Abstract
Exposure to psychological trauma is the precipitating factor for PTSD. In addition, a history of chronic or traumatic stress exposure is a predisposing risk factor. We have developed a Chronic plus Acute Prolonged Stress (CAPS) treatment for rats that models some of the characteristics of stressful events that can lead to PTSD in humans. We have previously shown that CAPS enhances acute fear responses and impairs extinction of conditioned fear. Further, CAPS reduced the expression of glucocorticoid receptors in the medial prefrontal cortex. In this study we examined the effects of CAPS exposure on behavioral stress coping style, anxiety-like behaviors, and acute stress reactivity of the hypothalamic-pituitary-adrenal (HPA) axis. Male Sprague-Dawley rats were exposed to CAPS treatment, consisting of chronic intermittent cold stress (4°C, 6hrs/day, 14 days) followed on day 15 by a single 1-hr session of sequential acute stressors (social defeat, immobilization, swim). After CAPS or control treatment, different groups were tested for shock probe defensive burying, novelty suppressed feeding, or evoked activation of adrenocorticotropic hormone (ACTH) and corticosterone release by an acute immobilization stress. CAPS resulted in a decrease in active burying behavior and an increase in immobility in the shock probe test. Further, CAPS-treated rats displayed increases in the latency to feed in the novelty suppressed feeding test, despite an increase in food intake in the home cage. CAPS treatment also reduced the HPA response to a subsequent acute immobilization stress. These results further validate CAPS treatment as a rat model of relevance to PTSD, and together with results reported previously, suggest that CAPS impairs fear extinction, shifts coping behavior from an active to a more passive strategy, increases anxiety, and alters HPA reactivity, resembling many aspects of human PTSD.
Keywords: PTSD, stress, coping style, anxiety, HPA-axis
INTRODUCTION
Post-traumatic stress disorder (PTSD) is a disabling illness that occurs after exposure to a severe stress, e.g., a life-threatening event or witnessing such an event. PTSD is characterized by three classes of symptoms: re-experiencing, avoidance, and hyper-arousal (American Psychiatric Association, 2000). Re-experiencing involves intrusions of vivid memories and dreams, and even dissociations, related to the traumatic event. Avoidance of situations or stimuli that serve as reminders of the traumatic event may also be manifest as a general emotional and social detachment. Hyper-arousal is expressed as elevated anxiety, enhanced startle, irritability, sleep disturbance, and difficulty concentrating. Rape, physical attacks or abuse, threats with a weapon, and combat are some of the events typically associated with PTSD (Kessler et al., 1995). Chronic PTSD represents a significant health concern, not only because of the disabling nature of the symptoms, but also because of the long-term consequences on physical health, including higher rates of chronic disease, such as cardiovascular disease, diabetes, asthma, and obesity, as well as higher rates of substance abuse (Centers for Disease Control and Prevention, 2006; Sareen et al., 2005). Chronic stress is also a risk factor, and possibly a causal factor, in the development of depressive and anxiety disorders (Breslau et al., 1999; Gilmer et al., 2005; Jordanova et al., 2007; Kendler et al., 1999; Koenen et al., 2007, 2002).
The complex nature of stress is particularly salient in wartime situations, in which there is a chronic state of environmental stress punctuated by intense, acute traumatic events. To model this, we developed a stress treatment that we have termed Chronic plus Acute Prolonged Stress (CAPS; not to be confused with the “CAPS” assessment used in human PTSD research). CAPS treatment combines 14-days of exposure to a chronic mild environmental stressor (chronic intermittent cold stress), followed on day 15 by a single session of intense acute stressors adapted from the Single Prolonged Stress (SPS) model (Yamamoto et al., 2009).
We have shown previously that CAPS treatment impaired fear extinction (Green et al., 2011), arguably an important component of human PTSD that may contribute to treatment resistance. For instance, PTSD patients show impairments in extinction (Blechert et al., 2007; Wessa and Flor, 2007), and they are incapable of suppressing fear responses in the presence of a safety signal, despite awareness of the safety signal and its meaning (Jovanovic et al., 2009). We also showed that CAPS treatment resulted in a down-regulation of glucocorticoid receptors (GR) in the medial prefrontal cortex (mPFC) (Green et al., 2011). This could have contributed to the impairments observed during extinction testing, as glucocorticoids are known to be involved in learning and memory, including fear and extinction learning (Gourley et al., 2009; de Quervain et al., 2009; Roozendaal et al., 2004; Roozendaal et al., 2006). The mPFC, particularly the infralimbic cortex, is a key region involved in extinction learning (Milad and Quirk, 2002; Milad et al., 2004; Morgan et al., 1993; Quirk et al., 2000; Sutker et al., 1995). Furthermore, humans with PTSD display dysregulated hypothalamic-pituitary-adrenal (HPA) axis activity, although the nature of this dysregulation remains unclear (Boscarino, 1996; Hoffman et al., 1989; Lemieux and Coe, 1995; Mason et al., 1986; Pitman and Orr, 1990; Yehuda et al., 1995, 1993,1990).
Having defined some key components of PTSD in this model, in the present experiments, we continued to explore the effects of CAPS on other measures of PTSD-like symptomotology, including coping style/defensive behavior and generalized anxiety, as well as acute HPA stress reactivity. In our previous study, we observed that CAPS, particularly when combined with early life stress, resulted in persistent freezing during fear conditioning, at a point when other rats were shifting to a more active escape strategy (rearing and jumping) (Green et al., 2011). Thus, using the shock probe-defensive burying test in the present experiment, we tested the hypothesis that CAPS would produce a shift from an active coping strategy (burying) to a passive coping strategy (immobility). Likewise, we examined if CAPS would increase anxiety-like behavior in the novelty suppressed feeding test (NSFT). Finally, we tested if CAPS treatment produced changes in the HPA response evoked by an acute stressor.
2. EXPERIMENTAL PROCEDURES
2.1 Animals
In total, 103 adult male Sprague-Dawley rats were used in these experiments. The rats were born in our animal facility, and after weaning, they were pair-housed with a same-sex littermate until postnatal day (PD) 46–60, depending on the experiment, at which time they were singly housed prior to starting the adult stress or unstressed control treatments. The rats were housed in Plexiglas cages (25 × 45 × 15 cm) on a 12/12 hr light-dark cycle (lights on at 07:00) with food and water available ad libitum. In addition, for the social defeat procedure, 12 adult male Long–Evans rats (Harlan, Indianapolis, IN), weighing at least 400 g, were used as defeaters. They were housed, together with an ovariectomized female, in large resident cages (60 × 60 × 35 cm) in a separate room on the same 12/12 hr light cycle. All experiments were conducted during the light phase. All procedures were conducted according to NIH guidelines for the care and use of laboratory animals and were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio. All efforts were made to minimize animal pain, suffering or discomfort, and to minimize the number of rats used.
2.2 CAPS
CAPS treatment consisted of 2 weeks of chronic intermittent cold stress followed by a single 1-hr session of acute prolonged stress on day 15. For cold stress, rats were transported in their home cage with food, water and bedding into a cold room at 4°C for 6 hrs per day for 14 days. The acute prolonged stress on day 15 consisted of 20 min social defeat, followed immediately by 30 min immobilization, and then 10 min swim stress. For social defeat, the ovariectomized Long-Evans female was removed from the resident cage, and the test rat was placed in the cage with the resident Long Evans male rat. Typically within 10–30 sec, the resident would attack and defeat the smaller “intruder” Sprague-Dawley test rat. Once defeat occurred, defined by the test rat assuming a supine posture and the resident showing a dominant posture for at least 4 sec, the test rat was placed under a wire mesh cage for 20 min, thus protecting it from further physical contact but allowing continued sensory exposure to the dominant rat. Immobilization involved taping the rat’s torso and limbs gently but snugly in a prone position on a flat platform, allowing no movement, for 30 min. Finally, swim stress was accomplished by placing the rat in a cylindrical tank (30 cm diameter × 60 cm height) filled to a depth of 30 cm with water at approximately 23°C. Control rats were handled briefly for approximately 30 sec.
2.3 Experiment 1: Shock-probe Defensive Burying Test
CAPS treatment was initiated between PD 51–53 (n = 9/group). One day following the end of CAPS (or the comparable time for controls), rats were tested in the shock probe defensive burying test to evaluate potential shifts in active and passive behavioral coping strategies in response to acute stress. The rats were placed into a modified cage containing 5 cm of bedding, with a shock probe protruding 6 cm into one end of the cage. The probe was set to deliver 2 mA of current when the probe was touched. After the rat made contact with the probe and received a shock, the current was shut off and the 15 min test began. Behavior was recorded using a CCD camera mounted above the cage and stored to video files for offline scoring and analysis. The dependent measures analyzed were the amount of time spent immobile and the amount of time spent engaged in actively burying the probe. Burying was defined as behavior consisting of burrowing into the bedding with the snout and upper body, then plowing, pushing, or shoveling the bedding toward the probe, and also flicking or spraying bedding material toward the probe. Immobility was defined as a lack of movement other than that required for breathing (slight scanning movements of the head were permitted). Behavior clearly identified as resting behavior (e.g., laying on side, legs extended) was excluded from immobility measures. As a proportional measure of preferred response, the bury time ratio was calculated as (time spent burying)/(time spent burying + time spent immobile).
2.4 Experiment 2: Novelty Suppressed Feeding Test (NSFT)
CAPS was initiated on PD 47 (n = 14–15/group). Following CAPS (or the comparable time period for controls), the rats were left undisturbed for 2 days. Beginning on the 3rd day, the animals were food deprived for 48 hrs (water was available ad libitum). The test was conducted on the 5th day post-CAPS, as described by Bodnoff and colleagues (1988), with minor modification. The rats were transferred to the behavior room and allowed 1 hr to acclimate. The rats were then individually placed into a corner of an unfamiliar black Plexiglas open field (100 × 100 × 40cm) facing the center where food pellets were placed. The latency to begin feeding and the amount of food consumed during the 12 min test were recorded. Latency to feed was defined as the time from when the rats were placed into the open field until they began to eat the pellets (not just approach or play with them). Following the test, the rats were returned to their home cage, where food consumption was monitored for another 30 min to determine if there were any changes in appetitive behavior. Food consumption was determined by subtracting the weight of any remaining food from the total weight of food placed in the open field and the home cage.
2.5 Experiment 3: Evoked HPA Responses to Acute Immobilization Stress
CAPS was initiated between PD 46–60 (n = 7–14/group). Group assignments were matched to balance the range of ages at which CAPS was initiated across all groups. Three days prior to the acute prolonged stress (Day 12 of CAPS or the comparable time for controls), all rats underwent jugular catheterization surgery. Rats were anesthetized with a mixture of ketamine 43 mg/ml, acepromazine 1.4 mg/ml, xylazine 8.6 mg/ml, administered i.m. at 1.0 ml/kg, and a catheter comprised of silastic and PE50 tubing was inserted into the jugular vein, then passed subcutaneously and exteriorized via an incision at the back of the neck and plugged. Every 3rd day until testing the catheter was flushed with approximately 0.2 mL of sterile heparinized saline (50 IU/ml) to maintain patency.
Separate groups were tested 1 or 5 days following the termination of CAPS. On the test day, the rats were transported to a quiet room, the catheter was connected via a fluid filled line to a syringe for remote blood collection without disturbing the animal, and approximately 0.1 mL of heparinized saline was administered to ensure patency. The rats were then given 90 minutes to acclimatize after transport. For blood sampling, 0.4 mL of blood was withdrawn via the catheter and replaced with 0.4 mL of sterile saline. Two baseline blood samples were collected 15 min apart. The rats were then immobilized for 30 min as described in Section 2.2. Two blood samples were collected during the stress, one at 5 min after the onset of stress, and one at 30 min. Following the 30 min stress sample, the rats were returned to their home cages and allowed to recover, during which time 4 blood samples were collected at 15, 30, 60, and 90 min post-stress. Blood was collected into tubes containing 10 μl of 0.5 M EDTA. Plasma was separated immediately by centrifugation at 10,000 rpm for 15 min at 4°C, and stored at −80°C until assayed.
Because the CAPS protocol includes a single 30-min immobilization stress, it was possible that any changes observed during the acute stress exposure on test day could be due to habituation or sensitization to the second presentation of immobilization stress, rather than an effect of CAPS specifically. Therefore, a control experiment was conducted in which 2 separate groups of rats (n = 8–10) were exposed to a single 30 min immobilization stress rather than the full CAPS procedure, 3 days after catheterization surgery. A third group was briefly handled but not immobilized. Then, 1 or 5 days later, all rats were exposed to an acute 30-min test immobilization, and blood samples were collected as above.
Plasma levels of ACTH and CORT were analyzed by radioimmunoassay. ACTH was determined from duplicate 100μl samples according to the manufacturer’s instructions (MP Biomedicals, Orangeburg, NY). The detection limit was 6 pg/ml, and the inter-assay variability was 10%. CORT was measured in diluted plasma samples according to the manufacturer’s instructions (MP Biomedicals). Detection limit was 8 ng/ml, and inter-assay variability was 8%.
2.6 Statistical Analyses
For the shock-probe defensive burying data, differences in immobility time, active burying time, and bury-time ratio were analyzed by t-test. Likewise, in the novelty suppressed feeding test, differences between groups in latency to feed and amount of food consumed were analyzed by t-test. Plasma hormone measures were analyzed by 2-way analysis of variance (ANOVA; group x sample, with repeated measures). In all analyses, significance was determined at p < 0.05. After ANOVA, sources of any significant main effects or interactions were determined by analysis with the Newman-Keuls post-hoc test.
3. RESULTS
3.1 Experiment 1: Shock-probe Defensive Burying Test
CAPS-treated rats displayed significantly less burying behavior than control rats (Fig. 1A, t(16) = 2.258, p < 0.05) and significantly more immobility (Fig. 1B, t(16) = 2.963, p < 0.01). Consequently, CAPS-treated rats displayed a significantly lower bury-time ratio than control rats (Fig. 1C, t(16) = 4.169, p < 0.001). This reduction in bury ratio reflects a shift from a predominantly active behavioral coping strategy to a predominantly passive coping strategy.
Figure 1.
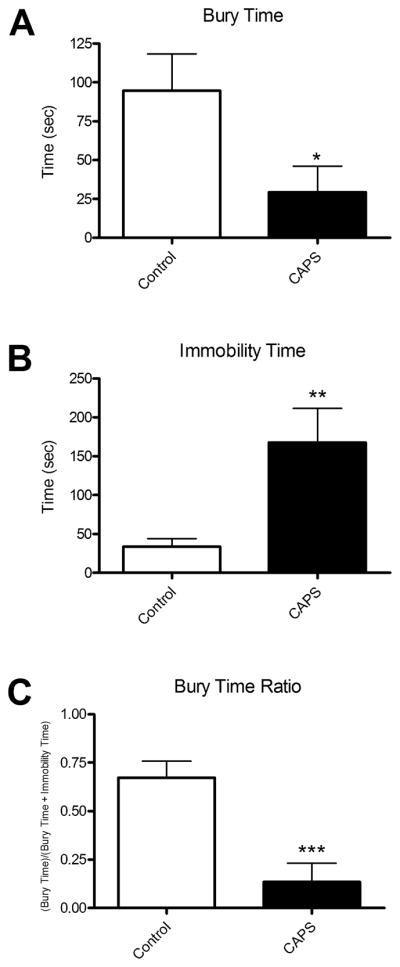
Effect of CAPS on active defensive burying behavior and passive immobility in the shock-probe defensive burying test. A) On the shock-probe test, CAPS-treated rats displayed significantly less active burying behavior (29.33 ± 16.77 s) than unstressed control rats (94.67 ± 23.58 s). B) CAPS-treated rats displayed significantly more immobility (167.60 ± 43.98 s) in response to a single, brief mild shock than did the non-stressed controls (33.67 ± 10.39 s). C) Consequently, CAPS-treated rats had a lower bury ratio (0.13 ± 0.10) than control rats (0.67 ± 0.09), reflecting a shift from a predominantly active behavioral coping strategy (ratio > 0.5) in the control group to a predominantly passive strategy (ratio < 0.5) following CAPS treatment. *p < 0.05, **p < 0.01, ***p < 0.001. Data expressed as mean ± SEM, n=9/group.
3.2 Experiment 2: Novelty Suppressed Feeding Test
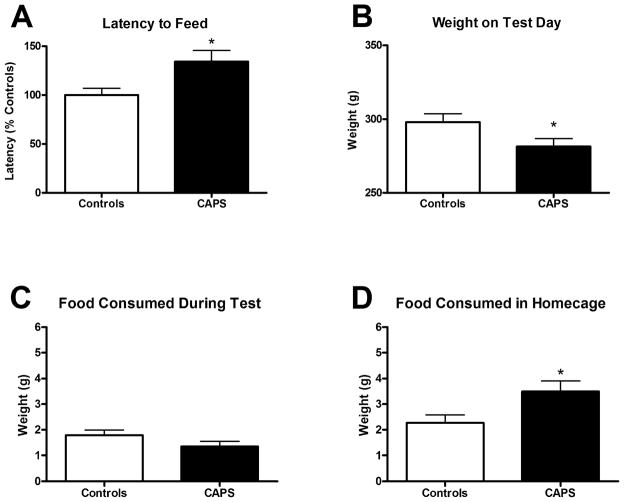
CAPS-treated rats displayed a significantly longer latency to feed (Fig. 2A, t(27) = 2.532, p < 0.05). CAPS treatment reduced weight gain during the treatment, as these rats had lower mean body weight (281.5 ± 5.3 g) than controls (298 ± 5.6 g) prior to testing (Fig. 2B, t(27) = 2.135, p < 0.05), as expected after chronic cold stress. Nonetheless, there was no difference in the amount of food consumed during the test period (Fig. 2C; t(27) = 1.54, p > 0.05), and CAPS-treated rats consumed slightly more food than controls in their home cage (Fig. 2D, t(27) = 2.451, p < 0.05). The fact that CAPS-treated rats consumed equivalent amounts of food during the test, and more food than controls in the home cage indicates that the increase in latency to feed was not due to a reduction in appetite, but to an increase in anxiety in the novel environment.
Figure 2.
Effect of CAPS on latency to feed in the novelty suppressed feeding test. A) CAPS-treated rats displayed a longer latency to begin eating (134.3 ± 11.4 s) compared to controls (100.0 ± 6.9 s). B) As expected, CAPS-treated rats had lower mean body weight on test day (281.5 ± 5.3 g) compared to controls (298 ± 5.6 g). C) CAPS-treated rats and control rats consumed an equivalent amount of food during the test (1.35 ± 0.20 g and 1.79 ± 0.20 g, respectively). D) In fact, CAPS-treated rats displayed increased food consumption in the home cage after the test (3.5 ± 0.4 g) compared to control rats (2.3 ± 0.3 g), perhaps related to the reduction in body weight gain during CAPS treatment (panel B). Thus, the increase in latency to feed was not attributable to a loss of appetite, but to an increase in anxiety in the novel environment. *p < 0.05. Latency expressed as mean percent of controls ± SEM. Feeding expressed as mean ± SEM, n = 14–15/grp.
3.3 Experiment 3: Evoked HPA Response to Acute Immobilization Stress
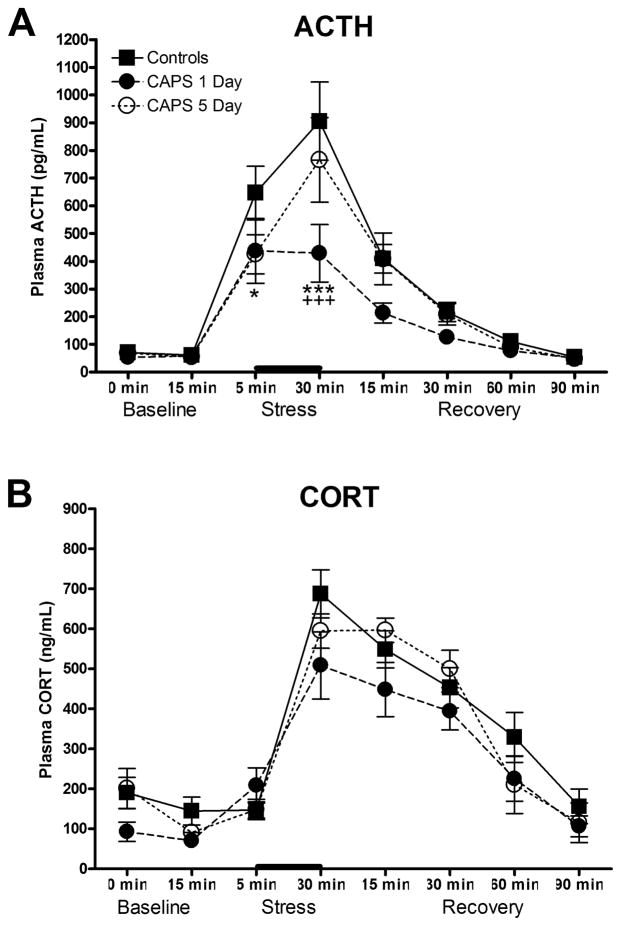
Acute immobilization stress induced a significant increase in both ACTH and CORT (F(7,168) = 47.8, p < 0.0001; F(7,182) = 62.05, p < 0.0001, respectively; significance not indicated in Fig. 3 for clarity). There was a significant main effect of Group on ACTH release (Fig. 3A, F(2,24) = 3.469, p < 0.05) and a Group x Sample interaction (F(14, 168) = 2.345, p < .01). Specifically, CAPS-treated rats displayed a blunted ACTH response to acute stress. Post-hoc analyses revealed that the 1-day post-CAPS group was significantly different from the control group on the 5- and 30-min stress samples, and significantly different from the 5-day post-CAPS group on the 30-min stress sample. The effect on the 5-min stress sample for the 5-day post-CAPS group approached significance (p = 0.0505). Thus, the most robust effect of CAPS on the subsequent ACTH response to an acute immobilization stress occurred one day after the CAPS procedure, and by 5 days, the ACTH response was returning towards that seen in control rats. Although CORT levels in the 1-day post-CAPS group were slightly but consistently lower than in controls, this was not a significant reduction (Fig. 3B, F(2,26) = 1.542, p > 0.05 for main effect of stress; F(14, 182) = 1.228, p > 0.05 for stress x sample interaction).
Figure 3.
Effect of CAPS on HPA stress reactivity in response to acute immobilization stress. A) CAPS-treated rats displayed a blunted ACTH response to a subsequent acute stressor (bar), particularly in the group tested on day 1 post-CAPS. B) There were no significant differences between groups on the acute CORT response to immobilization stress, although there was a modest decrease in the group tested on day 1 post-CAPS. *p < 0.05 1-day post-CAPS vs Controls, *** p < 0.001 1-day post-CAPS vs Controls, +++p < 0.001 1-day post-CAPS vs 5-days post-CAPS. Data expressed as mean ± SEM, n = 7–14/grp.
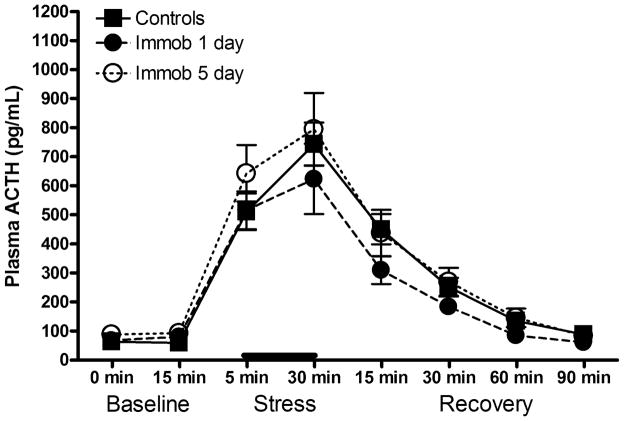
The control experiment, conducted to ensure that the blunted ACTH response in CAPS-treated rats was not due simply to habituation to a second exposure to immobilization stress, showed no effect of prior immobilization on ACTH release in response to the test immobilization stress (Fig. 4, F(2,24) = 1.162, p > 0.05), nor was there a group x sample interaction (F(14,168) = 0.871, p > 0.05). Because the effect of CAPS on the ACTH response in experiment 3 was seen only at 1-day post-CAPS, we also analyzed the results of the control experiment for day 1 only, excluding day 5. There was still no effect of prior immobilization (F(1,17) = 1.161, p > 0.05) nor an interaction (F(7,119) = 1.224, p > 0.05). Thus, these results suggest that the changes in evoked ACTH response following exposure to CAPS were due to the CAPS treatment specifically, and not due simply to a second exposure to immobilization. Because the effect of CAPS on CORT did not achieve significance in experiment 3, CORT was not analyzed in the control experiment.
Figure 4.
Lack of effect of a single prior exposure to acute immobilization stress on the subsequent ACTH response to a second immobilization stress. There was no effect of prior immobilization on ACTH release evoked by a second immobilization stress (bar) administered either 1 or 5 days later, suggesting that the effect seen in Fig. 3 was due to CAPS exposure, specifically. Data expressed as mean ± SEM, n= 8–10/grp.
4. DISCUSSION
In the present set of studies, CAPS-treated rats displayed a shift from an active to a passive coping style, and an increase in anxiety-related behavior. These behavioral effects co-occurred with a blunted ACTH response to acute stress. In addition to these changes, we have previously reported that CAPS impaired fear extinction and reduced GR expression in the mPFC (Green et al., 2011).
4.1 Passive Coping
In our previous report (Green et al., 2011), prenatal stress (prior to CAPS exposure) did not alter the preference for active coping relative to passive coping on the shock-probe defensive burying test. However, after CAPS exposure, we noted a different behavioral profile during fear conditioning. After multiple tone-shock pairings, control rats begin to show a decrease in freezing, which appears to be due to a shift in behavioral response to the tone, away from the passive freezing response to more active escape behaviors, including rearing and jumping. In that study, rats exposed to prenatal stress and CAPS as adults did not display this shift. Rather, they continued to display high levels of freezing. While this observation is anecdotal, and behavior observed during fear-conditioning is not a validated measure of coping style, this led us to hypothesize that CAPS might produce a shift from active coping to a more passive coping strategy. This hypothesis was tested explicitly in the present study using the shock probe defensive burying test, in which rats can exhibit 2 qualitatively different types of behavioral responses to the shock probe in varying proportions—an active response (burying the probe) and a passive response (immobility). Control rats displayed a slight preference for active coping behavior (burying), whereas CAPS-treated rats displayed a substantial shift to a strong preference for passive coping behavior (immobility). The increase in immobility cannot be explained by an overall decrease in locomotor activity, as we previously showed that CAPS-treated rats displayed no change in exploration in an open field (Green et al., 2011).
Coping style can mitigate the physiological impact of stress, and there is evidence from both animal and human research that active coping is more adaptive. Previous research has shown that when a rat is given the option to engage in an active coping response, such as chewing on a dowel during immobilization, the stress response is reduced (e.g., Hori et al., 2004; Ono et al., 2008). By contrast, when a rat is deprived of an active response option, such as removing the bedding during the shock probe test so the rat cannot bury, the physiological stress response is increased (Bondi et al., 2007). Likewise, rats that show a low bury response have higher CORT responses during the test (for review see Koolhaas et al., 1999).
A shift to immobility in the shock probe test resembles the “learned helplessness” phenomenon described in both the human and animal psychological literature. Passive responding and failure to engage active coping responses has long been demonstrated in a number of animal models, originally in dogs (e.g., Seligman et al., 1968) and then rodents (e.g., Maier, 1984). The learned helplessness model shares some characteristics with the CAPS model. For example, rodents exposed to inescapable tail shock display less aggression in a shock-elicited aggression test (similar to our finding of reduced active burying in the shock-probe defensive burying test) and reduced intruder attack by alpha males (Maier, 1984). These rats also display cognitive deficits, including more errors in tests involving learning contingencies (Maier, 1984) and delayed contextual fear extinction (Baratta et al., 2007). Again, this is similar to our previous finding that CAPS treatment impaired fear extinction.
Passivity may contribute to maladaptive stress responses. Consistent with this, studies with animals and humans have shown that active, stimulus-based, or problem-oriented coping styles, as opposed to more passive, emotion-based, avoidant coping styles, buffer HPA activation in response to the stressor/stimulus, increase the ability to eliminate the threat, and improve long-term mental and physical health outcomes (for review see, Koolhaas et al., 1999; Olff et al., 2005). On the other hand, in humans, the negative thought patterns related to many affective disorders often contribute to the perception that there is no way out of a stressful situation and little or no control over one’s situation and environment. Emotional withdrawal is a key symptom in the diagnosis of PTSD (American Psychiatric Association, 2000), and studies have shown that individuals previously exposed to traumatic events show greater levels of introversion, social isolation, and emotional blunting (e.g., Bunce et al., 1995). A predisposition for withdrawal may actually contribute to the development of PTSD, and greater symptom expression over time. For example, traumatized individuals who show a shift toward passive coping styles, including withdrawal, are more likely to develop PTSD at 3 months post-trauma (Gutner et al., 2006). Furthermore, men who were abused as children and display high levels of introversion and withdrawal are more likely to meet thresholds for clinical diagnosis of PTSD in adulthood (O’Leary, 2009). Similarly, individuals who report peri-traumatic feelings of helplessness are more likely to develop PTSD (Beck et al., 2006; Hari et al., 2010; O’Donnell et al., 2010).
4.2 Anxiety
We previously examined the effect of CAPS on anxiety-related behavior in an open field and found no differences. In the present experiment, we examined potential anxiogenic effects of CAPS using a more robust test of anxiety involving an approach-avoidance conflict, the novelty suppressed feeding test. In this test, food deprived rats must enter an anxiety-provoking environment to obtain food. Previous studies with the NSFT have shown that chronic stress results in longer latency to approach the food and begin eating, particularly in more passive, “low responder” rats (Stedenfeld et al., 2011), and that antidepressant treatment reduces the expression of anxiety-related behaviors in this test (Furmaga et al., 2011; Ibarguen-Vargas et al., 2009). Similarly, CAPS-treated rats displayed an increased latency to feed in the novel environment, reflecting greater anxiety, despite an increase in total food intake.
Anxiety is an important component of most animal models of human stress-related psychiatric disorders, as anxiety is a key element of such disorders, including PTSD. Further, PTSD is highly comorbid with other anxiety disorders, and also with depression (Kessler et al., 1995; Rush et al., 2005). These disorders are all notable for an extensive degree of overlap in symptomatology, including, for example, irritable mood, difficulty concentrating, and sleep disturbances. Further, antidepressants are also effective pharmacological treatment for many anxiety disorders (for review, see Morilak and Frazer, 2004). Thus, there are likely to be common neurobiological mechanisms and similar psychopathological processes underlying these shared symptoms.
4.3 Acute HPA stress-reactivity
CAPS treatment reduced the acute ACTH stress response, especially on day 1 after the termination of CAPS. The control experiment confirmed that this was not merely due to habituation to the prior exposure to immobilization stress on day 15 of CAPS treatment. There was also a slight but non-significant suppression of the CORT response to acute stress, and during the post-stress recovery period. It is not clear why the effect of CAPS on the acute CORT response was less robust than on the ACTH response. It may simply be due to differences in the temporal sensitivity of these measures. ACTH is rapidly and dynamically reactive. However, with CORT being slower to respond and slower to clear, a sample at any given time point represents a cumulative response. Thus, differences may have been obscured. On the other hand, it is possible that the adrenal glands may have been sensitized by the previous stress exposure, resulting in greater CORT release in response to ACTH, thus compensating in part for the reduction in evoked ACTH levels. Previous research has shown that chronic stress increases adrenal mass, which may contribute to such sensitization (e.g., Blanchard et al., 1998; Hauger et al., 1990). Another possibility may be related to intensity of the stress induced by immobilization. The HPA response to immobilization was very robust, and may have masked a modest difference between CORT responses in Control and CAPS-treated rats. It may be informative to employ a milder probe stimulus in future studies. Finally, it is important to note that the baseline CORT levels in this experiment were higher than those reported in our previous study (Green et al., 2011). This is likely attributable to differences in methodology. In the present study, rats were exposed to surgery, and then on the test day to handling and a novel environment, all of which can elevate baseline CORT levels, even with a period of acclimation. In the previous study, CORT levels were measured in trunk blood samples collected by rapid decapitation immediately after removal from their home cages.
The changes observed in acute HPA axis stress reactivity are interesting in light of the human PTSD literature. Evidence suggests that HPA activity is reduced in PTSD. However, the full HPA axis profile of individuals with PTSD is not clear, and there are many inconsistencies in the literature (for discussion, see de Kloet et al., 2006). Some studies have shown urinary and plasma cortisol levels to be lower in PTSD patients compared to controls (Boscarino, 1996; Mason et al., 1986; Yehuda et al., 1995, 1993, 1990), and these hormone levels may be negatively correlated with symptom severity (Olff et al., 2006). Further, individuals with lower CORT levels at the time of the post-trauma emergency room visit are more likely to develop PTSD (Delahanty and Nugent, 2006). Reduced hormonal responses may be due to a sensitized negative feedback mechanism, as PTSD patients tend to show greater ACTH suppression by dexamethasone (e.g., Duval et al., 2004). The present results are in line with these findings.
Few animal models of stress have replicated the HPA-axis characteristics of human PTSD, as the typical effect of chronic stress exposure in rodent models is sensitization of the HPA response to acute stress, if any change is observed at all. Rimanoczy and colleagues (2003) showed that prenatal stress exposure to morphine resulted in a suppressed ACTH response to restraint in adulthood, while maintaining a normal CORT response, similar to the effect seen in our study. Similarly, the SPS model, from which the acute component of our CAPS model was adapted, enhanced HPA suppression in response to dexamethasone treatment (Yamamoto et al., 2009). Further, rats exposed to SPS also display a blunted CORT response to a subsequent acute stressor (Harvey et al., 2006).
By comparison, varying alterations in ACTH and/or CORT have been reported in studies employing the widely-used Chronic Variable Stress (CVS)/Chronic Mild Stress (CMS) model. Most have shown either no change or an increase in basal ACTH (e.g., Choi et al., 2008a,b; Kioukia-Fougia et al., 2002; Ostrander et al., 2006) and no change or an increase in basal CORT (e.g., Choi et al., 2008a,b; Christiansen et al., 2012; Ostrander et al., 2006; Wu and Wang, 2010). Blunting of circadian cycles have been reported (Christiansen et al., 2012). Changes in HPA response to acute stress challenge after CVS/CMS are variable. One study reported an increased ACTH response to acute restraint stress, but no change in CORT response (Choi et al., 2008b). In another, an increase in ACTH response to a mild novelty stress was seen 1 day after CVS, which returned to normal on day 4 post-CVS, followed by a decrease in ACTH response on day 7, returning to baseline by day 30 (Ostrander et al., 2006). As in the present study (and in Choi et al., 2008b), the CORT response did not match the ACTH response. There was no change in the CORT response one day post-CVS, a decrease at days 4 and 7, then a return to normal by day 30. By contrast, when this same group challenged with a systemic stressor (hypoxia), the effect was similar to that seen in the present study, a decrease in ACTH response one day post-CVS, which returned to normal on day 4, with no change in CORT. ACTH then increased on day 7 post-CVS, again with no comparable change in CORT. Other factors that can affect changes in hormonal response after stress are anhedonia-like traits (Christiansen et al., 2012) and strain differences (Wu and Wang, 2010). In most chronic stress models, regardless of the nature of the change in HPA response, it is important to note that, as in the present study, effects were transient, and changes in ACTH and CORT responses are often dissociated.
These results would suggest that an HPA regulatory process that blunts the ACTH response to a subsequent acute stressor emerges in response to chronic stress, then dissipates over time when the stress ceases. In humans with PTSD, even after termination of the primary stressor, the cognitive process of re-experiencing may become a secondary chronic stressor on its own, maintaining the dysregulatory process. Thus, animal models may be particularly useful in revealing mechanisms by which pathological processes after traumatic stress are initiated, and in identifying unique mechanisms by which HPA responses may be inhibited in PTSD, as opposed to the hyperactive HPA axis often seen in other chronic stress-related mood disorders, such as depression.
Despite the transient effect of CAPS on the HPA response to acute stress, behavioral effects were evident at all time points. We examined the effects of CAPS on shock-probe defensive burying behavior on day 1 post-stress, comparable to when we observed the greatest ACTH suppression. However, the need for food restriction in the novelty-suppressed feeding test, and the desire to avoid confounding stress with food deprivation, necessitated testing on day 5 post-stress. In both cases, at 1 day and 5 days post-stress, we observed behavioral effects of CAPS treatment. In general, then, it appears that although the HPA effects begin to recover by day 5, the behavioral effects are evident at day 1 (increased passive coping in shock probe defensive burying), day 2 (increased freezing in fear conditioning, Green et al., 2011), and still present at day 5 (increased anxiety in NSFT, and impaired fear extinction, Green et al., 2011).
4.4 Conclusion
Valid animal models of human psychopathology must be based on a theoretical framework that shares a fundamental aspect of the human disorder, and they must show behavioral and biochemical features that resemble those in the human disorder (Willner, 1986). One requirement for a diagnosis of PTSD is experience of a traumatic event (American Psychiatric Association, 2000). This was modeled by the CAPS treatment in the present study, involving a low-level chronic “state” of stress, followed by a highly salient and intense acute stress experience, which may model the kinds of experiences that initiate PTSD, particularly in combat veterans. Chronic stress is correlated with vulnerability to PTSD (Breslau et al., 1999; Koenen et al., 2007, 2002), and in combat situations, chronic stress, punctuated by acute traumatic events, is the norm. Further, once the trauma has been experienced, an exaggerated and persistent fear response is arguably the fundamental aspect of PTSD (American Psychiatric Association, 2000), and this may be prolonged by impairments in extinction learning (Blechert et al., 2007; Wessa and Flor, 2007; Jovanovic et al., 2009). In our previous report, we showed that CAPS exposure enhanced freezing during fear conditioning and impaired extinction. Further, in the present study, CAPS resulted in other PTSD-like symptoms, including: anxiety; a shift from effective active coping to less adaptive passive coping; and HPA axis dysregulation.
The brain mechanisms that underlie these effects remain to be elucidated. CAPS is a combination of chronic metabolic stress (chronic cold) and a single session of intense acute stress that was adapted from the single-prolonged stress model (SPS; Yamamoto et al., 2009, 2010). Each of these components may have neurobiological consequences that contribute to the resulting phenotype. SPS has been shown to increase inhibitory avoidance, decrease extinction, and increase acoustic startle (Yamamoto et al., 2010; Ganon-Elazar and Akirov, 2012; Knox et al., 2012). This may be due, in part, to reduced excitatory neurotransmitter tone in the PFC and hippocampus, as SPS decreased glutamate and creatine in the PFC, and increased glycine transporter expression in the ventral hippocampus (Yamamoto et al., 2010; Knox et al, 2010). Chronic cold has been shown to impair cognitive flexibility and to decrease serotonin release in the orbital frontal cortex (Lapiz-Bluhm et al., 2009). Changes in prefrontal executive function could compromise the ability to regulate or select from among possible responses in fear- or anxiety-provoking situations. Chronic cold stress alone has been shown to sensitize the ACTH response to immobilization stress (Ma and Morilak, 2005), whereas SPS increased negative feedback inhibition of ACTH release (Liberzon et al., 1997), similar to the blunted ACTH response in the present study. Thus, the phenotype of CAPS-treated rats appears to be a combination of acute and chronic stress effects, perhaps involving changes in modulatory neurotransmission in the prefrontal cortex, consistent with our previous observations of altered GR expression following CAPS treatment (Green et al., 2011). This further suggests that drugs that modulate monoaminergic transmission, glucocorticoid activity, or excitatory amino acid signaling may represent viable strategies for treatment and symptom management of PTSD. Interestingly, it was recently reported that the SPS-induced increase in glycine transporter expression in the hippocampus was normalized with repeated extinction training, perhaps identifying a mechanism by which therapeutically effective behavioral interventions can also mitigate the effects of chronic stress (Yamamoto et al., 2010).
In sum, the CAPS model may prove useful as a valid animal model with which to investigate neurobiological mechanisms underlying pathophysiological changes associated with PTSD, or mechanisms of novel therapeutic strategies for PTSD.
Highlights.
Characterizes a Chronic plus Acute Prolonged Stress (CAPS) rat model of PTSD
CAPS produces a shift from active coping to passive coping
CAPS increases anxiety-related behavior
CAPS blunts the ACTH response to a subsequent acute stressor
CAPS may be useful to study mechanisms and novel treatments of disorders such as PTSD
Acknowledgments
This work was supported by a NIMH National Research Service Award individual postdoctoral fellowship F32 MH090693 (to MKR), by NIMH research grant R01 MH053851 (to DAM), and by funding provided to the STRONG STAR Multidisciplinary PTSD Research Consortium by the Department of Defense through the U.S. Army Medical Research and Materiel Command, Congressionally Directed Medical Research Programs, Psychological Health and Traumatic Brain Injury Research Program award W81XWH-08-2-0118. The views expressed in this paper are solely those of the authors and do not reflect an endorsement by or official policy of the Department of Defense or the U.S. Government.
Abbreviations
- ACTH
adrenocorticotropic hormone
- CAPS
chronic plus acute prolonged stress
- CORT
corticosterone
- GR
glucocorticoid receptor
- HPA
hypothalamic-pituitary-adrenal
- mPFC
medial prefrontal cortex
- NSFT
novelty suppressed feeding test
- PD
postnatal day
- PTSD
posttraumatic stress disorder
- SPS
single prolonged stress
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington, D.C: 2000. text rev. [Google Scholar]
- Baratta MV, Christianson JP, Gomez DM, Zarza CM, Amat J, Masini CV, Watkins LR, Maier SF. Controllable versus uncontrollable stressors bi-directionally modulate conditioned but not innate fear. Neurosci. 2007;146:1495–1503. doi: 10.1016/j.neuroscience.2007.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck JG, Palyo SA, Canna MA, Blanchard EB, Gudmundsdottir B. What factors are associated with the maintenance of PTSD after a motor vehicle accident? The role of sex differences in a help-seeking population. J Behav Ther. 2006;37:256–266. doi: 10.1016/j.jbtep.2005.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard RJ, Nikulina JN, Sakai RR, McKittrick C, McEwen B, Blanchard DC. Behavioral and endocrine change following chronic predatory stress. Physiol Behav. 1998;63:561–569. doi: 10.1016/s0031-9384(97)00508-8. [DOI] [PubMed] [Google Scholar]
- Blechert J, Michael T, Vriends N, Margraf J, Wilhelm FH. Fear conditioning in posttraumatic stress disorder: evidence for delayed extinction of autonomic, experiential, and behavioral responses. Behav Res Ther. 2007;45:2019–2033. doi: 10.1016/j.brat.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Bodnoff SR, Suranyi-Cadotte B, Aitken DH, Quirion R, Meaney MJ. The effects of chronic antidepressant treatment in an animal model of anxiety. Psychopharmacology. 1988;95:298–302. doi: 10.1007/BF00181937. [DOI] [PubMed] [Google Scholar]
- Bondi CO, Barrera G, Lapiz MDS, Bedard T, Mahan A, Morilak DA. Noradrenergic facilitation of shock-probe defensive burying in lateral septum of rats, and modulation by chronic treatment with desipramine. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:482–495. doi: 10.1016/j.pnpbp.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Boscarino JA. Posttraumatic stress disorder, exposure to combat, and lower plasma cortisol among Vietnam veterans: findings and clinical implications. J Clin Consult Psychol. 1996;64:191–201. doi: 10.1037//0022-006x.64.1.191. [DOI] [PubMed] [Google Scholar]
- Breslau N, Chilcoat HD, Kessler RC, Davis GC. Previous exposure to trauma and PTSD effects of subsequent trauma: Results from the Detroit area survey of trauma. Am J Psychiatry. 1999;156:902–907. doi: 10.1176/ajp.156.6.902. [DOI] [PubMed] [Google Scholar]
- Bunce SC, Larsen RJ, Peterson C. Life after trauma: personality and daily life experiences of traumatized people. J Pers. 1995;63:165–188. doi: 10.1111/j.1467-6494.1995.tb00806.x. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System Survey Data. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, GA: 2006. [Google Scholar]
- Choi DC, Evanson NK, Furay AR, Ulrich-Lai YM, Ostrander MM, Herman JP. The anteroventral bed nucleus of the stria terminalis differentially regulates hypothalamic-pituitary-adrenocortical axis responses to acute and chronic stress. Endocrinology. 2008a;149:818–826. doi: 10.1210/en.2007-0883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DC, Furay AR, Evanson NK, Ulrich-Lai YM, Nguyen MMN, Ostrander MM, Herman JP. The role of the posterior medial bed nucleus of the stria terminalis in modulating hypothalamic-pituitary-adrenocortical axis responsiveness to acute and chronic stress. Psychoneuroendocrinology. 2008b;33:659–699. doi: 10.1016/j.psyneuen.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen S, Bouzinova EV, Palme R, Wiborg O. Circadian activity of the hypothalamic-pituitary-adrenal axis is differentially affected in the rat chronic mild stress model of depression. Stress. 2012:1–11. doi: 10.3109/10253890.2011.654370. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- de Kloet CS, Vermetten E, Geuze E, Kavelaars A, Heijnen CJ, Westenberg HGM. Assessment of HPA-axis function in posttraumatic stress disorder: pharmacological and non-pharmacological challenge tests, a review. J Psychiatr Res. 2006;40:550–567. doi: 10.1016/j.jpsychires.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Delahanty DL, Nugent NR. Predicting PTSD prospectively based on prior trauma history and immediate biological responses. Ann NY Acad Sci. 2006;1071:27–40. doi: 10.1196/annals.1364.003. [DOI] [PubMed] [Google Scholar]
- de Quervain DJF, Aerni A, Schelling G, Roozendaal B. Glucocorticoids and the regulation of memory in health and disease. Front Neuroendocrinol. 2009;30:358–370. doi: 10.1016/j.yfrne.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Duval F, Crocq M, Guillon M, Mokrani M, Monreal J, Bailey P, Macher J. Increased adrenocorticotropin suppression after dexamethasone administration in sexually abused adolescents with posttraumatic stress disorder. Ann NY Acad Sci. 2004;1032:273–275. doi: 10.1196/annals.1314.036. [DOI] [PubMed] [Google Scholar]
- Furmaga H, Shah A, Frazer A. Serotonergic and noradrenergic pathways are required for the anxiolytic-like and antidepressant-like behavioral effects of repeated vagal nerve stimulation in rats. Biol Psychiatry. 2011;70:937–945. doi: 10.1016/j.biopsych.2011.07.020. [DOI] [PubMed] [Google Scholar]
- Ganon-Elazar E, Akirav I. Cannabinoids prevent the development of behavioral and endocrine alterations in a rat model of intense stress. Neuropsychopharmacology. 2012;37:456–466. doi: 10.1038/npp.2011.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmer WS, Trivedi MH, Rush AJ, Wisniewski SR, Luther J, Howland RH, Yohanna D, Khan A, Alpert J. Factors associated with chronic depressive episodes: a preliminary report from the STAR-D project. Acta Psychiatr Scand. 2005;112:425–433. doi: 10.1111/j.1600-0447.2005.00633.x. [DOI] [PubMed] [Google Scholar]
- Gourley SL, Kedves AT, Olausson P, Taylor JR. A history of corticosterone exposure regulates fear extinction and cortical NR2B, GluR2/3, and BDNF. Neuropsychopharmacology. 2009;34:707–716. doi: 10.1038/npp.2008.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MK, Rani CS, Joshi A, Soto-Piña AE, Martinez PA, Frazer A, Strong R, Morilak DA. Prenatal stress induces long term stress vulnerability, compromising stress response systems in the brain and impairing extinction of conditioned fear after adult stress. Neurosci. 2011;192:438–51. doi: 10.1016/j.neuroscience.2011.06.041. [DOI] [PubMed] [Google Scholar]
- Gutner CA, Rizvi SL, Monson CM, Resick PA. Changes in coping strategies, relationship to the perpetrator, and posttraumatic distress in female crime victims. J Trauma Stress. 2006;19:813–823. doi: 10.1002/jts.20158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari R, Begre S, Schmid JP, Saner H, Gander ML, von Kanel R. Change over time in posttraumatic stress caused by myocardial infarction and predicting variables. J Psychosom Res. 2010;69:143–150. doi: 10.1016/j.jpsychores.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Harvey BH, Brand L, Jeeva Z, Stein DJ. Cortical/hippocampal monoamines, HPA-axis changes and aversive behavior following stress and restress in an animal model of post-traumatic stress disorder. Physiol Behav. 2006;87:881–890. doi: 10.1016/j.physbeh.2006.01.033. [DOI] [PubMed] [Google Scholar]
- Hauger RL, Lorang M, Irwin M, Aguilera G. CRF receptor regulation and sensitization of ACTH responses to acute ether stress during chronic intermittent immobilization stress. Brain Res. 1990;532:34–40. doi: 10.1016/0006-8993(90)91738-3. [DOI] [PubMed] [Google Scholar]
- Hoffman L, Burges Watson P, Wilson G, Montgomery J. Low plasma beta-endorphin in posttraumatic stress disorder. Aust N Z J Psychiatry. 1989;23:269–273. doi: 10.3109/00048678909062145. [DOI] [PubMed] [Google Scholar]
- Hori N, Yuyama N, Tamura K. Biting suppresses stress-induced expression of corticotropin-releasing factor (CRF) in the rat hypothalamus. J Dent Res. 2004;83:124–128. doi: 10.1177/154405910408300208. [DOI] [PubMed] [Google Scholar]
- Ibarguen-Vargas Y, Surget A, Vourc’h P, Leman S, Andres CR, Gardier AM, Belzung C. Deficit in BDNF does not increase vulnerability to stress but dampens antidepressant-like effects in the unpredictable chronic mild stress. Behav Brain Res. 2009;202:245–251. doi: 10.1016/j.bbr.2009.03.040. [DOI] [PubMed] [Google Scholar]
- Jordanova V, Stewart R, Goldberg D, Bebbington E, Brugha T, Singleton N, Lindesay JEB, Jenkins R, Prince M, Meltzer H. Age variation in life events and their relationship with common mental disorders in a national survey population. Soc Psychiatry Psychiatr Epidemiol. 2007;42:611–616. doi: 10.1007/s00127-007-0209-9. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Fennell JE, Keyes M, Fiallos AM, Myers KM, Davis M, Duncan EJ. Posttraumatic stress disorder may be associated with impaired fear inhibition: relation to symptom severity. Psychiatry Res. 2009;167:151–160. doi: 10.1016/j.psychres.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156:837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson C. Posttraumatic stress disorder in the national comorbidity survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kioukia-Fougia N, Antoniou K, Bekris S, Liapi C, Christofidis I, Papadopoulou-Diafoti Z. The effects of stress exposure on the hypothalamic-pituitary-adrenal axis, thymus, thyroid hormones, and glucose levels. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:823–830. doi: 10.1016/s0278-5846(01)00297-4. [DOI] [PubMed] [Google Scholar]
- Knox D, Perrine SA, George SA, Galloway MP, Liberzon I. Single prolonged stress decreases glutamate, glutamine, and creatine concentrations in the rat medial prefrontal cortex. Neurosci Lett. 2010;480:16–20. doi: 10.1016/j.neulet.2010.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox D, George SA, Fitzpatrick CJ, Rabinak CA, Maren S, Liberzon I. Single prolonged stress disrupts retention of extinguished fear in rats. Learn Mem. 2012;19:43–49. doi: 10.1101/lm.024356.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen K, Harley R, Lyons MJ, Wolfe J, Simpson JC, Go J. A twin registry study of familial and individual risk factors for trauma exposure and posttraumatic stress disorder. J Nerv Ment Dis. 2002;190:209–218. doi: 10.1097/00005053-200204000-00001. [DOI] [PubMed] [Google Scholar]
- Koenen KC, Moffitt TE, Poulton R, Martin J, Caspi A. Early childhood factors associated with the development of post-traumatic stress disorder: Results from a longitudinal birth cohort. Psychol Med. 2007;37:181–192. doi: 10.1017/S0033291706009019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, Van Reenen CG, Hopster H, De Jong IC, Ruis MAW, Blokhuis HJ. Coping style in animals: current status in behavior and stress-physiology. Neurosci Biobehav Rev. 1999;23:925–935. doi: 10.1016/s0149-7634(99)00026-3. [DOI] [PubMed] [Google Scholar]
- Lapiz-Bluhm MD, Soto-Piña AE, Hensler JG, Morilak DA. Chronic intermittent cold stress and serotonin depletion induce deficits of reversal learning in an attentional set-shifting test in rats. Psychopharmacology. 2009;202:329–341. doi: 10.1007/s00213-008-1224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux AM, Coe CL. Abuse-related posttraumatic stress disorder: evidence for chronic neuroendocrine activation in women. Psychosom Med. 1995;57:105–115. doi: 10.1097/00006842-199503000-00002. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Krstov M, Young EA. Stress-restress: Effects on ACTH and fast feedback. Psychoneuroendocrinology. 1997;22:443–453. doi: 10.1016/s0306-4530(97)00044-9. [DOI] [PubMed] [Google Scholar]
- Ma S, Morilak DA. Chronic intermittent cold stress sensitises the hypothalamic-pituitary-adrenal response to a novel acute stress by enhancing noradrenergic influence in the rat paraventricular nucleus. J Neuroendocrinol. 2005;17:761–769. doi: 10.1111/j.1365-2826.2005.01372.x. [DOI] [PubMed] [Google Scholar]
- Maier SF. Learned helplessness and animal models of depression. Prog Neuro-Psychopharmacol & Biol Psychiat. 1984;8:435–446. [PubMed] [Google Scholar]
- Mason JW, Giller EL, Kosten TR, Ostroff R, Harkness L. Urinary-free cortisol in posttraumatic stress disorder. J Nerv Ment Dis. 1986;174:145–149. doi: 10.1097/00005053-198603000-00003. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Milad MR, Vidal-Gonzalez I, Quirk GJ. Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behav Neurosci. 2004;118:389–394. doi: 10.1037/0735-7044.118.2.389. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Romanski LM, LeDoux JE. Extinction of emotional learning: Contribution of medial prefrontal cortex. Neurosci Lett. 1993;163:109–113. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Frazer A. Antidepressants and brain monoaminergic systems: a dimensional approach to understanding their behavioural effects in depression and anxiety disorders. Int J Neuropsychopharmacol. 2004;7:193–218. doi: 10.1017/S1461145704004080. [DOI] [PubMed] [Google Scholar]
- O’Donnell ML, Creamer M, McFarlane AC, Silove D, Bryant RA. Should A2 be a diagnostic requirement for posttraumatic stress disorder in DSM-V? Psychiatry Res. 2010;176:257–260. doi: 10.1016/j.psychres.2009.05.012. [DOI] [PubMed] [Google Scholar]
- O’Leary PJ. Men who were sexually abused in childhood: coping strategies and comparisons in psychological functioning. Child Abuse Negl. 2009;33:471–479. doi: 10.1016/j.chiabu.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Olff M, Guzelcan Y, de Vries G, Assies J, Gersons BPR. HPA- and HPT-axis alterations in chronic posttraumatic stress disorder. Psychoneuroendocrinology. 2006;31:1220–1230. doi: 10.1016/j.psyneuen.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Olff M, Langeland W, Gersons BPR. Effects of appraisal and coping on the neuroendocrine response to extreme stress. Neurosci Behav Rev. 2005;29:457–467. doi: 10.1016/j.neubiorev.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Ono Y, Kataoka T, Miyake S, Cheng SJ, Tachibana A, Sasaguri KI, Onozuka M. Chewing ameliorates stress-induced suppression of hippocampal long-term potentiation. Neurosci. 2008;154:1352–1359. doi: 10.1016/j.neuroscience.2008.04.057. [DOI] [PubMed] [Google Scholar]
- Ostrander MM, Ulrich-Lai YM, Choi DC, Richtand NM, Herman JP. Hypoactivity of the hypothalamo-pituitary-adrenocortical axis during recovery from chronic variable stress. Endocrinology. 2006;147:2008–2017. doi: 10.1210/en.2005-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman RK, Orr S. Twenty-four hour urinary cortisol and catecholamine excretion in combat-related posttraumatic stress disorder. Biol Psychiatry. 1990;27:245–247. doi: 10.1016/0006-3223(90)90654-k. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimanoczy A, Slamberova R, Riley MA, Vathy I. Adrenocorticotropin stress response but not glucocorticoid-negatve feedback is altered by prenatal morphine exposure in adult male rats. Neuroendocrinology. 2003;78:312–320. doi: 10.1159/000074884. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Hahn EL, Nathan SV, de Quervain DJF, McGaugh JL. Glucocorticoid effects on memory retrieval require concurrent noradrenergic activity in the hippocampus and basolateral amygdala. J Neurosci. 2004;24:8161–8169. doi: 10.1523/JNEUROSCI.2574-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Okuda S, Van der Zee EA, McGaugh JL. Glucocorticoid enhancement of memory requires arousal-induced noradrenergic activation in the basolateral amygdala. PNAS. 2006;103:6741–6746. doi: 10.1073/pnas.0601874103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Zimmerman M, Wisniewski SR, Fava M, Hollon SD, Warden D, Biggs MM, Shores-Wilson K, Shelton RC, Luther JF, Thomas B, Trivedi MH. Comorbid psychiatric disorders in depressed outpatients: Demographic and clinical features. J Affect Disord. 2005;87:43–55. doi: 10.1016/j.jad.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Sareen J, Cox BJ, Clara I, Asmundson GJG. The relationship between anxiety disorders and physical disorders in the U.S. national comorbidity survey. Depress Anxiety. 2005;21:193–202. doi: 10.1002/da.20072. [DOI] [PubMed] [Google Scholar]
- Seligman MEP, Maier SF, Geer JH. Alleviation of learned helplessness in the dog. J Abnorm Psychol. 1968;73:256–262. doi: 10.1037/h0025831. [DOI] [PubMed] [Google Scholar]
- Stedenfeld KA, Clinton SM, Kerman IA, Akil H, Watson SJ, Sved AF. Novelty-seeking behavior predicts vulnerability in a rodent model of depression. Physiol Behav. 2011;103:210–216. doi: 10.1016/j.physbeh.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutker PB, Vasterling JJ, Brailey K, Allain AN. Memory, attention, and executive deficits in POW survivors: contributing biological and psychological factors. Neuropsychology. 1995;9:118–125. [Google Scholar]
- Wessa M, Flor H. Failure of extinction of fear responses in posttraumatic stress disorder: Evidence from second-order conditioning. Am J Psychiatry. 2007;164:1684–1692. doi: 10.1176/appi.ajp.2007.07030525. [DOI] [PubMed] [Google Scholar]
- Willner P. Validation criteria for animal models of human mental disorders: learned helplessness as a paradigm case. Prog Neuropsychopharmacol Biol Psychiatry. 1986;10:677–690. doi: 10.1016/0278-5846(86)90051-5. [DOI] [PubMed] [Google Scholar]
- Wu HH, Wang S. Strain differences in the chronic mild stress animal model of depression. Behav Brain Res. 2010;213:94–102. doi: 10.1016/j.bbr.2010.04.041. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Morinobu S, Takei S, Fuchikami M, Matsuki A, Yamawaki S, Liberzon I. Single prolonged stress: Toward an animal model of posttraumatic stress disorder. Depress Anxiety. 2009;26:1110–1117. doi: 10.1002/da.20629. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Morinobu S, Iwamoto Y, Ueda Y, Takei S, Fujita Y, Yamawaki S. Alterations in the hippocampal glycinergic system in an animal model of posttraumatic stress disorder. J Psychiatr Res. 2010;44:1069–1074. doi: 10.1016/j.jpsychires.2010.03.013. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Boisoneau D, Mason JW, Giller EL. Glucocorticoid receptor number and cortisol excretion in mood, anxiety, and psychotic disorder. Biol Psychiatry. 1993;34:18–25. doi: 10.1016/0006-3223(93)90252-9. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Kahana B, Binder-Brynes K, Southwick SM, Mason JW, Giller EL. Low urinary cortisol excretion in holocaust survivors with posttraumatic stress disorder. Am J Psychiatry. 1995;152:982–986. doi: 10.1176/ajp.152.7.982. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Southwick SM, Nussbaum G, Wahby V, Giller EL, Mason JW. Low urinary cortisol excretion in patients with posttraumatic stress disorder. J Nerv Ment Dis. 1990;187:366–369. doi: 10.1097/00005053-199006000-00004. [DOI] [PubMed] [Google Scholar]