Abstract
Acute studies suggest that adiponectin may reduce sympathetic activity and blood pressure (BP) via actions on the central nervous system (CNS). However, the chronic effects of adiponectin on energy expenditure and cardiovascular function are still poorly understood. We tested if chronic intracerebroventricular (ICV) infusion of adiponectin (1 or 7 µg/day) in Sprague-Dawley rats fed a high fat diet (HFD) for 8 weeks and at the high dose (7 µg/day) in spontaneously hypertensive rats (SHR), a hypertensive model associated with sympathetic overactivity, evoked chronic reductions in BP and heart rate (HR). We also determined if chronic ICV adiponectin infusion alters appetite, whole body oxygen consumption (VO2), and insulin and leptin levels. Neither dose of adiponectin infused for 7 days significantly altered BP or HR in the HFD group (115±2 to 112±2 mmHg and 384±6 to 379±6 bpm at 1 µg/day; 109±3 to 111±3 mmHg and 366±5 and 367±5 bpm at 7µg/day). The higher dose slightly reduced food intake (14±1 to 11±1 g/day), whereas VO2, insulin and leptin levels were not affected by the treatment. In SHRs, ICV adiponectin infusion reduced appetite (22±2 to 12±2 g/day) and insulin levels (~55%), but did not alter BP (162±4 to 164±3 mmHg) or HR (312±5 to 322±8 bpm). These results suggest that adiponectin, acting via its direct actions on the CNS, has a small effect to reduce appetite and insulin levels, but it has no long-term action to reduce BP or HR, or to alter whole body metabolic rate.
Keywords: adiponectin, blood pressure, heart rate, appetite, insulin sensitivity
1. INTRODUCTION
In the past two decades we have witnessed a major change in the field of adipocyte biology in which the traditional view of the adipose tissue as purely an energy storage compartment has been radically transformed by the fact that the adipose tissue is an important and dynamic endocrine organ producing a variety of bioactive molecules referred to as adipokines [11, 13, 20]. Among these, adiponectin is the most abundant and its regulation appears to contrast with that of other adipokines [20]. For instance, adiponectin levels are reduced under conditions such as obesity, hypertension and insulin resistance which has led to the suggestion of an association between hypoadiponectinemia and metabolic syndrome [1, 4, 6, 14, 19].
Previous acute studies have shown that adiponectin modulates energy balance via activation of AdipoR1 and AdipoR2 receptors present mainly in the arcuate and lateral hypothalamic nuclei [7, 8]. Central nervous system (CNS) single-dose administration of adiponectin in rodents has been shown to reduce body weight by increasing energy expenditure [15] and reducing appetite [2]. However, Kubota et al. (2007) [9] observed increased food consumption and decreased thermogenesis in mice treated with adiponectin. Thus, the acute effects of adiponectin to reduce food intake and to modulate thermogenesis have not been uniform. The chronic CNS effects of adiponectin on food intake have, to our knowledge, not been reported. These observations suggest that the overall impact of adiponectin on energy balance and body weight homeostasis is still not clearly understood.
In addition to its potential effects on energy homeostasis, recent evidence suggests that adiponectin may also modulate blood pressure (BP). When maintained on a high salt diet adiponectin KO mice develop hypertension which can be ameliorated with adiponectin replacement [11]. Also, reconstitution of adiponectin through adenoviral infection in genetically obese KKAy mouse reduces their elevated systolic BP [11]. Moreover, previous studies suggest that the effects of adiponectin to acutely reduce renal sympathetic nervous system (SNS) activity and to lower blood pressure are, at least in part, mediated by adiponectin’s action on the CNS [5, 18]. However, whether adiponectin exerts sustained effects in the CNS to promote long-term reductions in the BP is still unclear.
Since obesity is associated with reduced circulating levels of adiponectin and acute studies suggest that adiponectin may reduce BP by suppressing sympathetic activity, we investigated whether chronic intracerebroventricular (ICV) infusion of adiponectin exerts long-term effects on BP and heart rate (HR) in normotensive rats fed a high fat-high fructose diet, and we also tested whether the cardiovascular effects of adiponectin are exacerbated in spontaneously hypertensive rats, a well known model of hypertension associated with high sympathetic activity.
2. METHODS
The experimental procedures and protocols of this study conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center.
2.1 Animals
Male Sprague-Dawley (SD) and spontaneous hypertensive rats (SHR) at 8 weeks of age were purchased from Harlan (Madison, WI) and Taconic Farms (Albany, NY), respectively.
2.1.1 High fat-high fructose diet (HFD)
SD rats were fed high fat diet (Diet No. D12451, Research Diets, New Brunswick, NJ; 4.7 kcal/g) and degassed 7UP (Cadbury Schweppes, Plano, TX; 0.416 kcal/mL) to drink. The rats were maintained on the HFD for 8 weeks before experiments began.
2.2 Surgical Procedures
2.2.1 Telemetry pressure transmitter implantation
SD rats (450 ± 8 g) and SHR (341 ± 1 g) were anesthetized with 50 mg/kg sodium pentobarbital (Nembutal), and atropine sulfate (0.1 mg/kg) was administered to prevent excess airway secretions. Using aseptic techniques, a laparotomy was performed and the catheter of the pressure telemetry transmitter (Model TA11PAC40, Data Sciences International) was inserted into the abdominal aorta, distal to the kidneys according to the manufacturer’s instructions. The catheter was fixed in the aorta with a small drop of cyanoacrylate adhesive, and the transmitter was secured to the abdominal wall by sutures. Mean 24-hour BP and HR data were derived from the average BP and HR values measured by bursts of 10 seconds every 10 minutes using the software (Dataquest 4.0) provided by the manufacturer.
2.2.2 Intracerebroventricular cannulation
Immediately after telemetry probe implantation, a stainless steel cannula (26 gauge, 10 mm long) was implanted into the right lateral cerebral ventricle using the coordinates previously described [10]. The steel cannula was secured in place with 2 stainless steel screws, a metal cap, and dental acrylic. A stylet was inserted to seal the cannula until use. During stereotaxic manipulation, anesthesia was maintained with 0.5% isofluorane. After 7 days to recover from surgery, accuracy of the cannula placement was tested by measuring the dipsogenic response (immediate drinking of at least 5 ml of water in 10 minutes) to an intracerebroventricular (ICV) injection of 100 ng of angiotensin II.
Immediately after surgery, rats were housed individually in standard metabolic cages or in an oxygen consumption system (AccuScan system, Harvard Apparatus) for the duration of the study for measurements of oxygen consumption (VO2), carbon dioxide production (VCO2) and respiratory quotient (RQ). The rats were allowed to recover for 7 to 10 days before control measurements were initiated. VO2, VCO2 and RQ were determined daily (for 2 minutes every 10-minute interval) and expressed as the average of the last 2 days of control, adiponectin infusion and recovery periods. RQ was calculated by the formula: VCO2/ VO2.
2.3 Experimental Protocols
Mean arterial pressure (MAP), heart rate (HR), food and water intake and oxygen consumption (VO2) were measured 24-hours/day and average values were recorded daily.
2.3.1 Chronic ICV adiponectin infusion
Eight days after surgeries, adiponectin was infused ICV for 7 days at 2 doses (1 or 7 µg/day, 1.0 µl/hr dissolved in saline; n=6 and 4, respectively) in SD rats fed HFD using osmotic min-pumps (model 2001, Durect Corp.) implanted subcutaneously in the scapular region as previously described (20). These doses were based on previous acute studies [15]. To test whether the effects of adiponectin on cardiovascular regulation are enhanced in conditions of increased sympathetic activation, we also infused adiponectin at the higher dose (7 µg/day, 1 µl/hr, n=4) for 7 days in SHRs, a model of hypertension associated with high sympathetic activity.
Fasting blood samples (200 µl) were collected from each animal by tail snip on day 5 of the control period, on day 7 of adiponectin infusion, and on day 5 after stopping adiponectin infusion (recovery period). The blood was centrifuged using a refrigerated centrifuge and the plasma used for determination of glucose (glucose oxidase method, Beckman Glucose Analyzer), insulin and leptin concentrations (rat ELISA kits from Crystal Chem and R&D Systems, respectively). The intra and inter-assay sensitivity of these commercially available kits are <10% for intra and inter-assay for the rat insulin ELISA kit and <5% and <7% for intra and inter-assay sensitivity for the rat leptin ELISA kit.
2.4 Statistical Analyses
The results are expressed as mean ±SEM. The data obtained were analyzed by GraphPad Prism 5 software using 1-way ANOVA with repeated measures followed by Bonferroni’s post hoc test for comparisons between control and experimental values within each group when appropriated. Statistical significance was accepted at a level of P<0.05.
3. RESULTS
3.1 Effect of chronic ICV adiponectin treatment on food intake, body weight and metabolic rate
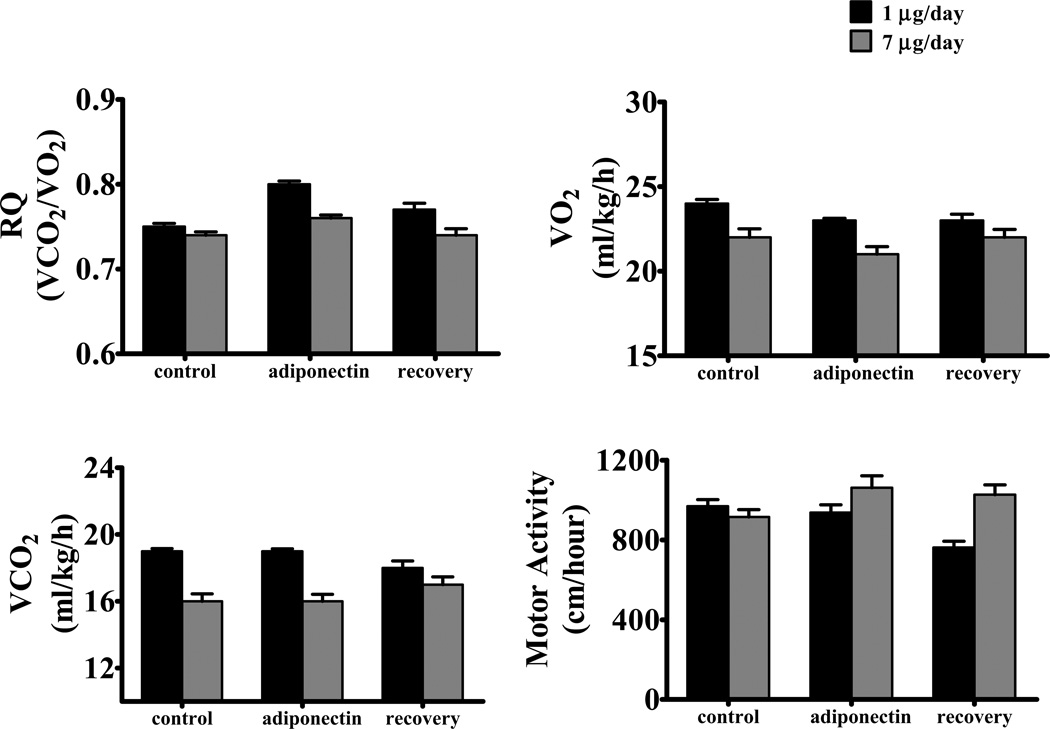
Chronic ICV adiponectin at the dose of 1 µg/day did not significantly alter caloric intake or body weight in SD fed a HFD for 8 weeks (Table 1). This dose of adiponectin also did not alter carbon dioxide production (VCO2) or motor activity (Figure 1). Although there was a tendency for a reduction in VO2 as well as for increased RQ values, these changes did not reach statistical significance (Figure 1). Similar findings were observed when SD rats fed a HFD were infused with a higher dose of adiponectin (7 µg/day) (Table 1 and Figure 1).
Table 1.
Responses to chronic ICV adiponectin infusion on body weight, food intake and plasma hormones in Sprague-Dawley (SD) rats fed a high fat-high sucrose diet and in spontaneously hypertensive rats (SHR) fed standard chow.
| Experimental Groups | Body Weight (g) |
Caloric Intake (kcal) |
Leptin (ng/mL) |
Glucose (mg/dL) |
Insulin (µL/mL) |
|---|---|---|---|---|---|
| SD (n=6) | |||||
| Control | 455 ± 12 | 81 ± 4 | 1.4 ± 0.2 | 132 ± 2 | 14 ± 8 |
| ADP (1µg/day) | 445 ± 10 | 75 ± 3 | 1.6 ± 0.4 | 145 ± 12 | 8 ± 2 |
| Recovery | 466 ± 11 | 73 ± 5 | 1.5 ± 0.2 | 128 ± 15 | 10 ± 2 |
| SD (n=4) | |||||
| Control | 448 ± 6 | 79 ± 7 | 1.5 ± 0.2 | 120 ± 8 | 10 ± 2 |
| ADP (7µg/day) | 449 ± 6 | 68 ± 4 | 1.5 ± 0.2 | 112 ± 3 | 11 ± 1 |
| Recovery | 456 ± 8 | 77 ± 5 | 1.6 ± 0.3 | 121 ± 2 | 14 ± 7 |
| SHR (n=4) | |||||
| Control | 341 ± 11 | 95 ± 10 | 1.4 ± 0.3 | 146 ± 11 | 30 ± 3 |
| ADP (7µg/day) | 326 ± 10 | 52 ± 7* | 1.6 ± 0.5 | 155 ± 14 | 13 ± 2* |
| Recovery | 341 ± 10 | 75 ± 7 * | 1.3 ± 0.2 | 183 ± 10 | 28 ± 4 |
Values represent Mean ± SEM.
indicates p<0.05 vs. control period within group.
Figure 1.
(A) respiratory coefficient (RQ), (B) oxygen consumption (VO2), (C) carbon dioxide production (VCO2) and (D) motor activity in Sprague-Dawley rats fed high fat-high fructose diet during chronic ICV adiponectin infusion at 1 µg/day (black bar, n=4) or 7 µg/day (gray bar, n=4). Data expressed as mean ± S.E.M.
In SHR, however, chronic ICV adiponectin infusion at the higher dose of 7 µg/day reduced caloric intake and caused a 15 g weight loss (Table 1). Caloric intake increased after cessation of adiponectin treatment, which likely explains the rapid weight gain in the 5-day recovery period where it was no longer reduced compared to control values. We did not evaluate metabolic rate and motor activity in the SHR group.
3.2 Effect of chronic ICV adiponectin treatment on glucose, insulin and leptin levels
Chronic central adiponectin infusion for 7 days at either low or high doses in SD rats fed HFD did not significantly alter fasting plasma glucose, insulin or leptin concentrations (Table 1). However, ICV adiponectin infusion in SHR reduced insulin levels without causing significant changes in plasma glucose or leptin levels (Table 1). It is important to note that baseline fasting insulin levels were about 2 to 3 times higher in SHR compared to SD rats (Table 1). This suggests that central adiponectin infusion may have more pronounced impact on insulin sensitivity in animals with impaired insulin sensitivity. In addition, there was a tendency for increased plasma glucose levels in SHRs after adiponectin infusion was stopped despite the return of insulin levels back to control values (Table 1).
3.3 Effects of chronic ICV adiponectin treatment on MAP and HR
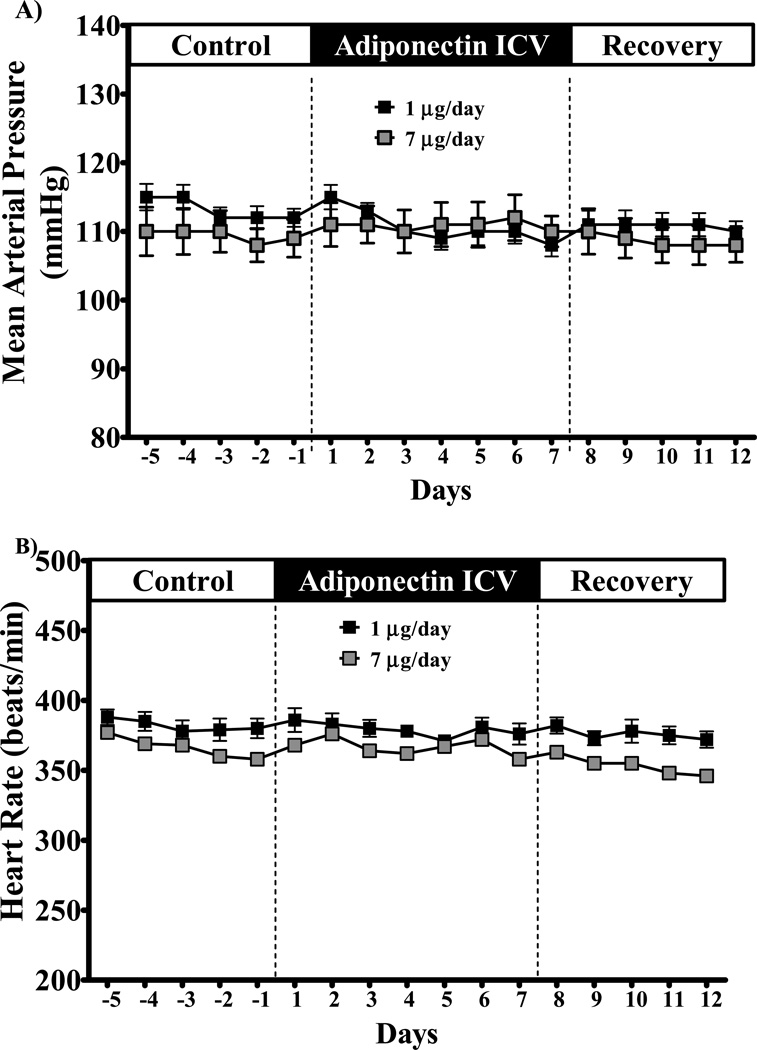
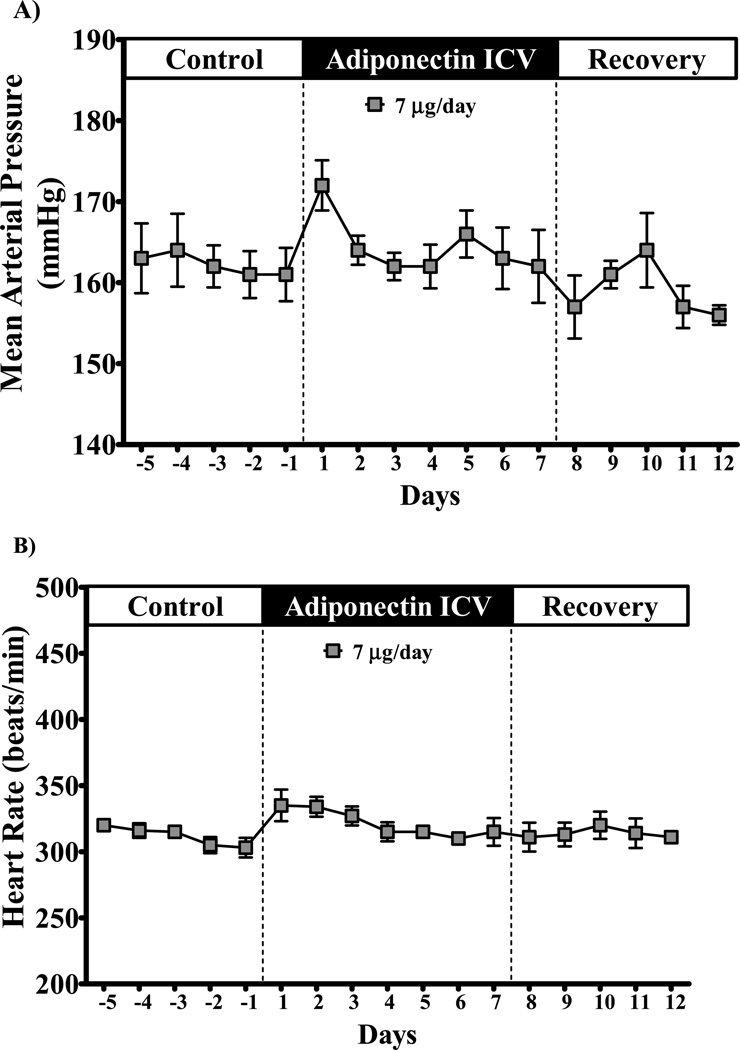
Chronic ICV adiponectin infusion at 1 or 7 µg/day did not alter MAP or HR in SD rats fed a HFD (Figure 2). Central adiponectin infusion also did not change MAP and HR in SHRs (Figure 3). However, SHRs exhibited a tendency for increased MAP and HR during the first 1 to 3 days of infusion which quickly returned to baseline levels (Figure 3). These results suggest that adiponectin does not appear to play an important role in long-term control of BP and HR by direct activation of its receptors in the CNS.
Figure 2.
(A) mean arterial pressure and (B) heart hate of Sprague-Dawley rats fed high fat-high fructose diet during chronic ICV adiponectin infusion at 1 µg/day (black squares, n = 6) or 7 µg/day (gray squares, n = 4). Data expressed as mean ± S.E.M.
Figure 3.
Mean arterial pressure (A) and heart hate (B) of spontaneous hypertensive rats (SHR) during chronic ICV infusion of adiponectin (7 µg/day). Data expressed as mean ± S.E.M.
4. DISCUSSION
In the present study we showed that chronic delivery of adiponectin into the CNS of normotensive as well as hypertensive rats did not significantly alter BP or HR. Except for a small, non-significant, reduction in whole-body oxygen consumption we also found no major alterations in motor activity, food intake, body weight and fasting insulin, glucose and leptin levels in SD rats fed high fat-high fructose diet. However, in the SHR group chronic ICV adiponectin treatment markedly reduced food intake and caused modest weight loss that was accompanied by a significant reduction in fasting insulin levels suggesting an improvement of glucose homeostasis.
Previous studies have shown that acute adiponectin injection into the CNS may alter food intake and energy expenditure [9, 15]. Our results, however, suggest that chronic activation of adiponectin receptors in the CNS does not appear to play a major role in regulating body weight homeostasis either by altering appetite or energy expenditure in a HFD rodent model. Of note, chronic adiponectin reduced food intake in SHRs. The mechanisms by which adiponectin reduces food intake in SHRs but not in SD rats are still unclear. One possible explanation is that despite the high fat-high fructose feeding SD rats remain sensitive to the endogenous circulating levels of adiponectin and further increases in adiponectin by exogenous infusion into the CNS exert little additional effect. However, SHRs exhibit impaired adiponectin signaling [16], and increasing brain adiponectin levels beyond the endogenous levels by direct ICV infusion may have resulted in more pronounced effect in these animals.
ICV adiponectin treatment also improved insulin sensitivity in SHRs and had a tendency to reduce fasting plasma insulin levels in HFD SD rats. It is important to note that despite been fed standard chow and weighing less than HFD rats, SHRs exhibited 2-fold greater fasting insulin levels at baseline which corroborates previous studies that showed insulin resistance in SHRs [16]. Therefore, one may speculate that adiponectin treatment may exert its beneficial effects on metabolic parameters such as insulin sensitivity in states of established insulin-resistance, while these effects are not as evident under normal circumstances. Another factor that may help explain the lack of a major impact of adiponectin treatment on energy balance and metabolic profile in HFD rats is that we delivered adiponectin directly into the CNS. It is possible that most of the effects of adiponectin are mediated by its peripheral actions rather than being mediated via activation of its receptors in the brain. Our study, however, was designed to investigate only the brain effects of adiponectin on energy balance and metabolic function and additional studies are needed to test this hypothesis.
A unique aspect of our study is that we evaluated the chronic effects of central adiponectin delivery on long-term control of BP and HR in both normotensive and hypertensive rats using state-of the-art telemetry technique to measure BP and HR 24-hours a day. Contrary to our original hypothesis, we observed no significant impact of chronic increases in CNS adiponectin levels on BP and HR. Previous studies have shown that adiponectin deficiency predisposes to salt-sensitive hypertension, whereas acute adiponectin injection in the CNS reduces renal SNS activity and lowers BP [18]. One potential explanation for lack of a chronic brain-mediated effect of adiponectin on cardiovascular regulation may be that long-term activation of adiponectin receptors in the CNS leads to compensatory changes that offset adiponectin’s ability to lower SNS activity. Adiponectin may also exert opposing effects on BP regulation by acting in different areas of the brain. For example, injections of adiponectin into the area postrema in anesthetized rats depolarizes neuronal cells causing a significantly increase in blood pressure [3], while microinjection of adiponectin in the medial nucleus of the tractus solitarius decreases BP [5]. Thus, acute ICV injection of adiponectin may exert a rapid effect to reduce SNS activity and BP by acting in certain areas of the brain but when more homogenous activation of adiponectin receptors occurs with prolonged delivery of adiponectin, as in the case of the present study, then no sustained effect of adiponectin on SNS activity and BP is observed. Since adiponectin is produced by adipocytes and reaches the brain via diffusion from the blood into the cerebral spinal fluid, it is likely that many areas of the brain involved in the regulation of cardiovascular function are exposed to adiponectin. Therefore, our observations using chronic intracerebroventricular delivery of adiponectin may better represent the long-term physiologic effects of adiponectin on BP and HR regulation.
It is also possible that adiponectin may exert a more prolonged effect on cardiovascular function by its peripheral actions including improved endothelial function. For instance, adiponectin KO mice develop larger infarcts associated with myocardial cell apoptosis and TNF-α production that can be reversed by adenovirus-mediated delivery of adiponectin in these mice [17]. Adiponectin KO mice also exhibit reduced mRNA levels of endothelial nitric oxide synthase in aorta and kidneys, suggesting impaired nitric oxide (NO) production, and adiponectin treatment increased NO production in vascular endothelial cells [12]. Taken together, adiponectin may exert rapid beneficial effects on BP regulation via a brain-mediated mechanism leading to acute reduction in SNS activity, while its long-term effects on cardiovascular function are mediated by a more slow progressive improvement on endothelial function. Our studies suggest, however, that direct CNS actions of adiponectin do not play a major role in long-term blood pressure regulation.
5. CONCLUSIONS
Overall, our results suggest that adiponectin, acting via its direct actions on the CNS, has a small effect to reduce appetite and insulin levels in insulin-resistant spontaneously hypertensive rats, but it has no long-term action to reduce BP or HR in normotensive or hypertensive models, or to alter whole body metabolic rate.
HIGHLIGHTS.
-
✓
Chronic CNS adiponectin infusion does not alter appetite or energy expenditure in Sprague-Dawley rats.
-
✓
Chronic CNS adiponectin infusion reduces appetite and improves insulin sensitivity in SHRs.
-
✓
Chronic CNS adiponectin treatment does not exert major chronic effects on BP and HR.
ACKNOWLEDGMENTS
We thank Haiyan Zhang for the measurement of fasting insulin and leptin levels.
SOURCES OF FUNDING
The authors’ research was supported by a National Heart, Lung and Blood Institute grant PO1HL-51971 and by a Scientist Development Grant from the American Heart Association to Alexandre A. da Silva and Jussara M. do Carmo.
GLOSSARY
- BP
blood pressure
- HR
heart rate
- VO2
oxygen consumption
- VCO2
carbon dioxide production
- RQ
respiratory quotient
- SNS
sympathetic nerve activity
- ICV
intracerebroventricular
- CNS
central nervous system
- HFD
high fat-high fructose diet
- SD
Sprague-Dawley
- SHR
spontaneously hypertensive rat
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST/DISCLOSURES
None.
REFERENCES
- 1.Berg AH, Scherer PE. Adipose Tissue, Inflammation, and Cardiovascular Disease. Circ Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 2.Coope A, Milanski M, Araujo P, Tambascia M, Saad M, Genoleze B, Velloso L. AdipoR1 mediates the anorexigenic and insulin/leptin-like actions of adiponectin in the hypothalamus. FEBS Letters. 2008;582:1471–1476. doi: 10.1016/j.febslet.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 3.Fry M, Smith PM, Hoyda TD, Duncan M, Ahima RS, Sharkey KA, Ferguson AV. Area Postrema Neurons Are Modulated by the Adipocyte Hormone Adiponectin. J Neurosci. 2006;26:9695–9702. doi: 10.1523/JNEUROSCI.2014-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K, Nishida M, Kihara S, Sakai N, Nakajima T, Hasegawa K, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Hanafusa T, Matsuzawa Y. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 5.Hoyda TD, Smith PM, Ferguson AV. Adiponectin acts in the nucleus of the solitary tract to decrease blood pressure by modulating the excitability of neuropeptide Y neurons. Brain Res. 2009;1256:76–84. doi: 10.1016/j.brainres.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K. Adiponectin and adiponectin receptors in obesity-linked insulin resistance. Novartis Found Symp. 2007;286:164–176. doi: 10.1002/9780470985571.ch15. [DOI] [PubMed] [Google Scholar]
- 7.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 8.Kos K, Harte AL, da Silva NF, Tonchev A, Chaldakov G, James S, Snead DR, Hoggart B, O'Hare JP, McTernan PG, Kumar S. Adiponectin and resistin in human cerebrospinal fluid and expression of adiponectin receptors in the human hypothalamus. J Clin Endocrinol Metab. 2007;92:1129–1136. doi: 10.1210/jc.2006-1841. [DOI] [PubMed] [Google Scholar]
- 9.Kubota N, Yano W, Kubota T, Yamauchi T, Itoh S, Kumagai H, Kozono H, Takamoto I, Okamoto S, Shiuchi T, Suzuki R, Satoh H, Tsuchida A, Moroi M, Sugi K, Noda T, Ebinuma H, Ueta Y, Kondo T, Araki E, Ezaki O, Nagai R, Tobe K, Terauchi Y, Ueki K, Minokoshi Y, Kadowaki T. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab. 2007;6:55–68. doi: 10.1016/j.cmet.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Kuo JJ, da Silva AA, Tallam LS, Hall JE. Role of adrenergic activity in pressor responses to chronic melanocortin receptor activation. Hypertension. 2004;43:370–375. doi: 10.1161/01.HYP.0000111836.54204.93. [DOI] [PubMed] [Google Scholar]
- 11.Ohashi K, Kihara S, Ouchi N, Kumada M, Fujita K, Hiuge A, Hibuse T, Ryo M, Nishizawa H, Maeda N, Maeda K, Shibata R, Walsh K, Funahashi T, Shimomura I. Adiponectin replenishment ameliorates obesity-related hypertension. Hypertension. 2006;47:1108–1116. doi: 10.1161/01.HYP.0000222368.43759.a1. [DOI] [PubMed] [Google Scholar]
- 12.Ohashi K, Ouchi N, Matsuzawa Y. Adiponectin and hypertension. Am J Hypertens. 2011;24:263–269. doi: 10.1038/ajh.2010.216. [DOI] [PubMed] [Google Scholar]
- 13.Ouchi N, Kihara S, Funahashi T, Matsuzawa Y, Walsh K. Obesity, adiponectin and vascular inflammatory disease. Curr Opin Lipidol. 2003;14:561–566. doi: 10.1097/00041433-200312000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Ouchi N, Shibata R, Walsh K. Cardioprotection by Adiponectin. Trends Cardiovasc Med. 2006;16:141–146. doi: 10.1016/j.tcm.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qi Y, Takahashi N, Hileman SM, Patel HR, Berg AH, Pajvani UB, Scherer PE, Ahima RS. Adiponectin acts in the brain to decrease body weight. Nat Med. 2004;10:524–529. doi: 10.1038/nm1029. [DOI] [PubMed] [Google Scholar]
- 16.Rodríguez A, Catalán V, Becerril S, Gil MJ, Mugueta C, Gómez-Ambrosi J, Frühbeck G. Impaired adiponectin-AMPK signalling in insulin-sensitive tissues of hypertensive rats. Life Sciences. 2008;83:540–549. doi: 10.1016/j.lfs.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 17.Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2–dependent mechanisms. Nat Med. 2005;11:1096–1103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanida M, Shen J, Horii Y, Matsuda M, Kihara S, Funahashi T, Shimomura I, Sawai H, Fukuda Y, Matsuzawa Y, Nagai K. Effects of adiponectin on the renal sympathetic nerve activity and blood pressure in rats. Exp Biol Med. 2007;232:390–397. [PubMed] [Google Scholar]
- 19.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Weyer PAT. Hypoadiponectinemia in Obesity and Type 2 Diabetes: Close Association with Insulin Resistance and Hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 20.Yang WS, Chuang LM. Human genetics of adiponectin in the metabolic syndrome. J Mol Med (Berl) 2006;84:112–121. doi: 10.1007/s00109-005-0011-7. [DOI] [PubMed] [Google Scholar]