Abstract
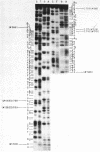
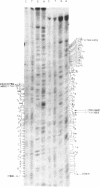
In order to investigate the 5' terminal structural heterogeneity of the 16S size class of SV40 late RNA, we have bound an SV40 DNA fragment labeled at its 5' termini with P32 to the .939-.945 map unit region of late lytic cytoplasmic polyadenylated RNA, used reverse transcriptase to prepare cDNA copies of the 5' termini of this RNA, separated the cDNA products on an 8% polyacrylamide-7 M urea gel and subjected these products to nucleic acid sequence analysis. A number of discrete cDNAs were obtained. Analysis of these cDNAs has suggested the presence of three categories of 16S species all containing the same body extending from residues 1381-2592 (.939-.170 m.u.) but differring in the structure of their leader segments. Members of the first category contain leaders which are colinear with SV40 DNA, have a common 3' terminus at residue 444 and extend varying distances in a 5' direction. The most abundant 16S species contains a leader of 203 nucleotides and is a member of this group. RNAs of the second category contain leaders with an internal gap between residues 211-352. The single RNA comprising the third category contains a leader with a tandem repetition of nucleotides 351-443 at the 3' terminus of its leader.
Full text
PDF


















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acheson N. H., Buetti E., Scherrer K., Weil R. Transcription of the polyoma virus genome: synthesis and cleavage of giant late polyoma-specific RNA. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2231–2235. doi: 10.1073/pnas.68.9.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloni Y., Dhar R., Laub O., Horowitz M., Khoury G. Novel mechanism for RNA maturation: the leader sequences of simian virus 40 mRNA are not transcribed adjacent to the coding sequences. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3686–3690. doi: 10.1073/pnas.74.9.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloni Y. Methylated SV40 mRNAs. FEBS Lett. 1975 Jul 1;54(3):363–367. doi: 10.1016/0014-5793(75)80940-9. [DOI] [PubMed] [Google Scholar]
- Berget S. M., Moore C., Sharp P. A. Spliced segments at the 5' terminus of adenovirus 2 late mRNA. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3171–3175. doi: 10.1073/pnas.74.8.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack C., Tonegawa S. Variable and constant parts of the immunoglobulin light chain gene of a mouse myeloma cell are 1250 nontranslated bases apart. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5652–5656. doi: 10.1073/pnas.74.12.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Mandel J. L., Chambon P. Ovalbumin gene is split in chicken DNA. Nature. 1977 Nov 24;270(5635):314–319. doi: 10.1038/270314a0. [DOI] [PubMed] [Google Scholar]
- Casey J., Davidson N. Rates of formation and thermal stabilities of RNA:DNA and DNA:DNA duplexes at high concentrations of formamide. Nucleic Acids Res. 1977;4(5):1539–1552. doi: 10.1093/nar/4.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celma M. L., Dhar R., Pan J., Weissman S. M. Comparison of the nucleotide sequence of the messenger RNA for the major structural protein of SV40 with the DNA sequence encoding the amino acids of the protein. Nucleic Acids Res. 1977 Aug;4(8):2549–2550. [PMC free article] [PubMed] [Google Scholar]
- Chow L. T., Gelinas R. E., Broker T. R., Roberts R. J. An amazing sequence arrangement at the 5' ends of adenovirus 2 messenger RNA. Cell. 1977 Sep;12(1):1–8. doi: 10.1016/0092-8674(77)90180-5. [DOI] [PubMed] [Google Scholar]
- Dhar R., Subramanian K. N., Pan J., Weissman S. M. Structure of a large segment of the genome of simian virus 40 that does not encode known proteins. Proc Natl Acad Sci U S A. 1977 Mar;74(3):827–831. doi: 10.1073/pnas.74.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doel M. T., Houghton M., Cook E. A., Carey N. H. The presence of ovalbumin mRNA coding sequences in multiple restriction fragments of chicken DNA. Nucleic Acids Res. 1977 Nov;4(11):3701–3713. doi: 10.1093/nar/4.11.3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiers W., Contreras R., Haegemann G., Rogiers R., Van de Voorde A., Van Heuverswyn H., Van Herreweghe J., Volckaert G., Ysebaert M. Complete nucleotide sequence of SV40 DNA. Nature. 1978 May 11;273(5658):113–120. doi: 10.1038/273113a0. [DOI] [PubMed] [Google Scholar]
- Ghosh P. K., Reddy V. B., Swinscoe J., Choudary P. V., Lebowitz P., Weissman S. M. The 5'-terminal leader sequence of late 16 S mRNA from cells infected with simian virus 40. J Biol Chem. 1978 May 25;253(10):3643–3647. [PubMed] [Google Scholar]
- Goodman H. M., Olson M. V., Hall B. D. Nucleotide sequence of a mutant eukaryotic gene: the yeast tyrosine-inserting ochre suppressor SUP4-o. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5453–5457. doi: 10.1073/pnas.74.12.5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groner Y., Carmi P., Aloni Y. Capping structures of simian virus 40 19S and 16S mRNAs. Nucleic Acids Res. 1977 Nov;4(11):3959–3968. doi: 10.1093/nar/4.11.3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegeman G., Fiers W. Characterization of the 5'-terminal capped structures of late simian virus 40-specific mRNA. J Virol. 1978 Mar;25(3):824–830. doi: 10.1128/jvi.25.3.824-830.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegeman G., Fiers W. Evidence for 'splicing' of SV40 16S mRNA. Nature. 1978 May 4;273(5657):70–73. doi: 10.1038/273070a0. [DOI] [PubMed] [Google Scholar]
- Hsu M. T., Ford J. Sequence arrangement of the 5' ends of simian virus 40 16S and 19S mRNAs. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4982–4985. doi: 10.1073/pnas.74.11.4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J., Flavell R. A. The rabbit beta-globin gene contains a large large insert in the coding sequence. Cell. 1977 Dec;12(4):1097–1108. doi: 10.1016/0092-8674(77)90172-6. [DOI] [PubMed] [Google Scholar]
- Khoury G., May E. Regulation of early and late simian virus 40 transcription: overproduction of early viral RNA in the absence of a functional T-antigen. J Virol. 1977 Jul;23(1):167–176. doi: 10.1128/jvi.23.1.167-176.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klessig D. F. Two adenovirus mRNAs have a common 5' terminal leader sequence encoded at least 10 kb upstream from their main coding regions. Cell. 1977 Sep;12(1):9–21. doi: 10.1016/0092-8674(77)90181-7. [DOI] [PubMed] [Google Scholar]
- Krzyzek R. A., Collett M. S., Lau A. F., Perdue M. L., Leis J. P., Faras A. J. Evidence for splicing of avian sarcoma virus 5'-terminal genomic sequences into viral-specific RNA in infected cells. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1284–1288. doi: 10.1073/pnas.75.3.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laub O., Aloni Y. Transcription of simian virus 40. V. Regulattion of simian virus 40 gene expression. J Virol. 1975 Nov;16(5):1171–1183. doi: 10.1128/jvi.16.5.1171-1183.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavi S., Groner Y. 5'-Terminal sequences and coding region of late simian virus 40 mRNAs are derived from noncontiguous segments of the viral genome. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5323–5327. doi: 10.1073/pnas.74.12.5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Prives C. L., Aviv H., Paterson B. M., Roberts B. E., Rozenblatt S., Revel M., Winocour E. Cell-free translation of messenger RNA of simian virus 40: synthesis of the major capsid protein. Proc Natl Acad Sci U S A. 1974 Feb;71(2):302–306. doi: 10.1073/pnas.71.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy V. B., Dhar R., Weissman S. M. Nucleotides sequence of the genes for the simian virus 40 proteins VP2 and VP3. J Biol Chem. 1978 Jan 25;253(2):621–630. [PubMed] [Google Scholar]
- Reddy V. B., Thimmappaya B., Dhar R., Subramanian K. N., Zain B. S., Pan J., Ghosh P. K., Celma M. L., Weissman S. M. The genome of simian virus 40. Science. 1978 May 5;200(4341):494–502. doi: 10.1126/science.205947. [DOI] [PubMed] [Google Scholar]
- Rothenberg E., Donoghue D. J., Baltimore D. Analysis of a 5' leader sequence on murine leukemia virus 21S RNA: heteroduplex mapping with long reverse transcriptase products. Cell. 1978 Mar;13(3):435–451. doi: 10.1016/0092-8674(78)90318-5. [DOI] [PubMed] [Google Scholar]
- Tilghman S. M., Tiemeier D. C., Polsky F., Edgell M. H., Seidman J. G., Leder A., Enquist L. W., Norman B., Leder P. Cloning specific segments of the mammalian genome: bacteriophage lambda containing mouse globin and surrounding gene sequences. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4406–4410. doi: 10.1073/pnas.74.10.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilghman S. M., Tiemeier D. C., Seidman J. G., Peterlin B. M., Sullivan M., Maizel J. V., Leder P. Intervening sequence of DNA identified in the structural portion of a mouse beta-globin gene. Proc Natl Acad Sci U S A. 1978 Feb;75(2):725–729. doi: 10.1073/pnas.75.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S., Brack C., Hozumi N., Schuller R. Cloning of an immunoglobulin variable region gene from mouse embryo. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3518–3522. doi: 10.1073/pnas.74.8.3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S., Maxam A. M., Tizard R., Bernard O., Gilbert W. Sequence of a mouse germ-line gene for a variable region of an immunoglobulin light chain. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1485–1489. doi: 10.1073/pnas.75.3.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela P., Venegas A., Weinberg F., Bishop R., Rutter W. J. Structure of yeast phenylalanine-tRNA genes: an intervening DNA segment within the region coding for the tRNA. Proc Natl Acad Sci U S A. 1978 Jan;75(1):190–194. doi: 10.1073/pnas.75.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg R. A., Warnaar S. O., Winocour E. Isolation and characterization of simian virus 40 ribonucleic acid. J Virol. 1972 Aug;10(2):193–201. doi: 10.1128/jvi.10.2.193-201.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellauer P. K., Dawid I. B. The structural organization of ribosomal DNA in Drosophila melanogaster. Cell. 1977 Feb;10(2):193–212. doi: 10.1016/0092-8674(77)90214-8. [DOI] [PubMed] [Google Scholar]
- Westphal H., Lai S. P. Quantitative electron microscopy of early adenovirus RNA. J Mol Biol. 1977 Nov 5;116(3):525–548. doi: 10.1016/0022-2836(77)90082-1. [DOI] [PubMed] [Google Scholar]
- White R. L., Hogness D. S. R loop mapping of the 18S and 28S sequences in the long and short repeating units of Drosophila melanogaster rDNA. Cell. 1977 Feb;10(2):177–192. doi: 10.1016/0092-8674(77)90213-6. [DOI] [PubMed] [Google Scholar]
- Zain B. S., Dhar R., Weissman S. M., Lebowitz P., Lewis A. M., Jr Preferred site for initiation of RNA transcription by Escherichia coli RNA polymerase within the simian virus 40 DNA segment of the nondefective adenovirus-simian virus 40 hybrid viruses Ad2 + ND 1 and Ad2 + ND 3 . J Virol. 1973 May;11(5):682–693. doi: 10.1128/jvi.11.5.682-693.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]