginning of the first exposure to hypoxia and were restored rapidly to approximately 80% of the baseline within 60s after hypoxia and then gradually recovered to baseline thereafter (Figure 1). Similarly, maternal hemodynamics recovered rapidly to close to the baseline within 60s after the second and third periods of hypoxia with a decrease to approximately 80% of baseline followed by a gradual recovery to baseline. There were no statistical differences in maternal heart rate, stroke volume, or CO, between the control group, caffeine group, and MSX-3 group. However, CPT group did display the delayed heart rate reduction during the second and third periods of hypoxia (P<0.05 vs. Control, ANOVA). There were no significant differences in maternal blood pressure among the four maternal groups (Figure 2).
Figure 1. Maternal hemodynamic effects of repeated maternal hypoxia with adenosine receptor antagonists.
Dams were repeatedly exposed to 100% nitrogen for 45 seconds followed by a 10 to 20 minute recovery period.(A): Maternal heart rate, (B): Maternal stroke volume, (C): Maternal cardiac output.
Figure 2. Maternal blood pressure responses to hypoxia, caffeine, and Adenosine receptor antagonist treatments.
Maternal blood pressure was consecutively measured using a tail cuff blood pressure system. There were no significant differences in one-minute mean maternal blood pressure among the four groups.
Fetal hemodynamics
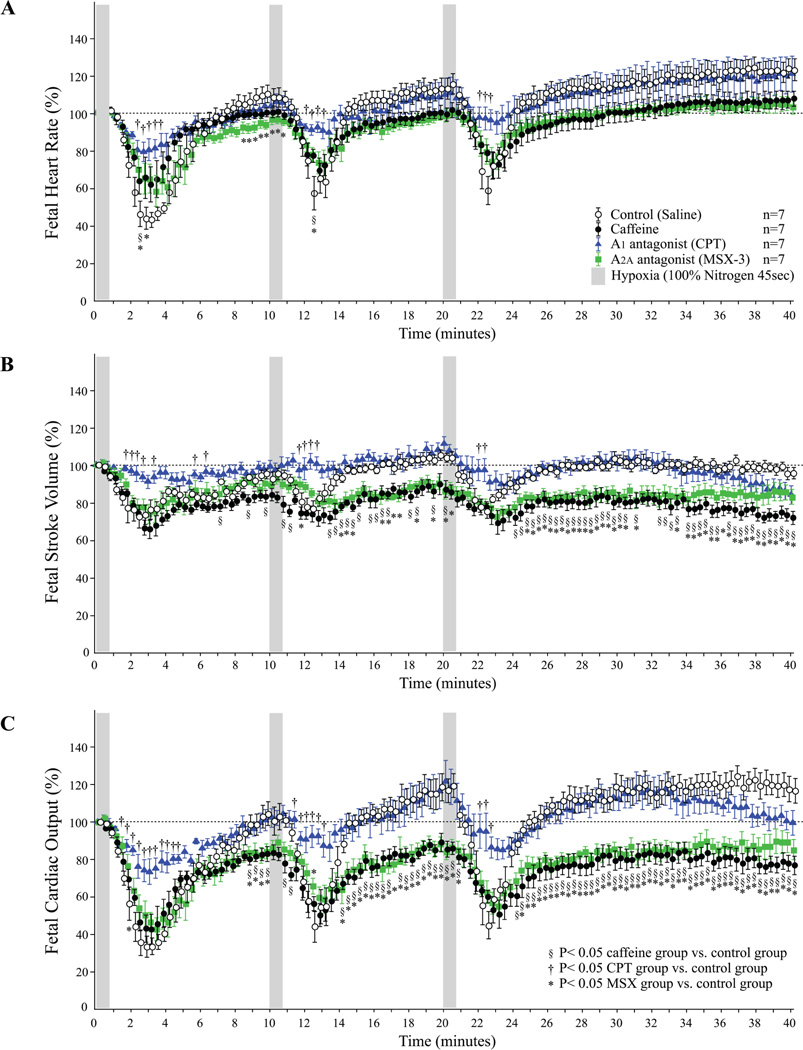
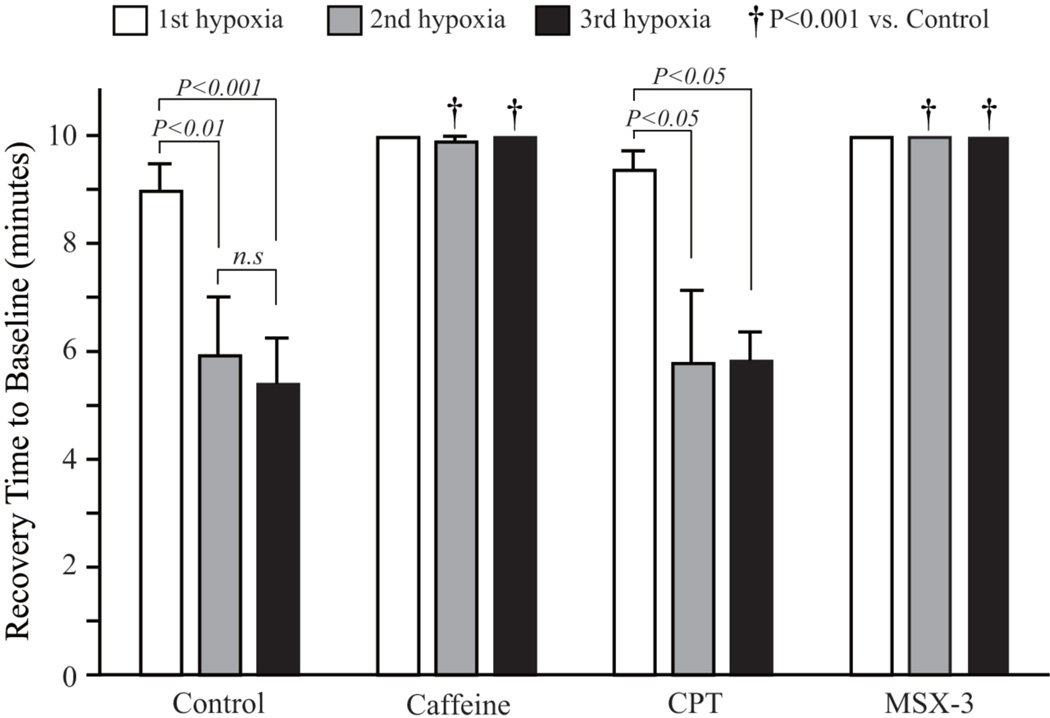
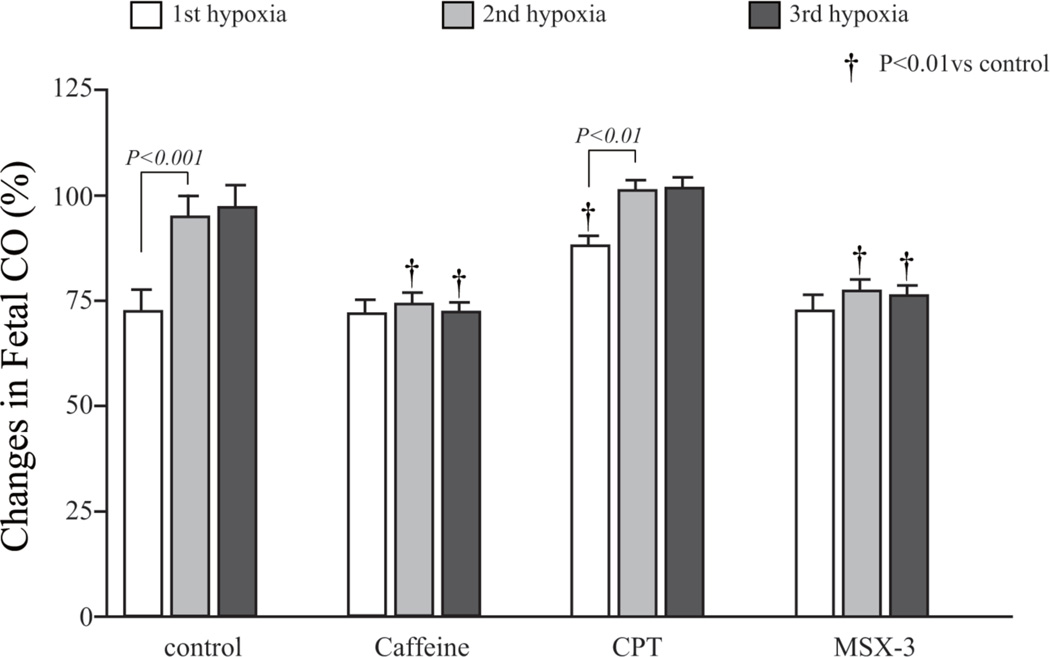
A decrease in fetal HR, SVβ, and CO in response to maternal hypoxia appeared slowly after the decrease in the maternal heart rate, stroke volume and CO, respectively. The maximum drop was found approximately 3 minutes after the start of the hypoxia (Figure 3). In the control group, repeated hypoxia resulted in a shortening of the recovery time. The recovery time to baseline after the second and third exposures to hypoxia was statistically shorter than the recovery time after initial hypoxia (Figure 4). Repeated hypoxia also resulted in the blood flow exceeding the baseline following recovery. The CO overshoot was caused by an increase in fetal heart rate over baseline in addition to the restoration of stroke volume to the baseline (Figure 3). There were no significant differences in the lowest fetal CO after hypoxia between the control group and the caffeine or selective adenosine A2A receptor antagonist, MSX-3, group. However, recovery was negatively impacted and no restoration to baseline occurred in either the caffeine or MSX-3 groups. There were statistical differences in the fetal CO during the recovery periods between the control and the caffeine group or MSX-3 group (Figure 3C). The differences in fetal CO in the caffeine and MSX-3 groups were caused by the combination of the lower stroke volumes and lower heart rates. In addition, shortening of the recovery time and overshoot in CO in response to repeated hypoxia were not observed in the caffeine or MSX-3 groups (Figure 3C, Figure 4). No significant differences between the control, caffeine, and MSX-3 groups were found in the 10-minute mean percentage change in fetal CO after the first hypoxia. However, the 10-minute mean percentage changes in recovery time in the caffeine and MSX-3 groups were less than those in the control group after the second and third exposures to hypoxia (Figure 4). In the CPT, selective adenosine A1 receptor antagonist, group, the decrease in fetal heart rate and stroke volume were less than those in the control group (Figure 3A and 3B); as a result, the drop in fetal CO in the CPT group was significantly less than that in the control group (Figure 3C). Mean percentage decrease in fetal CO of CPT treated group 10 minutes after the first hypoxia was less than the control group.(Figure 5). Control and CPT groups did not change the fetal CO from baseline after 2nd and 3rd hypoxia exposure, whereas the fetal CO of caffeine and MSX3 groups remained decreased (Figure 5).
Figure 3. Fetal hemodynamic effects of repeated maternal hypoxia with adenosine receptor antagonists.
Dams were repeatedly exposed to 100% nitrogen for 45 seconds followed by a 10 to 20 minute recovery period.(A): Fetal heart rate, (B): Fetal stroke volume, (C): Fetal cardiac output.
Figure 4. Fetal dorsal aortic flow recovery time to baseline following hypoxia, caffeine, and Adenosine receptor antagonist treatments.
Recovery time was defined as the time from the start of hypoxia to the point in time when fetal dorsal aortic flow recovered to the baseline. If no restoration to the baseline occurred, the recovery time was defined as 10 minutes.
Figure 5. Mean percentage changes in fetal cardiac output following the onset of hypoxia.
The mean percentage change of the fetal cardiac output after the first hypoxia in the CPT group was higher than that in the control group. Control and CPT groups showed a similar trend of changes in fetal CO, while Caffeine and adenosine A2A antagonist, MSX-3, treated group did not recover the fetal CO to the baseline levels following 2nd and 3rd hypoxia exposure.
Discussion
Our previous studies in a mouse animal model showed that developing fetal CV function is vulnerable to transient maternal hypoxia (7), and a modest amount of maternal caffeine exposure has adverse effects on developing fetal CV function mediated by adenosine A2A receptor inhibition by caffeine (8). Maternal caffeine exposure induces variable reduction in regional fetal arterial blood flow (−46% for the internal carotid artery, −25% for the dorsal aorta, and −15% for the umbilical artery on GD 11.5) suggesting that regional changes in blood flow may be due to regional variations in vascular resistance (8). In the present study, we evaluated the effects of adenosine antagonists on the early fetal CV system during intermittent maternal hypoxia. It is speculated that the intermittent maternal hypoxia increases the adenosine concentration in the feto-placental circulation. Hence, it was expected that maternal hypoxia might trigger increased maternal and/or fetal adenosine to modulate the effects of hypoxia on fetal CV function. In the present study, an initial exposure to maternal hypoxia was shown to negatively impact fetal hemodynamics. However, repeated episodes of maternal hypoxia accelerated the recovery of fetal cardiac output to the baseline and resulted in overshoot beyond the baseline. The results of inhibition of A2A receptor, which blunted the recurrent hypoxia mediated recovery, suggest that adenosine is likely to exert favorable effects on the fetal CV system via A2A receptor activation (Figure 6A). Caffeine retarded the recovery of fetal dorsal aortic flow following hypoxia, similar to the effects of treatment with an A2A receptor antagonist (Figure 6B). Although caffeine is a non-selective competitive antagonist of adenosine receptors, the results of the present study suggested that caffeine has a negative effect on fetal CV function via A2A receptor inhibition during maternal hypoxia.
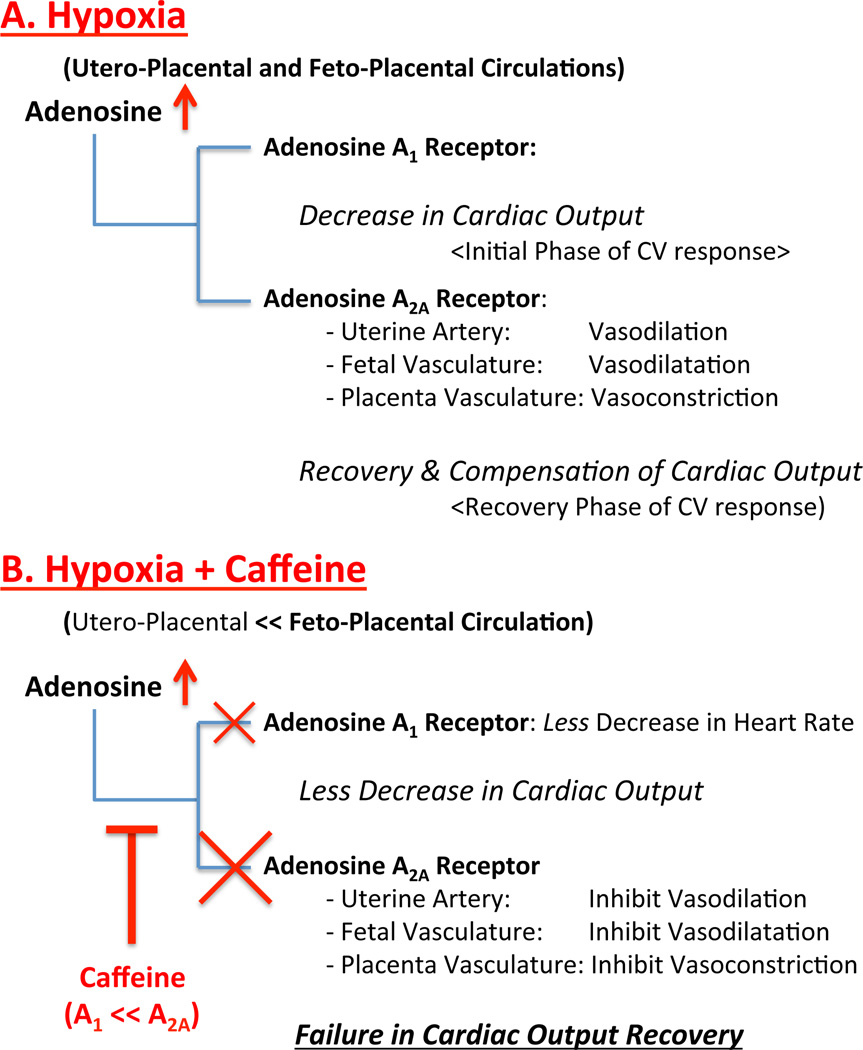
Figure 6. Schematic diagram of maternal hypoxia and caffeine exposure effects on utreoplacental and fetoplacental circulations.
Maternal hypoxia increases adenosine concentration within uteroplacental (maternal) and fetoplacental (fetal) circulations in which both adenosine A1 and A2A receptors are activated (panel A). Adenosine A1 receptor activation decreases maternal and fetal heart rate and cardiac output (initial hypoxia response), and A2A receptor activation increases uterine and fetal arterial blood flow by vasodilation with relatively decreased placenta vascular flow (vasoconstriction) resulting in fetal cardiac output recovery (recovery phase of hypoxia). Caffeine, a non-selective adenosine antagonist, inhibits adenosine receptor activation. While initial decrease in cardiac output of utero- and feto-placental circulations by combined hypoxia and caffeine exposure is less than hypoxia only, inhibition of adenosine A2A receptor by caffeine leads to decrease in uterine and fetal arterial blood flow (inhibition of vasodilation) and blood flow steal to the placental vasculature (blood flow redistribution within fetoplacental circulation by placental vasodilation,) resulting in failure in cardiac output recovery of fetoplacental circulation (panel B).
We have previously shown that adenosine A1 receptor inhibition showed no effect on fetal CV function without hypoxic stress (8). However, in the present study, A1 receptor inhibition significantly prevented the decrease in fetal cardiac output induced by maternal hypoxia. This difference may be due to the action of adenosine A1 receptor inhibition on the maternal response to hypoxia, and the effects of A1 receptor inhibition on fetal heart rate without hypoxia were in agreement with those reported previously (25, 27). Our results of CPT treatment suggest that adenosine A1 receptor antagonists might be of use in cases of fetal distress in the setting of maternal hypoxia and caffeine exposure. However, adenosine functions as a counterregulatory hormone in the myocardium by decreasing work and thereby protecting the myocardium from ischemia (31). Further studies are required to confirm the possible efficacy of A1 receptor antagonists in fetal distress.
Previous studies showed that adenosine A1 and A2A receptors are present and regulates fetal CV function from early organogenesis period (8, 25), and the caffeine and CPT (and MSX-3 may be) can cross the placenta (32). However, it remains to be investigated whether maternal hypoxia and caffeine exposure effects on developing fetal CV function are mediated by adenosine receptors in the maternal utero-placental circulation and/or the feto-placental circulation, and further studies are warranted to define the e underlying mechanisms by which maternal hypoxia and caffeine exposure effect developing fetal CV function and growth.
In conclusion, we demonstrated the in vivo response of the early developing fetus to combined maternal hypoxia and caffeine exposure and our results suggest that caffeine exposure affects fetal CV response to the acute maternal hypoxia via adenosine A2A receptor inhibition during the critical period of primary CV morphogenesis.
Acknowledgements
This study was supported by NIH RFA-R01 H065129 (BBK).
Abbreviations
- CV
cardiovascular
- GD
gestational day
- CPT
8-cyclopentyl-1, 3-dimethylxanthine
- MSX-3
3-(3-hydroxypropyl)-8-(m-methoxystyryl)-7-methyl-1-propargylxanthine phosphate disodium salt
- CO
cardiac output
Footnotes
Disclosure
There is no financial conflict of interest related to the current research.
Contributor Information
Nobuo Momoi, Email: momo@fmu.ac.jp, Cardiovascular Development Research Program, Children’s Hospital of Pittsburgh of University of Pittsburgh Medical Center./ Department of Pediatrics, Fukushima Medical University (current affiliation).
Joseph P. Tinney, Email: joseph.tinney@louisville.edu, Cardiovascular Innovation Institute, Department of Pediatrics, University of Louisville.
Bradley B. Keller, Email: brad.keller@louisville.edu, Cardiovascular Innovation Institute, Department of Pediatrics, University of Louisville.
Kimimasa Tobita, Email: kit3@pitt.edu, Cardiovascular Development Research Program, Children’s Hospital of Pittsburgh of University of Pittsburgh Medical Center, Department of Developmental Biology, School of Medicine, University of Pittsburgh.
References
- 1.Sadler TW. Susceptible periods during embryogenesis of the heart and endocrine glands. Environmental health perspectives. 2000;108(Suppl 3):555–561. doi: 10.1289/ehp.00108s3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keller BB. Function and biomechanics of developing cardiovascular systems. Formation of the Heart and Its Regulation. 2001 [Google Scholar]
- 3.Tobita KTJ, Keller BB. Cardiovascular Phenotype Analysis of Murine Embryos. In: HBaw RA, editor. Cardiovascular Physiology in the Genetically Engineered Mouse. 2nd Edition. New York: Kluwer Academic Publishers; 2002. pp. 353–376. [Google Scholar]
- 4.Morrison JL. Sheep models of intrauterine growth restriction: fetal adaptations and consequences. Clinical and experimental pharmacology & physiology. 2008;35(7):730–743. doi: 10.1111/j.1440-1681.2008.04975.x. [DOI] [PubMed] [Google Scholar]
- 5.Rees S, Harding R, Walker D. An adverse intrauterine environment: implications for injury and altered development of the brain. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 2008;26(1):3–11. doi: 10.1016/j.ijdevneu.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 6.Martin CB., Jr Normal fetal physiology and behavior, and adaptive responses with hypoxemia. Seminars in perinatology. 2008;32(4):239–242. doi: 10.1053/j.semperi.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Furukawa S, Tinney JP, Tobita K, Keller BB. Hemodynamic vulnerability to acute hypoxia in day 10.5–16.5 murine embryos. The journal of obstetrics and gynaecology research. 2007;33(2):114–127. doi: 10.1111/j.1447-0756.2007.00499.x. [DOI] [PubMed] [Google Scholar]
- 8.Momoi N, Tinney JP, Liu LJ, Elshershari H, Hoffmann PJ, Ralphe JC, et al. Modest maternal caffeine exposure affects developing embryonic cardiovascular function and growth. American journal of physiology. Heart and circulatory physiology. 2008;294(5):H2248–H2256. doi: 10.1152/ajpheart.91469.2007. [DOI] [PubMed] [Google Scholar]
- 9.Fredholm BB. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell death and differentiation. 2007;14(7):1315–1323. doi: 10.1038/sj.cdd.4402132. [DOI] [PubMed] [Google Scholar]
- 10.Koos BJ, Doany W. Role of plasma adenosine in breathing responses to hypoxia in fetal sheep. Journal of developmental physiology. 1991;16(2):81–85. [PubMed] [Google Scholar]
- 11.Yoneyama Y, Wakatsuki M, Sawa R, Kamoi S, Takahashi H, Shin S, et al. Plasma adenosine concentration in appropriate- and small-for-gestational-age fetuses. American journal of obstetrics and gynecology. 1994;170(2):684–688. doi: 10.1016/s0002-9378(94)70248-9. [DOI] [PubMed] [Google Scholar]
- 12.Slegel P, Kitagawa H, Maguire MH. Determination of adenosine in fetal perfusates of human placental cotyledons using fluorescence derivatization and reversed-phase high-performance liquid chromatography. Analytical biochemistry. 1988;171(1):124–134. doi: 10.1016/0003-2697(88)90132-7. [DOI] [PubMed] [Google Scholar]
- 13.Yoneyama Y, Sawa R, Suzuki S, Shin S, Power GG, Araki T. The relationship between uterine artery Doppler velocimetry and umbilical venous adenosine levels in pregnancies complicated by preeclampsia. American journal of obstetrics and gynecology. 1996;174(1 Pt 1):267–271. doi: 10.1016/s0002-9378(96)70406-4. [DOI] [PubMed] [Google Scholar]
- 14.Fredholm BB. Astra Award Lecture. Adenosine, adenosine receptors and the actions of caffeine. Pharmacology & toxicology. 1995;76(2):93–101. doi: 10.1111/j.1600-0773.1995.tb00111.x. [DOI] [PubMed] [Google Scholar]
- 15.Fredholm BB, Battig K, Holmen J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacological reviews. 1999;51(1):83–133. [PubMed] [Google Scholar]
- 16.Shryock JC, Belardinelli L. Adenosine and adenosine receptors in the cardiovascular system: biochemistry, physiology, and pharmacology. The American journal of cardiology. 1997;79(12A):2–10. doi: 10.1016/s0002-9149(97)00256-7. [DOI] [PubMed] [Google Scholar]
- 17.Tabrizchi R, Bedi S. Pharmacology of adenosine receptors in the vasculature. Pharmacology & therapeutics. 2001;91(2):133–147. doi: 10.1016/s0163-7258(01)00152-8. [DOI] [PubMed] [Google Scholar]
- 18.Janier MF, Vanoverschelde JL, Bergmann SR. Adenosine protects ischemic and reperfused myocardium by receptor-mediated mechanisms. The American journal of physiology. 1993;264(1 Pt 2):H163–H170. doi: 10.1152/ajpheart.1993.264.1.H163. [DOI] [PubMed] [Google Scholar]
- 19.Olsson RA, Pearson JD. Cardiovascular purinoceptors. Physiological reviews. 1990;70(3):761–845. doi: 10.1152/physrev.1990.70.3.761. [DOI] [PubMed] [Google Scholar]
- 20.Law WR, Raymond RM. Adenosine potentiates insulin-stimulated myocardial glucose uptake in vivo. The American journal of physiology. 1988;254(5 Pt 2):H970–H975. doi: 10.1152/ajpheart.1988.254.5.H970. [DOI] [PubMed] [Google Scholar]
- 21.Read MA, Boura AL, Walters WA. Vascular actions of purines in the foetal circulation of the human placenta. British journal of pharmacology. 1993;110(1):454–460. doi: 10.1111/j.1476-5381.1993.tb13832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sim MK, Maguire MH. Presence of adenosine in the human term placenta. Determination of adenosine content and pathways of adenosine metabolism. Circulation research. 1972;31(5):779–788. doi: 10.1161/01.res.31.5.779. [DOI] [PubMed] [Google Scholar]
- 23.Kubonoya K, Power GG. Plasma adenosine responses during repeated episodes of umbilical cord occlusion. American journal of obstetrics and gynecology. 1997;177(2):395–401. doi: 10.1016/s0002-9378(97)70204-7. [DOI] [PubMed] [Google Scholar]
- 24.Xu D, Kong HY, Liang BT. Expression and pharmacological characterization of a stimulatory subtype of adenosine receptor in fetal chick ventricular myocytes. Circulation research. 1992;70(1):56–65. doi: 10.1161/01.res.70.1.56. [DOI] [PubMed] [Google Scholar]
- 25.Porter GA, Jr, Rivkees SA. Ontogeny of humoral heart rate regulation in the embryonic mouse. American journal of physiology. Regulatory, integrative and comparative physiology. 2001;281(2):R401–R407. doi: 10.1152/ajpregu.2001.281.2.R401. [DOI] [PubMed] [Google Scholar]
- 26.Rivkees SA, Zhao Z, Porter G, Turner C. Influences of adenosine on the fetus and newborn. Molecular genetics and metabolism. 2001;74(1–2):160–171. doi: 10.1006/mgme.2001.3217. [DOI] [PubMed] [Google Scholar]
- 27.Hofman PL, Hiatt K, Yoder MC, Rivkees SA. A1 adenosine receptors potently regulate heart rate in mammalian embryos. The American journal of physiology. 1997;273(4 Pt: 1):R1374–R1380. doi: 10.1152/ajpregu.1997.273.4.R1374. [DOI] [PubMed] [Google Scholar]
- 28.Maternal caffeine intake during pregnancy and risk of fetal growth restriction: a large prospective observational study. BMJ. 2008;337:a2332. doi: 10.1136/bmj.a2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weng X, Odouli R, Li DK. Maternal caffeine consumption during pregnancy and the risk of miscarriage: a prospective cohort study. American journal of obstetrics and gynecology. 2008;198(3):279, e1–e8. doi: 10.1016/j.ajog.2007.10.803. [DOI] [PubMed] [Google Scholar]
- 30.Schindler CW, Karcz-Kubicha M, Thorndike EB, Muller CE, Tella SR, Ferre S, et al. Role of central and peripheral adenosine receptors in the cardiovascular responses to intraperitoneal injections of adenosine A1 and A2A subtype receptor agonists. British journal of pharmacology. 2005;144(5):642–650. doi: 10.1038/sj.bjp.0706043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cothran DL, Lloyd TR, Taylor H, Linden J, Matherne GP. Ontogeny of rat myocardial A1 adenosine receptors. Biology of the neonate. 1995;68(2):111–118. doi: 10.1159/000244226. [DOI] [PubMed] [Google Scholar]
- 32.Watson CS, White SE, Homan JH, Fraher L, Brien JF, Bocking AD. The adenosine A(1)-receptor antagonist 8-CPT reverses ethanol-induced inhibition of fetal breathing movements. Journal of applied physiology. 1999;87(4):1333–1338. doi: 10.1152/jappl.1999.87.4.1333. [DOI] [PubMed] [Google Scholar]