Summary
α-catenin is central to recruitment of actin networks to the cadherin-catenin complex (CCC) [1, 2], but how such networks are subsequently stabilized against applied stress during morphogenesis is poorly understood. To identify proteins that functionally interact with α-catenin in this process, we performed enhancer screening using a weak allele of the C. elegans α-catenin, hmp-1, and identified UNC-94/tropomodulin. Tropomodulins (Tmods) cap the minus ends of F-actin in sarcomeres [3]. They also regulate lamellipodia [4], can promote actin nucleation [5], and are required for normal cardiovascular development [6, 7] and neuronal growth cone morphology [8]. Tmods regulate the morphology of cultured epithelial cells [9], but their role in epithelia in vivo remains unexplored. We find that UNC-94 is enriched within a HMP-1-dependent junctional actin network at epidermal adherens junctions subject to stress during morphogenesis. Loss of UNC-94 leads to discontinuity of this network, and high-speed filming of hmp-1(fe4);unc-94(RNAi) embryos reveals large junctional displacements that depend on the Rho pathway. In vitro, UNC-94 acts in combination with HMP-1, leading to longer actin bundles than with HMP-1 alone. Our data suggest Tmods protect actin filaments recruited by α-catenin from minus-end subunit loss, enabling them to withstand the stresses of morphogenesis.
Keywords: cadherin, α-catenin, C. elegans, morphogenesis, tropomodulin, actin
Results and Discussion
C. elegans epidermal morphogenesis provides an excellent context in which to investigate the relationship between actin and α-catenin in vivo. The embryonic epidermis contains three types of cells: (1) dorsal cells, which eventually fuse into a syncytium; (2) lateral (seam) cells, arranged in a single row along the anterior-posterior axis on each side of the embryo; and (3) ventral cells A conserved CCC, including HMR-1/cadherin, HMP-2/β-catenin, HMP-1/α-catenin, and JAC-1/p120 catenin [10, 11] is crucial for epidermal morphogenesis [12, 13]. Actomyosin mediated contractile stresses are transmitted by circumferential actin filament bundles (CFBs) in dorsal and ventral epidermal cells. CFBs insert orthogonally at junctional boundaries between lateral (seam) epidermal cells and dorsal and ventral epidermal cells, and help to drive the four-fold elongation of the embryo. CFB anchorage at adherens junctions (AJs) requires the CCC [14–16].
Functional interactions between multiple pathways are important for both focal adhesions and hemidesmosomes [17, 18]. However, a systematic search for similar functional interactions has not been carried out for AJs. We performed such a search, using feeding RNAi against genes on Chromosome I to find lethal enhancers of a weak loss-of-function allele of hmp-1, fe4. hmp-1(fe4) mutants exhibit embryonic and early larval lethality; escapers develop into fertile adults with body shape defects [11]. The fe4 lesion results in an amino acid substitution in the VH3 domain of HMP-1 [11], which slightly weakens F-actin binding (S. Maiden and J. Hardin, in preparation).
We identified several genes implicated in regulating cell-cell adhesion, including the AF6/Afadin homolog (afd-1) and an Exocyst component (sec-8) [19–21], validating our approach. A full analysis, including results for the other five chromosomes, will be published elsewhere (Lynch et al., in review). Among the enhancers was UNC-94, a tropomodulin family member.
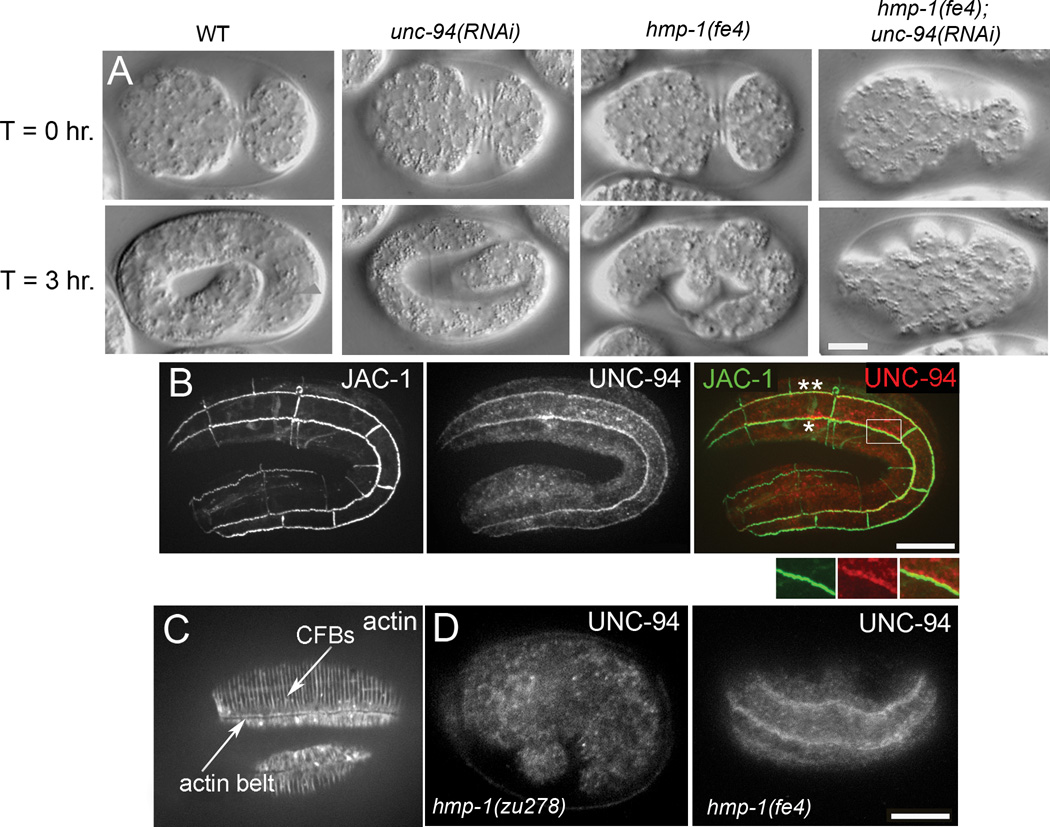
To examine the functional relationship between HMP-1 and UNC-94 we first performed 4D Nomarski microscopy on hmp-1(fe4); unc-94(RNAi) embryos (Fig. 1A). Wild-type embryos elongate to approximately 4-fold their initial length before hatching. unc-94(RNAi) embryos appear superficially wild-type, even though unc-94(RNAi) lowers UNC-94 protein levels to virtually undetectable levels (Supp. Fig. S1A). hmp-1(fe4) embryos exhibit defective elongation (Fig. 1A) and approximately 80% die as embryos and L1 larvae (n=95). hmp-1(fe4) embryos that hatch typically elongate to only twice their original length with severe body shape defects. In contrast, 100% of hmp-1(fe4);unc-94(RNAi) embryos exhibit embryonic lethality (n=93). Of these, 95% fail to elongate past the 1.5 fold stage and then retract to their original body length (Fig. 1A), compared with only 13% of hmp-1(fe4) embryos (see Supplemental Movie S1 for movies of representative embryos). Thus HMP-1 and UNC-94 together are essential for epidermal elongation.
Figure 1. hmp-1(fe4);unc-94(RNAi) embryos arrest during embryonic elongation and epidermal UNC-94 localization to junctions requires hmp-1 function.
A. Nomarski images of representative embryos undergoing elongation are shown at the indicated time intervals. Embryos are initially oriented with anterior to the left and the ventral side up at T=0 hr., and lateral views are shown for all other time points. T=0 hr. shows embryos at the completion of enclosure. Wild-type (WT), unc-94(RNAi), hmp-1(fe4), and hmp-1(fe4); unc-94(RNAi) embryos are shown. No obvious defects in elongation are evident in the unc-94(RNAi) embryo. hmp-1(fe4) embryos develop mild body shape defects during elongation. 95% of hmp-1(fe4);unc-94(RNAi) embryos (n=93) elongate to the 1.5 fold stage or less, then retract to their original length. See Supplemental video S1, available online. B. Wild-type embryo expressing JAC-1::GFP to mark adherens junctions and stained with an affinity purified rabbit anti-UNC-94 antibody. Seam:ventral (*) and seam:dorsal (**) cell borders are indicated. UNC-94 is first detected at epidermal cell borders around the 2-fold stage of elongation. The color merge shows JAC-1::GFP in green and UNC-94 in red. Enlargement of the boxed region shows UNC-94 staining overlapping JAC-1::GFP. C. Wild-type stained with phalloidin. Circumferential filament bundles (CFBs) and the junctional actin belt are indicated. D. UNC-94 staining in wild-type and hmp-1 mutants. In wild-type elongation-stage embryos, 23% have UNC-94 localization at epidermal cell borders (n = 79). In hmp-1(zu278) embryos, 0% have UNC-94 localization at epidermal cell borders (n = 87). In hmp-1(fe4) embryos, 35% have UNC-94 localization at epidermal cell borders (n = 63). Note that this staining was done via freeze-cracking, which is known to disrupt actin, but preserves the UNC-94 epitope recognized by anti-UNC-94 antibodies. Disruption of junctional actin caused by this technique may account for the percentage of embryos exhibiting UNC-94 localization at epidermal cell borders. Bars = 10 µm.
We next performed immunostaining with an antibody that we previously used to show that UNC-94 is found at body wall muscle cell:cell boundaries and the minus ends of sarcomeric thin filaments [22, 23]. UNC-94 is first detectable at the two-fold stage and is enriched at seam:ventral and seam:dorsal cell borders, the same borders where CFBs transmit stress during elongation (Fig. 1B; see Fig. 1C for the relationship between CFBs and junctional actin in a wild-type embryo). Although UNC-94 is near AJs, it is distal to them and extends into the cytoplasm, in the same location as the junctional actin band that runs parallel to AJs. Consistent with this localization pattern, UNC-94 does not coimmunoprecipitate with HMP-1 under conditions in which HMP-1 and HMP-2 do (Supp. Fig. S1B). Significantly, UNC-94 does not localize to epidermal junctions in hmp-1(zu278) homozygotes, which produce a truncated HMP-1 protein incapable of binding actin [16] (Fig. 1D), indicating that HMP-1’s actin-binding activity is required to mobilize actin filaments containing UNC-94 near epidermal cell borders. In contrast, UNC-94 localizes largely normally to epidermal cell borders in hmp-1(fe4) embryos (Fig. 1D), and localizes in pharyngeal cells in a HMP-1-independent manner (Supp. Fig. S1C). Thus HMP-1 acts upstream of UNC-94 at epidermal cell borders normally under tension, but this functional relationship is not mediated through direct physical binding.
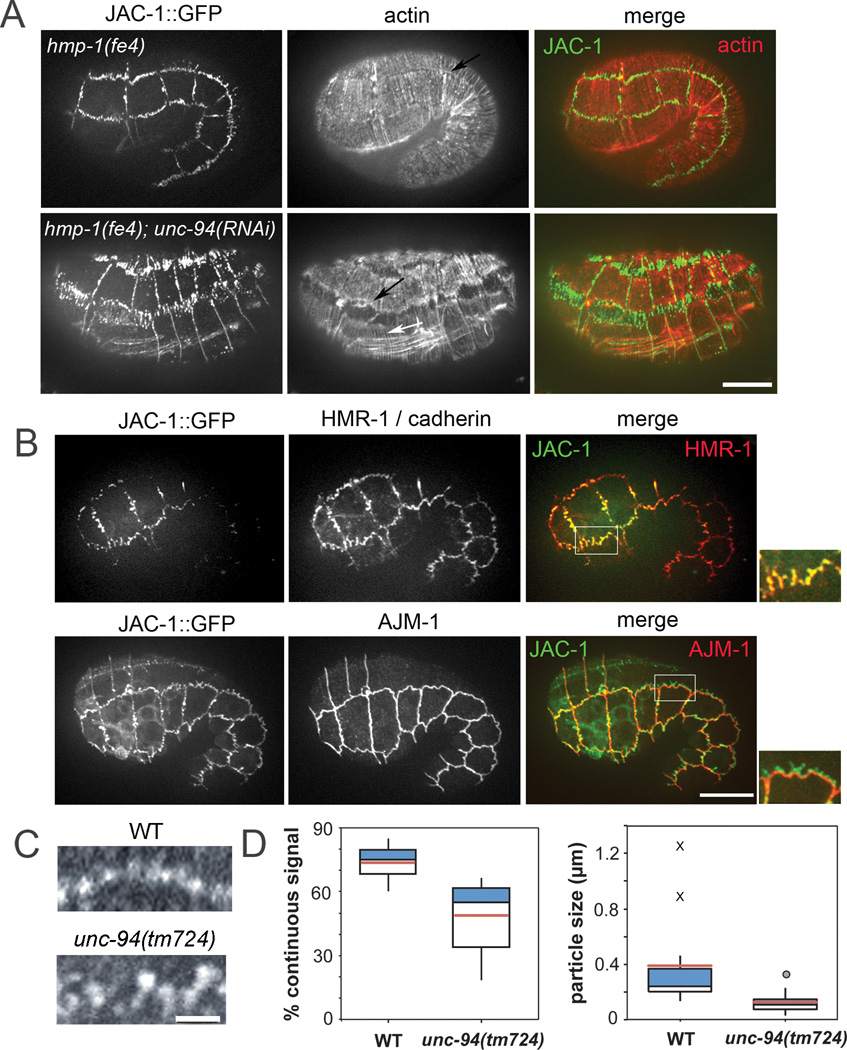
To better understand why hmp-1(fe4);unc-94(RNAi) embryos fail to elongate, we covisualized AJs and actin during this process using phalloidin staining and JAC-1/p120 catenin::GFP. Strikingly, seam:dorsal and seam:ventral epidermal cell borders in hmp-1(fe4);unc-94(RNAi) embryos are highly disrupted, appearing ripped apart to yield a characteristic zig-zag pattern (Fig. 2A). Junctions between other epidermal cells are no more perturbed than those in hmp-1(fe4) homozygotes. In areas of perturbed JAC-1::GFP, only some faint actin filaments are visible. Despite this, CFBs are still present in hmp-1(fe4);unc-94(RNAi) embryos, interfacing with the edges of the mislocalized JAC-1::GFP (Fig. 2A). The junctional-actin belt is still present, but more diffuse in hmp-1(fe4);unc-94(RNAi) embryos compared to hmp-1(fe4) (Fig. 2A, black arrows). The perturbed AJs and actin organization at these epidermal cell borders in hmp-1(fe4);unc-94(RNAi) embryos likely accounts for their failed elongation.
Figure 2. UNC-94 contributes to adherens junction stability.
A. Representative hmp-1(fe4) and hmp-1(fe4);unc-94(RNAi) embryos. Embryos are of similar age (the hmp-1(fe4);unc-94(RNAi) embryo has retracted). Embryos express JAC-1/p120catenin::GFP and are stained with phalloidin to visualize actin. Color merges show JAC-1::GFP in green and actin in red. In hmp-1(fe4);unc-94(RNAi) embryos, JAC-1::GFP is fragmented and mislocalized at seam:dorsal and seam:ventral borders. Actin is depleted in areas of disrupted JAC-1::GFP; however, CFBs (white arrow) and diffuse junctional actin (black arrow) are still visible. Anterior is to the left in all panels. Bar=10 µm. B. In hmp-1(fe4);unc-94(RNAi) embryos the cadherin-catenin complex is selectively perturbed. Pre-arrest hmp-1(fe4);unc-94(RNAi) embryos expressing JAC-1::GFP were stained for either HMR-1/cadherin or AJM-1. Color merges show that HMR-1 (red) co-localizes with JAC-1::GFP (green), and that AJM-1 is not perturbed in regions where JAC-1::GFP is mislocalized. For all images in A–D, anterior is to the left. C. Junctional proximal actin in representative wild-type (WT; top) and unc-94(tm724) embryos (bottom). Regular puncta of actin are connected along the junction in wild-type embryos, but gaps are present in junctions from unc-94(tm724) embryos. Bars=10 µm. D. Box plots of total percent junctional area in which actin signal is present (left) and average length of contiguous regions of actin (right). Blue: First quartile; white: third quartile; pink = mean. Circle = mild outlier; X = extreme outliers. In wild-type, there are occasional stretches of long, unbroken domains of positive signal along entire cells or multiple cells (X).
Immunostaining experiments demonstrate that HMR-1 co-localizes with mislocalized JAC-1::GFP in hmp-1(fe4);unc-94(RNAi) embryos, indicating that the entire CCC is affected (Fig. 2B, top). In contrast, AJM-1, a component of the more basal AJM-1/DLG-1 complex, is unaffected in hmp-1(fe4);unc-94(RNAi) embryos (Fig. 2B, bottom). Thus UNC-94 specifically regulates the CCC and its associated actin.
To better characterize the range of defects in unc-94(tm724), and unc-94(RNAi) embryos, we scored wild-type and unc-94 loss of function embryos stained with phalloidin based on the extent of F-actin disruption (Supp. Fig. S2). We found defects in both junctional actin and CFBs, suggesting that UNC-94 has a role not only in maintaining proper junctional actin, but also in anchoring of CFBs to the junctional actin band. To examine junctional proximal actin defects in more detail, we measured the extent to which junctional actin was contiguous at seam:dorsal and seam:ventral boundaries in wild-type and unc-94(tm724) embryos using phalloidin staining (Fig. 2C,D). In wild-type embryos, 73.7 ± 2.7% (mean ± SEM; n = 11 cells) of the junctional perimeter contained signal, compared with 48.9 ± 4.2% in unc-94(tm724) embryos (n = 16 cells; significantly different, p < 0.0002, heteroscedastic T-test). Similarly, the mean length of contiguous regions of actin at junctions was significantly greater in wildtype (0.41 ± 0.11 µm, n = 11 cells) vs. unc-94(tm724) (0.13 ± 0.02 µm, n = 16 cells; significantly different, p < 0.04). Such defects may have a common cause: defects in the junctional actin band may affect proper anchoring/spacing of CFBs. Vertebrate Tmod3 may similarly stabilize F-actin at lateral cell membranes in immortalized epithelial cell lines [9].
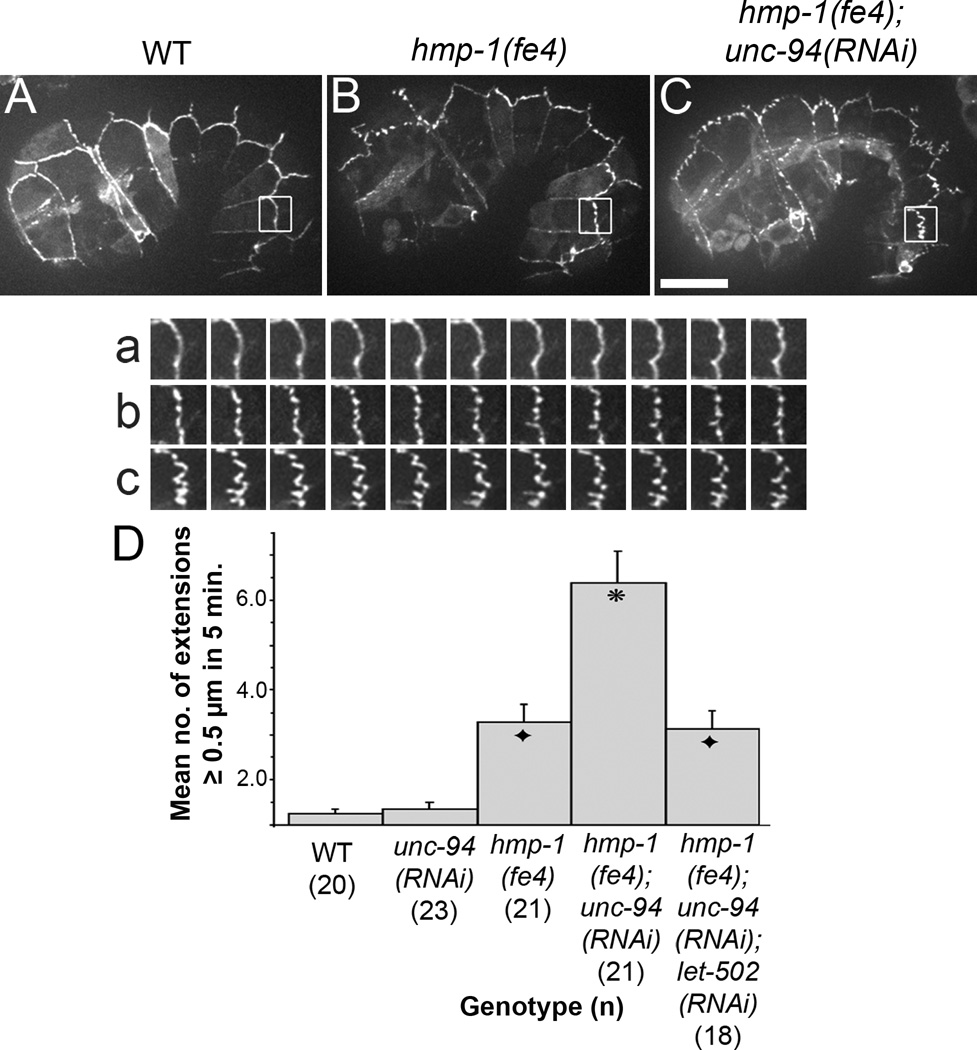
Taken together, these data suggest an important role for HMP-1 and UNC-94 in regulation of AJs and junctional actin at cell borders under stress during morphogenesis. Next we observed CCC dynamics in living, pre-arrested embryos in jac-1::gfp expressing embryos using high-speed filming (Fig. 3A–C). In wild-type embryos, JAC-1::GFP is restricted to the apicolateral contact zones between epidermal cells (Fig. 3A). unc-94(RNAi) and unc-94(tm724) embryos exhibit JAC-1::GFP dynamics similar to wild-type, though rarely some mislocalization occurs (Supp. Fig. S3A,B). In hmp-1(fe4) homozygotes, the JAC-1::GFP distribution is slightly fragmented and some JAC-1::GFP is transiently pulled away from the main area of the junction (Fig. 3B). Strong zygotic loss of hmp-1 function in zu278 homozygotes yields a similar mild effect (Supp. Fig. S3C), consistent with our previous report [24]. In contrast to single mutants, however, dislocation of JAC-1::GFP is greatly enhanced in hmp-1(fe4);unc-94(RNAi) embryos (Fig. 3C). Re-slicing images through the z-axis of these extended regions shows that they are linear and occur perpendicular to the AJ (data not shown). Their spacing and linearity is consistent with them being caused by pulling forces exerted by the CFBs. hmp-1(fe4);unc-94(RNAi) embryos form about twice as many JAC-1::GFP extensions (>= 0.5 µm long) as hmp-1(fe4) (Fig. 3D). Moreover, as demonstrated in Fig. 2C, the AJs of hmp-1(fe4);unc-94(RNAi) embryos become progressively more disrupted as time goes on, suggesting that applied stress results in dystrophic disruption of these junctions. Significantly, these regions extend into both seam and ventral/dorsal epidermal cells, with many more in the latter (Supp. Table S1). Tissue-specific rescue experiments further demonstrate that while UNC-94 plays a role in both seam cells and non-seam cells, there is a more stringent requirement in non-seam cells (Supp. Fig. S4A).
Figure 3. Adherens junctions of hmp-1(fe4);unc-94(RNAi) embryos exhibit abnormal dynamics.
(A, B, C) Images from spinning disk confocal movies of embryos expressing JAC-1::GFP are shown. a–c show an enlargement of the boxed regions in A – C at 30 second intervals for 5 minutes. Supplemental video S2, corresponding to a–c, is available online. In wild-type embryos, JAC-1::GFP localizes to epidermal cell borders; in hmp-1(fe4) embryos, there are occasional areas in which JAC-1::GFP is transiently extended away from its normal position. In hmp-1(fe4);unc-94(RNAi) embryos this behavior is more pronounced. Bar=10 µm. (D) Bar graph showing quantification of the number (mean ± SEM; n indicated in parentheses) of JAC-1::GFP extension longer than 5 µm formed at either the seam:dorsal or seam:ventral cell border during 5 minutes of filming. Embryos at comma to 1.5 fold stage were scored. Each extension was measured only once, at its longest length. Asterisk: significantly different from hmp-1(fe4) and hmp-1(fe4);unc-94(RNAi);let-502(RNAi) (Tukey test: p < 0.01). Black diamonds: not significantly different from hmp-1(fe4) (p > 0.5).
The phenotypes we observe are very similar to those described previously in rga-2/RhoGAP mutants [25], though they are less pervasive along the apicobasal axis. We therefore assessed whether reducing stress on epidermal junctions could ameliorate the JAC-1::GFP extensions observed in hmp-1(fe4);unc-94(RNAi) embryos, using RNAi against let-502/Rho kinase. hmp-1(fe4);unc-94,let-502(RNAi) embryos exhibit a significant decrease in the number of JAC-1::GFP extensions (Fig. 3D) and arrested embryos show less JAC-1::GFP mislocalization (cf. Supp. Fig. S3D vs. E). This suggests that the AJs in hmp-1(fe4);unc-94(RNAi) embryos are not able to withstand the stress transmitted by CFBs during elongation, and instead, become pulled in the direction of the exerted force. To investigate the effects of let-502 loss of function on junctions further, we used a temperature-sensitive let-502 mutant to assess whether LET-502 activity is required for recruitment of UNC-94 to cell borders, and found that this is not the case, although cell elongation along the anterior-posterior axis is required for compaction of the zone of UNC-94 expression along seam:non-seam borders (Supp. Fig. S4B).
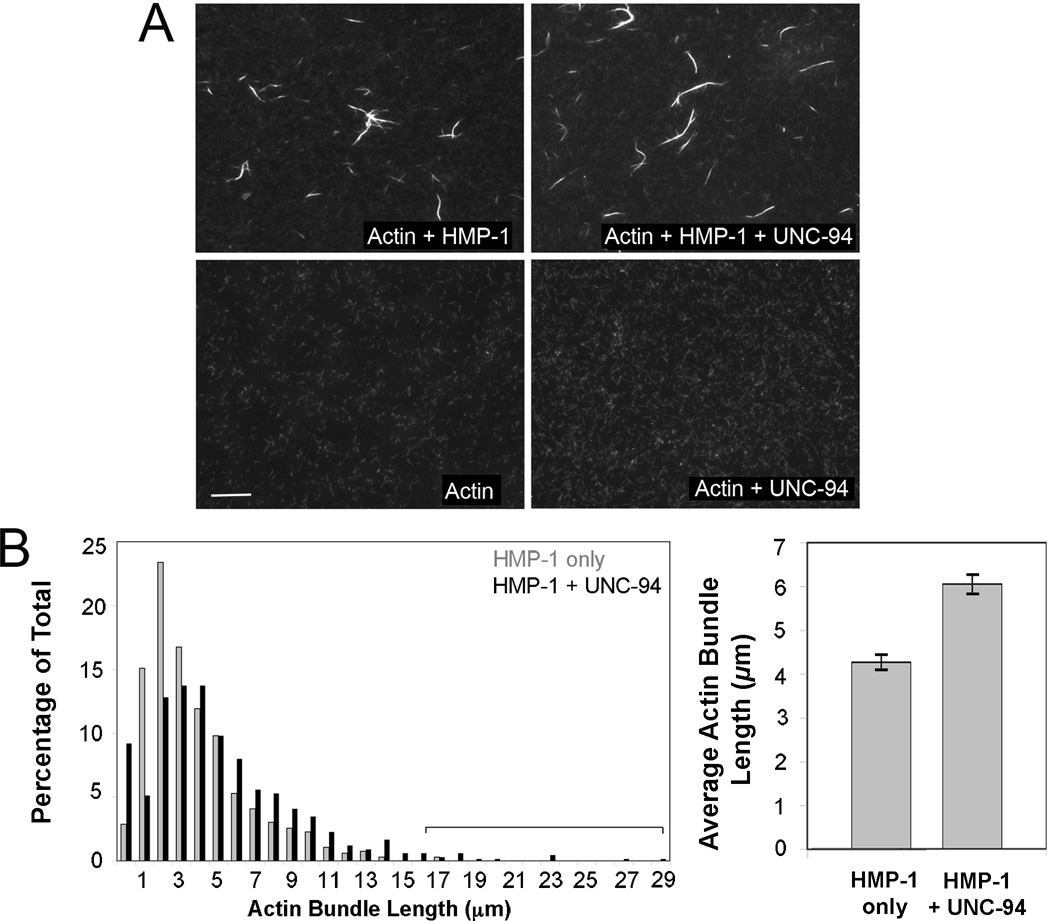
To gain mechanistic insight into how HMP-1 and UNC-94 act together to modulate actin networks, we performed in vitro actin binding and bundling assays. We previously showed that UNC-94 alone can inhibit latrunculin A induced depolymerization of plus-end capped C. elegans F-actin, and that UNC-94 blocks minus-end F-actin depolymerization induced by UNC-60B/ADF-cofilin [23]. We also showed previously that full-length HMP-1 alone can cosediment with actin filaments in vitro. This activity appears to be regulated by intramolecular interactions within the full-length protein, since the full-length protein cosediments less avidly than C-terminal fragments [16]. We therefore examined the combined effects of HMP-1 and UNC-94 on actin filament morphology in vitro using fluorescently labeled actin filaments capped at plus ends by CapZ, to which HMP-1, UNC-94, or both were added (Fig. 4A). HMP-1 added alone induced actin bundles like other α-catenins [26–28]. However, actin bundles generated in the presence of both HMP-1 and UNC-94 (n = 603, average length = 6.1 µm ± 2.7; S.D.) were 42% longer than those resulting from HMP-1 alone (n= 663, average length = 4.3 µm ± 2.8; significantly different, p<0.001, Fig. 4B), and this increase is in part due to an increase in long bundles (Fig. 4B, bracket). Taken together, these data indicate that HMP-1 and UNC-94 likely act together to generate robust actin filaments in the junctional actin band, which in turn resist mechanical deformation due to Rho-mediated actomyosin contractility.
Figure 4. HMP-1 and UNC-94 synergistically regulate actin bundles in vitro.
(A) Images showing fluorescently labeled, plus-end capped F-actin (5 µM) to which either HMP-1 (5 µM), UNC-94 (2.5 µM) or both have been added. Note that actin bundles form only when HMP-1 is present and that UNC-94 can lengthen HMP-1-generated actin bundles. Bar = 10 µm. (B) (Left) Histogram of actin bundle length when HMP-1 is added alone (n=663) or together with UNC-94 (n=603). Percentage of bundles exhibiting particular lengths (indicated in µm) is shown. The bracket indicates that when HMP-1 and UNC-94 are added together, there is an increase in the longest population of bundles filaments. (Right) Mean length of actin bundles formed by HMP-1 alone and HMP-1 plus UNC-94. Error bars, SEM. Actin bundles generated by HMP-1 plus UNC-94 are significantly longer than those generated by HMP-1 alone (p < 0.001, two tailed t-test).
The actin cytoskeleton and AJs cooperate to drive numerous epithelial morphogenetic events [2]. AJs recruit actin via α-catenin; initial recruitment may be modulated by Arp2/3-mediated actin branching or by processive plus-end proteins that stimulate more linear networks [2]. Once actin networks form at AJs, however, they must withstand stress and resist dissolution. Our results indicate a new role for Tmod at the minus ends of actin filaments in this process.
In the embryonic epidermis of C. elegans, UNC-94 is enriched at a subset of epidermal cell borders that interface with CFBs, within the dense network of junctional actin bundles that runs lateral to AJs at these cell borders. Our in vitro analysis indicates that UNC-94 can protect filaments bundled by HMP-1 from minus-end subunit loss, since this assay was performed under conditions that favor depolymerization from the minus end, rather than addition of monomers to plus ends. Since some Tmods promote actin nucleation [5], a non-mutually exclusive possibility is that UNC-94 also plays a supporting role in de novo formation of junctional actin filaments.
In hmp-1(fe4);unc-94(RNAi) embryos, inefficient actin recruitment by mutant α-catenin, coupled with minus-end subunit loss, may lead to a less robust junctional actin network, which in turn results in lateral instability of AJs. If CFBs are mechanically coupled to the junctional actin band near their tips (e.g., via actin crosslinking proteins, or through α-catenin itself), stress would tend to be distributed laterally throughout the junctional actin band, reducing stress at CFB insertion sites. We envision such reinforcement as functioning in much the same way that the roots of a tree protect them from being uprooted, by distributing stress laterally. This idea is supported by our observation that decreasing actomyosin contractility alleviates the junctional displacements observed in hmp-1(fe4);unc-94(RNAi) embryos. We propose that the retraction phenotype exhibited by hmp-1(fe4);tmd-1(RNAi) embryos (in which the embryos extend to ~1.5 fold and then retract to their original length) may be due to a combination of uneven CFB pulling forces from abnormally arranged CFBs and weakened ultrastructure of the junctional actin band. This could result in failure to translate the stress applied by CFBs into the epidermal cell shape changes that drive elongation.
Junctional actin bands are present in many epithelial cell types. The forces applied to them can be aligned predominantly along the junction or orthogonal to it, the latter being the case during C. elegans embryonic elongation (reviewed in [29]). Our work suggests that regulation of minus end actin dynamics via Tmod plays an important role in promoting stability of actin networks under such orthogonal stress. Future experiments aimed at teasing apart the ultrastructure and biochemical regulation of junctional actin bands should help to clarify their functions, and how multiple actin regulators contribute to the mechanical integrity of AJs.
Supplementary Material
Highlights.
Tropomodulin (Tmod) has previously unrecognized roles in cell-cell adhesion
Tmod accumulates at adherens junctions under tension in an α-catenin-dependent manner
Tmod acts with α-catenin to stabilize junctions against Rho-mediated contractility
Tmod and α-catenin together act to form more robust F-actin bundles
Acknowledgements
We thank Yuji Kohara for providing cDNAs, Shohei Mitani for providing the unc-94(tm724) allele, Ryan King and the Ivan Rayment lab for technical assistance in expression and purification of His-tagged UNC-94, and Michael Joyce for help with coIP assays. We thank Vida Praitis and Bill Bement for critical reading of the manuscript, and Theresa Grana for helpful comments. This work was supported by NIH grants NRSA GM067410 and R15 HD059952 to E.C., NIH grant R01 GM58038, Muscular Dystrophy Association grant 4218, and NSF grant IOB 0518081 to J.H., and NIH grant R01 AR48615 to S.O.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weis WI, Nelson WJ. Re-solving the cadherin-catenin-actin conundrum. J Biol Chem. 2006;281:35593–35597. doi: 10.1074/jbc.R600027200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niessen CM, Leckband D, Yap AS. Tissue organization by cadherin adhesion molecules: dynamic molecular and cellular mechanisms of morphogenetic regulation. Physiol Rev. 2011;91:691–731. doi: 10.1152/physrev.00004.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fischer RS, Fowler VM. Tropomodulins: life at the slow end. Trends Cell Biol. 2003;13:593–601. doi: 10.1016/j.tcb.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Littlefield RS, Fowler VM. Thin filament length regulation in striated muscle sarcomeres: pointed-end dynamics go beyond a nebulin ruler. Semin Cell Dev Biol. 2008;19:511–519. doi: 10.1016/j.semcdb.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamashiro S, Speicher KD, Speicher DW, Fowler VM. Mammalian tropomodulins nucleate actin polymerization via their actin monomer binding and filament pointed end-capping activities. J Biol Chem. 2010;285:33265–33280. doi: 10.1074/jbc.M110.144873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chu X, Chen J, Reedy MC, Vera C, Sung KL, Sung LA. E-Tmod capping of actin filaments at the slow-growing end is required to establish mouse embryonic circulation. Am J Physiol Heart Circ Physiol. 2003;284:H1827–H1838. doi: 10.1152/ajpheart.00947.2002. [DOI] [PubMed] [Google Scholar]
- 7.Fritz-Six KL, Cox PR, Fischer RS, Xu B, Gregorio CC, Zoghbi HY, Fowler VM. Aberrant myofibril assembly in tropomodulin1 null mice leads to aborted heart development and embryonic lethality. J Cell Biol. 2003;163:1033–1044. doi: 10.1083/jcb.200308164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fath T, Fischer RS, Dehmelt L, Halpain S, Fowler VM. Tropomodulins are negative regulators of neurite outgrowth. Eur J Cell Biol. 2011;90:291–300. doi: 10.1016/j.ejcb.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weber KL, Fischer RS, Fowler VM. Tmod3 regulates polarized epithelial cell morphology. J Cell Sci. 2007;120:3625–3632. doi: 10.1242/jcs.011445. [DOI] [PubMed] [Google Scholar]
- 10.Costa M, Raich W, Agbunag C, Leung B, Hardin J, Priess JR. A putative catenin-cadherin system mediates morphogenesis of the Caenorhabditis elegans embryo. J Cell Biol. 1998;141:297–308. doi: 10.1083/jcb.141.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pettitt J, Cox EA, Broadbent ID, Flett A, Hardin J. The Caenorhabditis elegans p120 catenin homologue, JAC-1, modulates cadherin-catenin function during epidermal morphogenesis. J Cell Biol. 2003;162:15–22. doi: 10.1083/jcb.200212136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cox EA, Hardin J. Sticky worms: adhesion complexes in C. elegans. J Cell Sci. 2004;117:1885–1897. doi: 10.1242/jcs.01176. [DOI] [PubMed] [Google Scholar]
- 13.Lynch AM, Hardin J. The assembly and maintenance of epithelial junctions in C. elegans. Front Biosci. 2009;14:1414–1432. doi: 10.2741/3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chisholm AD, Hardin J. Epidermal morphogenesis. WormBook. 2005:1–22. doi: 10.1895/wormbook.1.35.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, Gally C, Labouesse M. Tissue morphogenesis: how multiple cells cooperate to generate a tissue. Curr Opin Cell Biol. 2010;22:575–582. doi: 10.1016/j.ceb.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Kwiatkowski AV, Maiden SL, Pokutta S, Choi HJ, Benjamin JM, Lynch AM, Nelson WJ, Weis WI, Hardin J. In vitro and in vivo reconstitution of the cadherin-catenin-actin complex from Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2010;107:14591–14596. doi: 10.1073/pnas.1007349107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaidel-Bar R, Itzkovitz S, Ma'ayan A, Iyengar R, Geiger B. Functional atlas of the integrin adhesome. Nat Cell Biol. 2007;9:858–867. doi: 10.1038/ncb0807-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zahreddine H, Zhang H, Diogon M, Nagamatsu Y, Labouesse M. CRT-1/calreticulin and the E3 ligase EEL-1/HUWE1 control hemidesmosome maturation in C. elegans development. Curr Biol. 2010;20:322–327. doi: 10.1016/j.cub.2009.12.061. [DOI] [PubMed] [Google Scholar]
- 19.Grindstaff KK, Yeaman C, Anandasabapathy N, Hsu SC, Rodriguez-Boulan E, Scheller RH, Nelson WJ. Sec6/8 complex is recruited to cell-cell contacts and specifies transport vesicle delivery to the basal-lateral membrane in epithelial cells. Cell. 1998;93:731–740. doi: 10.1016/s0092-8674(00)81435-x. [DOI] [PubMed] [Google Scholar]
- 20.Ikeda W, Nakanishi H, Miyoshi J, Mandai K, Ishizaki H, Tanaka M, Togawa A, Takahashi K, Nishioka H, Yoshida H, et al. Afadin: A key molecule essential for structural organization of cell-cell junctions of polarized epithelia during embryogenesis. J Cell Biol. 1999;146:1117–1132. doi: 10.1083/jcb.146.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhadanov AB, Provance DW, Jr, Speer CA, Coffin JD, Goss D, Blixt JA, Reichert CM, Mercer JA. Absence of the tight junctional protein AF-6 disrupts epithelial cell-cell junctions and cell polarity during mouse development. Curr Biol. 1999;9:880–888. doi: 10.1016/s0960-9822(99)80392-3. [DOI] [PubMed] [Google Scholar]
- 22.Stevenson TO, Mercer KB, Cox EA, Szewczyk NJ, Conley CA, Hardin JD, Benian GM. unc-94 encodes a tropomodulin in Caenorhabditis elegans. Journal of molecular biology. 2007;374:936–950. doi: 10.1016/j.jmb.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamashiro S, Cox EA, Baillie DL, Hardin JD, Ono S. Sarcomeric actin organization is synergistically promoted by tropomodulin, ADF/cofilin, AIP1 and profilin in C. elegans. J Cell Sci. 2008;121:3867–3877. doi: 10.1242/jcs.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simske JS, Koppen M, Sims P, Hodgkin J, Yonkof A, Hardin J. The cell junction protein VAB-9 regulates adhesion and epidermal morphology in C. elegans. Nat Cell Biol. 2003;5:619–625. doi: 10.1038/ncb1002. [DOI] [PubMed] [Google Scholar]
- 25.Diogon M, Wissler F, Quintin S, Nagamatsu Y, Sookhareea S, Landmann F, Hutter H, Vitale N, Labouesse M. The RhoGAP RGA-2 and LET-502/ROCK achieve a balance of actomyosin-dependent forces in C. elegans epidermis to control morphogenesis. Development. 2007;134:2469–2479. doi: 10.1242/dev.005074. [DOI] [PubMed] [Google Scholar]
- 26.Dickinson DJ, Nelson WJ, Weis WI. A polarized epithelium organized by beta- and alpha-catenin predates cadherin and metazoan origins. Science. 2011;331:1336–1339. doi: 10.1126/science.1199633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 2005;123:903–915. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rimm DL, Koslov ER, Kebriaei P, Cianci CD, Morrow JS. Alpha 1(E)-catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc Natl Acad Sci U S A. 1995;92:8813–8817. doi: 10.1073/pnas.92.19.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gomez GA, McLachlan RW, Yap AS. Productive tension: force-sensing and homeostasis of cell-cell junctions. Trends Cell Biol. 2011;21:499–505. doi: 10.1016/j.tcb.2011.05.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.