Abstract
Glutamate-induced delayed calcium dysregulation (DCD) is causally linked to excitotoxic neuronal death. The mechanisms of DCD are not completely understood, but it has been proposed that the excessive influx of external Ca2+ is essential for DCD. The NMDA-subtype of glutamate receptor (NMDAR) and the plasmalemmal Na+/Ca2+ exchanger operating in the reverse mode (NCXrev) have been implicated in DCD. In experiments with “younger” neurons, 6-8 days in vitro (6-8 DIV), in which the NR2A-containing NMDAR expression is low, ifenprodil, an inhibitor of NR2B-containing NMDAR, completely prevented DCD whereas PEAQX, another NMDAR antagonist that preferentially interacts with NR2A-NMDAR, was without effect. With “older” neurons (13-16 DIV), in which NR2A- and NR2B-NMDARs are expressed to a greater extent, both ifenprodil and PEAQX applied separately failed to prevent DCD. However, combined application of ifenprodil and PEAQX completely averted DCD. Ifenprodil and ifenprodil-like NR2B-NMDAR antagonists Ro 25-6981 and Co 101244 but not PEAQX or AP-5 inhibited gramicidin- and Na+/NMDG-replacement-induced increases in cytosolic Ca2+ mediated predominantly by NCXrev. This suggests that ifenprodil, Ro 25-6981, and Co 101244 inhibit NCXrev. The ability of ifenprodil to inhibit NCXrev correlates with its efficacy in preventing DCD and emphasizes an important role of NCXrev in DCD. Overall our data suggest that both NR2A- and NR2B-NMDARs are involved in DCD in “older” neurons, and it is necessary to inhibit both NMDARs and NCXrev to prevent glutamate-induced DCD.
Keywords: neuron, glutamate, calcium dysregulation; NMDA receptor; Na+/Ca2+ exchanger
1. INTRODUCTION
Glutamate excitotoxicity is a key component in a variety of neuropathologies including stroke, traumatic brain injury, and age-related neurodegenerations (Bramlett and Dietrich, 2004; Hazell, 2007; Salinska et al., 2005). A sustained increase in cytosolic Ca2+ concentration ([Ca2+]c), or delayed Ca2+ dysregulation (DCD)1, represents a major detrimental factor in glutamate excitotoxicity (Nicholls and Budd, 1998; Tymianski et al., 1993a). Two major hypotheses concerning the mechanism of DCD postulate that in neurons exposed to glutamate an increase in [Ca2+]c occurs predominantly due to influx of external Ca2+ via activated N-methyl-D-aspartate (NMDA)-subtype of glutamate receptors (NMDAR) (Tymianski et al., 1993b) or via the reverse Na+/Ca2+ exchanger (NCXrev) (Hoyt et al., 1998; Kiedrowski, 1999). Significant efforts were aimed at developing neuroprotection based on NMDAR and NCXrev antagonists (Scatton, 1994). However, earlier studies with high-affinity NMDAR antagonists such as MK801 revealed serious problems associated with strong inhibition of vitally important glutamate neurotransmission leading to increased likelihood of neurodegenerations (Ikonomidou et al., 1999; Lipton, 2004). In contrast, the lack of selective and efficacious inhibitors of neuronal NCXrev significantly hindered development of neuroprotective strategies based solely on NCXrev inhibition (Jeffs et al., 2007).
NMDARs are formed by heteromeric complexes consisting of NR1 and NR2 subunits (McBain and Mayer, 1994). The NR1 subunit is abundantly expressed in CNS (Janssens and Lesage, 2001). The NR2 subunit that contains the glutamate binding site has four different isoforms, encoded by different genes NR2A-NR2D (Cull-Candy et al., 2001). Ifenprodil was found to be the first neuroprotective agent selective for NR2B-containing NMDARs (NR2B-NMDARs) (Carter et al., 1988; Carter et al., 1989; Williams, 1993). Importantly, ifenprodil increases the potency of protons to block the NMDAR (Mott et al., 1998) and protects neurons against glutamate excitotoxicity in an activity-dependent manner (Kew et al., 1996). This mechanism was proposed to significantly contribute to ifenprodil efficacy and the lack of unwanted side effects of this drug (Scatton, 1994).
In our previous study, we found that both NMDAR and NCXrev contribute to DCD in neurons exposed to glutamate and, consequently, both Ca2+ influx mechanisms have to be inhibited to prevent DCD (Brittain et al., 2012). Ifenprodil inhibits DCD in younger neurons exposed to glutamate (Stanika et al., 2009). This effect was attributed to ifenprodil-mediated inhibition of NR2B-NMDAR. However, whether ifenprodil inhibits NCXrev is unknown. In the present study, we hypothesized that ifenprodil as well as ifenprodil-like NR2B-selective NMDAR antagonists Ro 25-6981 and Co 101244, in addition to antagonizing NR2B-NMDAR, also inhibit NCXrev. The obtained results support this hypothesis and suggest that ifenprodil, Ro 25-6981, and Co 101244 suppress NCXrev activity.
2. MATERIALS AND METHODS
All animal experiments were carried out in accordance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978). All efforts were made to minimize animal suffering, to reduce the number of animals used, and to utilize alternatives to in vivo techniques, if available.
2-1. Materials
Glutamate, glycine, and gramicidin were purchased from Sigma (St. Louis, MO). Fura-2FF-AM and Fura-2-AM were from Teflabs (Austin, TX). Fluo-4FF-AM and SBFI-AM were from Invitrogen (Carlsbad, CA). Ifenprodil and PEAQX were from Sigma. Ro 25-6981 and Co 101244 were from Santa Cruz Biotechnology (Santa Cruz, CA).
2-2. Cell culturing
Primary cultures of hippocampal neurons were prepared from postnatal day 1 rat pups, according to Institutional Animal Care and Use Committee (IACUC) approved protocol. For fluorescence measurements, neurons were plated on glass-bottomed Petri dishes without preplated glia as previously described (Dubinsky, 1993). For all platings, 35μg/ml uridine plus 15μg/ml 5-fluoro-2′-deoxyuridine were added 24 hours after plating to inhibit proliferation of non-neuronal cells. Neuronal cultures were maintained in a 5% CO2 atmosphere at 37°C in Earl's MEM supplemented with 10% NuSerum (BD Bioscience, Bedford, MA), 27 mM glucose, and 26 mM NaHCO3 (Dubinsky et al., 1995).
2-3. Fluorescence imaging
In our experiments, we used “younger” hippocampal neurons grown for 6-8 days in vitro (6-8 DIV) and “older” neurons grown for 13-16 DIV. The standard bath solution contained 139 mM NaCl, 3 mM KCl, 0.8 mM MgCl2, 1.8 mM CaCl2, 10 mM NaHEPES, pH 7.4, 5 mM glucose, and 65 mM sucrose. Sucrose was used to maintain osmolarity similar to that in the growth medium (340 mosm)(Wang and Thayer, 1996; White and Reynolds, 1996). Fluorescence imaging was performed with a Nikon Eclipse TE2000-U inverted microscope using a Nikon objectives Plan Fluor 20× 0.45 NA or Super Fluor 40× 1.3 NA and an EM-CCD Hamamatsu C9100-12 camera (Hamamatsu Photonic Systems, Bridgewater, NJ) controlled by Simple PCI software 6.1 (Compix Inc., Sewickley, PA) or Photometrics Cool SNAPHQ camera (Roper Scientific, Tucson, AZ) controlled by MetaFluor software 6.3 (Molecular Devices, Downingtown, PA). The excitation light was delivered by a Lambda-LS system (Sutter Instruments, Novato, CA). To minimize photobleaching and phototoxicity, the images were taken every 15 seconds during the time-course of the experiment.
For fluorescence microscopy experiments, neurons were loaded with either 2.6μM Fura-2AM (Figures 1, 2 and Supplemental Figures 1, 2) or 2.6μM Fura-2FF-AM (Figures 4-7 and Supplemental Figure 5) for 60 minutes at 37°C in the presence of 0.015% Pluronic F-127. The excitation filters (340±5 and 380±7 nm) were controlled by a Lambda 10-2 optical filter changer (Sutter Instruments, Novato, CA). Fluorescence was recorded from individual neurons through a 505 nm dichroic mirror at 535±25 nm. The changes in [Ca2+]c were monitored by following Fura-2 or Fura-2FF F340/F380 ratio. Alternatively, the changes in [Ca2+]c were monitored simultaneously with changes in [Na+]c using a Ca2+-sensitive fluorescent dye Fluo-4FF-AM and a Na+-sensitive dye SBFI-AM (Supplemental Figure 4). Neurons were loaded simultaneously with 2.5μM Fluo-4FF-AM for 30 minutes and 9μM SBFI-AM for 1 hour at 37°C. The excitation wavelengths were 340±5 and 380±7 nm for SBFI and 480±20 nm for Fluo-4FF. Fluorescence was recorded from individual neurons through a 505 nm dichroic mirror at 535±25 nm. The changes in [Na+]c were monitored by following SBFI F340/F380 ratio. The changes in [Ca2+]c were monitored by following Fluo-4FF F480 and normalized as F/F0. Quantification of Fluo-4FF signals was carried out following manufacturer instructions (http://probes.invitrogen.com/media/pis/mp01240.pdf). [Ca2+]c and [Na+]c were calculated using Grynkiewicz method (Grynkiewicz et al., 1985) assuming Kd for Fura-2 is 0.225μm, for Fura-2FF is 5.5μM, for Fluo-4FF is 9.7μM, and for SBFI is 11.3 mM. In all experiments, the background was subtracted from fluorescence signals. Since Ca2+ binding and spectroscopic properties of fluorescent dyes can vary significantly in intracellular environment, the presented cytosolic Ca2+ concentrations should be considered estimates as stated previously by other investigators (Dietz et al., 2007; Stanika et al., 2009).
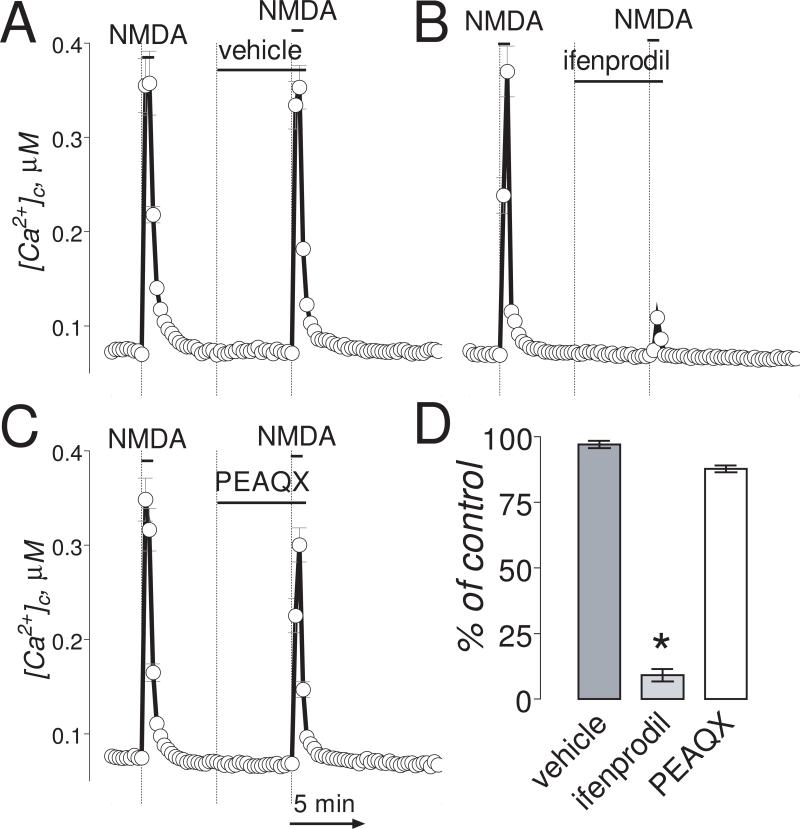
Figure 1. In “younger” neurons (6-8 DIV), ifenprodil completely inhibited Ca2+ influx induced by NMDA.
The bath solution was supplemented with 1μM tetrodotoxin and 5μM nifedipine. Neurons were loaded with 2.6μM Fura-2AM. In these experiments, we used two 30-second NMDA (30μM, plus 10μM glycine) pulses. The inhibitors or vehicle were applied 5 minutes before the second NMDA pulse and amplitude of [Ca2+]c increase was compared to [Ca2+]c increase in response to the first NMDA pulse. In A-C, where indicated, vehicle (0.2% DMSO), ifenprodil (1μM) or PEAQX (5μM) were applied. NMDA (30μM, plus 10μM glycine) was applied twice for 30 seconds as indicated. The Ca2+ influx into neurons was evaluated by measuring amplitude of the increases in [Ca2+]c. [Ca2+]c was calculated using the Grynkiewicz method (Grynkiewicz et al., 1985). The time scale shown in panel C is applicable to traces in A and B. In D, statistical analysis of the Ca2+ influx inhibition. Data are mean±SEM, *p<0.01 compared to vehicle, n=3.
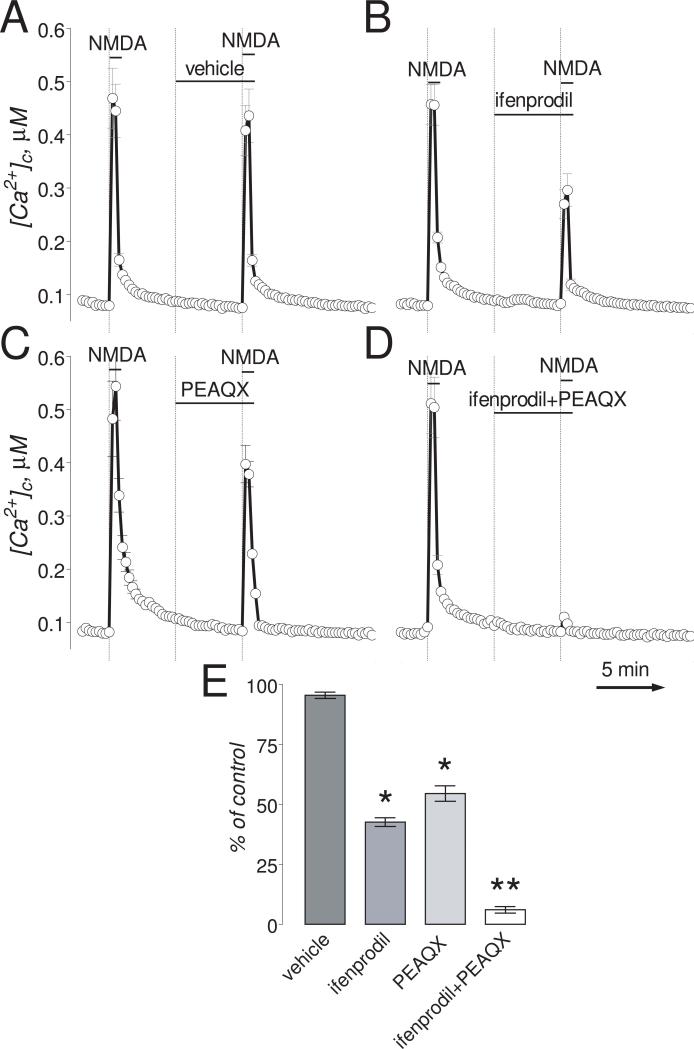
Figure 2. In “older” neurons (13-16 DIV), ifenprodil and PEAQX partially inhibited Ca2+ influx induced by NMDA. Combined application of ifenprodil and PEAQX completely blocked the [Ca2+]c increase.
The bath solution was supplemented with 1μM tetrodotoxin and 5μM nifedipine. Neurons were loaded with 2.6μM Fura-2AM. Where indicated, (A.) vehicle (0.2% DMSO), ifenprodil (50μM), PEAQX (5μM), or the combination of both ifenprodil (1μM) and PEAQX (0.1μM). NMDA (30μM, plus 10μM glycine) was applied twice for 30 seconds as indicated. The Ca2+ influx into neurons was evaluated by measuring amplitude of the increases in [Ca2+]c. [Ca2+]c was calculated using the Grynkiewicz method (Grynkiewicz et al., 1985). The time scale shown in panel D is applicable to traces in A-C. In E, statistical analysis of the Ca2+ influx inhibition. Data are mean±SEM, *p<0.05, **p<0.01 compared to vehicle, n=3.
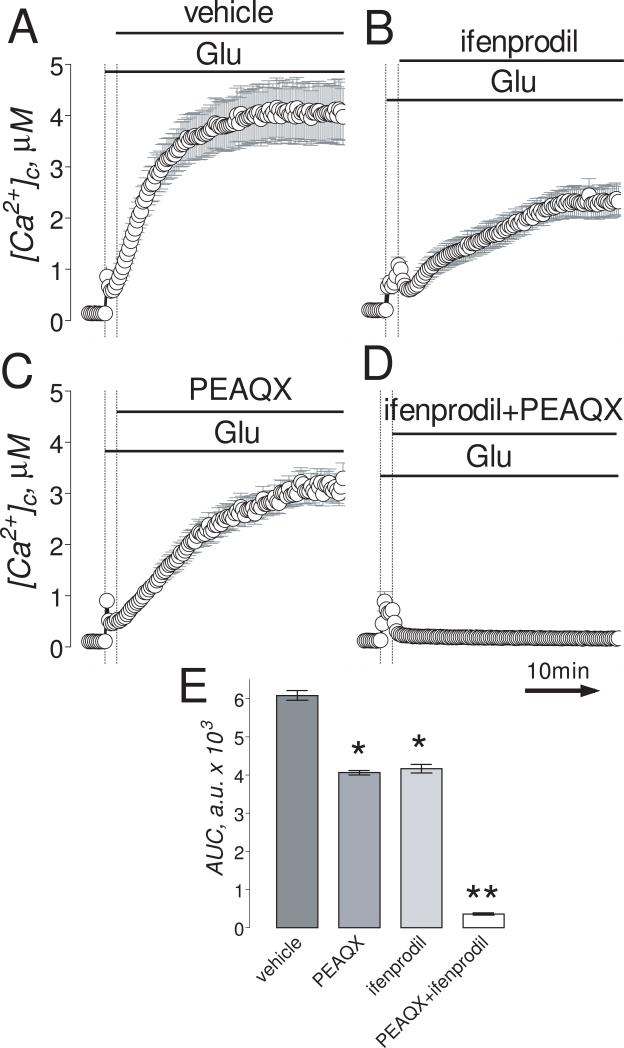
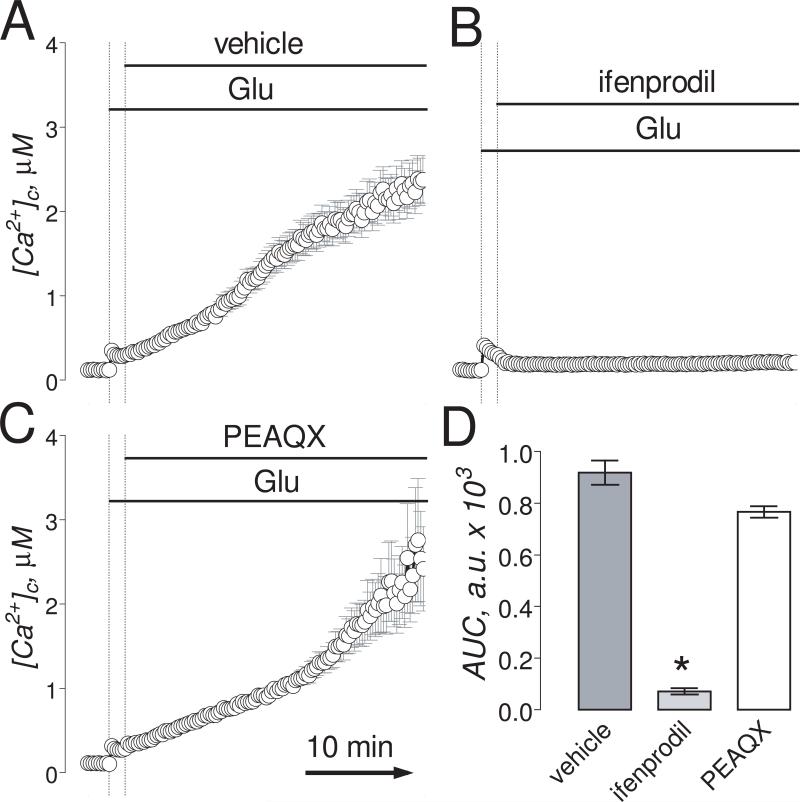
Figure 4. In “older” neurons (13-16 DIV), the combination of ifenprodil and PEAQX but not individual inhibitors applied separately completely prevented glutamate-induced sustained elevation in [Ca2+]c.
Neurons were loaded with 2.6μM Fura-2FF-AM. In A-D, neurons were exposed to 25μM glutamate (Glu, plus 10μM glycine). Where indicated, vehicle (0.2% DMSO), 50μM ifenprodil or 5μM PEAQX, or a combination of 1μM ifenprodil and 0.1μM PEAQX were applied. The time scale shown in panel D is applicable to traces in A-C. In E, statistical analysis of glutamate-induced [Ca2+]c changes over time. Data are mean±SEM, *p<0.05, **p<0.01 compared to vehicle, n=3.
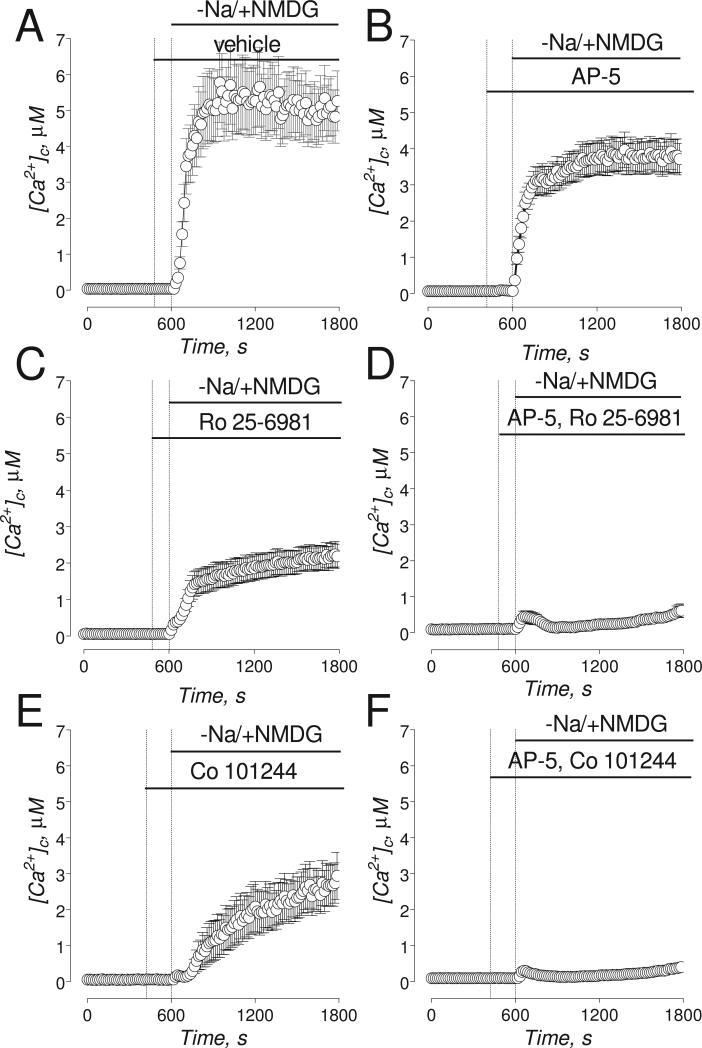
Figure 7. The effects of AP-5, Ro 25-6981, and Co 101244 on Na+/NMDG-induced increases in [Ca2+]c.
The experiments illustrated in this Figure were performed with “older” (13-16 DIV) neurons. Neurons were loaded with 2.6μM Fura-2FF-AM. In A-F, where indicated, NaCl in the bath solution was substituted for equimolar NMDG and neurons were treated with a vehicle of different NMDAR antagonists. In A, Na+/NMDG-induced increase in [Ca2+]c in neurons treated with a vehicle (0.2% DMSO). In B-F, where indicated, neurons were treated with 20μM AP-5 (B); 0.5μM Ro 25-6981 (C), a combination of 0.5μM Ro 25-6981 and 20μM AP-5 (D); 5μM Co 101244 (E); a combination of 5μM Co 101244 and 20μM AP-5 (F). In all experiments, the bath solution was supplemented with 1μM tetrodotoxin, 5μM nifedipine, and 1 mM ouabain.
2-4. Western Blot
Cultured hippocampal neurons were washed with PBS and lysed with a solution containing 50 mM Tris-HCl, pH 7.35, 2 mM EDTA, 5 mM dithiothreitol, and 1% Nonidet P-40, and supplemented with a Proteinase Inhibitor Cocktail (Roche, Indianapolis, IN). The lysate was centrifuged in Eppendorf microcentrifuge 5415D at 13,000 rpm for 10 minutes and total protein was determined in the supernatant using Bradford assay (Bio-Red Laboratories, Hercules, CA). Aliquots of this solution 30μg/line were loaded onto 3-8% Tris-Acetate gel (Invitrogen, Carlsbad, CA). Electrophoresis and protein transfer onto Hybond™-ECL™ nitrocellulose membrane (Amersham Biosciences, Piscataway, NJ) was performed as described for the NuPage electrophoresis system (Invitrogen). The membranes were blocked with 5% BSA and 0.15% Triton X-100 in phosphate-buffered saline (PBS), pH 7.0, incubated for an hour at room temperature with one of the following primary antibodies: anti-NR2B rabbit polyclonal antibody or anti-NR2A rabbit polyclonal antibody (Millipore, Temecula, CA) at 1:2000 dilution. Blots were developed using goat anti-rabbit or goat anti-mouse IgG (1:20000) coupled with horseradish peroxidase (Jackson ImmunoResearch Laboratories, West Grove, PA) and Supersignal West Pico chemiluminescent reagent (Pierce, Rockford, IL). Molecular weight marker HiMark™ Pre-Stained Standards (15μl, Invitrogen) were used to determine molecular weights of the bands. Anti-actin monoclonal antibody (Millipore) at 1:2000 dilution were used as a loading control.
2-5. Electrophysiological patch-clamp experiments
Whole-cell patch-clamp recordings were conducted as described previously (Convery and Hancox, 1999; Smith et al., 2006) with minor modifications. Briefly, patch-clamp recordings were conducted at room temperature using a HEKA EPC-10 amplifier. Data were acquired using the Pulse program (HEKA Electronic, Lambrecht/Pfalz, Germany). The composition of the electrode solution used for recording voltage ramp currents mediated by NCX was as follows: 25 mM NaCl, 120 mM K-Aspartate, 20 mM tetraethylammonium-Cl, 10 mM HEPES, 0.01 mM K-EGTA, 4.5 mM MgCl2, and 4 mM Na-ATP, pH 7.3 adjusted with KOH (Smith et al., 2006). The external solution used for recording currents was as follows: 129 mM NaCl, 10 mM CsCl (to block K+ channels), 3 mM KCl, 0.8 mM MgCl2, 1.8 mM CaCl2, 5 mM glucose, 10 mM Na-HEPES, pH 7.2, 35 mM sucrose, 0.005 mM nifedipine (to block voltage-gated Ca2+ channels), 0.02 mM ouabain (to inhibit Na+/K+-ATPase), 0.001 mM tetrodotoxin (to block Na+ channels). A perfusion Fast-Step system (Warner Instruments, LLC, Hamden, CT) was used to deliver drugs focally onto isolated hippocampal neurons in the whole-cell configuration. All drugs were diluted in the bath solution. The bath solution across the cells was perfused at approximately 1 ml/min using gravity flow.
2-6. Toxicity experiments
Untreated neurons and neurons treated with 1μM ifenprodil were incubated with 5μM nifedipine, 1μM tetrodotoxin, and 1 mM ouabain for 10 minutes prior to Na+/NMDG replacement and then for 15 minutes following Na+/NMDG replacement. After that, cells were returned to the original Na+-containing bath solution without ouabain. Ifenprodil (1μM) was present in the bath solutions throughout the experiment. The cells were placed into the incubator at 37°C for 6 hours. After 6 hours, cell death was determined by Trypan Blue exclusion method by counting unstained (alive) and stained (dead) cells (~50 cells per field, two fields per dish, three dishes per condition) (Li et al., 2009). The experiments were performed in triplicate.
2-7. Statistics
Statistical analysis consisted of unpaired t-test or one-way ANOVAs followed by Bonferroni's post hoc test (GraphPad Prism® 4.0, GraphPad Software Inc., San Diego, CA). Every experiment was performed using at least three separate neuronal platings. The data are mean ± SEM of at least 3 independent experiments.
3. RESULTS
The NMDAR is one of the major Ca2+ influx pathways contributing to DCD in neurons exposed to glutamate (Tymianski et al., 1993b). Indeed, in “younger” neurons (6-8 DIV), ifenprodil (1μM) completely inhibited the increase in [Ca2+]c induced by NMDA (Fig. 1A,B) with an IC50=0.11±0.07μM (Suppl. Fig. 1A,B). PEAQX (5μM), another NMDAR antagonist that preferentially antagonizes NR2A-containing NMDARs (Auberson et al., 2002; Feng et al., 2004), failed to inhibit the NMDA-induced increase in [Ca2+]c (Fig. 1C,D and Suppl. Fig. 1C,D). In “older” neurons (13-16 DIV), ifenprodil (50μM) and PEAQX (5μM) applied separately only partly (45-55%) inhibited NMDA-induced increases in [Ca2+]c (Fig. 2A-C) with IC50=0.29±0.14μM and 0.13±0.04μM, respectively (Suppl. Fig. 2).
If applied simultaneously, even at lower concentrations, ifenprodil (1μM) and PEAQX (0.1μM) practically completely inhibited NMDA-induced increases in [Ca2+]c (Fig. 2D). This peculiar inhibition of the NMDA effect correlated with the expression of NR2A and NR2B subunits in “younger” and “older” neurons used in our experiments. Consistent with previously reported results (Stanika et al., 2009), expression of NR2B in younger “neurons” was much higher compared to NR2A (Suppl. Fig. 3). In addition, in “younger” neurons, expression of NR2A was much lower compared to “older” neurons, consistent with earlier published data (Brewer et al., 2007). This may explain why ifenprodil alone was effective in inhibiting the NMDA effect in “younger” neurons (Fig. 1A,B) and why both inhibitors, ifenprodil and PEAQX, were necessary to inhibit the NMDA effect in “older” neurons (Fig. 2D).
Consistent with the high efficacy in inhibiting NMDAR in “younger” neurons, ifenprodil (1μM), added 90 seconds after glutamate (100μM, plus 10μM glycine), completely prevented glutamate-induced DCD (Fig. 3A,B,D). PEAQX (5μM), on the other hand, was ineffective (Fig. 3C,D). In “older” neurons, both ifenprodil (50μM) and PEAQX (5μM) applied separately slightly slowed down the increases in [Ca2+]c induced by 25μM glutamate (plus 10μM glycine) (Fig. 4A-C). However, applied together, even at 50-fold lower concentrations, ifenprodil (1μM) and PEAQX (0.1μM) completely prevented DCD in “older” neurons (Fig. 4D,E). These results suggest that inhibition of NMDAR is necessary and sufficient to prevent glutamate-induced DCD.
Figure 3. In “younger” neurons (6-8 DIV), ifenprodil but not PEAQX inhibited glutamate-induced sustained elevation in [Ca2+]c.
Neurons were loaded with 2.6μM Fura-2FF-A-M. In AC, neurons were exposed to 100μM glutamate (Glu, plus 10μM glycine). Where indicated, 1μM ifenprodil or 5μM PEAQX were applied. The time scale shown in panel C is applicable to traces in A and B. In D, statistical analysis of glutamate-induced [Ca2+]c changes over time. Here and in other figures, glutamate-induced changes in [Ca2+]c over time were analyzed by using the area under the curve (AUC) as it has been done previously (Chang et al., 2006). Data are mean±SEM, *p<0.01 compared to vehicle, n=3.
In our previous study, we found that both NMDAR and NCXrev significantly contributed to DCD in hippocampal neurons exposed to glutamate and that inhibition of only NMDAR or NCXrev was not sufficient to prevent DCD (Brittain et al., 2012). This in fact contradicts to our conclusion based on the results obtained with ifenprodil and PEAQX (see above). Based on the results of our previous study (Brittain et al., 2012), either ifenprodil, or PEAQX, or both should inhibit NCXrev in addition to NMDAR. To investigate the effect of ifenprodil and PEAQX on NCXrev, we induced NCX reversal using either gramicidin application (Newell et al., 2007) or Na+/NMDG replacement (Wu et al., 2008). In addition to ifenprodil and PEAQX, we tested ifenprodil-like NR2B-selective NMDAR antagonists Ro 25-6981 and Co 101244. In our previous study, we found that gramicidin depolarized the plasma membrane, induced the release of endogenous glutamate in the low micromolar range, and increased both cytosolic Na+ concentration ([Na+]c) and [Ca2+]c (Brittain et al., 2012). The Na+/NMDG replacement also led to a minuscule release of endogenous glutamate (1.8±0.3μM, n=6, versus 0.18±0.07μM before Na+/NMDG replacement, n=11, p<0.001). In addition, Na+/NMDG replacement decreased [Na+]c, and increased [Ca2+]c (Suppl. Fig. 4). The increase in [Ca2+]c induced by Na+/NMDG replacement depended on elevation of [Na+]c induced by pre-incubation with ouabain (1 mM) consistent with the reported earlier requirement for ouabain pre-treatment to reverse NCX by Na+/NMDG replacement in cultured cortical neurons (Wu et al., 2008). Without ouabain, Na+/NMDG replacement did not produce an increase in [Ca2+]c (not shown).
Similar to experiments with gramicidin (Brittain et al., 2012), glutamate-pyruvate transaminase (GPT, 25μg/ml) prevented the increase in external glutamate concentration (0.16±0.05μM, n=3, compared to 1.8±0.3μM glutamate released after Na+/NMDG replacement without GPT, n=6, p<0.001), but failed to prevent the increase in [Ca2+]c (Suppl. Fig. 5) suggesting that although the released endogenous glutamate might contribute to the [Ca2+]c increase, it does not play a major role. In calcium imaging experiments, we found that in contrast to AP-5 (Brittain et al., 2012), ifenprodil prevented both gramicidin- and Na+/NMDG-induced increases in [Ca2+]c (Fig. 5A,B and E,F) suggesting that in addition to NR2B-NMDARs ifenprodil inhibited NCXrev.
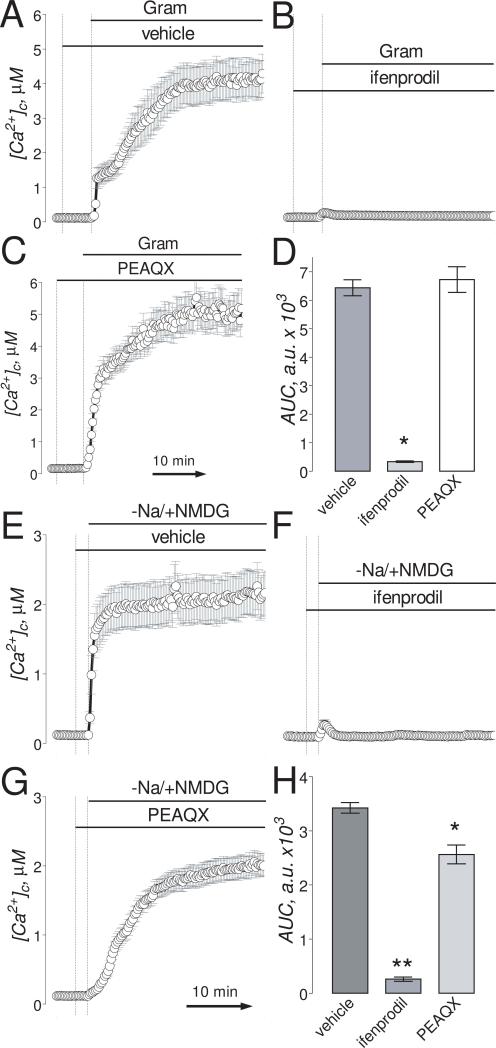
Figure 5. Ifenprodil but not PEAQX inhibited the increases in [Ca2+]c mediated by reversal of Na+/Ca2+ exchanger triggered by gramicidin or by Na+/NMDG replacement.
The experiments illustrated in this Figure were performed with “older” (13-16 DIV) neurons. Neurons were loaded with 2.6μM Fura-2FF-AM. In A-C, where indicated, neurons were treated with 5μM gramicidin (Gram), 1μM ifenprodil, or 5μM PEAQX. In E-G, where indicated, NaCl in the bath solution was substituted for equimolar NMDG and neurons were treated with 1μM ifenprodil, or 5μM PEAQX. In all experiments, the bath solution was supplemented with 1μM tetrodotoxin, 5μM nifedipine, and 1 mM ouabain. The time scale shown in panel C is applicable to traces in A and B. The time scale shown in panel G is applicable to traces in E and F. In D, statistical analysis of gramicidin-induced [Ca2+]c changes over time. Data are mean±SEM, *p<0.01 compared to vehicle, n=3. In H, statistical analysis of Na+/NMDG-induced [Ca2+]c changes over time. Data are mean±SEM, *p<0.05, **p<0.01 compared to vehicle (0.2% DMSO), n=3.
We also determined the extent of neuronal death in response to Na+/NMDG replacement and evaluated neuroprotection afforded by ifenprodil. We used protocol similar to that employed in calcium imaging experiments (Fig. 5E,F). Na+/NMDG replacement resulted in 59.5±1.4% of neurons dying within 6 hours (Suppl. Fig. 6). Ifenprodil (1μM) protected neurons and decreased neuronal death rate to 26±2.7% that was close to death rate of neurons treated for 15 minutes with a vehicle (0.2% DMSO), 5μM nifedipine, 1μM TTX, and 1 mM ouabain (20.0±2.3%).
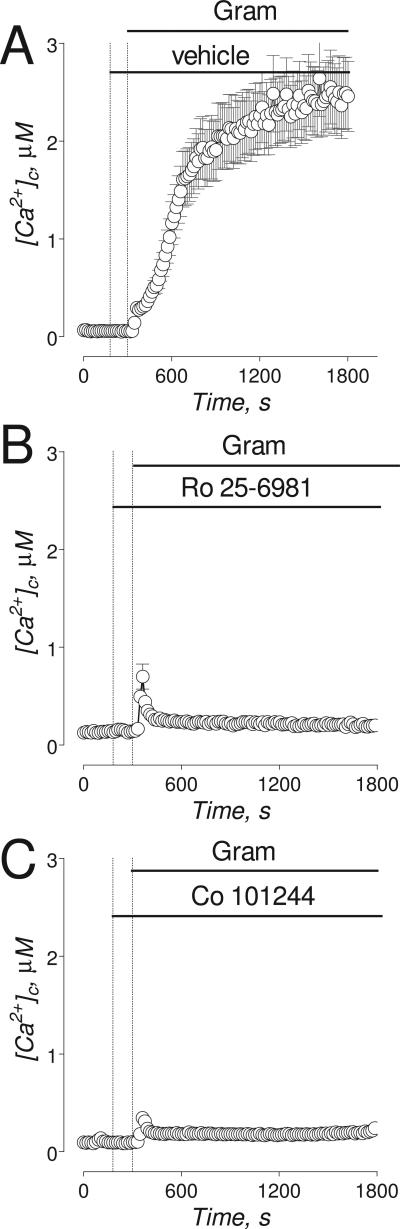
PEAQX, similar to AP-5, was ineffective in preventing gramicidin- and Na+/NMDG-induced increases in [Ca2+]c (Fig. 5C, G). Both Ro 25-6981 and Co 101244, similar to ifenprodil, prevented gramicidin-induced increases in [Ca2+]c suggesting that these NR2B-NMDAR antagonists also inhibit NCXrev (Fig. 6). Ro 25-6981 and Co 101244 were less efficacious than ifenprodil in inhibiting Na+/NMDG-induced increases in [Ca2+]c (Fig. 7A,C,E) whereas AP-5 was less efficacious than all tested NR2B-NMDAR antagonists (Fig. 7B). However, in combination with AP-5 both Ro 25-6981 and Co 101244 completely inhibited Na+/NMDG-induced [Ca2+]c increases (Fig. 7D,F).
Figure 6. The effects of Ro 25-6981 and Co 101244 on gramicidin-induced increases in [Ca2+]c.
The experiments illustrated in this Figure were performed with “older” (13-16 DIV) neurons. Neurons were loaded with 2.6μM Fura-2FF-AM. In A-F, where indicated, 5μM gramicidin (Gram) was applied to neurons. In A, gramicidin-induced increase in [Ca2+]c in neurons treated with a vehicle (0.2% DMSO). In B and C, where indicated, neurons were treated with 0.5μM Ro 25-6981 (B), or 5μM Co 101244 (C). In all experiments, the bath solution was supplemented with 1μM tetrodotoxin, 5μM nifedipine, and 1 mM ouabain.
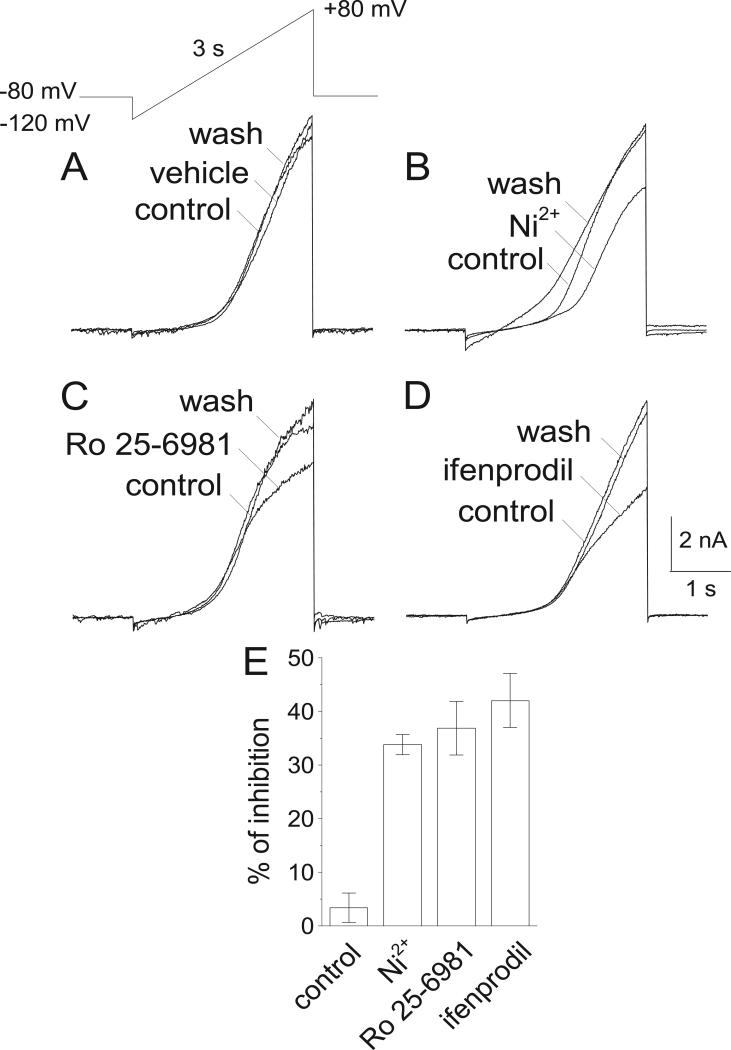
To confirm that ifenprodil and ifenprodil-like NR2B-selective NMDAR antagonists can inhibit NCXrev, we performed electrophysiological patch-clamp experiments with cultured hippocampal neurons (13-16 DIV). We employed the whole-cell configuration and a voltage-ramp protocol used previously (Convery and Hancox, 1999; Smith et al., 2006). As a positive control, we used 5 mM Ni2+, an inhibitor of NCXrev (Convery and Hancox, 1999; Smith et al., 2006). Ni2+ (5 mM) decreased the ion currents induced by the voltage ramp, suggesting that, at least in part, these currents were mediated by NCXrev (Fig 8A,B,E). Both ifenprodil (5μM) and Ro 25-6981 (0.5μM) acted similarly to Ni2+ and significantly decreased the ion currents, suggesting that both NR2B-selective NMDAR antagonists inhibited NCXrev (Fig. 8C-E).
Figure 8. Effect of Ni2+, Ro 25-6981, and ifenprodil on NCXrev-mediated ion currents recorded with cultured hippocampal neurons.
In A, the ascending voltage ramp protocol employed in these experiments and three overlapping current traces obtained prior to and after switching perfusion channels to demonstrate the lack of its effect on NCXrev-mediated ion currents. 0.2% DMSO was used as a vehicle. In B-D, the effects of 5 mM Ni2+ (B), 0.5μM Ro 25-6981 (C), and 5μM ifenprodil (D) on NCXrev-mediated ion currents. The effects of inhibitors were calculated as percentage from the peak current without inhibitor taken as 100%. The time scale shown in panel D is applicable to all traces in B-D. For further details, see Materials and Methods.
4. DISCUSSION
Excitotoxic neuronal death is causally linked to DCD induced by glutamate (Manev et al., 1989; Tymianski et al., 1993b). Accordingly, prevention or attenuation of DCD has been considered a promising strategy to alleviate the consequences of prolonged glutamate exposure (Tymianski et al., 1993c; Tymianski et al., 1994). The NMDAR is one of the major routes for Ca2+ influx in glutamate-exposed neurons (Tymianski et al., 1993b) and inhibition of NMDAR increases survival of neurons in vitro (Brustovetsky et al., 2004). However, strong inhibition of glutamate neurotransmission with high-affinity NMDAR antagonists such as MK801 leads to unacceptable side-effects (Lipton, 2006). In contrast, ifenprodil, an activity-dependent NMDAR antagonist, effectively inhibits NMDAR activated by high concentrations of glutamate and, at the same time, retains the basal level of glutamate neurotransmission and is therefore considered to be more promising and clinically relevant neuroprotector (Kew et al., 1996).
In our experiments with “younger” neurons that predominantly express NR2B subunit, ifenprodil alone completely inhibited NMDA-induced increases in [Ca2+]c and prevented glutamate-induced DCD according to ifenprodil selectivity to NR2B-containg NMDARs (Kew et al., 1996). PEAQX, on the other hand, was ineffective with “younger” neurons. Although PEAQX cannot be considered a selective antagonist of NR2A-containing NMDARs (Neyton and Paoletti, 2006), it is well established that PEAQX preferentially antagonizes NR2A-conatining NMDARs with at least 12-fold higher potency compared to NR2B-NMDARs (Feng et al., 2004). This explains inefficiency of PEAQX in our experiments with “younger” neurons that express predominantly NR2B-NMDARs. In “older” neurons that express greater amount of NR2A-containing NMDARs, ifenprodil alone appeared to be less efficacious. Only combined application of ifenprodil and PEAQX completely inhibited NMDA-induced increase in [Ca2+]c and prevented glutamate-induced DCD in “older” neurons, emphasizing important role of NMDARs in the collapse of Ca2+ homeostasis in neurons exposed to excitotoxic glutamate.
In addition to NMDAR, Ca2+ influx via NCXrev significantly contributes to glutamate-induced DCD (Hoyt et al., 1998; Kiedrowski, 1999). In our previous study, we provided evidence that inhibition of only NMDAR was insufficient to prevent DCD and that NCXrev inhibition is required to protect neurons against glutamate-induced DCD (Brittain et al., 2012). In the present study, ifenprodil alone (with “younger” neurons) or in combination with PEAQX (with “older” neurons) completely prevented glutamate induced DCD. Consequently, we hypothesized that ifenprodil as well as other ifenprodil-like NR2B-selective NMDAR antagonist such as Ro 25-6981 and Co 101244, in addition to NMDAR also inhibit NCXrev. The experiments described in this paper support our hypothesis and suggest that ifenprodil, Ro 25-6981, and Co 101244 indeed inhibit NCXrev.
In the forward mode, the NCX mediates an exchange of 1 intracellular Ca2+ for 3 extracellular Na+ ions driven by Na+ concentration gradient across the plasma membrane and facilitated by plasma membrane potential (Blaustein and Lederer, 1999). Plasma membrane depolarization and collapse of the Na+ gradient lead to NCX reversal. Both, a decrease in Na+ gradient and membrane depolarization take place following glutamate exposure, resulting in NCX reversal and massive Ca2+ influx into neurons (Czyz et al., 2002; Kiedrowski et al., 1994). However, because NMDAR also significantly contributes to glutamate-induced, sustained elevation in [Ca2+]c (Tymianski et al., 1993b), it is difficult to determine the exact target(s) of the pharmacological agents in experiments with neurons exposed to glutamate. Accordingly, to test whether ifenprodil, Ro 25-6981, and Co 101244 inhibit NCXrev, we used two experimental approaches that did not involve application of exogenous glutamate.
In our experiments, the reversal of NCX was triggered by collapse of Na+ gradient across the plasma membrane and plasma membrane depolarization induced by gramicidin, a monovalent cation ionophore that does not transport Ca2+ (Czyz and Kiedrowski, 2002). However, gramicidin could damage mitochondria (Luvisetto and Azzone, 1989; Rottenberg and Koeppe, 1989) involved in Ca2+ buffering in neurons (Wang and Thayer, 1996; White and Reynolds, 1997). To circumvent this potentially confounding variable, NCX reversal was triggered by Na+/NMDG-replacement in the bath solution (Wu et al., 2008). Both gramicidin and Na+/NMDG replacement produced an increase in [Ca2+]c that was inhibited by ifenprodil. Both manipulations led to the release of endogenous glutamate in low micromolar concentration range probably due to reversal of Na+-glutamate co-transporter. This might contribute to the increase in [Ca2+]c due to Ca2+ influx via activated NMDAR, particularly, under conditions when the forward mode of NCX is shut down due to elimination or reversal of Na+ gradient across the plasma membrane. In our previous study, we used GPT, which converts glutamate and pyruvate into α-ketoglutarate and alanine (Matthews et al., 2000; Matthews et al., 2003), to prevent the increase in glutamate concentration in the bath solution induced by gramicidin (Brittain et al., 2012). In the present study, we used GPT to prevent the increase in external glutamate following Na+/NMDG replacement. In both cases, GPT prevented the rises in glutamate concentration in the bath solution but failed to prevent the increases in [Ca2+]c. This suggests that although NMDAR stimulation by the released endogenous glutamate might contribute to the gramicidin- and Na+/NMDG-induced increases in [Ca2+]c, it does not play a major role in these [Ca2+]c increases. This conclusion is supported by the fact that AP-5, a potent NMDAR inhibitor, failed to inhibit [Ca2+]c increases induced by gramicidin or Na+/NMDG replacement. On the other hand, in the experiments with Na+/NMDG replacement, the magnitude of [Ca2+]c elevations depended on [Na+]c and became significantly increased with increasing [Na+]c in the presence of ouabain, an inhibitor of Na+/K+-ATPase. The similar dependence was reported earlier (Wu et al., 2008). This suggests that the mechanism of Na+/NMDG-induced increase in [Ca2+]c most likely utilized the reverse Na+ gradient. Taken together, our results suggest that the reversal of NCX is the most likely mechanism of the increases in [Ca2+]c induced by gramicidin or Na+/NMDG replacement. Consequently, inhibition of these [Ca2+]c increases with ifenprodil, Ro 25-6981, and Co 101244 strongly suggests that these NR2B-NMDAR antagonists also inhibit NCXrev whereas the lack of inhibition with PEAQX and AP-5 indicates that these agents do not inhibit NCXrev. This is consistent with the fact that all three tested NR2B-NMDAR antagonists – ifenprodil, Ro 25-6981, and Co 101244 – have very similar chemical structures that differ significantly from chemical structures of AP-5 and PEAQX.
Na+/Ca2+ exchange is electrogenic and it is possible to measure electrical currents across the plasma membrane generated by NCX (Kimura et al., 1986; Mechmann and Pott, 1986). Most of electrophysiological studies of NCX have been done with cardiac cells (Convery and Hancox, 1999; Kimura et al., 1987; Ohtsuka et al., 2004; Reppel et al., 2007; Smith et al., 2006). Recently, we applied this approach to cultured neurons and recorded neuronal NCX-mediated ion currents (Brittain et al., 2012; Brustovetsky et al., 2011). In these studies, we used voltage-ramp protocol, in which the membrane potential was changed from -120 mV to +80 mV. Due to the electrogenic nature of Na+/Ca2+ exchange and favorable Na+ and Ca2+ concentrations inside and outside of the cell, under positive voltage NCX operates in the reverse mode, transporting 3 sodium ions out of the cell for every calcium ion transported in. The result is a net outward current and the peak current recorded at +80 mV is used as a measure of NCXrev activity (Brittain et al., 2012). These currents are sensitive to Ni2+, which is usually used to attribute the currents to NCX (Convery and Hancox, 1999; Kimura et al., 1987; Smith et al., 2006). In the experiments with cultured neurons, the inhibitory effect of Ni2+ appeared to be smaller than in the experiments with cardiac cells and varied from about 33-48% (Brittain et al., 2012) to about 30% in the present study. The reason for this variability is not known, but could reflect differences in background “leak” currents.
In addition to NCX, Ni2+ can also inhibit voltage-dependent Ca2+ channels (VDCC) (Lenz et al., 1998; Zhang et al., 1993) and, therefore, inhibition of these channels could be partially responsible for the Ni2+ effect on ion currents. However, it is unlikely that inhibition of VDCC contributes significantly to the Ni2+ effect on the outward currents observed during the slow ramp depolarizations. The reversal potential for Ca2+ in our experimental conditions is close to +95 mV (Hille, 1992). Calcium currents through VDCC would generate an inward current, not an outward current. VDCC are inhibited by the inclusion of nifedipine in the bath solution and likely inactivated by the slow ramp depolarization. Furthermore, when the plasma membrane is depolarized to +80 mV the driving force for Ca2+ influx through VDCC is low (Reppel et al., 2007). Consequently, contribution of Ca2+ influx through these channels to the overall current measured at +80 mV is most likely negligible.
The ability of ifenprodil, Ro 25-6981, and Co 101244 to inhibit NCXrev is not unique among NMDAR antagonists. In our recent study (Brittain et al., 2012), we found evidence that MK801 and memantine, non-competitive NMDAR antagonists, most likely also inhibit NCXrev in hippocampal neurons. Recently, PPADS, a P2X receptor antagonist, was found to inhibit the NCXrev in guinea pig airway smooth muscle (Flores-Soto et al., 2011). On the other hand, we showed recently that KB-R7943, a widely used inhibitor of NCXrev (Iwamoto et al., 1996), also antagonized NMDAR (Brustovetsky et al., 2011), suggesting that not only NMDAR antagonists (MK801, memantine, ifenprodil, and ifenprodil-like agents) can inhibit NCXrev, but also NCXrev inhibitor can block NMDAR. These findings strongly support the notion that pharmacological agents used in studies of NMDAR and NCXrev might be not as selective as has been assumed previously.
The ability of ifenprodil alone or in combination with PEAQX to prevent glutamate-induced DCD in neurons correlates with ifenprodil's efficacy to inhibit NCXrev. It is conceivable that other neuroprotective agents, used against glutamate excitotoxicity and originally aimed at different molecular targets, may also exert neuroprotection, at least in part, by inhibiting NCXrev. Accordingly, further studies are necessary to investigate the ability of different neuroprotective agents to inhibit NCXrev. The results presented in this paper improve our understanding of the mechanisms of ifenprodil neuroprotective action and support our hypothesis about significant contribution of both NMDAR and NCXrev in glutamate-induced DCD in neurons.
Supplementary Material
HIGHLIGHTS.
➢ Ifenprodil alone or together with PEAQX inhibits glutamate-induced Ca dysregulation
➢ Ifenprodil is more effective in younger (6-8 DIV) than in older (13-16 DIV) neurons
➢ Ifenprodil but not AP-5 or PEAQX inhibits reverse Na/Ca exchanger (NCXrev)
➢ Ifenprodil-like NMDAR antagonists Ro 25-6981 and Co 101244 also inhibit NCXrev
➢ Both NMDAR and NCXrev are essential for the glutamate-induced Ca2+ dysregulation
Acknowledgements
This study was supported by the NIH/NINDS R01 NS050131, in part by R01 NS078008, and by Biomedical Research grant from Indiana University School of Medicine to N.B. R.K. was supported by an AHA National Scientist Development Grant SDG 5280023. M.K.B. was supported by AHA pre-doctoral fellowship 10PRE4300005 (Midwest Affiliate).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations: DCD, delayed Ca2+ dysregulation; NMDA, N-methyl-D-aspartate; NMDAR, NMDA receptor; NCX, Na+/Ca2+ exchanger; NCXrev, reverse NCX; PEAQX, ({[(1S)-1-(4-bromophenyl)ethyl]amino}-(2,3-dioxo-1,4-dihydroquinoxalin-5-yl)methyl)phosphonic acid; NMDG, N-methyl-D-glucamine; GPT, glutamate pyruvate transaminase; AP-5, D-(-)-2-amino-5-phosphonopentanoic acid; PPADS, pyridoxal phosphate-6-azo(benzene-2,4-disulfonic acid); TTX, tetrodotoxin.
References
- 1.Auberson YP, Allgeier H, Bischoff S, Lingenhoehl K, Moretti R, Schmutz M. 5-Phosphonomethylquinoxalinediones as competitive NMDA receptor antagonists with a preference for the human 1A/2A, rather than 1A/2B receptor composition. Bioorg.Med.Chem.Lett. 2002;12:1099–1102. doi: 10.1016/s0960-894x(02)00074-4. [DOI] [PubMed] [Google Scholar]
- 2.Blaustein MP, Lederer WJ. Sodium/calcium exchange: its physiological implications. Physiol Rev. 1999;79:763–854. doi: 10.1152/physrev.1999.79.3.763. [DOI] [PubMed] [Google Scholar]
- 3.Bramlett HM, Dietrich WD. Pathophysiology of cerebral ischemia and brain trauma: similarities and differences. J.Cereb.Blood Flow Metab. 2004;24:133–150. doi: 10.1097/01.WCB.0000111614.19196.04. [DOI] [PubMed] [Google Scholar]
- 4.Brewer LD, Thibault O, Staton J, Thibault V, Rogers JT, Garcia-Ramos G, Kraner S, Landfield PW, Porter NM. Increased vulnerability of hippocampal neurons with age in culture: temporal association with increases in NMDA receptor current, NR2A subunit expression and recruitment of L-type calcium channels. Brain Res. 2007;1151:20–31. doi: 10.1016/j.brainres.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 5.Brittain MK, Brustovetsky T, Sheets PL, Brittain JM, Khanna R, Cummins TR, Brustovetsky N. Delayed calcium dysregulation in neurons requires both the NMDA receptor and the reverse Na+/Ca2+ exchanger. Neurobiol.Dis. 2012;46:109–117. doi: 10.1016/j.nbd.2011.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brustovetsky T, Brittain MK, Sheets PL, Cummins TR, Pinelis V, Brustovetsky N. KB-R7943, an inhibitor of the reverse Na+ /Ca2+ exchanger, blocks N-methyl-D-aspartate receptor and inhibits mitochondrial complex I. Br.J.Pharmacol. 2011;162:255–270. doi: 10.1111/j.1476-5381.2010.01054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brustovetsky T, Purl K, Young A, Shimizu K, Dubinsky JM. Dearth of glutamate transporters contributes to striatal excitotoxicity. Exp.Neurol. 2004;189:222–230. doi: 10.1016/j.expneurol.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 8.Carter C, Benavides J, Legendre P, Vincent JD, Noel F, Thuret F, Lloyd KG, Arbilla S, Zivkovic B, Mackenzie ET. Ifenprodil and SL 82.0715 as cerebral anti-ischemic agents. II. Evidence for N-methyl-D-aspartate receptor antagonist properties. J.Pharmacol.Exp.Ther. 1988;247:1222–1232. [PubMed] [Google Scholar]
- 9.Carter C, Rivy JP, Scatton B. Ifenprodil and SL 82.0715 are antagonists at the polyamine site of the N-methyl-D-aspartate (NMDA) receptor. Eur.J.Pharmacol. 1989;164:611–612. doi: 10.1016/0014-2999(89)90275-6. [DOI] [PubMed] [Google Scholar]
- 10.Chang DT, Rintoul GL, Pandipati S, Reynolds IJ. Mutant huntingtin aggregates impair mitochondrial movement and trafficking in cortical neurons. Neurobiol.Dis. 2006;22:388–400. doi: 10.1016/j.nbd.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Convery MK, Hancox JC. Comparison of Na+-Ca2+ exchange current elicited from isolated rabbit ventricular myocytes by voltage ramp and step protocols. Pflugers Arch. 1999;437:944–954. doi: 10.1007/s004240050866. [DOI] [PubMed] [Google Scholar]
- 12.Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr.Opin.Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- 13.Czyz A, Baranauskas G, Kiedrowski L. Instrumental role of Na+ in NMDA excitotoxicity in glucose-deprived and depolarized cerebellar granule cells. J.Neurochem. 2002;81:379–389. doi: 10.1046/j.1471-4159.2002.00851.x. [DOI] [PubMed] [Google Scholar]
- 14.Czyz A, Kiedrowski L. In depolarized and glucose-deprived neurons, Na+ influx reverses plasmalemmal K+-dependent and K+-independent Na+/Ca2+ exchangers and contributes to NMDA excitotoxicity. J.Neurochem. 2002;83:1321–1328. doi: 10.1046/j.1471-4159.2002.01227.x. [DOI] [PubMed] [Google Scholar]
- 15.Dietz RM, Kiedrowski L, Shuttleworth CW. Contribution of Na(+)/Ca(2+) exchange to excessive Ca(2+) loading in dendrites and somata of CA1 neurons in acute slice. Hippocampus. 2007;17:1049–1059. doi: 10.1002/hipo.20336. [DOI] [PubMed] [Google Scholar]
- 16.Dubinsky JM. Intracellular calcium levels during the period of delayed excitotoxicity. Journal of Neuroscience. 1993;13:623–631. doi: 10.1523/JNEUROSCI.13-02-00623.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dubinsky JM, Kristal BS, Elizondo-Fournier M. An obligate role for oxygen in the early stages of glutamate-induced, delayed neuronal death. J.Neurosci. 1995;15:7071–7078. doi: 10.1523/JNEUROSCI.15-11-07071.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng B, Tse HW, Skifter DA, Morley R, Jane DE, Monaghan DT. Structure-activity analysis of a novel NR2C/NR2D-preferring NMDA receptor antagonist: 1-(phenanthrene-2-carbonyl) piperazine-2,3-dicarboxylic acid. Br.J.Pharmacol. 2004;141:508–516. doi: 10.1038/sj.bjp.0705644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flores-Soto E, Reyes-Garcia J, Sommer B, Chavez J, Barajas-Lopez C, Montano LM. PPADS, a P2X receptor antagonist, as a novel inhibitor of the reverse mode of the Na(+)/Ca(2+) exchanger in guinea pig airway smooth muscle. Eur.J.Pharmacol. 2011 doi: 10.1016/j.ejphar.2011.11.018. doi:10.1016/j.ejphar.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 20.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J.Biol.Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 21.Hazell AS. Excitotoxic mechanisms in stroke: an update of concepts and treatment strategies. Neurochem.Int. 2007;50:941–953. doi: 10.1016/j.neuint.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 22.Hille B. Ionic Channels of Excitable Membranes. 2nd Edition 1992. pp. 1–607.
- 23.Hoyt KR, Arden SR, Aizenman E, Reynolds IJ. Reverse Na+/Ca2+ exchange contributes to glutamate-induced intracellular Ca2+ concentration increases in cultured rat forebrain neurons. Mol.Pharmacol. 1998;53:742–749. [PubMed] [Google Scholar]
- 24.Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- 25.Iwamoto T, Watano T, Shigekawa M. A novel isothiourea derivative selectively inhibits the reverse mode of Na+/Ca2+ exchange in cells expressing NCX1. J.Biol.Chem. 1996;271:22391–22397. doi: 10.1074/jbc.271.37.22391. [DOI] [PubMed] [Google Scholar]
- 26.Janssens N, Lesage AS. Glutamate receptor subunit expression in primary neuronal and secondary glial cultures. J.Neurochem. 2001;77:1457–1474. doi: 10.1046/j.1471-4159.2001.00369.x. [DOI] [PubMed] [Google Scholar]
- 27.Jeffs GJ, Meloni BP, Bakker AJ, Knuckey NW. The role of the Na(+)/Ca(2+) exchanger (NCX) in neurons following ischaemia. J.Clin.Neurosci. 2007;14:507–514. doi: 10.1016/j.jocn.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 28.Kew JN, Trube G, Kemp JA. A novel mechanism of activity-dependent NMDA receptor antagonism describes the effect of ifenprodil in rat cultured cortical neurones. J.Physiol. 1996;497(Pt 3):761–772. doi: 10.1113/jphysiol.1996.sp021807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiedrowski L. N-methyl-D-aspartate excitotoxicity: relationships among plasma membrane potential, Na(+)/Ca(2+) exchange, mitochondrial Ca(2+) overload, and cytoplasmic concentrations of Ca(2+), H(+), and K(+). Mol.Pharmacol. 1999;56:619–632. doi: 10.1124/mol.56.3.619. [DOI] [PubMed] [Google Scholar]
- 30.Kiedrowski L, Brooker G, Costa E, Wroblewski JT. Glutamate impairs neuronal calcium extrusion while reducing sodium gradient. Neuron. 1994;12:295–300. doi: 10.1016/0896-6273(94)90272-0. [DOI] [PubMed] [Google Scholar]
- 31.Kimura J, Miyamae S, Noma A. Identification of sodium-calcium exchange current in single ventricular cells of guinea-pig. J.Physiol. 1987;384:199–222. doi: 10.1113/jphysiol.1987.sp016450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kimura J, Noma A, Irisawa H. Na-Ca exchange current in mammalian heart cells. Nature. 1986;319:596–597. doi: 10.1038/319596a0. [DOI] [PubMed] [Google Scholar]
- 33.Lenz RA, Wagner JJ, Alger BE. N- and L-type calcium channel involvement in depolarization-induced suppression of inhibition in rat hippocampal CA1 cells. J.Physiol. 1998;512(Pt 1):61–73. doi: 10.1111/j.1469-7793.1998.061bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li V, Brustovetsky T, Brustovetsky N. Role of cyclophilin D-dependent mitochondrial permeability transition in glutamate-induced calcium deregulation and excitotoxic neuronal death. Exp.Neurol. 2009;218:171–182. doi: 10.1016/j.expneurol.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lipton SA. Failures and successes of NMDA receptor antagonists: molecular basis for the use of open-channel blockers like memantine in the treatment of acute and chronic neurologic insults. NeuroRx. 2004;1:101–110. doi: 10.1602/neurorx.1.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lipton SA. Paradigm shift in neuroprotection by NMDA receptor blockade: memantine and beyond. Nat.Rev.Drug Discov. 2006;5:160–170. doi: 10.1038/nrd1958. [DOI] [PubMed] [Google Scholar]
- 37.Luvisetto S, Azzone GF. Nature of proton cycling during gramicidin uncoupling of oxidative phosphorylation. Biochemistry. 1989;28:1100–1108. doi: 10.1021/bi00429a026. [DOI] [PubMed] [Google Scholar]
- 38.Manev H, Favaron M, Guidotti A, Costa E. Delayed increase of Ca2+ influx elicited by glutamate: role in neuronal death. Mol.Pharmacol. 1989;36:106–112. [PubMed] [Google Scholar]
- 39.Matthews CC, Zielke HR, Parks DA, Fishman PS. Glutamate-pyruvate transaminase protects against glutamate toxicity in hippocampal slices. Brain Res. 2003;978:59–64. doi: 10.1016/s0006-8993(03)02765-3. [DOI] [PubMed] [Google Scholar]
- 40.Matthews CC, Zielke HR, Wollack JB, Fishman PS. Enzymatic degradation protects neurons from glutamate excitotoxicity. J.Neurochem. 2000;75:1045–1052. doi: 10.1046/j.1471-4159.2000.0751045.x. [DOI] [PubMed] [Google Scholar]
- 41.McBain CJ, Mayer ML. N-methyl-D-aspartic acid receptor structure and function. Physiol Rev. 1994;74:723–760. doi: 10.1152/physrev.1994.74.3.723. [DOI] [PubMed] [Google Scholar]
- 42.Mechmann S, Pott L. Identification of Na-Ca exchange current in single cardiac myocytes. Nature. 1986;319:597–599. doi: 10.1038/319597a0. [DOI] [PubMed] [Google Scholar]
- 43.Mott DD, Doherty JJ, Zhang S, Washburn MS, Fendley MJ, Lyuboslavsky P, Traynelis SF, Dingledine R. Phenylethanolamines inhibit NMDA receptors by enhancing proton inhibition. Nat.Neurosci. 1998;1:659–667. doi: 10.1038/3661. [DOI] [PubMed] [Google Scholar]
- 44.Newell EW, Stanley EF, Schlichter LC. Reversed Na+/Ca2+ exchange contributes to Ca2+ influx and respiratory burst in microglia. Channels (Austin.) 2007;1:366–376. doi: 10.4161/chan.5391. [DOI] [PubMed] [Google Scholar]
- 45.Neyton J, Paoletti P. Relating NMDA receptor function to receptor subunit composition: limitations of the pharmacological approach. J.Neurosci. 2006;26:1331–1333. doi: 10.1523/JNEUROSCI.5242-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nicholls DG, Budd SL. Mitochondria and neuronal glutamate excitotoxicity. Biochim.Biophys.Acta. 1998;1366:97–112. doi: 10.1016/s0005-2728(98)00123-6. [DOI] [PubMed] [Google Scholar]
- 47.Ohtsuka M, Takano H, Suzuki M, Zou Y, Akazawa H, Tamagawa M, Wakimoto K, Nakaya H, Komuro I. Role of Na+-Ca2+ exchanger in myocardial ischemia/reperfusion injury: evaluation using a heterozygous Na+-Ca2+ exchanger knockout mouse model. Biochem.Biophys.Res Commun. 2004;314:849–853. doi: 10.1016/j.bbrc.2003.12.165. [DOI] [PubMed] [Google Scholar]
- 48.Reppel M, Sasse P, Malan D, Nguemo F, Reuter H, Bloch W, Hescheler J, Fleischmann BK. Functional expression of the Na+/Ca2+ exchanger in the embryonic mouse heart. J.Mol.Cell Cardiol. 2007;42:121–132. doi: 10.1016/j.yjmcc.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 49.Rottenberg H, Koeppe RE. Mechanism of uncoupling of oxidative phosphorylation by gramicidin. Biochemistry. 1989;28:4355–4360. doi: 10.1021/bi00436a035. [DOI] [PubMed] [Google Scholar]
- 50.Salinska E, Danysz W, Lazarewicz JW. The role of excitotoxicity in neurodegeneration. Folia Neuropathol. 2005;43:322–339. [PubMed] [Google Scholar]
- 51.Scatton B. Excitatory amino acid receptor antagonists: a novel treatment for ischemic cerebrovascular diseases. Life Sci. 1994;55:2115–2124. doi: 10.1016/0024-3205(94)00392-0. [DOI] [PubMed] [Google Scholar]
- 52.Smith GL, Elliott EE, Kettlewell S, Currie S, Quinn FR. Na(+)/Ca(2+) exchanger expression and function in a rabbit model of myocardial infarction. J.Cardiovasc.Electrophysiol. 2006;17(Suppl 1):S57–S63. doi: 10.1111/j.1540-8167.2006.00384.x. [DOI] [PubMed] [Google Scholar]
- 53.Stanika RI, Pivovarova NB, Brantner CA, Watts CA, Winters CA, Andrews SB. Coupling diverse routes of calcium entry to mitochondrial dysfunction and glutamate excitotoxicity. Proc.Natl.Acad.Sci.U.S.A. 2009;106:9854–9859. doi: 10.1073/pnas.0903546106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tymianski M, Charlton MP, Carlen PL, Tator CH. Secondary Ca2+ overload indicates early neuronal injury which precedes staining with viability indicators. Brain Res. 1993a;607:319–323. doi: 10.1016/0006-8993(93)91523-u. [DOI] [PubMed] [Google Scholar]
- 55.Tymianski M, Charlton MP, Carlen PL, Tator CH. Source specificity of early calcium neurotoxicity in cultured embryonic spinal neurons. J.Neurosci. 1993b;13:2085–2104. doi: 10.1523/JNEUROSCI.13-05-02085.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tymianski M, Charlton MP, Carlen PL, Tator CH. Properties of neuroprotective cell-permeant Ca2+ chelators: effects on [Ca2+]i and glutamate neurotoxicity in vitro. J.Neurophysiol. 1994;72:1973–1992. doi: 10.1152/jn.1994.72.4.1973. [DOI] [PubMed] [Google Scholar]
- 57.Tymianski M, Wallace MC, Spigelman I, Uno M, Carlen PL, Tator CH, Charlton MP. Cell-permeant Ca2+ chelators reduce early excitotoxic and ischemic neuronal injury in vitro and in vivo. Neuron. 1993c;11:221–235. doi: 10.1016/0896-6273(93)90180-y. [DOI] [PubMed] [Google Scholar]
- 58.Wang GJ, Thayer SA. Sequestration of glutamate-induced Ca2+ loads by mitochondria in cultured rat hippocampal neurons. J.Neurophysiol. 1996;76:1611–1621. doi: 10.1152/jn.1996.76.3.1611. [DOI] [PubMed] [Google Scholar]
- 59.White RJ, Reynolds IJ. Mitochondrial depolarization in glutamate-stimulated neurons: an early signal specific to excitotoxin exposure. J.Neurosci. 1996;16:5688–5697. doi: 10.1523/JNEUROSCI.16-18-05688.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.White RJ, Reynolds IJ. Mitochondria accumulate Ca2+ following intense glutamate stimulation of cultured rat forebrain neurones. J.Physiol. 1997;498(Pt 1):31–47. doi: 10.1113/jphysiol.1997.sp021839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Williams K. Ifenprodil discriminates subtypes of the N-methyl-D-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol.Pharmacol. 1993;44:851–859. [PubMed] [Google Scholar]
- 62.Wu MP, Kao LS, Liao HT, Pan CY. Reverse mode Na+/Ca2+ exchangers trigger the release of Ca2+ from intracellular Ca2+ stores in cultured rat embryonic cortical neurons. Brain Res. 2008;1201:41–51. doi: 10.1016/j.brainres.2008.01.042. [DOI] [PubMed] [Google Scholar]
- 63.Zhang JF, Randall AD, Ellinor PT, Horne WA, Sather WA, Tanabe T, Schwarz TL, Tsien RW. Distinctive pharmacology and kinetics of cloned neuronal Ca2+ channels and their possible counterparts in mammalian CNS neurons. Neuropharmacology. 1993;32:1075–1088. doi: 10.1016/0028-3908(93)90003-l. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.