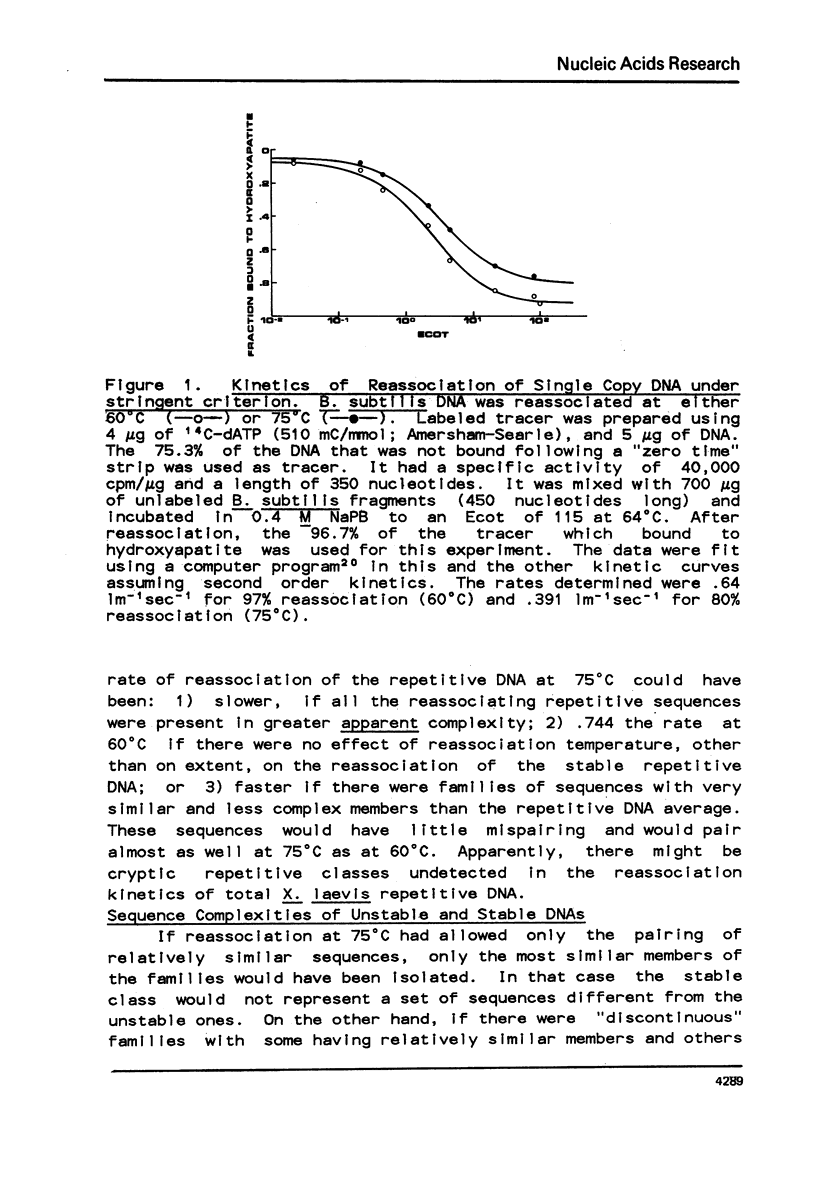
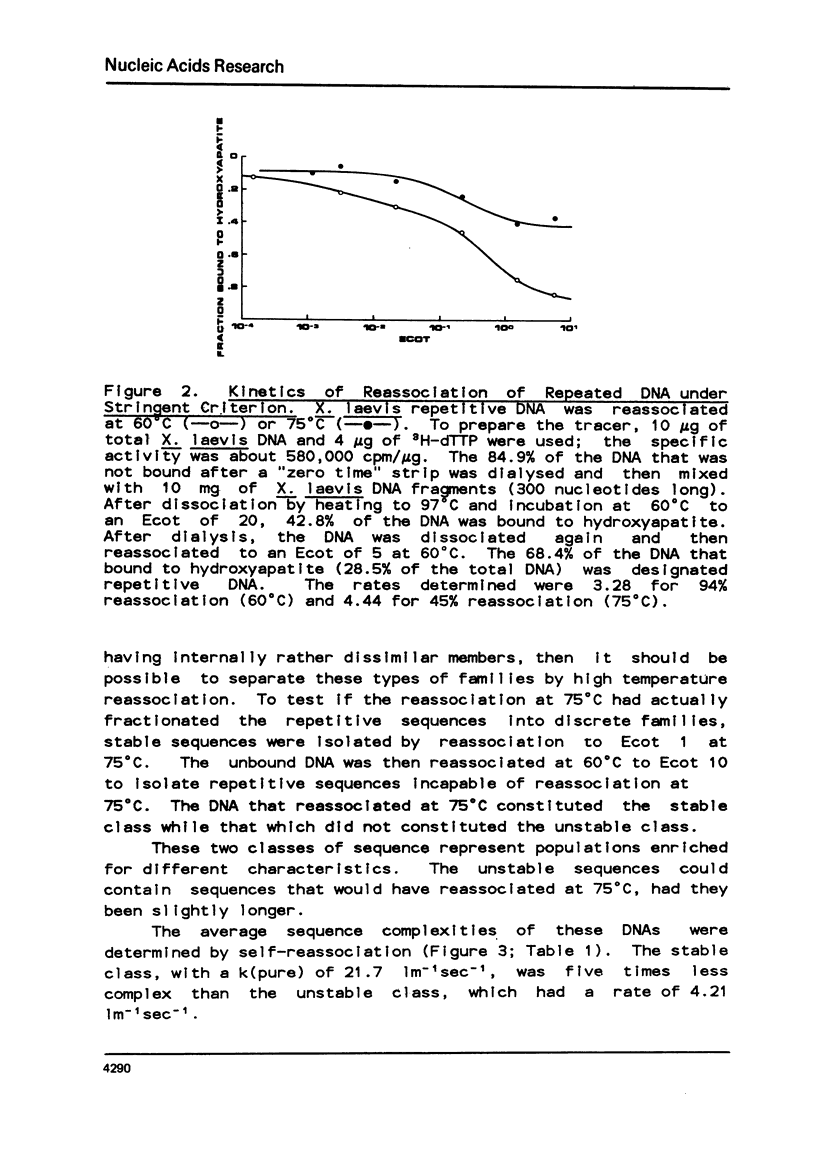
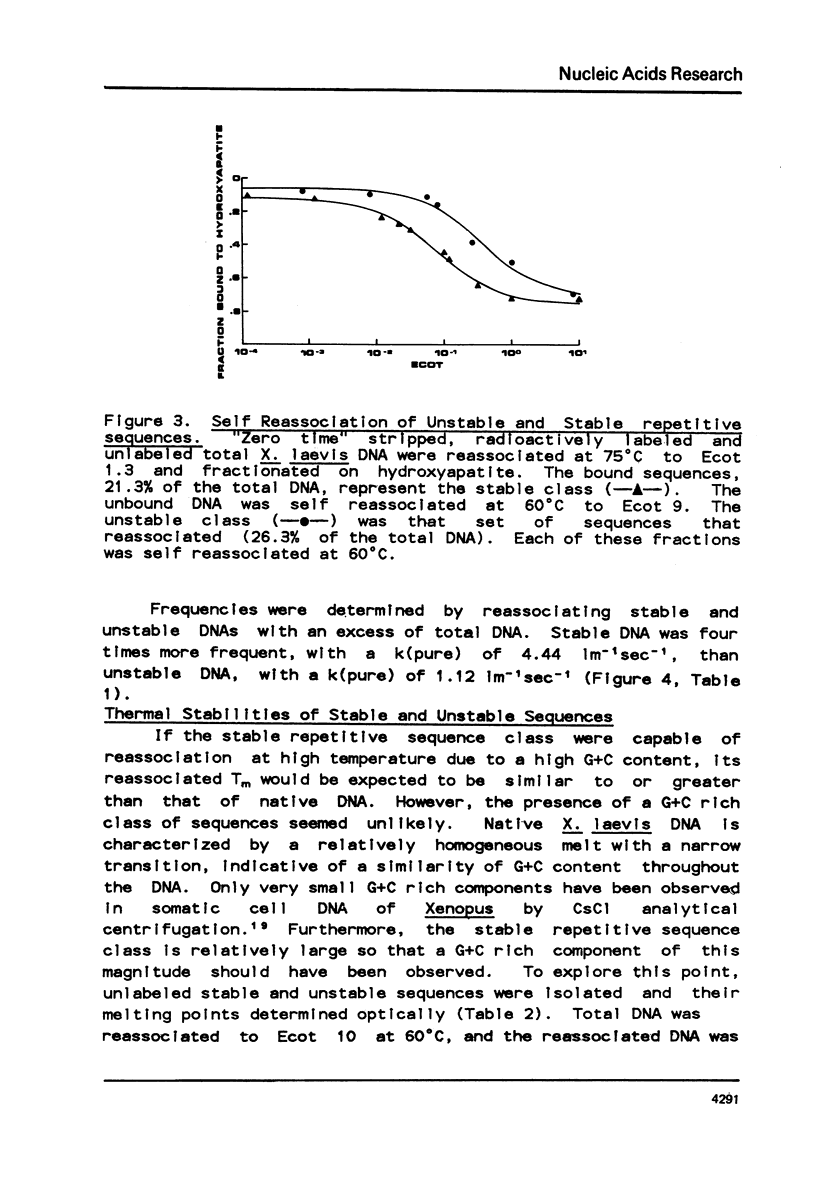
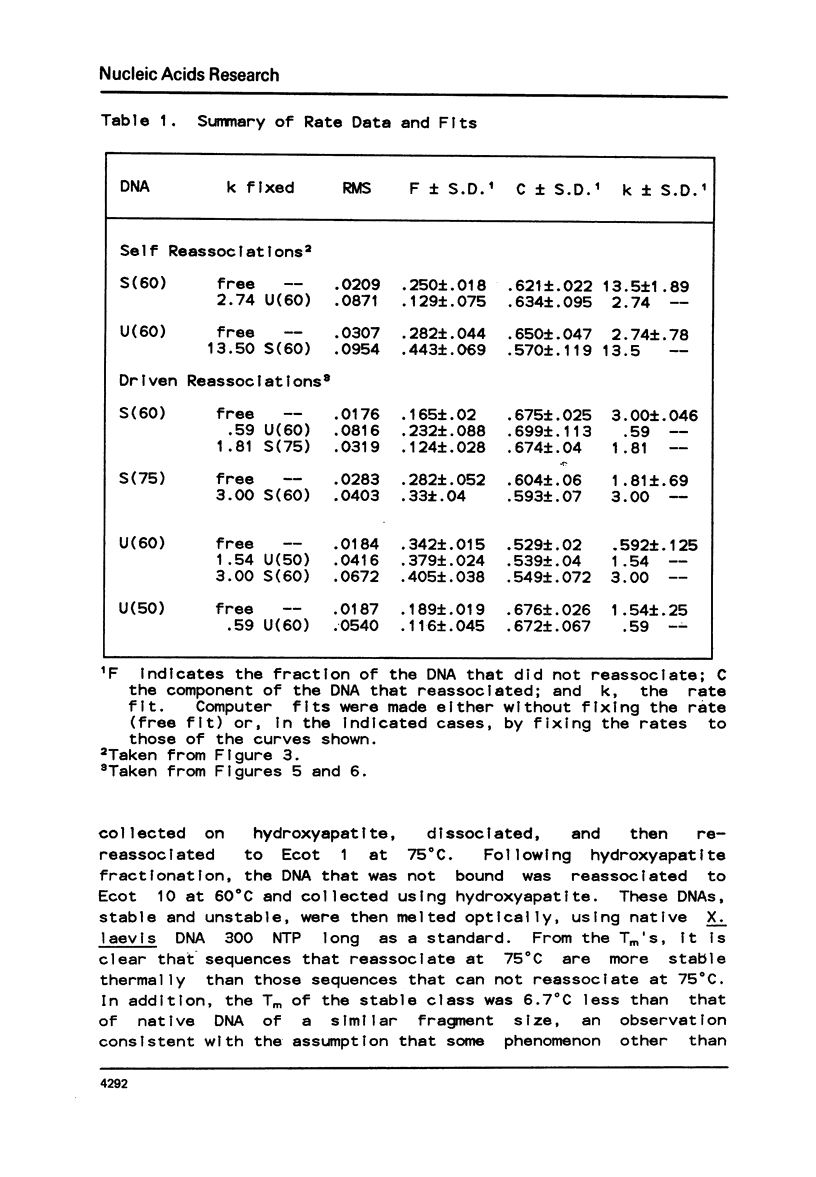
Abstract
Sequences that did or did not reassociate at 75°C (stable and unstable, respectively) were isolated from total repetitive Xenopuslaevis DNA. Sequence complexities or frequencies were determined by self (minicot) or DNA excess (slave minicot) reassociations at 60°C. Stable sequences were five times shorter and four times more frequent than unstable sequences. Reassociations at 75°C or at 50°C were used to establish apparent sequence frequencies at these criteria. Interspersion curves at either 60°C or 75°C and low Cot reassociation of long fragments of total X.laevis DNA at either 60°C or 75°C, followed by S1 digestion and agarose chromatography, were used to determine genome arrangement of the stable and unstable classes of sequence. Reassociation at high temperature was found to permit the fractionation of repetitive sequences into two populations of differing characteristics.
Full text
PDF





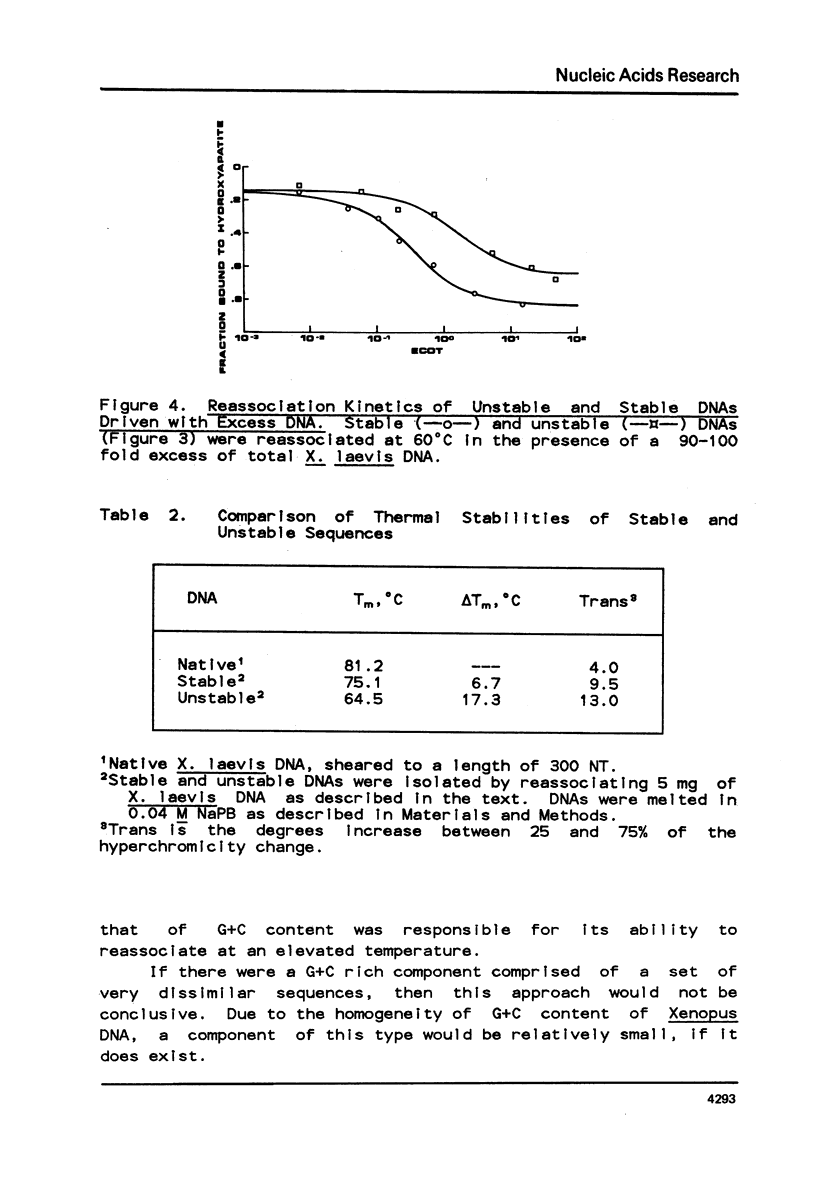

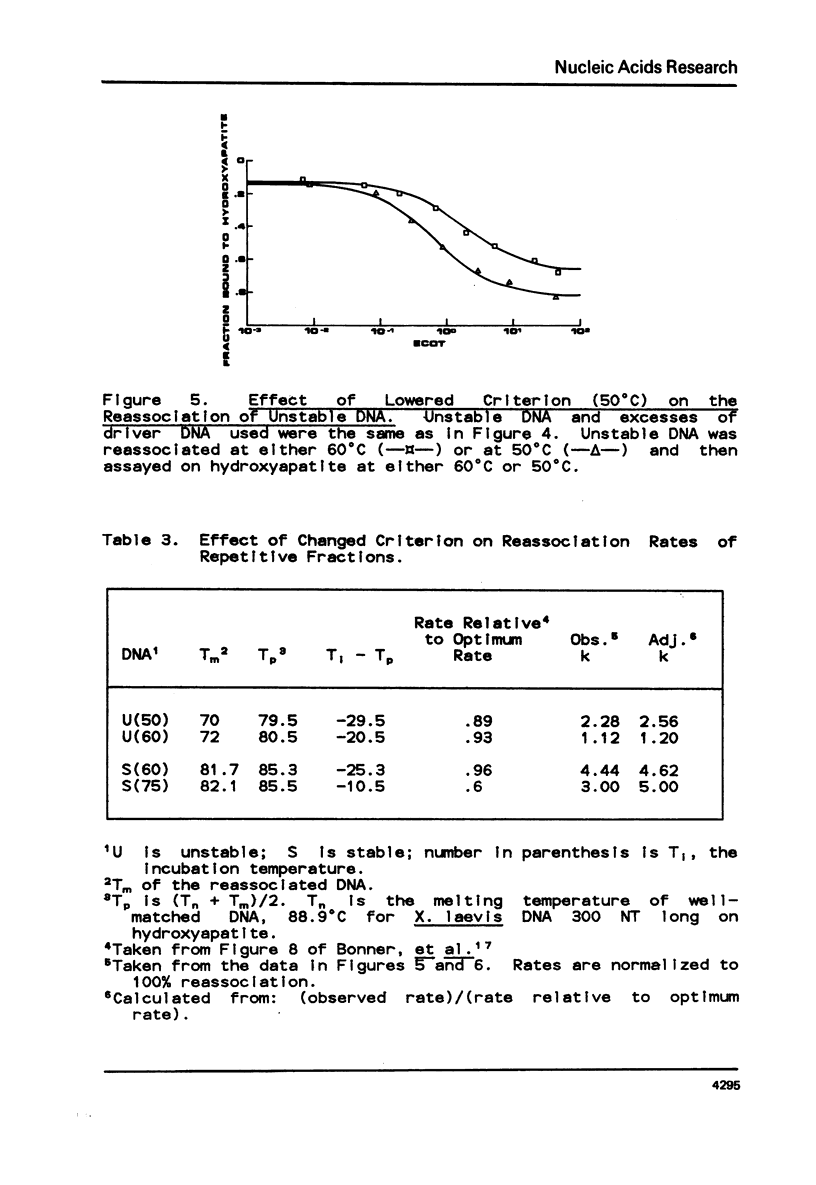

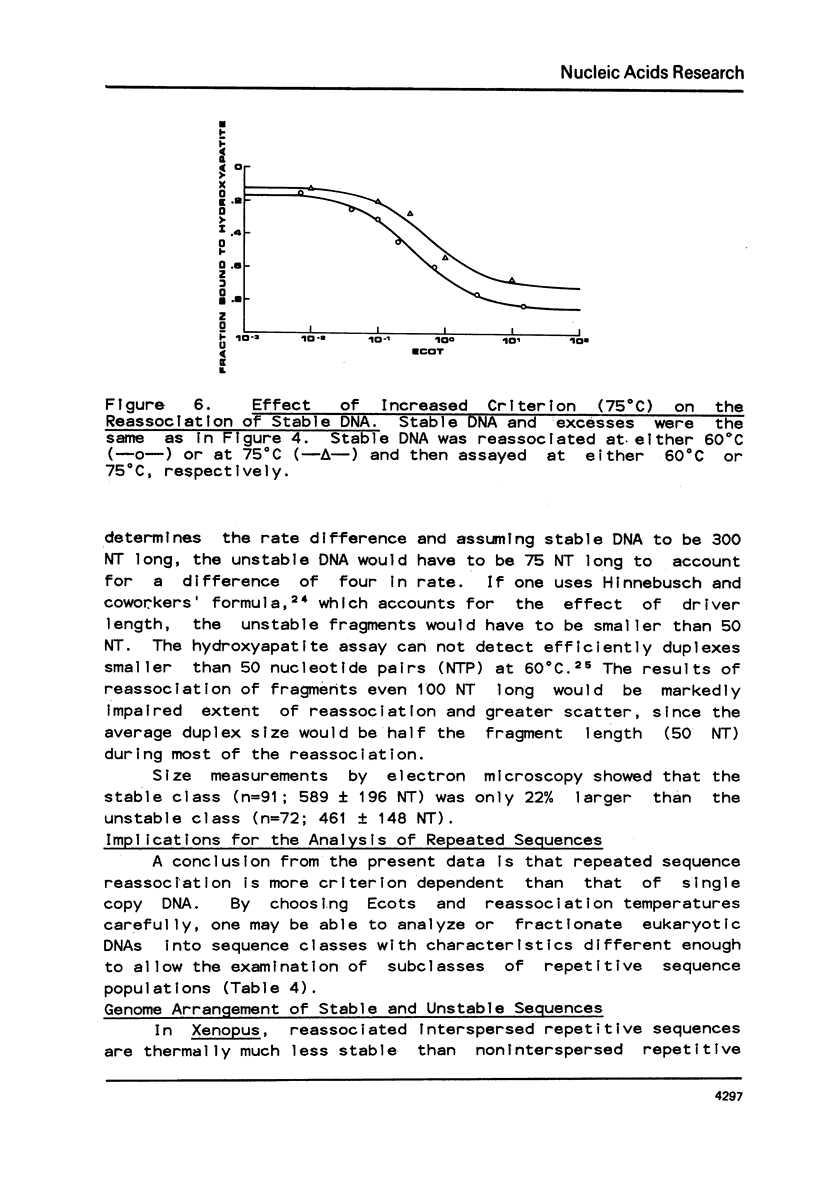
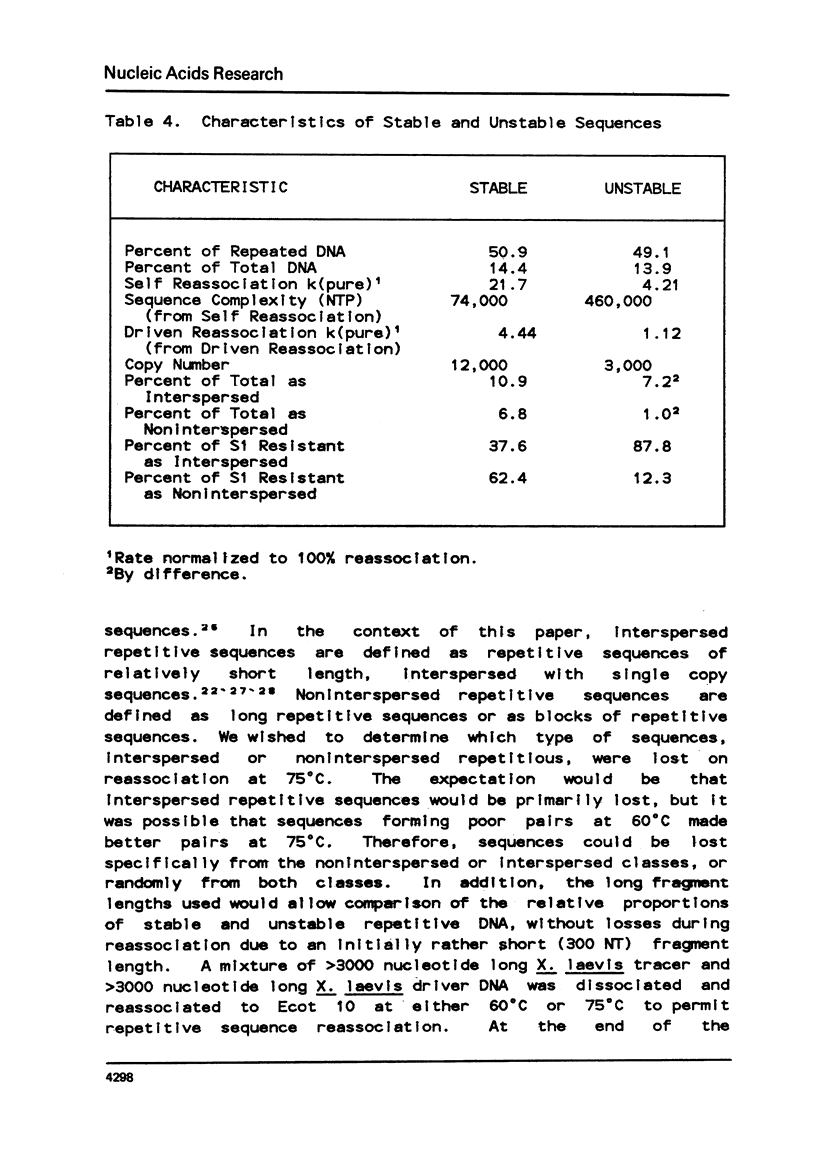



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner T. I., Brenner D. J., Neufeld B. R., Britten R. J. Reduction in the rate of DNA reassociation by sequence divergence. J Mol Biol. 1973 Dec 5;81(2):123–135. doi: 10.1016/0022-2836(73)90184-8. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Graham D. E., Eden F. C., Painchaud D. M., Davidson E. H. Evolutionary divergence and length of repetitive sequences in sea urchin DNA. J Mol Evol. 1976 Dec 31;9(1):1–23. doi: 10.1007/BF01796119. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Graham D. E., Neufeld B. R. Analysis of repeating DNA sequences by reassociation. Methods Enzymol. 1974;29:363–418. doi: 10.1016/0076-6879(74)29033-5. [DOI] [PubMed] [Google Scholar]
- Brown D. D., Dawid I. B. Specific gene amplification in oocytes. Oocyte nuclei contain extrachromosomal replicas of the genes for ribosomal RNA. Science. 1968 Apr 19;160(3825):272–280. doi: 10.1126/science.160.3825.272. [DOI] [PubMed] [Google Scholar]
- Case S. T., Baker R. F. Investigation into the use of Aspergillus oryzae S1 nuclease in the presence of solvents which destabilize or prevent DNA secondary structure: formaldehyde, formamide, and glyoxal. Anal Biochem. 1975 Apr;64(2):477–484. doi: 10.1016/0003-2697(75)90457-1. [DOI] [PubMed] [Google Scholar]
- Davidson E. H., Graham D. E., Neufeld B. R., Chamberlin M. E., Amenson C. S., Hough B. R., Britten R. J. Arrangement and characterization of repetitive sequence elements in animal DNAs. Cold Spring Harb Symp Quant Biol. 1974;38:295–301. doi: 10.1101/sqb.1974.038.01.033. [DOI] [PubMed] [Google Scholar]
- Davidson E. H., Hough B. R., Amenson C. S., Britten R. J. General interspersion of repetitive with non-repetitive sequence elements in the DNA of Xenopus. J Mol Biol. 1973 Jun 15;77(1):1–23. doi: 10.1016/0022-2836(73)90359-8. [DOI] [PubMed] [Google Scholar]
- Eden F. C., Graham D. E., Davidson E. H., Britten R. J. Exploration of long and short repetitive sequence relationships in the sea urchin genome. Nucleic Acids Res. 1977;4(5):1553–1567. doi: 10.1093/nar/4.5.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D. E. The isolation of high molecular weight DNA from whole organisms or large tissue masses. Anal Biochem. 1978 Apr;85(2):609–613. doi: 10.1016/0003-2697(78)90262-2. [DOI] [PubMed] [Google Scholar]
- Hayes F. N., Lilly E. H., Ratliff R. L., Smith D. A., Williams D. L. Thermal transitions in mixtures of polydeoxyribodinucleotides. Biopolymers. 1970;9(9):1105–1117. doi: 10.1002/bip.1970.360090911. [DOI] [PubMed] [Google Scholar]
- Hinnebusch A. G., Clark V. E., Klotz L. C. Length dependence in reassociation kinetics of radioactive tracer DNA. Biochemistry. 1978 Apr 18;17(8):1521–1529. doi: 10.1021/bi00601a026. [DOI] [PubMed] [Google Scholar]
- Hough B. R., Smith M. J., Britten R. J., Davidson E. H. Sequence complexity of heterogeneous nuclear RNA in sea urchin embryos. Cell. 1975 Jul;5(3):291–299. doi: 10.1016/0092-8674(75)90104-x. [DOI] [PubMed] [Google Scholar]
- Kelly R. B., Cozzarelli N. R., Deutscher M. P., Lehman I. R., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. XXXII. Replication of duplex deoxyribonucleic acid by polymerase at a single strand break. J Biol Chem. 1970 Jan 10;245(1):39–45. [PubMed] [Google Scholar]
- Marsh J. L., McCarthy B. J. Effect of reaction conditions on the reassociation of divergent deoxyribonucleic acid sequences. Biochemistry. 1974 Jul 30;13(16):3382–3388. doi: 10.1021/bi00713a031. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Davidson E. H., Britten R. J. A program for least squares analysis of reassociation and hybridization data. Nucleic Acids Res. 1977 Jun;4(6):1727–1737. doi: 10.1093/nar/4.6.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice N. R., Straus N. A. Relatedness of mouse satellite deoxyribonucleic acid to deoxyribonucleic acid of various Mus species. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3546–3550. doi: 10.1073/pnas.70.12.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachat F. H., Hogness D. S. Repetitive sequences in isolated Thomas circles from Drosophila melanogaster. Cold Spring Harb Symp Quant Biol. 1974;38:371–381. doi: 10.1101/sqb.1974.038.01.040. [DOI] [PubMed] [Google Scholar]
- Sutton W. D., McCallum M. Related satellite DNA's in the genus Mus. J Mol Biol. 1972 Nov 28;71(3):633–652. doi: 10.1016/s0022-2836(72)80028-7. [DOI] [PubMed] [Google Scholar]
- Vogt V. M. Purification and further properties of single-strand-specific nuclease from Aspergillus oryzae. Eur J Biochem. 1973 Feb 15;33(1):192–200. doi: 10.1111/j.1432-1033.1973.tb02669.x. [DOI] [PubMed] [Google Scholar]
- Welker N. E., Campbell L. L. Unrelatedness of Bacillus amyloliquefaciens and Bacillus subtilis. J Bacteriol. 1967 Oct;94(4):1124–1130. doi: 10.1128/jb.94.4.1124-1130.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westmoreland B. C., Szybalski W., Ris H. Mapping of deletions and substitutions in heteroduplex DNA molecules of bacteriophage lambda by electron microscopy. Science. 1969 Mar 21;163(3873):1343–1348. doi: 10.1126/science.163.3873.1343. [DOI] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]


