Abstract
Here, we show that the local incorporation of osmotically active hyaluronan into previously compressed collagen constructs results in further rapid dehydration/compression of collagen layers, channel formation and generation of new interfaces; these novel structures, at the nano–micro (i.e. meso-scale) were formed within native collagen gels, in a highly predictable spatial manner and offer important new methods of fabricating scaffolds (e.g. tubes and open-spirals) with potential for use in tissue regeneration such as in peripheral nerves and small vessels. This paper tests the possibility that the local fluid content of a dense collagen network can be controlled by incorporation of an osmotically active (native) macromolecule—hyluronan. This is an exemplar physiological, osmotic swelling agent. Hyaluronan is commonly secreted by cells deep in connective tissues, so is a good candidate for this role in a cell-driven system balancing mechanical compaction of bulk tissue collagen. These constructs may have potential as functional in vitro models representing developmental and pathological processes.
Keywords: collagen, biomaterials, tissue regeneration, hyaluronan
1. Introduction
In recent years, a new paradigm has been developing for the rapid fabrication of native tissues by layer engineering. This has taken the form of two streams: one of predominantly cell layers [1] and the other of matrix-rich layers [2,3]. Both involve the production of thin sheets (tens of micrometres) of their respective tissues by reproducible, rapid or mass production technologies with subsequent layer assembly into complex multi-layered tissues. The great advantage of layer tissue engineering is its ability to mimic native tissue three-dimensional structure and composition, rapidly, reproducibly and at tissue-like resolution (i.e. layer composition can be changed at 50–100 µm intervals). We have developed matrix-layer engineering based on rapid fabrication of dense cell–collagen tissue sheets and their multi-layer assembly into either stacks (linear) or spirals (radial layering) [2].
The basic process underlying this technology radiation is collagen plastic compression. In this process, a hyper-hydrated gel of native collagen containing living cells has a large proportion of its fluid content rapidly expelled (normally in one direction). This produces a controllable, dense living tissue layer in minutes [2,4]. This plastic compression is characterized by minimal re-swelling and fluid return across the (physiological) range of fluid loss used. Conventionally, it would be expected that plastic deformation of a viscoelastic, water-rich material such as a collagen gel would continue until the collagen density increased to a point where deformation became elastic. Hence, the process will be plastic to the point where hydration of the collagen itself (driven by its exposed fixed charge density) begins to drive water re-uptake. Beyond this point of water loss, the material would be expected to re-swell as soon as the compressive load is removed, making this the elastic deformation phase. This plastic–elastic transition is potentially important both for its use in tissue fabrication and as a route to understanding the natural manner in which cells produce connective tissue structure [5,6]. The cell relevance comes from the balance of cell-generated loads versus protein hydration levels that must also exist in natural tissues, particularly in those that are newly forming or repairing.
In natural connective tissues, dense fibrillar collagen almost always occurs along with extra-fibrillar, charged polysaccharides. These are commonly present as glycosaminoglycans (GAGs) either as part of proteoglycans or free as hyaluronan, which exert strong swelling pressures owing to their extremely high level of fixed charge and natural hydration. This swelling tendency is far greater than that of collagen such that they generate a relative dehydration effect, in some cases reducing the diameter of adjacent collagen fibrils [7]. The question can then be asked, does this plastic–elastic collagen compaction transition play an important part in how cells produce and maintain local (meso-scale) tissue structure? For example, cell forces generated on dilute collagen gels (and presumably on newly deposited; e.g. repair) collagen bundles undoubtedly permanently compact the collagen However, when those same cells secrete GAGs in any form their physico-chemical influence will inevitably shift the plastic–elastic transition by reducing the immediate and local availability of free water. In some configurations, this GAG swelling would be expected to contribute to mechanical compression, in others it would tend to strip water from the fibrillar collagen [7]. We have investigated the potential of this effect here, using our model of compressed collagen materials, both in terms of a tool that can be used to generate microstructure during rapid tissue processing and as a means to explain natural, cell-associated remodelling events in the extracellular matrix.
In our model system, we have adopted the osmotically active GAG hyaluronan (hyaluronic acid: HA). Hyaluronan is a negatively charged GAG, found in locations such as skin, vitreous humour and synovial fluid [8]. It has a very high fixed charge density, giving it marked osmotic swelling, with powerful hydration- and gel-forming properties in aqueous media [9,10]. Along with its known biological properties and clinical use, this dramatic ability to compete for and bind to water makes HA an ideal candidate to test the role of local fluid flow on collagen meso-scale structure [9,11]. Dry HA has a very large potential to draw in aqueous fluid and to physically swell to many times its original volume forming first a stiff, then a more viscous gel. This has two immediate consequences: first, the generation of mechanical forces on any surrounding structures that initially contained the dry HA, second, there is a very rapid movement of water out of the surrounding structures into the HA gel to feed this swelling. In a spatially confined system, this redistribution of fluid can have significant local (in our case meso-scale) effects.
The first model in this study involved the integration of dry HA mats into a three-dimensional spiral construct of compressed collagen. This is applied in one of two spatial configurations, at the core or as a layer through the spiral (figure 1), testing the effects of spatially confined rapid HA swelling. Key outputs here were changes in three-dimensional structure and collagen-layer thickness (i.e. collagen water content). In a second model, the same principle of confined GAG swelling was tested using HA-coated beads embedded within compressed collagen gels. In this case, the idea was to model point-production of HA within a collagen gel, comparable to that which would occur when cells in collagen-rich extracellular matrix secrete HA into their immediate surroundings.
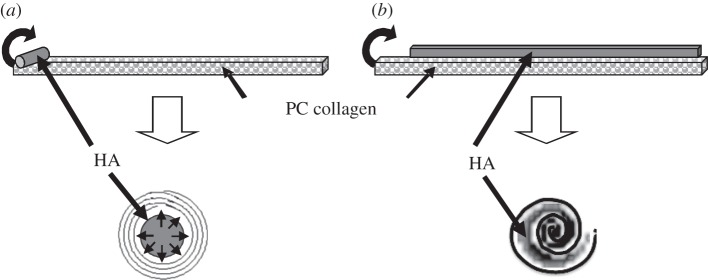
Figure 1.
A diagram showing the placement of the hyaluronan mat and method of co-rolling with the collagen sheet to form spirals with (a) HA at the core of the spiral and (b) an HA–collagen sandwich spiral structure.
2. Material and methods
2.1. Formation of acellular collagen gels and spiral assembly of three-dimensional constructs
Collagen gels were prepared from 2.4 ml sterile type I rat-tail collagen (2.16 mg ml−1 protein in 0.6% acetic acid; First Link, West Midlands, UK) and 0.6 ml of 10× concentration Dulbecco's modified Eagle's medium (DMEM), which was neutralized with NaOH [12]. Once cast and set into a rectangular well (22 × 33 mm), the gels were compacted. A 165 µm thickness stainless-steel metal mesh (approx. 300 µm mesh size) and a layer of nylon mesh (approx. 50 µm mesh size) were placed on a double layer of absorbent paper. The collagen gel was placed on the nylon mesh, covered with a second nylon mesh and loaded with a 125 g weight for 5 min at room temperature, to produce a flat collagen sheet protected between two nylon meshes [2].
Plastic-compressed (PC) collagen sheets were rolled off their short axis immediately after compression to produce tightly spiralled rods [2]. In the case of constructs for local re-swelling, either an 8 mg strip of freeze-dried hyaluronan (Sigma Aldrich Ltd., Irvine, UK) was placed along the short edge of the PC collagen sheet (figure 1a) prior to rolling, to produce an HA core, or alternatively a dried sheet of HA (again 8 mg total) over the whole of the PC collagen sheet such that the two layers were rolled together to create a collagen-HA sandwich roll (figure 1b). The effects of immediate contact of the HA depot with collagen were assessed by fixing the constructs in 1.5 per cent glutaraldehyde without further treatment. Where longer term effects of swelling/rehydration were studied, spiral constructs were incubated at 37°C in distilled water for periods of 1 min to 24 h at which point they were fixed for scanning electron microscopy (SEM). Note that the HA insert was only partially confined, as the ends of the spiral were open, allowing gradual leakage and loss of HA over hours as it was re-hydrated.
2.2. Scanning electron microscopy
All gels were fixed in 1.5 per cent glutaraldehyde (in 0.1 M sodium cacodylate buffer pH 7.2, overnight), treated with 1 per cent tannic acid in 0.05 M Na cacodylate buffer (1 h) and dehydrated through an ascending alcohol series to hexamethyldisilazane with air drying. Specimens were coated with gold palladium (Au/Pd Target, Emitech, Ashford, UK) for 2 min and viewed in a JEOL SEM (JEOL JSM 5500 LV) the collagen-layer thickness was measured at a minimum of 30 random points along the length of each spiral construct to give the mean and standard deviation of its layer dimensions.
2.3. Quantitative analysis of cell viability (live–dead staining)
Human neo-natal fibroblasts (HDFn) were prepared by routine explant of human dermis (obtained from the operating theatre with full ethical approval, following surgery for circumcision) as previously described [12]. HDFn were maintained in DMEM (4.5 g l−1 glucose; Sigma Aldrich Ltd.) containing 10 per cent (v/v) foetal calf serum (First Link Ltd., Birmingham, UK) and 1 per cent (v/v) penicillin G streptomycin solution (P-S; prepared with 10 μg ml−1 streptomycin sulphate; Invitrogen Corporation, Paisley, UK) in a humidified atmosphere of air containing 5 per cent CO2 at 37°C.
Two million HDFn cells were seeded onto the collagen gels [1] and assembled as in figure 1a. One hour after assembly of the spiralled constructs, cell viability was determined using the live/dead viability/cytotoxicity kit-L-7013 (Invitrogen Corporation), following the protocol set out by the manufacturers. Samples were incubated at 37°C for 2 h in a covered Petri dish and then unrolled. The flat collagen sheets were mounted onto microscope slides. Samples were viewed under a fluorescence microscope (Olympus BX50) and the number of live and dead cells (green and red fluorescence, respectively) counted at sites adjacent to and at 30 mm distant from the HA depot to give a mean value and standard deviation.
2.4. Gel expansion cell assay using cell-sized hyaluronic acid-coated microspheres
Streptavidin-coated 10 µm polymer microspheres (Bangs Laboratories, IN, USA) were brought to room temperature. After preliminary washing with phosphate-buffered saline (PBS), beads were washed and centrifuged with 0.1 M borate buffer at pH 8.0 three times and diluted to a final concentration of 0.05 per cent solids. Microspheres were coated with biotinylated HA (Sigma Aldrich Ltd.) at a binding ratio of 100 µg of biotinylated HA per 1.5 × 106 microspheres. The binding mixture was incubated at room temperature for 30 min. Microspheres were then centrifuged and re-suspended in PBS. Control beads were made in a similar manner without adding HA. Microsphere-populated compressed gels were made as described earlier, with the addition of microspheres to the gel-mix immediately before neutralization with NaOH to give a concentration (after compression) of 1 × 106 beads ml−1. Gels were synthesized in triplicate and incubated in DMEM (Gibco) as for cell culture, for increasing time periods. Thickness of the compressed gel-bead constructs was measured at time points by gel imaging using a 63× objective lens (Zeiss LD Plan Neofluor Korr NA 0.75) on an inverted Axiovert 100M (Zeiss) microscope. Image stacks were captured at 2 μm steps with an ORCA-ER CCD digital camera (Hamamatsu Photonics UK) with Openlab acquisition software (Perkin Elmer/Improvision), with image analysis by Volocity (Improvision/Perkin Elmer) or Image J (http://rsbweb.nih.gov/ij/) packages. Thickness measurements were made at five different sites along each of the triplicate gels over a period of 3 days. Staining of HA-coated beads in gels involved fixing gels overnight in 4 per cent paraformaldehyde and staining overnight in 0.25 per cent Alcian blue (in 3% acetic acid).
3. Results
To test the effects of hyaluronan-induced fluid flows on gross and micron-scale construct morphology, dry hyaluronan was inserted into specific spatial positions between the layers of the three-dimensional collagen spirals (figure 1). Untreated spiral structures were routinely produced from approximately 40 μm thick compressed collagen sheets (final 12% w/v collagen). Without addition of hyaluronan, these retained essentially the same morphology over the 24 h experimental period, i.e. comprising a tightly spiralled, continuous 40 µm layer, 2.2 mm diameter by 20 mm in length (figure 2a). Where a strip of dry HA was placed at the core of the spiral (figures 1a and 2b), the earliest gross effect was the slow appearance of viscous hyaluronan escaping from the ends of the construct, apparent to the naked eye as a refractive index change of the surrounding fluid. After 5 min immersion, the HA core was hydrated and swelled to form the continuous channel through the axis of the construct, shown in figure 2b, as a thin-walled tube.
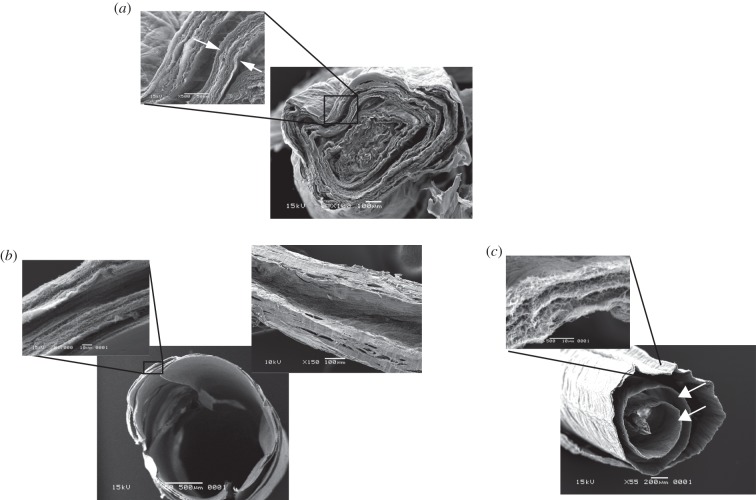
Figure 2.
SEM images showing the structure of (a) unmodified (HA-free) PC collagen spiral showing the tight concentric layers of collagen with zero core space and minimal inter-layer separation (even this is probably exaggerated through dehydration shrinkage during SEM preparation). Inset shows high magnification detail (note the thickness of one layer (arrows). (b) Construct made with a core strip of HA and incubated for 5 min, at which point the construct had formed a clear wide-bore lumen (i.e. formatting a tube: left-hand transverse, or trans-axial image). This channel ran along the full-length of the construct (right hand, longitudinal or axial plane image). The high magnification detail inset of compressed collagen layers shows how these had become dramatically thinner in this process. Note the overall diameter of this tube construct was similar to the untreated solid collagen spiral. (c) Construct made with co-axially spiralled layers of collagen and HA (i.e. rolled as a sandwich). The main image shows the resultant ‘clock-spring’ structure after 30 min swelling (post-spiral formation) with the continuous inter-collagen space between each collagen layer (arrows) running right across the diameter. Note again the dramatic reduction in thickness of individual collagen layers after HA swelling. Inset shows detail of a single collagen layer with its component lamellae. Overall gross diameter of constructs in panels (a–c) was largely the same.
Following this treatment, the different collagen layers within a completed spiral were not easy to distinguish in SEM specimens as their thickness was so dramatically reduced to produce the core channel. This made it difficult to translate and compare the layer structures in terms of with-without hyaluronan treatment, between figure 2a,b. The inherent anisotropic structure of the collagen spiral layers without hyaluronan treatment was reduced by the reduction in their thickness to just a few micrometres. In addition, those layers that were originally located near the spiral core, without hyaluronan treatment, were pushed together and outwards to form the edges of the construct, making them harder to distinguish. The non-hyaluronan spiral of figure 2a has around three clear outer layers (40+ µm thick) with a further two to three less distinct layers towards the core of this specimen. Figure 2b (inset), can be seen to comprise five distinct, but much thinner layers, now closely opposed to the outer edge. In other words, after treatment (figure 2b), there were the same number of spiral layers (five) but they are much thinner and pushed out to the circumference.
This configuration of five layers tightly wound into an outer edge allows us to make a correlation with the original total sheet length (without treatment). Given that the HA-treated layers have a negligible thickness relative to the whole spiral, we can assume that each HA-treated layer is one circumference of the spiral in length (i.e. each layer is the length of the construct circumference). Given the spiral diameter of 2.2 mm, the circumference will be 6.9 mm and so the five ‘negligibly thick’ layers will be a total of 34.5 mm in length. This estimate of total collagen sheet length agrees well with the actual, cast length of the collagen sheets, of 33 mm. In other words, the original sheet length would be approximately the same as the final length of the five thin, circumferential layers after hyaluronan treatment. To put it another way, in this configuration, the collagen layers had dramatically been reduced in thickness, but not in length.
The second configuration was to insert the hyaluronan mat continuously between the collagen layers (figure 1b), which again swelled rapidly to generate a new three-dimensional morphology. In this case, each spiral layer was pushed apart and thinned to give structures resembling a clock-spring in cross section (figure 2c). It is clear that this situation is very different in that the individual collagen layers were physically separated from each other by the inserted HA layer, inevitably minimizing any interlayer friction which would have been significant without that layer (as in figure 2b). Because of this, it is suggested that these layers are free to shrink in their long axis, as well as in thickness. Image analysis of the cross-sectional length of the clock-spring layer, in figure 2c, after hyaluronan, indicated a total visible sheet length of 9.7 mm. However, as we concluded previously, the expected length of the initial collagen sheet (i.e. as it was cast) is 33 mm, consistent with a shrinkage of approximately 60 per cent in the length plane. This supports the idea that the HA treatment shrank the sheet dimensions on all planes, where it was possible. This reduction in total sheet length would explain the reduction in the number of spiral layers that were apparent in this configuration.
Both morphologies suggested that water had been removed from the collagen layers, while the HA depot swelling had pushed the layers apart. In both formats, swelling was largely complete in 10 min. In addition, the overall diameter of spiral constructs was essentially the same as time-zero or non-HA constructs.
In both configurations, the mechanism appeared to be driven by a rapid redistribution of fluids under the influence of the hyaluronan. Fluid was drawn in rapidly to hydrate and swell the hyaluronan deposit. Confinement of the HA depot caused that fluid to be drawn primarily from the collagen layer. As the HA swelled and further hydrated, its viscosity fell, allowing the HA to escape, leaving the spaces and cavities (i.e. the morphology) identified in figure 2.
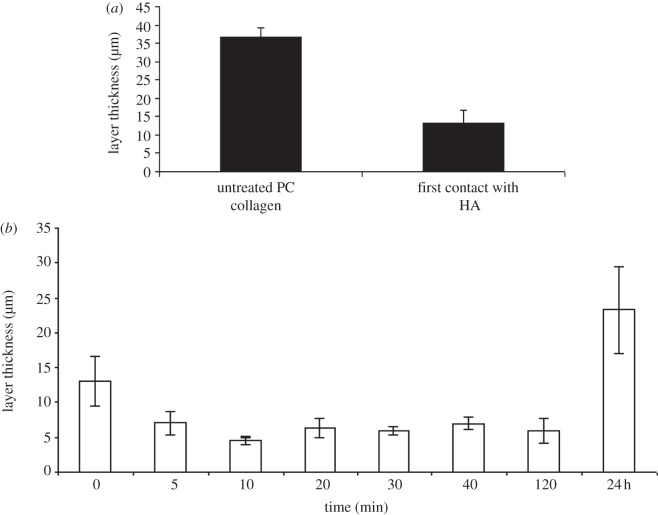
This mechanism was tested by measuring the thickness of the collagen layers with time after the start of co-swelling with the HA depot, as shown in figure 3a, using the HA core configuration (figure 1b). Prior to HA treatment, mean layer thickness of PC collagen was 36.5 ± 2.7 µm. Immediately after contact with HA, i.e. directly after the two layers were assembled together but before water immersion, collagen-layer thickness was reduced to 13.1 ± 3.6 µm, almost a threefold decrease. On immersion for only 5 min in water, the mean collagen-layer thickness surrounding the HA core was further halved. The minimum layer thickness, and thus greatest collagen density, of 4.6 ± 0.6 µm, was reached after 10 min. Where constructs were left over longer periods for the HA to leach out completely, at the 24 h time point, collagen layers had regained some of their initial thickness ending at 23.3 ± 6.4 µm. Importantly, despite this element of elastic recoil and partial return of initial dimensions the tube morphology was still left (i.e. the channel remained patent even at 24 h), although with a thicker outer wall. Critically, the gross outside diameter of the constructs remained relatively constant (approx. 2.2 mm) throughout the first few hours of treatment, demonstrating that the void volume of the central channel was largely created at the expense of the collagen-layer thickness, rather than by gross expansion of the whole rolled construct.
Figure 3.
(a) Comparison of the layer thickness pre- and post-immediate contact with HA. (b) Collagen layer thickness surrounding the core of HA as a function of time of water immersion.
A key question for both the use of this HA-driven morphogenic mechanism and its potential relevance to natural tissue-shape generation [13] is the effect on local cell viability. Such local cell viability was tested by seeding constructs with human dermal fibroblasts and live–dead staining these 1 h after assembly of the spiralled constructs. Percentage cell viability was determined both adjacent to and at 30 mm distant from the HA depot. Mean live cell viability was 88.0 ± 6.8% in direct contact with the HA depot, compared with 89.9 ± 1.2% live cells 30 mm remote to the depot, i.e. not directly exposed to HA. It was concluded that there was no detectable cell damage/death over and above that owing to the basic processing.
In other common biomimetic processes developed to engineer living tissues, the idea of morphogenetic uses in man-made materials for hyaluronan-driven fluid redistribution also suggests reinterpretations of natural processes. In this case, HA is a natural extracellular matrix component, produced at times by cells embedded naturally in a collagen network. The question can be asked, then, could natural tissue matrix cells control (microsculpture) local three-dimensional structure and collagen density by local HA secretion into their pre-existing collagen mesh? Such a mechanism might be expected in tissues such as the fibro-cartilages (e.g. meniscus) where proteoglycans and HA naturally swell the collagen network, or in regions of the eye adjacent to hyaluronan-rich tissues.
To test this idea, in principle, we developed an experimental system of HA-coated microspheres, seeded into compressed, tissue-like collagen gels. The aim here was to model the physical–spatial consequences of natural connective tissue cells at the point that they begin to express a pericellular zone of HA, on matrix structure. The effect of seeding cell-sized HA-coated beads (figure 4) into standard compacted gels was monitored in terms of construct thickness over time (figure 5). This comparison demonstrated a large and significant overall expansion of the collagen layer, making it 40 per cent thicker after 24 h and 50 per cent by 3 days. By contrast, collagen layers containing HA-free microspheres maintained a constant thickness throughout this period. Consequently, this system had produced a ‘tissue swelling’ dynamic over 24 h similar to that expected, for example, for native meniscal chondrocytes in a fibrocartilage collagen matrix. Importantly, in this system, the swelling shape-change was stable over the 3-day monitoring period as the microsphere–HA was fully retained within the nano-fibrillar collagen mesh and unable to leach out.
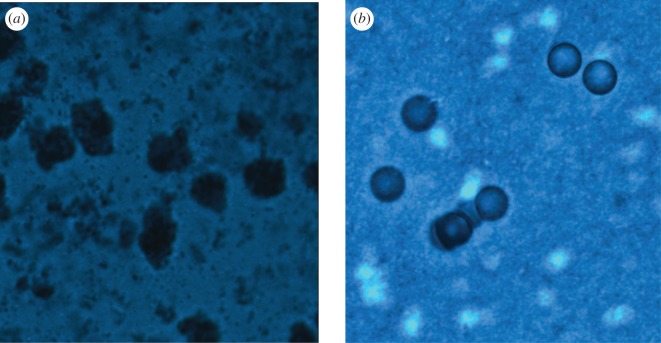
Figure 4.
Polystyrene microspheres (10 µm diameter) embedded in compressed collagen gel stained with Alcian blue. (a) Biotin/avidin HA-coated beads. (b) Uncoated control beads, both embedded in collagen. Staining on gels was performed after 3 days.
Figure 5.
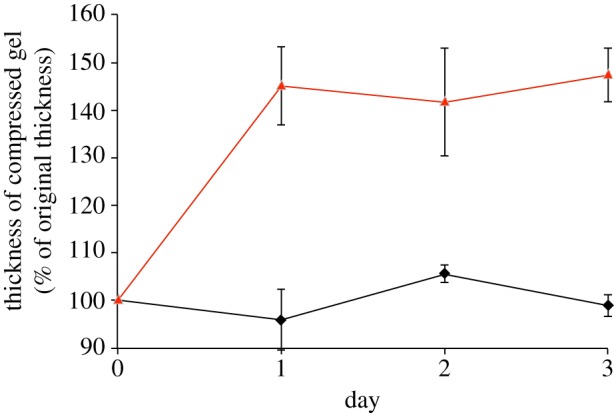
Expansion of hyaluronan-coated microsphere-populated collagen gels over time. Lines representing hyaluronan-free and hyaluronan-coated microspheres are coloured black and red respectively.
4. Discussion
On a gross scale, the three-dimensional spiral assembly of constructs was formed using the technique of plastic compression [2] in which fluid is mechanically expressed from native collagen gels to produce approximately 40-μm thick films (12% w/v collagen). This was adapted here such that dry HA was deposited in one of two patterns, designed to produce three-dimensional structures that mimic those of natural tissue (figure 1a). The first was to produce a tube and the second a spiral (watch spring-like) scaffold (figure 1c).
These results are consistent with the predicted uptake of fluid by HA depots, resulting in formation of internal three-dimensional spaces and cavities in the collagen construct forming either tubes through the length of the spiral core or open clock-spring structures where each collagen layer was separated by a fluid layer. Clearly, in tissue engineering terms, these shapes are proof-of-principle in that they are predictable reflections of the HA depot position. Channels would have more immediate biomedical function than clock-spring structures.
However, the detailed mechanism by which internal shape was generated was informative. Internal cavities were not formed as initially expected, by the HA depots expanding to simply push out a shape in the existing collagen, such as air inflating micro-balloons. For this to occur, there would have been a net inflow of fluid from outside the constructs and a corresponding increase in their overall construct diameter. In fact, constructs did not increase in their overall dimensions, but instead the thickness of the collagen layers decreased in proportion to channel/cavity formation. This means that fluid taken up by the swelling HA depots had been sequestered largely from the collagen gel itself, proportionally increasing the collagen density. Estimates of collagen density based on extrapolation from the initial collagen concentration and using SEM measurements of final layer thickness must incorporate a degree of error owing to gel shrinkage during SEM preparation. However, the minimal layer thickness of 4.6 µm, identified after 10 min incubation, could indicate a potential collagen density more than 80 per cent (w/v). This transient level would indeed be a supra-physiological collagen density, with tissues such as native tendon reaching only approximately 30 per cent collagen (w/v) [14].
In addition, re-swelling of these highly dehydrated collagen layers by 24 h is consistent with the idea that this had taken the collagen material beyond its plastic, and into an elastic deformation phase. In other words, once the hyaluronan depot had swelled and disappeared, the collagen was able to take up water to regain much of its original thickness. In fact, the collagen-layer thickness after re-swelling (24 h) was 65 per cent of that when it was initially prepared (36.5 µm). The initial plastic compression, prior to HA contact, increased the collagen density from 0.2 per cent to 11.6 ± 0.4% [4]. Assuming such a nominal initial collagen density of 12 per cent [4], this suggests that the elastic–plastic transition [15] collagen density (i.e. that density to which the collagen returned 24 h after HA treatment) is 18–19% collagen (w/v). In other words, where it is possible to produce collagen densities greater than approximately 18 per cent, for example, by physical expression of fluid, it would slowly re-swell back to this balance level, once the driver of dehydration had been removed. Interestingly, many dense connective tissues, such as dermis [16] have a collagen density in the range of 17–20%. Then, it may be that this transition point has physiological significance.
The case of tendon–ligament, with collagen densities reported at 25–30% [14], may indicate that these tissues are specially adapted, perhaps reflecting that in situ under normal anatomy, these tissues are almost never under zero tensile load [17]. Indeed, it has been proposed that interstitial fluid flow, generated within and through such tissues by normal axial loading, is essential to function [18]. This concept of an interdependence between fluid content, collagen–GAG content and external loading [19] predicts that proteoglycan in tendon may be more important for maintaining fluid content under load. This would be consistent with evidence of Fessel & Snedeker [20] who concluded that tendon GAGs do not act as molecular cross-linkers between adjacent collagen fibrils. Given the very rapid fluid redistribution to the HA depot, it was a very real possibility that adjacent cells would be damaged. Clearly, if fluid shear membrane damage and cell death was a result, then it would make such HA remodelling difficult to use in tissue engineering processes. Critically, there was no detectable loss of cell viability after full HA re-swelling and collagen layer remodelling, even for cells directly adjacent to the HA depot. HA is indeed a natural connective tissue component (although not at these concentrations), which is likely to remain most entirely extracellular. Clearly, from the studies here the HA depots had no significant toxic effects over the time course used. This means that the technique can safely be used as a morphogenic, fabrication stage in engineering of cell-seeded constructs. The cell-friendly nature of this effect also leaves open the strong possibility that local GAG deposition is a natural mechanism for fluid redistribution, spatial remodelling and morphogenesis.
The second model system here (HA-coated micro-beads) was designed to test the idea that in a mesh of native collagen, local resident cells might expand the general tissue dimensions by local release of HA to be confined by the collagen. The micro-bead model confirmed that HA-coated cell-sized particles, trapped in the collagen, increased the overall thickness of the collagen layer. Unlike the spiral model which did not fully retain the HA depots, in this model, HA was confined within the collagen mesh and so the resulting increase in mesh thickness owing to HA fluid retention was stable with time. This functional model for tissue expansion may have applications as a cell-based in vitro model for a range of morphogenesis, tissue engineering and pathological disease processes which are dependent on HA mediated swelling pressure and tissue expansion. Examples of such processes include synovial cartilage formation, where GAG synthesis by chondrocytes generates swelling pressure [5,6] and in Graves orbitopathy, where increased HA synthesis leads to pathological expansion of orbital soft tissues leading to visual morbidity [21]. Compressed collagen gels could therefore provide for a functional in vitro model for investigating how potential drugs and growth factors modulating HA synthesis may impact on functional tissue expansion.
In conclusion, this experimental mimic suggests that some of the natural tissue morphologies associated with collagen network swelling and GAG content may be a result of natural cell secretion of GAG. Specifically, it may be that known patterns of native cartilage tissues acquire their structure (and mechano-function) by ‘sequential cell deposition’ of compacted collagen, followed by GAG or proteoglycans. Certainly, it now becomes possible to biomimetically engineer complex, predictable micro–nano structures into dense collagen matrices. The rapid tissue-fabrication potential of this approach is considerable, independent of its natural occurrence in cell-physiology of matrix growth, repair and remodelling.
Acknowledgements
This study was supported by the BBSCR/EPSRC UK research council programme on ‘Tissue Bioreactor Science’. We are grateful to Dr P. Sarathchandra and Dr F. Foroughi for their assistance. The microsphere work was funded by the Medical Research Council and Wellcome Trust (M.B.) and the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology (D.G.E.). The views expressed in this publication are those of the authors and not necessarily those of the Department of Health.
References
- 1.Shimizu T., Sekine H., Yang J., Isoi Y., Yamato M., Kikuchi A., Kobayashi E., Okano T. 2006. Polysurgery of cell sheet grafts overcomes diffusion limits to produce thick, vascularized myocardial tissues. FASEB J. 20, 708–710 [DOI] [PubMed] [Google Scholar]
- 2.Brown R., Wiseman M., Chuo C. B., Cheema U., Nazhat S. 2005. Ultra-rapid engineering of biomimetic tissues: a plastic compression fabrication process for nano-micro structures. Adv. Funct. Mater. 15, 1762–1770 10.1002/adfm.200500042 (doi:10.1002/adfm.200500042) [DOI] [Google Scholar]
- 3.Brown R. A., Phillips J. B. 2007. Cell responses to biomimetic protein scaffolds used in tissue repair and engineering. Int. Rev. Cytol. 262, 75–150 10.1016/S0074-7696(07)62002-6 (doi:10.1016/S0074-7696(07)62002-6) [DOI] [PubMed] [Google Scholar]
- 4.Cheema U., Nazhat S., Alp B., Foroughi N., Anandagoda N., Mudera V., Mudera V. 2007. Fabricating tissues: review of farming versus engineering strategies. Biotechnol. Bioprocess. 12, 9–14 10.1007/BF02931797 (doi:10.1007/BF02931797) [DOI] [Google Scholar]
- 5.Maroudas A. I. 1976. Balance between swelling pressure and collagen tension in normal and degenerate cartilage. Nature 260, 808–809 10.1038/260808a0 (doi:10.1038/260808a0) [DOI] [PubMed] [Google Scholar]
- 6.Urban J. P., Maroudas A. 1981. Swelling of the intervertebral disc in vitro. Connect Tissue Res. 9, 1–10 10.3109/03008208109160234 (doi:10.3109/03008208109160234) [DOI] [PubMed] [Google Scholar]
- 7.Katz E. P., Wachtel E. J., Maroudas A. 1986. Extrafibrillar proteoglycans osmotically regulate the molecular packing of collagen in cartilage. Biochim. Biophys. Acta 882, 136–139 10.1016/0304-4165(86)90065-6 (doi:10.1016/0304-4165(86)90065-6) [DOI] [PubMed] [Google Scholar]
- 8.Kogan G., Soltes L., Stern R., Gemeiner P. 2007. Hyaluronic acid: a natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol. Lett. 29, 17–25 10.1007/s10529-006-9219-z (doi:10.1007/s10529-006-9219-z) [DOI] [PubMed] [Google Scholar]
- 9.Laurent T. C., Fraser J. R. 1992. Hyaluronan. FASEB J. 6, 2397–2404 [PubMed] [Google Scholar]
- 10.Rehakova M., Bakos D., Vizarova K., Soldan M., Jurickova M. 1996. Properties of collagen and hyaluronic acid composite materials and their modification by chemical crosslinking. J. Biomed. Mater. Res. 30, 369–372 (doi:10.1002/(SICI)1097-4636(199603)30:3<369::AID-JBM11>3.0.CO;2-F) [DOI] [PubMed] [Google Scholar]
- 11.Park S. N., Park J. C., Kim H. O., Song M. J., Suh H. 2002. Characterization of porous collagen/hyaluronic acid scaffold modified by 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide cross-linking. Biomaterials 23, 1205–1212 10.1016/S0142-9612(01)00235-6 (doi:10.1016/S0142-9612(01)00235-6) [DOI] [PubMed] [Google Scholar]
- 12.Eastwood M., Mudera V. C., McGrouther D. A., Brown R. A. 1998. Effect of precise mechanical loading on fibroblast populated collagen lattices: morphological changes. Cell Motil. Cytoskeleton 40, 13–21 (doi:10.1002/(SICI)1097-0169(1998)40:1<13::AID-CM2>3.0.CO;2-G) [DOI] [PubMed] [Google Scholar]
- 13.Griffith L. G., Swartz M. A. 2006. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol. 7, 211–224 10.1038/nrm1858 (doi:10.1038/nrm1858) [DOI] [PubMed] [Google Scholar]
- 14.Frank C., Amiel D., Woo S. L., Akeson W. 1985. Normal ligament properties and ligament healing. Clin. Orthop. Relat. Res. 196, 15–25 [PubMed] [Google Scholar]
- 15.Abou Neel E. A., Cheema U., Knowles J. C., Brown R. A., Nazhat S. N. 2006. Use of multiple unconfined compression for control of collagen gel scaffold density and mechanical properties. Soft Matter 2, 986–992 10.1039/b609784g (doi:10.1039/b609784g) [DOI] [PubMed] [Google Scholar]
- 16.Candlish J., Chandra N. 1967. Studies of the chemical composition of a healing skin wound in rats, and of the concentrations of some constituents of tissues distant from the healing wound. Biochem. J. 102, 767–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown R. 2006. Cytomechanics in connective tissue repair and engineering. In Tissue repair, contraction and the myofibroblast (eds Chaponnier C., Desmoulière A., Gabbiani G.), pp. 9–24 Georgetown, TX: Landes Bioscience; [DOI] [PubMed] [Google Scholar]
- 18.Chen C. T., Malkus D. S., Vanderby R., Jr 1998. A fiber matrix model for interstitial fluid flow and permeability in ligaments and tendons. Biorheology 35, 103–118 10.1016/S0006-355X(99)80001-8 (doi:10.1016/S0006-355X(99)80001-8) [DOI] [PubMed] [Google Scholar]
- 19.Sander E. A., Nauman E. A. 2003. Permeability of musculoskeletal tissues and scaffolding materials: experimental results and theoretical predictions. Crit. Rev. Biomed. Eng. 31, 1–26 10.1615/CritRevBiomedEng.v31.i12.10 (doi:10.1615/CritRevBiomedEng.v31.i12.10) [DOI] [PubMed] [Google Scholar]
- 20.Fessel G., Snedeker J. G. 2009. Evidence against proteoglycan mediated collagen fibril load transmission and dynamic viscoelasticity in tendon. Matrix Biol. 28, 503–510 10.1016/j.matbio.2009.08.002 (doi:10.1016/j.matbio.2009.08.002) [DOI] [PubMed] [Google Scholar]
- 21.Hufnagel T. J., Hickey W. F., Cobbs W. H., Jakobiec F. A., Iwamoto T., Eagle R. C. 1984. Immunohistochemical and ultrastructural studies on the exenterated orbital tissues of a patient with Graves’ disease. Ophthalmology 91, 1411–1419 [DOI] [PubMed] [Google Scholar]