Abstract
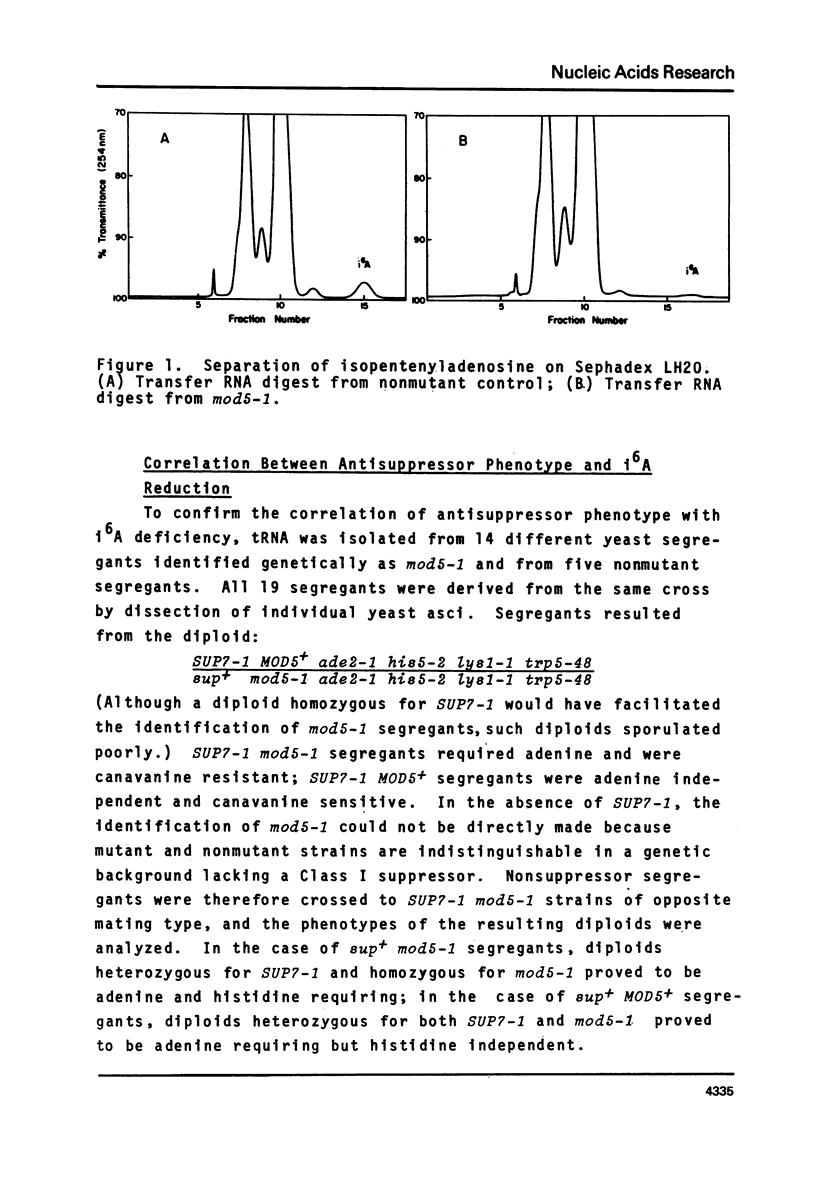
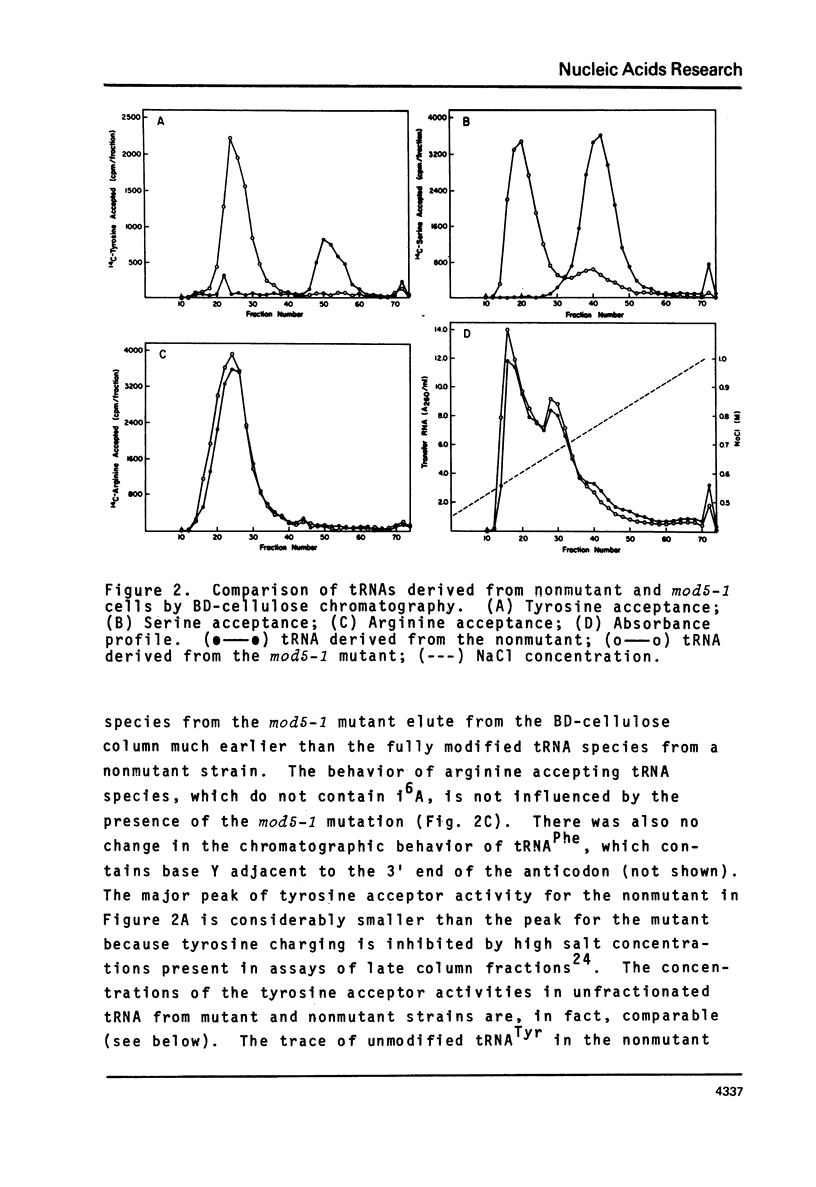
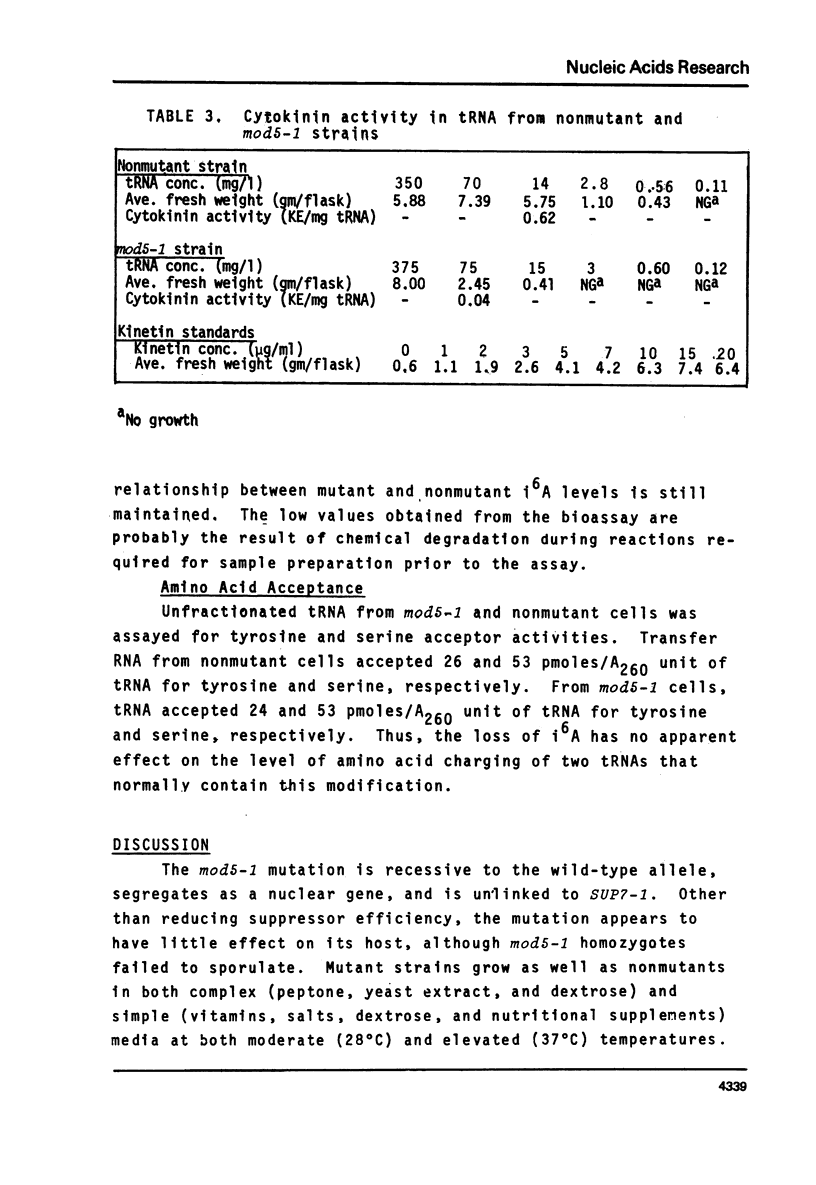
We have isolated a mutant of Saccharomyces cerevisiae that contains 1.5% of the normal tRNA complement of isopentenyladenosine (i6A). The mutant was characterized by the reduction in efficiency of a tyrosine inserting UAA nonsense suppressor. The chromatographic profiles of tRNATyr and tRNASer on benzoylated DEAE-cellulose are consistent with the loss of i6A by these species. Transfer RNA from the mutant exhibits 6.5% of the cytokinin biological activity expected for yeast tRNA. Transfer RNAs from the mutant that normally contain i6A accept the same levels of amino acids in vitro as the fully modified species. With the exception of i6A, the level of modified bases in unfractionated tRNA from the mutant appears to be normal. The loss of i6A apparently affects tRNA's role in protein synthesis at a step subsequent to aminoacylation.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong D. J., Burrows W. J., Evans P. K., Skoog F. Isolation of cytokinins from tRNA. Biochem Biophys Res Commun. 1969 Oct 22;37(3):451–456. doi: 10.1016/0006-291x(69)90936-x. [DOI] [PubMed] [Google Scholar]
- Armstrong D. J., Skoog F., Kirkegaard L. H., Hampel A. E., Bock R. M., Gillam I., Tener G. M. Cytokinins: distribution in species of yeast transfer RNA. Proc Natl Acad Sci U S A. 1969 Jun;63(2):504–511. doi: 10.1073/pnas.63.2.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capecchi M. R., Hughes S. H., Wahl G. M. Yeast super-suppressors are altered tRNAs capable of translating a nonsense codon in vitro. Cell. 1975 Nov;6(3):269–277. doi: 10.1016/0092-8674(75)90178-6. [DOI] [PubMed] [Google Scholar]
- Fittler F., Hall R. H. Selective modification of yeast seryl-t-RNA and its effect on the acceptance and binding functions. Biochem Biophys Res Commun. 1966 Nov 22;25(4):441–446. doi: 10.1016/0006-291x(66)90225-7. [DOI] [PubMed] [Google Scholar]
- Furuichi Y., Wataya Y., Hayatsu H., Ukita T. Chemical modification of tRNA-Tyr-yeast with bisulfite. A new method to modify isopentenyladenosine residue. Biochem Biophys Res Commun. 1970 Dec 9;41(5):1185–1191. doi: 10.1016/0006-291x(70)90211-1. [DOI] [PubMed] [Google Scholar]
- Gefter M. L., Russell R. L. Role modifications in tyrosine transfer RNA: a modified base affecting ribosome binding. J Mol Biol. 1969 Jan 14;39(1):145–157. doi: 10.1016/0022-2836(69)90339-8. [DOI] [PubMed] [Google Scholar]
- Gesteland R. F., Wolfner M., Grisafi P., Fink G., Botstein D., Roth J. R. Yeast suppressors of UAA and UAG nonsense codons work efficiently in vitro via tRNA. Cell. 1976 Mar;7(3):381–390. doi: 10.1016/0092-8674(76)90167-7. [DOI] [PubMed] [Google Scholar]
- Gillam I., Millward S., Blew D., von Tigerstrom M., Wimmer E., Tener G. M. The separation of soluble ribonucleic acids on benzoylated diethylaminoethylcellulose. Biochemistry. 1967 Oct;6(10):3043–3056. doi: 10.1021/bi00862a011. [DOI] [PubMed] [Google Scholar]
- Gilmore R. A., Stewart J. W., Sherman F. Amino acid replacements resulting from super-suppression of nonsense mutants of iso-1-cytochrome c from yeast. J Mol Biol. 1971 Oct 14;61(1):157–173. doi: 10.1016/0022-2836(71)90213-0. [DOI] [PubMed] [Google Scholar]
- Gilmore R. A. Super-suppressors in Saccharomyces cerevisiae. Genetics. 1967 Aug;56(4):641–658. doi: 10.1093/genetics/56.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman H. M., Olson M. V., Hall B. D. Nucleotide sequence of a mutant eukaryotic gene: the yeast tyrosine-inserting ochre suppressor SUP4-o. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5453–5457. doi: 10.1073/pnas.74.12.5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean H., Söll D. G., Crothers D. M. Studies of the complex between transfer RNAs with complementary anticodons. I. Origins of enhanced affinity between complementary triplets. J Mol Biol. 1976 May 25;103(3):499–519. doi: 10.1016/0022-2836(76)90214-x. [DOI] [PubMed] [Google Scholar]
- Hawthorne D. C., Mortimer R. K. Genetic mapping of nonsense suppressors in yeast. Genetics. 1968 Dec;60(4):735–742. doi: 10.1093/genetics/60.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht S. M., Kirkegaard L. H., Bock R. M. Chemical modifications of transfer RNA species. Desulfurization with Raney nickel. Proc Natl Acad Sci U S A. 1971 Jan;68(1):48–51. doi: 10.1073/pnas.68.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball M. E., Soll D. The phenylalanine tRNA from Mycoplasma sp. (Kid): a tRNA lacking hypermodified nucleosides functional in protein synthesis. Nucleic Acids Res. 1974 Dec;1(12):1713–1720. [PMC free article] [PubMed] [Google Scholar]
- Litwack M. D., Peterkofsky A. Transfer ribonucleic acid deficient in N6-(delta 2-isopentenyl)adenosine due to mevalonic acid limitation. Biochemistry. 1971 Mar 16;10(6):994–1001. doi: 10.1021/bi00782a010. [DOI] [PubMed] [Google Scholar]
- McCready S. J., Cox B. S. Antisuppressors in yeast. Mol Gen Genet. 1973 Aug 28;124(4):305–320. doi: 10.1007/BF00267660. [DOI] [PubMed] [Google Scholar]
- Olson M. V., Montgomery D. L., Hopper A. K., Page G. S., Horodyski F., Hall B. D. Molecular characterisation of the tyrosine tRNA genes of yeast. Nature. 1977 Jun 16;267(5612):639–641. doi: 10.1038/267639a0. [DOI] [PubMed] [Google Scholar]
- Piper P. W., Wasserstein M., Engbaek F., Kaltoft K., Celis J. E., Zeuthen J., Liebman S., Sherman F. Nonsense suppressors of Saccharomyces cerevisiae can be generated by mutation of the tyrosine tRNA anticodon. Nature. 1976 Aug 26;262(5571):757–761. doi: 10.1038/262757a0. [DOI] [PubMed] [Google Scholar]
- Randerath E., Yu C. T., Randerath K. Base analysis of ribopolynucleotides by chemical tritium labeling: a methodological study with model nucleosides and purified tRNA species. Anal Biochem. 1972 Jul;48(1):172–198. doi: 10.1016/0003-2697(72)90181-9. [DOI] [PubMed] [Google Scholar]
- Randerath K., Randerath E., Chia L. S., Nowak B. J. Base analysis of ribopolynucleotides by chemical tritium labeling: an improved mapping procedure for nucleoside trialcohols. Anal Biochem. 1974 May;59(1):263–271. doi: 10.1016/0003-2697(74)90032-3. [DOI] [PubMed] [Google Scholar]
- Skoog F., Armstrong D. J., Cherayil J. D., Hampel A. E., Bock R. M. Cytokinin activity: localization in transfer RNA preparations. Science. 1966 Dec 9;154(3754):1354–1356. doi: 10.1126/science.154.3754.1354. [DOI] [PubMed] [Google Scholar]
- Sprinzl M., Grüter F., Gauss D. H. Collection of published tRNA sequences. Nucleic Acids Res. 1978 May;5(5):r15–r27. [PMC free article] [PubMed] [Google Scholar]


