Abstract
In the current study, the mechanical and hypothermic damage induced by vibration and cold storage on human mesenchymal stem cells (hMSCs) stored at 2–8°C was quantified by measuring the total cell number and cell viability after exposure to vibration at 50 Hz (peak acceleration 140 m s−2 and peak displacement 1.4 mm), 25 Hz (peak acceleration 140 m s−2, peak displacement 5.7 mm), 10 Hz (peak acceleration 20 m s−2, peak displacement 5.1 mm) and cold storage for several durations. To quantify the viability of the cells, in addition to the trypan blue exclusion method, the combination of annexin V-FITC and propidium iodide was applied to understand the mode of cell death. Cell granularity and a panel of cell surface markers for stemness, including CD29, CD44, CD105 and CD166, were also evaluated for each condition. It was found that hMSCs were sensitive to vibration at 25 Hz, with moderate effects at 50 Hz and no effects at 10 Hz. Vibration at 25 Hz also increased CD29 and CD44 expression. The study further showed that cold storage alone caused a decrease in cell viability, especially after 48 h, and also increased CD29 and CD44 and attenuated CD105 expressions. Cell death would most likely be the consequence of membrane rupture, owing to necrosis induced by cold storage. The sensitivity of cells to different vibrations within the mechanical system is due to a combined effect of displacement and acceleration, and hMSCs with a longer cold storage duration were more susceptible to vibration damage, indicating a coupling between the effects of vibration and cold storage.
Keywords: stem cells, mechanical stress, vibration, regenerative medicine, hypothermia, viability
1. Introduction
Recently, challenges faced during the shipping of cell and tissue therapeutic products from factory site to bedside have drawn significant attention in the emerging regenerative medicine industry [1]. Traditionally, transportation solutions for living microorganisms involve lyophilization (freeze drying) or cryopreservation. However, most cell therapeutic applications require the supply of living cells for immediate and easy administration by the clinical end-user, preferably without the presence of a cryoprotectant [2,3]. A cold chain shipping system maintaining the temperature of products in the range of 2–8°C may be more appropriate than the frozen shipping of cryopreserved products. A survey for therapeutic product transportation [4] has identified that the two most commonly used transportation temperature ranges are 2–8°C or 18–24°C (room temperature). As the scope of this work is to understand the biological consequences following current industrial practices for cold shipping and cold storage, the process of storage and transport at 2–8°C was simulated.
Some logistics companies (for example, Tegrant Corporation (http://www.greenboxsystems.com/index.htm) and ZeoCool (http://www.zeocool.co.uk/)) have made significant investments in packaging and container designs to maintain the transportation temperatures in the desired range. However, in such circumstances, in addition to maintaining the temperature, the consequences of mechanical stresses on the biological system due to the vibration of the cell suspension during cold transportation must be understood. It has been demonstrated that osteoblasts [5–7] and stem cells derived from adipose tissue [8,9] and bone marrow [10] are sensitive to vibration. Depending on the frequency and amplitude of vibration as well as the duration of such stimulation, beneficial effects on cell differentiation have been observed, although vibration-induced cell apoptosis has also been reported [11]. However, all these studies have investigated the influence of vibrations only on cells attached onto substrates. In addition, most of the studies concering the effects of vibration on cells have been conducted either under standard cell culture conditions (37°C) or at room temperature. Therefore, it is not possible to directly correlate the effects of vibration reported in the literature to the situation encountered in cold transportation of cell suspensions.
While vibration effects are unlikely to be an issue in the transportation of cryopreserved cells — a ‘block of ice’—in suspension, it may be important because a deviation from an allowed vibration dose may affect cell viability and therapeutic effect owing to the sensitivity of cells to mechanical stress [12]. This is analogous to the mechanical damage of cold-transported fruit as a consequence of transportation vibration, an important problem that requires careful management in agricultural transport for export [13]. A survey of the literature indicates that management of transport vibrations is one of the crucial factors in the successful transportation of living organisms as goods, and several investigations have been devoted to this subject, in particular to the impact of vibration on goods caused by various road roughnesses [14], vehicle and package resonances [15] and package handling [16]. Understanding the critical levels of vibrations and forces for cellular products will assist in the design of appropriate packaging. The efficient design of appropriate packaging for cell transportation is important because over-packaging is uneconomic and raises sustainable development issues; conversely, under-packaging can result in damage and loss of sales, and compromised therapeutic effects can lead to potential variations in the outcomes of clinical trials [17].
ASTM Standard D4169 [18] and D4728–06 [19] provide a useful review of standard practices for performance testing of shipping containers and systems, and a starting point for test design. While they provide a good starting point for investigations of perishable products, living cells used as therapeutic products for implantation are significantly more complicated, with regulatory requirements being higher. It is therefore essential to understand the threshold of vibrations that could cause damage, the extent and recoverability of the damage, and the effect of mechanical stress on biological functions of cells. As human mesenchymal stem cells (hMSCs) reside in different connective tissues and in the skeletal system and contribute to tissue homeostasis, considerable research has been carried out to understand the effects of mechanical stress on the function and phenotype of hMSCs attached to substrates in vitro [12,20,21]. It is also well understood that cells in suspension culture are subject to damage induced by hydrodynamic force owing to agitation in stirred tanks [22,23]. However, the authors are aware of little work on the response of suspended hMSCs to mechanically imposed stresses other than that addressing their delivery through catheters [24]. This study investigates the mechanical damage to hMSCs induced by vibration and cold storage by measuring the total cell number and viability of the cells. To establish the threshold of vibrations that cause damage, vibrations with larger accelerations, displacements and durations than those ultimately expected in practice were applied to indicate the likelihood of damage. In order to characterize not only cell viability but also to understand the mode of cell death, in addition to the trypan blue exclusion method used in previous work [25], the cell apoptosis and death markers annexin V-FITC and propidium iodide (PI) were also used in this work.
2. Material and methods
As the purpose of this study was to investigate the effects of vibration-induced mechanical stress on cell viability during cold transportation, the experiment was set up in a cold room, with temperature maintained at 2–8°C, and vibrations emulating different transport vibration situations were used. Samples kept in the same cold storage conditions were used as controls and also for the study of cold storage effects on cell viability.
2.1. The mechanical system and vibration conditions
2.1.1. The mechanical system
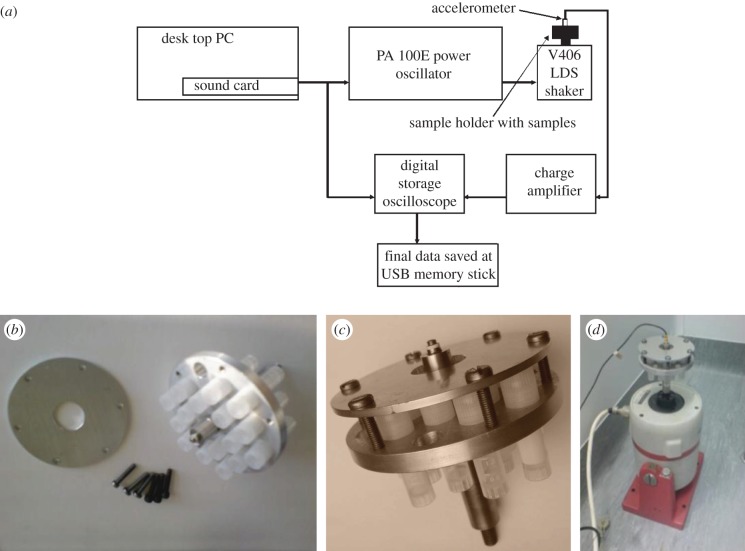
The mechanical system (figure 1a) used as the experimental platform was assembled by connecting a portable vibrator shaker V406 (LDS UK), to a PA 100E power amplifier (LDS UK) taking in electrical signals generated by a PC sound card. The vibration experienced by the samples was measured with an accelerometer (Bruel & Kjaer type 8301/SN 578616 –11.86 pC g−1) connected to a charge amplifier (Bruel & Kjaer type 2635). The signal from the charge amplifier was displayed on a Tektronix TDS 2012B two channel digital storage oscilloscope. The displacement, velocity and acceleration experienced by the sample were collected with time and saved to allow further analysis.
Figure 1.
(a) A schematic of the set-up of the experimental mechanical system; (b) images of a sample holder with the plastic vials for cell suspension; (c) sample holder assembled and (d) sample holder mounted vertically on the shaker with accelerometer attached. (Online version in colour.)
A sample holder was fabricated to hold 18 cryogenic vials containing the cell suspension. The design allowed vial sets to be retrieved or replaced with dummies during trials, without affecting the balance of the holder (figure 1b). The vials were secured by a top plate (figure 1c) to prevent movement during vibration. The accelerometer was mounted in the centre of the holder, and the whole assembly was mounted on the shaker (figure 1d).
2.1.2. Multiple frequency combination and initial single frequency experiments
Owing to the lack of data within the literature on the effect of vibration on the viability of suspended cells, initial experiments—using a multiple frequency combination and a range of single frequencies—were carried out to identify a working range and to establish further experimental parameters. On the basis of the results from previous vibration experiments, using human dermal fibroblasts (HDF) [25], in the initial experiment hMSCs were exposed to vibration at multiple frequencies in order to scan for observable effects at conditions relevant to possible vehicle and package resonant vibrations. hMSCs were subjected to 24 h repetitions of the following series of vibrations:
three 20 ms half sine shock pulses with a 0.98 s rest interval following each shock pulse. Such a series train of pulse gave an acceleration of 140 m s−2, displacement 5.7 mm and a repetition rate 1 Hz similar to those caused by road bumps [14] and drops [16];
the previous vibration pattern then followed by bursts of vibration lasting 3 s with frequencies of 10, 25, 50, 100, 500, 1000, 5000 Hz and with parameters defined fully in the table 1 in order to explore the effects of a range of potential vibration parameters. In the multiple frequency experiment, a ‘rest’ interval was needed to allow the Visual Basic program to switch between vibrations with different frequencies. This ‘rest’ time, (50–60 ms), was the time needed to load the wav file for the next frequency; and
repeating (1) and (2) for 24 h.
In parallel, initial single frequency experiments were also carried out by exposing samples to vibration at 10, 25, 50, 100 and 500 Hz, respectively, for 24 h (see table 1 for vibration parameters)
Table 1.
Comparison of the amplitudes of displacement, velocities, accelerations and input energies of different sine waves and half sine shock pulse used in multiple frequency test and tests at individual frequencies.
| frequency (Hz) | max. displacement (m) | max. acceleration (m s−2) | max. velocity (m s−1) | energy∼(max.velocity)2 (m2/s2) |
|---|---|---|---|---|
| 5000 | 0.00000016 | 160 | 0.00503 | 0.000025 |
| 1000 | 0.0000015 | 60 | 0.00942 | 0.000089 |
| 500 | 0.0000066 | 65 | 0.02073 | 0.00043 |
| 100 | 0.00028 | 110 | 0.17592 | 0.03094925 |
| 50 | 0.0014 | 140 | 0.43981 | 0.19343284 |
| 25 | 0.0057 | 140 | 0.89533 | 0.80161133 |
| 10 | 0.0051 | 20 | 0.32043 | 0.10267731 |
| 20 ms half sine shock pulse | 0.0057 | 140 | 0.89533 | 0.80161133 |
2.1.3. Vibration at 10, 25 and 50 Hz and cold storage experiments
Following on the initial single frequency experiment, 25 and 50 Hz were chosen, respectively, as representative of conditions with strong and moderate effects as observed in the initial experiment. In order to indentify the effects of duration of vibration on cell viability, exposure of hMSCs to vibration of the chosen frequencies were scheduled at 24 or 48 h. To further understand the effects of pre-vibration cold storage, some cell suspensions were kept in cold storage for up to 144 h before being vibrated at 50 Hz. Investigation of cold storage effects without vibration was also carried out in a parallel study by measuring cell viability for samples collected at 24 h intervals during 96 h cold storage. The details of vibration parameters, including displacement, acceleration, velocity and energy input, were listed in table 1. A high value of acceleration, 140 m s−2, was chosen for vibration at 25 and 50 Hz in order to include conditions representative of not only challenging transport situations but also to allow the simulation of potential nonlinear parametric sloshing phenomena, such as rotational motion, chaotic motion and free surface disintegration [26]. To further identify the vibration parameters leading to the cell damage observed at 25 Hz, cells were also exposed to a 10 Hz vibration with a similar displacement to that at 25 Hz but with a much reduced acceleration (table 1). The acceleration for the 10 Hz vibration was necessarily reduced to 20 m s−2 from 140 m s−2 as sine wave frequency f, displacement x and acceleration a are connected via the expression
and it is consequently impossible to have accelerations and displacements at 10 Hz similar to those at 25 Hz for this mechanical system.
2.2. Cell suspension preparation
hMSCs harvested from normal human bone marrow and cultured to passage 2 (Lonza) were used as cell sources to establish a working cell bank at passage 4 sufficient to allow for the use of similar input cells for all experiments. Cells were seeded at a density of 1900 cells cm−2 and maintained in T-175 culture flasks. The culture medium used was Lonza hMSCs growth medium (MSCGM) supplemented with mesenchymal cell growth supplement (MCGS, Lonza). During culture, the medium was changed every 2–3 days to ensure the presence of sufficient nutrients and when the culture reached 80–90%, confluence cells were removed from the surface of the culture flasks by trypsinization. Samples for the vibration experiments were prepared by aliquoting 1 ml of cell suspension in cell culture medium with a concentration of 106 cells ml−1 into a 2 ml sterile cryogenic vial (Nalgene) with a 10 mm headspace. To reflect those conditions anticipated for cell transport as a therapeutic, no hypothermic storage media were used during cold storage so that no manipulation of the supplied cells would be needed to remove the hypothermic media at clinical sites such that the received products can be delivered to the patient. The vials loaded with cell suspension were capped, sealed and mounted on the shaker and corresponding control samples were also kept in the same environment.
2.3. Cells viability evaluation and surface antigen expression analysis
Cell viability and surface antigen expression were analysed immediately after the vibration and cold storage experiments. Two automated cell counting systems based on the trypan blue exclusion method were used. The first, the Cedex (Innovartis), was suitable for counting cells at high concentrations but required the whole sample volume [27]; the other, the Countess Automated Cell Counter (Invitrogen), only required 20 μl sample volumes and consequently allowed further analysis of mode of cell death for the same sample.
The cell suspension was mixed by repeated gentle pipetting to break up any clumps and to redistribute the cells throughout the medium evenly. For the trypan blue staining, cell suspension was mixed with an equal volume of 0.4 per cent trypan blue and cells were counted immediately using the built-in image analysis software of the Cedex or cell counter in order to achieve accurate cell number measurements minimizing the effects of cell clumping.
Cell viability measured by trypan blue exclusion method revealed the percentage of cells without an intact membrane alone. In order to further understand whether the loss of membrane integrity was attributable to the vibration-induced mechanical stress, or other mode of cell death, samples vibrated at 10, 25 and 50 Hz and exposed to different cold storage duration were further analysed by both the trypan blue exclusion method and flow cytometer apoptosis analysis (Beckman Coulter Cell Lab QuantaSC Flow Cytometer). For annexin V apoptosis analysis, following the protocol from the supplier, a combination of annexin V-FITC and PI (Invitrogen, UK) allows the distinction between early apoptotic cells (annexin V+), apoptotic (annexin V+ and PI+), dead cells (PI+) and viable cells (unstained).
As has been reported that re-warming of cells taken from cold storage resulted in apoptosis due to the activation of the apoptotic process for cells partially injured by cold stress [28], samples were immediately analysed after cold storage and vibration experiments to avoid introduction of any further cellular stress owing to re-warming. This reflects the timing and conditions for handling of such a cellular therapeutic product in clinical practice.
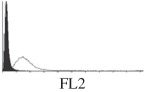
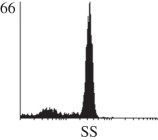
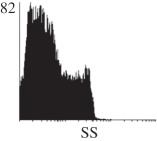
Samples were centrifuged at 300 g for 5 min, and directly conjugated antibodies were added after re-suspension at the manufacturer's recommended concentrations, followed by incubation at room temperature for 45 min in the dark. Cells were also incubated with monoclonal antibodies specific to mesenchymal stem cell surface markers CD29 (FITC), CD44 (FITC), CD105 (PE) and CD166 (PE) (Beckman Coulter, UK) (table 2). All monoclonal antibodies were used in conjunction with their respective isotype controls, and autofluorescence and non-specific binding of the cells obtained with isotype control were used as negative controls. For each sample run 10 000 events within the population of interest were recorded, and analysed cell populations of interest were gated according to side scatter (SS) and particle size. Analysis of a cell suspension prepared on day 0 was used to set the voltage to the appropriate level to obtain a signal for both autofluorescence and non-specific binding below the first log decade of the fluorescent signal axis and the setting established used for the rest of the experiments. Cyflogic v. 1.2.1 (CyFlo Ltd, Turku, Finland) was used to display the profiles of the negative control and samples as an overlay histogram.
Table 2.
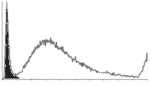
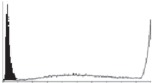
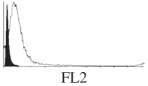
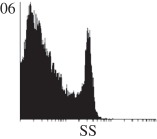
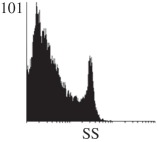
Histogram of hMSC CD markers and SS vibrated at different vibration conditions and after different cold storage durations. The shaded area in the overlaid histogram of CD markers represents the negative control.
| CD29 | CD44 | CD105 | CD166 | side scatter | |
|---|---|---|---|---|---|
| T0 | 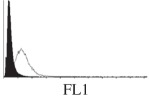 |
 |
 |
 |
 |
| T24 h |  |
 |
negative | negative | 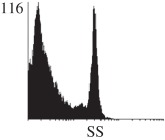 |
| T24 h at 25 Hz | 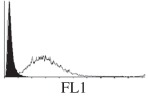 |
 |
negative | negative | 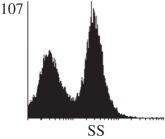 |
| T48 h | 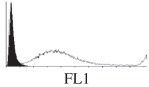 |
 |
 |
 |
 |
| T24 h + 24 h at 10 Hz | 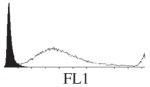 |
 |
 |
 |
 |
| T72 h | 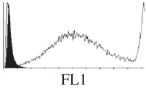 |
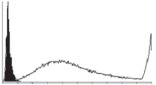 |
 |
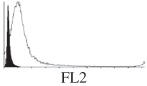 |
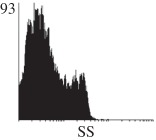 |
| T96 h | 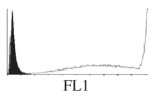 |
out of range set | negative | 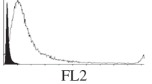 |
 |
2.4. Analysis of significance of experimental data
Samples for all experiments were prepared in triplicate, and data were normalized and presented as the ratio between the vibrated and the control samples. In order to establish whether there were statistically significant differences between the results obtained from different conditions, the confidence interval of the ratio of two means [29] were calculated and presented as error bars on plotted data. This gives 95 per cent percent confidence that vibrated samples are significantly different from the controls if the line crossing vibrated/control = 1, representing the null hypothesis that the vibrated and the control samples are exactly the same, is outside the error bar.
3. Experimental results
3.1. Multiple frequency combination and initial single frequency experiments
Twenty-four hours of vibration with the multiple frequency combination and vibration at a single frequency of 25 Hz caused the largest decrease in total cell population and cell viability (figure 2a,b). Moderate effects were observed in the 50 Hz single frequency experiment and as vibration frequencies increased from 100 to 500 Hz and also at 10 Hz the decrease in cell viability and total cell numbers owing to vibration were less apparent. To further confirm and verify the effects noticed, experiments at 10, 25 and 50 Hz were repeated with controlled cold storage duration and vibration duration.
Figure 2.
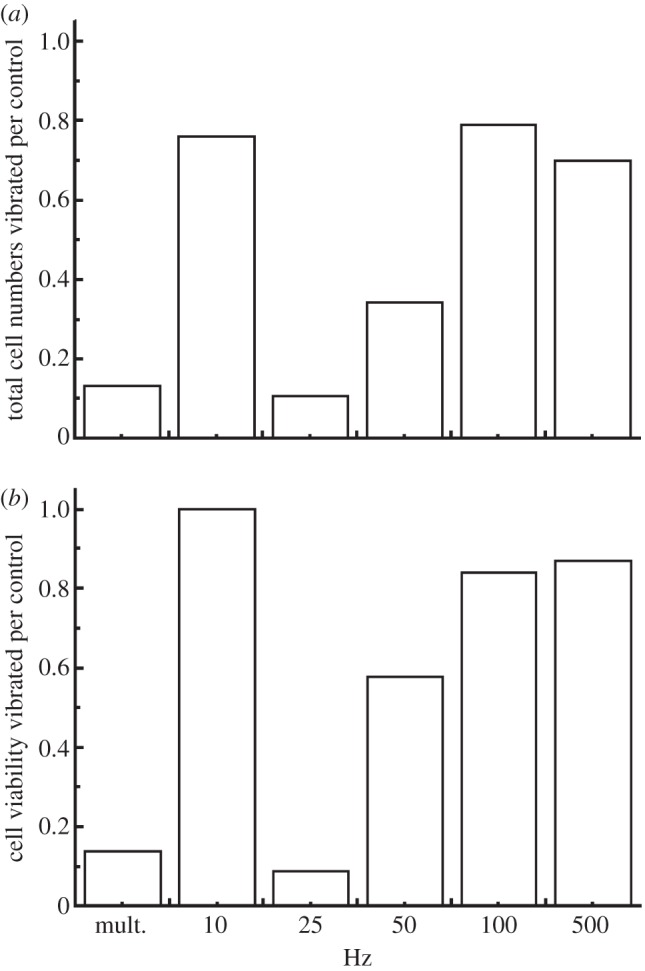
(a) Total cell numbers and (b) viability relative to the control after 24 h vibration at multiple frequency combination (mult.) and 10, 25, 50, 100 and 500 Hz, respectively.
3.2. Vibration at 50 Hz, peak acceleration 140 m s−2, peak displacement 1.4 mm
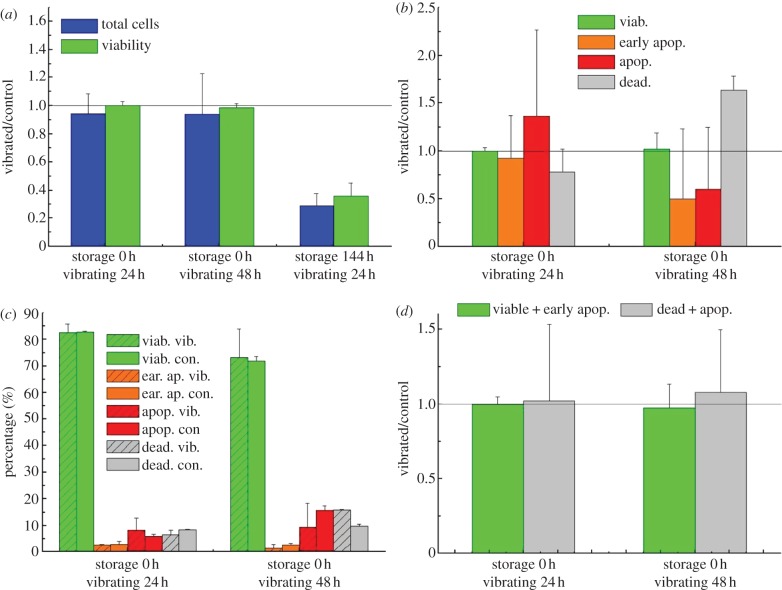
Total cell numbers and cell viability relative to the control showed a small, but not statistically significant decrease (figure 3a) for both 24 and 48 h vibration with no pre-vibration cold storage. However when samples were stored for 144 h before being exposed to 24 h of vibration, the ratios of the total cell numbers and cell viability between the vibrated to the control samples were markedly reduced in comparison with those without pre-vibration cold storage (figure 3a) and significantly lower than 1 for total cell number and viability.
Figure 3.
Cell number and viability relative to the control after 24 or 48 h vibration at 50 Hz and for those with 144 h pre-vibration storage: evaluated with (a) the trypan blue exclusion method; (b) Flow cytometry measurements presented as values relative to the control and (c) absolute percentage of subpopulations; combination of viable and early apoptotic, and (d) dead and apoptotic. (Online version in colour.)
Further flow cytometer analysis of subpopulations at different stages of cell death revealed no significant difference in viable cell population between the vibrated and the control (figure 3b), and between 24 and 48 h vibration. Trends indicating a presence of more apoptotic and dead cells were observed after 24 h and 48 h vibration. The percentage of dead cells after 48 h of vibration was significantly greater when compared with the control (figure 3b). As the percentages of early apoptotic and apoptotic cells were much smaller than those of viable cells for both 24 and 48 h (figure 3c), it is not unexpected that the error bar based on the normalized data appears large even for small variations between the samples within the groups. When both viable and early apoptotic (PI negative), and dead and apoptotic (PI positive) components of the populations were combined (figure 3d), the viability measured relative to the control is similar to that quantified by the trypan blue exclusion method (figure 3a).
3.3. Vibration at 25 Hz, peak acceleration 140 m s−2, peak displacement 5.7 mm
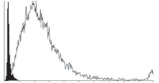
Trypan blue measurements (figure 4a) showed that with vibration, total cell numbers and cell viability reduces to 4 and 28 per cent of the control, respectively. Flow cytomeric dot plots of vibrated samples and the control (figure 5) revealed that most of the cells analysed were PI positive but annexin V negative after exposure to vibration, indicating the loss of membrane integrity and subsequent cell death. Cells without exposure to vibration were scattered across the four regions of the dot plot with more of the population in the apoptotic region (both annexin V and PI positive). Further quantitative analysis of the flow cytometry measurements (figure 4b) indicated that the proportion of the dead cells in the vibrated samples was more than 10 times that in the control. It also showed a moderate decrease in the percentage of viable and early apoptotic cells, but the percentage of apoptotic cells was much less than that observed in the control. When both viable and early apoptotic cells, and apoptotic and dead cells, were combined (figure 4c), the percentage of dead cells was still three to four times of that in the control, and the percentage of viable cells was approximately 68 per cent of the control.
Figure 4.
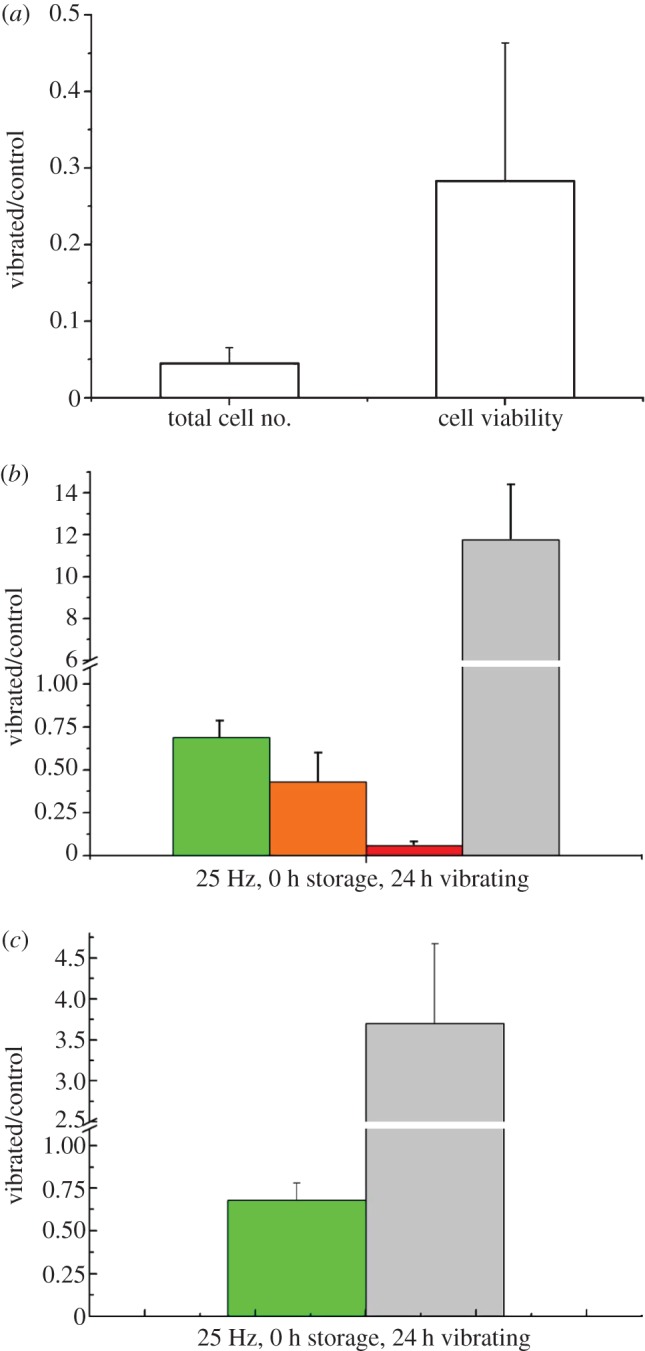
Cell numbers and viability relative to the control after 24 h vibration at 25 Hz evaluated with (a) the trypan blue exclusion method. Flow cytometry measurements presented as (b) relative value of subpopulations and as a combination of viable and early apoptotic, and (c) dead and apoptotic subpopulations. (b) Green bars, viable; orange bars, early apoptotic; red bars, apoptotic; grey bars, dead. (c) Green bars, viable + early apoptotic; grey bars, dead + apoptotic. (Online version in colour.)
Figure 5.
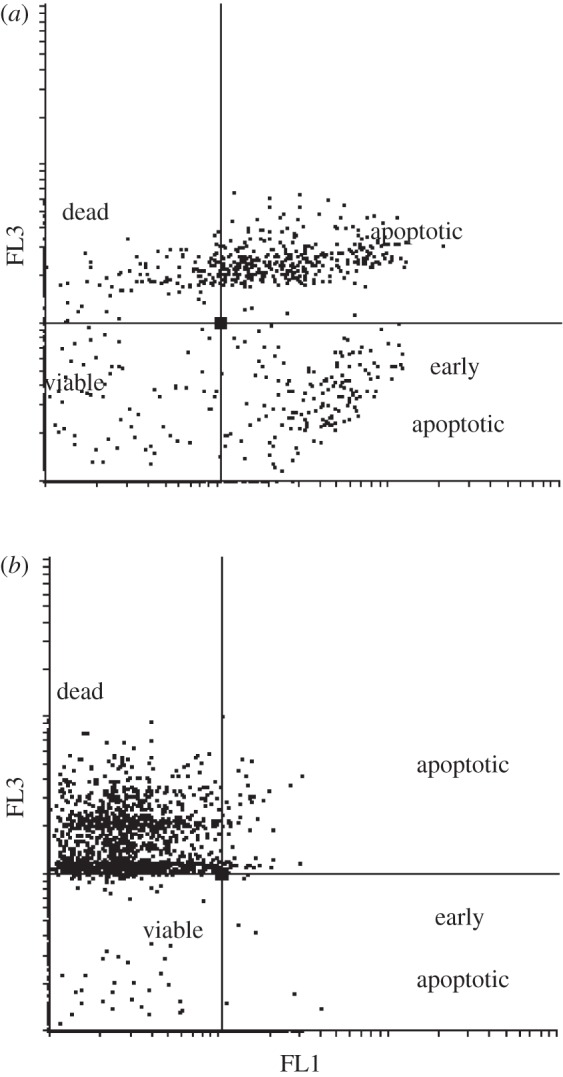
Flow cytometric dot plots of annexin V-FITC (FL1) versus propidium iodide (FL3) from one representative (a) un-vibrated and (b) vibrated sample.
3.4. Vibrations at 10 Hz, peak acceleration 20 m s−2, peak displacement 5.1 mm
Vibration at 10 Hz tended to decrease total cell number and cell viability slightly, but the observed change was not significant relative to the control (figure 6a). When the subpopulations of viable and early apoptotic, and dead and late apoptotic cells (figure 6b), were combined, there was still no significant difference between the vibrated sample and the control, as shown by the outcomes of measurement using the flow cytometer.
Figure 6.
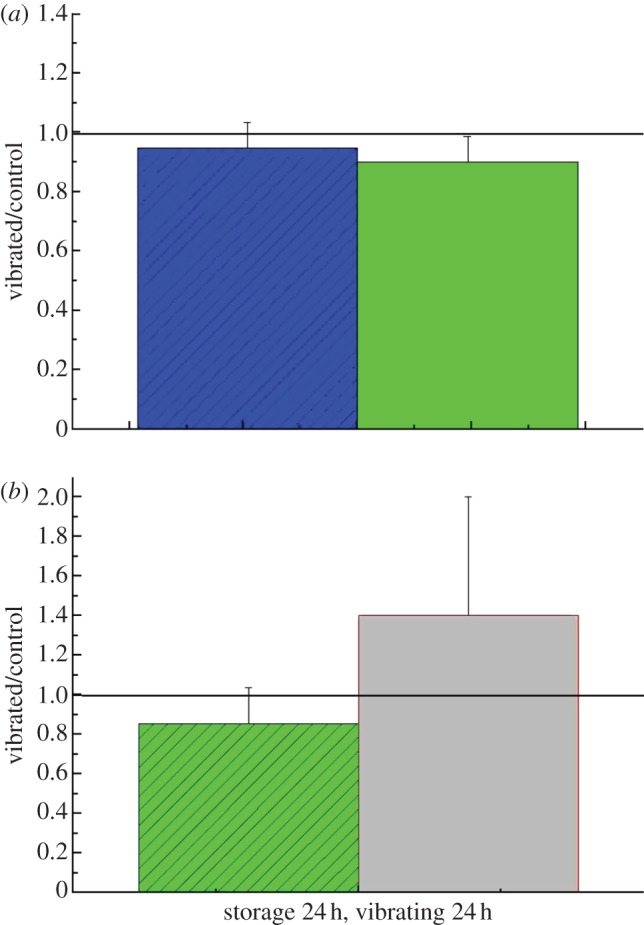
(a) Cell numbers and viability relative to the control after 24 h cold storage and vibration at 10 Hz evaluated with the trypan blue exclusion method. (b) Flow cytometry measurements presented as a relative value of combination of viable and early apoptotic, and dead and apoptotic subpopulations. (a) Blue bars, total cell number; green bars, viability. (b) Green bars, viable + early apoptotic; grey abrs, apoptotic + dead. (Online version in colour.)
3.5. Effect of cold storage
The results of flow cytometry measurements to quantify the effect of up to 96 h of cold storage on cell viability are presented in figure 7. This shows that the number of dead cells (PI+) in the population increased slightly after 24 h, however, from 48 to 72 h, a marked increase in dead cells from 20 per cent to nearly 40 per cent of the cell population was observed. The percentage of early apoptotic and apoptotic cells did not change significantly. Further viability measurements using the Countess by the trypan blue method (figure 7b) agreed with the flow cytometry measurements (figure 7a), particularly for 72 and 96 h of the cold storage.
Figure 7.
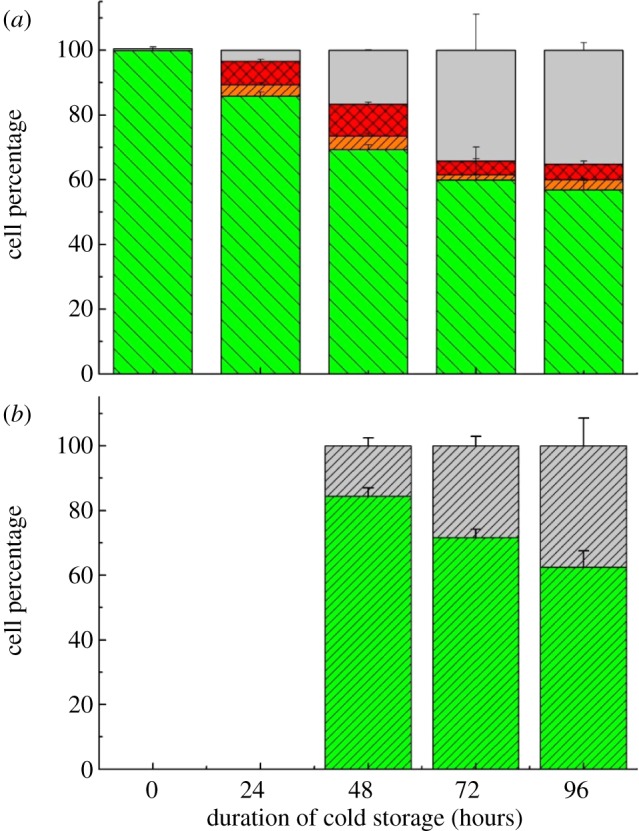
Samples exposed to different cold storage durations: (a) flow cytometry measurements of percentage of subpopulations and (b) trypan blue measurements of viable and dead cells. (a) Grey bars, dead; red bars with stripes, apoptotic; orange bars with stripes, early apoptotic; green bars, viable. (b) Green bars with stripes, viable; grey bars with stripes, dead. (Online version in colour.)
3.6. Evaluation of cell surface markers
The hMSCs at T0 were positive for CD29, CD44, CD105 and CD166, with the strong expression of CD105 and CD44 and moderate expression of CD29 and CD166. After 24 h cold storage, moderate expression of CD29 and CD44 was still noticed, but no expression of CD105 and CD166 could be detected. As the cold storage duration increased to 48 and 72 h, the expression of CD29 and CD44 became more significant and was ultimately, at 96 h, above the range set for the instrument. Moderate expression of CD105 and CD166 was shown after 48 and 72 h cold storage, but at 96 h only a moderate CD166 expression was observed and CD105 expression was negative. When cells were vibrated at 10 Hz, there was little difference in CD marker expression to cells solely kept in cold storage. For vibrations at 25 Hz, the expression of CD29 and CD44 was higher than that in the control. The SS also indicated a decrease in cell granularity with prolonged cold storage with and without vibration. After 24 and 48 h of cold storage, the SS showed a bimodal distribution indicating a mixture of populations of normal cells and cells with reduced granularity. The latter population increased with storage duration as evidenced by the increase of the intensity of the left-hand peak. When cold storage was prolonged over 72 h, the bimodal distribution was no longer observed and the single distribution illustrated the dramatic decrease of cell granularity.
4. Discussion
With the development of cell/tissue-based regenerative medicine products for clinical application, it has become more critical to establish a supply route to ensure the quality of these products and, hence, the success of their clinical application. Different transportation strategies have been investigated [30] and a significant amount of research has been carried out to understand the effects of cold storage and cryopreservation on the viability of cells [31–33]. The study carried out within the research reported here has investigated the effects of the combination of cold storage and mechanical stress during cold transport of an hMSC suspension without the presence of cryoprotectant.
4.1. Consequences of vibration on cell viability
Within the initial preliminary experiment, a marked reduction in cell viability was noticed for hMSCs, consistent with that observed also for human dermal fibroblasts [25]. The single frequency sweep experiment further revealed that the effects on cell viability were related to the parameters of the vibration, including frequency, displacement and acceleration rate. Findings from this initial experiment led to further investigations of changes in cell viability, total cell number and status of cell death induced by cold and mechanical stress when hMSCs with different pre-vibration cold storage durations were exposed to vibration conditions of specified parameters.
When the hMSC suspension was vibrated at 50 Hz, no significant effects on cell viability were noticed for cells vibrated immediately after preparation of the suspension. However, for cells stored for 144 h in cell culture medium at 2–8°C, a significant reduction of cell viability was observed. This showed that cells exposed to prolonged cold storage were more susceptible to the mechanical stress induced by vibration.
At 25 Hz, marked reductions in cell number and cell viability were observed. The trypan blue exclusion method showed a marked reduction in viability to 28 per cent of that of the control, while the annexin and PI method gave a result of 68 per cent of that of the control. As the total cell number was much reduced after vibration, the difference was likely due to the lack of reliability of the trypan blue exclusion method at lower cell densities [34].
A comparison of the results of 24 h vibration at 25 and 50 Hz showed that the conditions experienced at 25 Hz reduced the final total cell number to 0.4 per cent of the control, but that the total cell number was still around 95 per cent of the control for 50 Hz. A response similar to vibration at different frequencies was also observed in previous experiments with HDF [25]. The differences between the vibration parameters of these two conditions include frequency and displacement. In order to further understand the influence of displacement on cell viability, an experiment was also carried out at 10 Hz with a displacement similar to that for 25 Hz and also at 100 Hz with a much reduced displacement and similar acceleration. There was no significant effect on cell viability when samples were vibrated at 10 and 100 Hz, indicating the marked and moderate reduction on cell viability observed at 25 and 50 Hz, respectively, may be the consequences of combinational effects of displacement and acceleration.
4.2. Mode of cell death induced by vibration and cold storage
The influence of cold storage alone on cell viability and the function of cell therapeutic products has been extensively investigated, especially for haematopoietic stem cells derived from different tissues [31,35,36] and regenerative medicine products based on cord-/bone marrow-derived mesenchymal stem cells [37–39]. Solely considering the effects of cold storage, the evaluation of cell membrane integrity and apoptosis showed an increase in both annexin V+ and PI+ cell populations, especially after 24 and 48 h cold storage, indicating that cells are approaching cell death. The increase of the PI+ cell populations was further observed with a cold storage duration extended from 2 to 4 days, consistent with those reported for hypothermic conservation of haematopoietic progenitors [40]. Annexin V has been generally used as an indicator to identify apoptotic cells. However, annexin V expression is not exclusive to apoptotic cells as translocation of phosphatidylserine to the external cell surface also occurs during cell necrosis and oncosis [41,42]. Research into cold storage effects have shown that cold storage alone resulted in mainly necrosis [28,43]. Hence the observed increase of annexin V+ and PI+ cell populations with prolonged cold storage duration could indicate more cells proceeding into cell death through oncotic necrosis (also referred to as swelling necrosis), a form of passive or accidental cell death.
Although cell apoptosis induced by hypothermia/re-warming has been investigated extensively, especially for tissue preservation of major organs, such as liver or kidney tissues, there are still different opinions on whether cell death induced by hypothermia is through the mode of apoptosis [44] or necrosis [45]. Hence, the involvement of apoptosis in cold-induced cell death could not be excluded totally [43]; further characterization, perhaps using an apoptosis DNA fragment test, is needed to identify and confirm the mode of cell death. In addition, the final stage of apoptosis, e.g. caspase activation, can only occur if the cells are metabolically active, i.e. returned to culture. Future research should therefore apply a real-time biochemical indicator of cell injury, e.g. heat shock proteins [46,47], to distinguish between the factors of vibration, cold storage and re-warming and their contribution to necrosis or apoptosis.
The effects of the addition of vibration to the cell samples shown at 50 Hz 48 h vibration were a transition from expression of annexin V+ to an expression dominated by PI+. The transition of cell population would further imply that as a consequence of cold-induced cell oncosis and eventually necrosis, prolonged cold storage induces a change in cell membrane and causes the cells to be more susceptible to vibration damage. The synergistic effect of cold storage and vibration was further confirmed by a marked reduction in cell viability and by the total cell number observed for samples with 144 h pre-vibration cold storage These cold storage effects on the cell membrane were also verified further by a rapid decrease of cell SS, indicating the decrease in cell granularity owing to the damage to the cell membrane and the subsequent release of cell organelles. Although cell granularity also diminishes as apoptosis proceeds, considering there was no further incubation to activate apoptosis before sample testing, the reduction of cell granularity would most likely to be the consequence of membrane rupture owing to the necrosis induced by cold storage [48].
4.3. Influence of cold storage and vibration on stem cell surface markers
The panel of mesenchymal stem cell surface markers investigated also revealed some sensitivity of the cells to the influence of cold storage and vibration conditions. Prolonged cold storage durations intensified CD29 and CD44 expression, attenuated CD105 expression and had no obvious effects on CD166. CD105, also called endoglin, is involved in angiogenesis and has been suggested as an appropriate marker for tumour-related angiogenesis [49]. Recently Levi et al. [50] identified the correlation of low expression of CD105 with an increased osteogenic potential for adipose-derived stromal cells. Considering that stem cells are more resistant to hypothermic environments than other somatic cells [51], the change of cell surface markers observed could be attributed to a subpopulation of hMSC surviving in the cold storage conditions. This finding is in accord with those observed by others that the tolerance of cells to cold storage is dependent on cell type [52,53] and that only a certain subpopulation of cells will survive prolonged cold storage. [31].
Increased CD29 and CD44 expression was noticed after 24 h of 25 Hz vibration, but not at 10 Hz. Heng et al. [24] have reported some non-significant change of gene expression of hMSCs phenotypic markers, depending on the shear stress experienced by cells during trans-catheter injection. Considering the relatively large displacement and acceleration at 25 Hz, the shear stresses that hMSCs have been exposed to could be much higher in this study. Moreover, the flow of medium within the vial during vibration may be more complex than the laminar flow in a catheter and also the duration of exposure to the hydrodynamic-induced shear stress was longer in the current study. It has been reported that CD44-positive haematopoietic progenitor cells can sense shear stress in laminar flow [54] and that the response of osteoblast-like cells to oscillatory shear stress was through the activation of CD29 (beta1 integrin) [55]. The increase of CD29 and CD44 expression at 25 Hz noticed in this study could be due to the higher shear stresses experienced by the cells as the consequence of different hydrodynamic behaviour within the system at the specified frequencies. Similar differing effects for shear stresses generated by laminar flow and orbital liquid motion have been reported for endothelial cells [56]. It is anticipated that the higher CD29 and CD44 expression may facilitate the binding of hMSCs with extracellular matrix and hence, encourage cell migration, recruitment and tissue regeneration after locally/systematic administration [57,58].
4.4. Correlation of vibration parameters with real-life cold chain shipping practice
Initially, two ‘extreme’ examples derived from the publications of Litak et al. and Garcia et al. were used as a benchmark to aid the establishment of vibration parameters. Litak et al. [14] reported shock pulses of 100 ms durations with peak accelerations of 20 or 50 m s−2 with or without damping when a van was crossing a rail track at 20 km h−1. Garcia et al. [16] also reported shock pulses with a maximum peak acceleration of 850 m s−2 and a duration of 20 ms when midsized (0.36 × 0.34 × 0.34 m) and lightweight (6.5 kg) packages were shipped between Europe and the USA. As the maximum displacement allowance of the shaker in the experimental arrangement used was 7 mm, it was not possible to simulate the acceleration rate and duration of these two extreme examples (note that 2 cm or 5 cm displacements are needed for 20 or 50 m s−2 accelerations and 3.5 cm for 850 m s−2). Also considering the cost of cell therapeutic products and the high patient risk if malfunctioning products were to be used, the worst case must be investigated in order to satisfy the demanding regulatory requirement. The 12 kg mass packaging system used (Greenbox, Tegrant Corporation) was designed to maintain cold chain temperatures during transportation and had a lower anti-shock performance when compared with the 6.5 kg mass system used in the study of Garcia et al. specially designed to control the effects of vibration. Hence, the vibration parameters chosen were a compromise between the vibration conditions of the two extreme examples, and experiments were carried out with the maximum displacement achievable by the experimental system used.
At the maximum displacement of 6 mm, a 20 ms shock pulse with an acceleration of 140 m s−2 could be generated. To identify the threshold of vibrations that could cause cell damage, vibration tests at different frequencies were carried out at acceleration rate 140 m s−2 whenever possible. For 10 Hz vibrations, the maximum acceleration is 20 m s−2 (similar to that reported by Litak et al.) and displacement is 5 mm, and these are similar to those used in the random vibrations test for shipping containers defined by IS0 13355 standard [19]. An acceleration of 140 m s−2 and duration 20 ms also represent the effects of some drops and disturbances caused by road bumps and trenches, especially when the vehicle speed is greater than 20 km h−1. The destructive effects of vibration noticed in this study imply that cell damage is more likely to occur in such cell suspension containing packages when vibrated at higher amplitudes during cold transportation.
In summary, with the development of stem cells and cell culture technology, increasing attention has been placed on packaging design and on identifying product dispensing routes facilitating the clinical end use. The findings reported here on the effects of vibration and cold storage on cell viability and total cell number highlight the importance of understanding the sensitivity of cells to their mechanical and thermal environment during transport and the necessity of improved package design for highly regulated cell/tissue-based therapeutic products. Currently, the evaluation and validation process for any specified format of shipping container purely depends upon in vitro and in vivo characterization of the shipped cells. Further work will concentrate on understanding the hydrodynamics of medium flow in the system to identify the thresholds for effects and to understand the underlying mechanisms giving rise to the observed biological consequences. This will inform a more rational and active approach to package design for cellular products, allowing decision-making based on real-time objective data rather than purely relying on consequence evaluation using cell characterization techniques to ensure the consistency and stability of the products following transport from the factory site to the bedside.
5. Conclusions
Flow cytometry and trypan blue exclusion measurements indicated that cold storage alone in cell culture medium caused decreases of cell viability, viable and total cell numbers, especially after 48 h. Cell death is most likely to be the consequence of membrane rupture owing to the necrosis induced by cold storage. The sensitivity of cells to different vibration conditions within the mechanical system is a combination of the effects of displacement and acceleration, and hMSCs with longer cold storage duration are more susceptible to vibration damage, indicating a coupling between the effects of vibration and cold storage.
Acknowledgements
This work was supported by UK Technology Strategy Board and EPSRC and industrial collaborators Intercytex, Paul Kemp and Penny Johnson and Cool Logistics, Richard Harrop. Y.L. was additionally supported by a UK Research Council Fellowship. We thank the referees for their most constructive comments. http://www.lboro.ac.uk/departments/mm/staff/liu-y.html; http://www.lboro.ac.uk/research/lcbe/
References
- 1.Whiteside T. L., et al. 2011. Shipping of therapeutic somatic cell products. Cytotherapy 13, 201–213 10.3109/14653249.2010.506507 (doi:10.3109/14653249.2010.506507) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathew A. J., Baust J. M., Van Buskirk R. G., Baust J. G. 2004. Cell preservation in reparative and regenerative medicine: evolution of individualized solution composition. Tissue Eng. 10, 1662–1671 10.1089/ten.2004.10.1662 (doi:10.1089/ten.2004.10.1662) [DOI] [PubMed] [Google Scholar]
- 3.Baust J. M., Snyder K. K., VanBuskirk R. G., Baust J. G. 2009. Changing paradigms in biopreservation. Biopreserv. Biobank 7, 3–12 10.1089/bio.2009.0701.jmb (doi:10.1089/bio.2009.0701.jmb) [DOI] [PubMed] [Google Scholar]
- 4.Pamphilon D. H., Selogie E., Szczepiorkowski Z. M. & BEST Collaborative Cellular 2010. Transportation of cellular therapy products: report of a survey by the cellular therapies team of the Biomedical Excellence for Safer Transfusion (BEST) collaborative. Vox Sang 99, 168–173 10.1111/j.1423-0410.2010.01329.x (doi:10.1111/j.1423-0410.2010.01329.x) [DOI] [PubMed] [Google Scholar]
- 5.Tanaka S. M., Li J. L., Duncan R. L., Yokota H., Burr D. B., Turner C. H. 2003. Effects of broad frequency vibration on cultured osteoblasts. J. Biomech. 36, 73–80 10.1016/S0021-9290(02)00245-2 (doi:10.1016/S0021-9290(02)00245-2) [DOI] [PubMed] [Google Scholar]
- 6.Pre D., Ceccarelli G., Benedetti L., Magenes G., Cusella De Angelis M. G. 2009. Effects of low-amplitude, high-frequency vibrations on proliferation and differentiation of SAOS-2 human osteogenic cell line. Tissue Eng. Part C Methods 15, 669–679 10.1089/ten.tec.2008.0599 (doi:10.1089/ten.tec.2008.0599) [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg N., Levy M., Francis M. 2002. Experimental model for stimulation of cultured human osteoblast-like cells by high frequency vibration. Cytotechnology 39, 125–130 10.1023/A:1023925230651 (doi:10.1023/A:1023925230651) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pre D., Ceccarelli G., Gastaldi G., Asti A., Saino E., Visai L., Benazzo F., Cusella De Angelis M. G., Magenes G. 2011. The differentiation of human adipose-derived stem cells (hASCs) into osteoblasts is promoted by low amplitude, high frequency vibration treatment. Bone 49, 295–303 10.1016/j.bone.2011.04.013 (doi:10.1016/j.bone.2011.04.013) [DOI] [PubMed] [Google Scholar]
- 9.Tirkkonen L., et al. 2011. The effects of vibration loading on adipose stem cell number, viability and differentiation towards bone-forming cells. J. R. Soc. Interface 8, 1736–1747 10.1098/rsif.2011.0211 (doi:10.1098/rsif.2011.0211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou Y., Guan X., Zhu Z., Gao S., Zhang C., Li C., Zhou K., Hou W., Yu H. 2011. Osteogenic differentiation of bone marrow-derived mesenchymal stromal cells on bone-derived scaffolds: effect of microvibration and role of Erk1/2 activation. Eur. Cells Mater. 22, 12–25 [DOI] [PubMed] [Google Scholar]
- 11.Gaston J., Quinchia Rios B., Bartlett R., Berchtold C., Thibeault S. L. 2012. The response of vocal fold fibroblasts and mesenchymal stromal cells to vibration. PLoS ONE 7, e30965. 10.1371/journal.pone.0030965 (doi:10.1371/journal.pone.0030965) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shav D., Einav S. 2010. The effect of mechanical loads in the differentiation of precursor cells into mature cells. Analysis of cardiac development: from embryo to old age. Ann. NY Acad. Sci. 1188, 25–31 10.1111/j.1749-6632.2009.05079.x (doi:10.1111/j.1749-6632.2009.05079.x) [DOI] [PubMed] [Google Scholar]
- 13.van Zeebroeck A., Tijskens E., Dintwa E., Kafashan J., Loodts J., De Baerdemaeker J., Ramon H. 2006. The discrete element method (DEM) to simulate fruit impact damage during transport and handling: model building and validation of DEM to predict bruise damage of apples. Postharvest Biol. Technol. 41, 85–91 10.1016/j.postharvbio.2006.02.007 (doi:10.1016/j.postharvbio.2006.02.007) [DOI] [Google Scholar]
- 14.Litak G., Borowiec M., Hunicz J., Koszalka G., Niewczas A. 2009. Vibrations of a delivery car excited by railway track crossing. Chaos Solitons Fractals 42, 270–276 10.1016/j.chaos.2008.11.020 (doi:10.1016/j.chaos.2008.11.020) [DOI] [Google Scholar]
- 15.ASTM Int'l 2008. ASTM standard D999–08 standard test methods for vibration testing of shipping containers. West Conshohocken, PA: ASTM International [Google Scholar]
- 16.Garcia-Romeu-Martinez M., Singh S. P., Cloquell-Ballester V., Saha K. 2007. Measurement and analysis of international air parcel shipping environment for DHL and FedEx between Europe and United States. Packag. Technol. Sci. 20, 421–429 10.1002/pts.775 (doi:10.1002/pts.775) [DOI] [Google Scholar]
- 17.Jarimopas B., Singh S. P., Saengnil W. 2005. Measurement and analysis of truck transport vibration levels and damage to packaged tangerines during transit. Packag. Technol. Sci. 18, 179–188 10.1002/pts.687 (doi:10.1002/pts.687) [DOI] [Google Scholar]
- 18.ASTM Int'l 2008. ASTM standard D 4169–08 standard practice for performance testing of shipping containers and systems. West Conshohocken, PA: ASTM International [Google Scholar]
- 19.ASTM Int'l 2006. ASTM standard D4728–06, standard test method for random vibration testing of shipping containers. West Conshohocken, PA: ASTM International [Google Scholar]
- 20.Haugh M. G., Meyer E. G., Thorpe S. D., Vinardell T., Duffy G. P., Kelly D. J. 2011. Temporal and spatial changes in cartilage-matrix-specific gene expression in mesenchymal stem cells in response to dynamic compression. Tissue Eng. Part A 17, 3085–3093 10.1089/ten.tea.2011.0198 (doi:10.1089/ten.tea.2011.0198) [DOI] [PubMed] [Google Scholar]
- 21.Liu C., Abedian R., Meister R., Haasper C., Hurschler C., Krettek C., von Lewinski G., Jagodzinski M. 2012. Influence of perfusion and compression on the proliferation and differentiation of bone mesenchymal stromal cells seeded on polyurethane scaffolds. Biomaterials 33, 1052–1064 10.1016/j.biomaterials.2011.10.041 (doi:10.1016/j.biomaterials.2011.10.041) [DOI] [PubMed] [Google Scholar]
- 22.Chisti Y. 2001. Hydrodynamic damage to animal cells. Crit. Rev. Biotechnol. 21, 67–110 10.1080/20013891081692 (doi:10.1080/20013891081692) [DOI] [PubMed] [Google Scholar]
- 23.Hu W., Berdugo C., Chalmers J. J. 2011. The potential of hydrodynamic damage to animal cells of industrial relevance: current understanding. Cytotechnology 63, 445–460 10.1007/s10616-011-9368-3 (doi:10.1007/s10616-011-9368-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heng B. C., Hsu S. H., Cowan C. M., Liu A., Tai J., Chan Y., Sherman W., Basu S. 2009. Transcatheter injection-induced changes in human bone marrow-derived mesenchymal stem cells. Cell Transplant. 18, 1111–1121 10.3727/096368909X12483162197006 (doi:10.3727/096368909X12483162197006) [DOI] [PubMed] [Google Scholar]
- 25.Nikolaev N. I., Liu Y., Williams D. J. 2010. Effects of dynamic mechanical forces on potential cell therapy products in cold transportation. In 6th World Congress of Biomechanics (WCB 2010), 1–6 August 2010 (eds Lim C. T., Goh J. C. H.), pp. 1137–1140 Berlin, Germany: Springer [Google Scholar]
- 26.Ibrahim R. A. 2005. Chapter 6: parametric sloshing: faraday waves. In Liquid sloshing dynamics: theory and applications (ed. Ibrahim R. A.), pp. 338–404 Cambridge, UK: Cambridge University Press [Google Scholar]
- 27.Liu Y., Hourd P., Williams D. 2007. Measurement system capability: a comparison of manual and automated cell counting systems. Tissue Eng. 13, 1722–1722 [Google Scholar]
- 28.Salahudeen A. K., Joshi M., Jenkins J. K. 2001. Apoptosis versus necrosis during cold storage and rewarming of human renal proximal tubular cells. Transplantation 72, 798–804 10.1097/00007890-200109150-00010 (doi:10.1097/00007890-200109150-00010) [DOI] [PubMed] [Google Scholar]
- 29.Motulsky H. 1995. Intuitive biostatistics. New York, NY: Oxford: Oxford University Press [Google Scholar]
- 30.Gorodetsky R., Levdansky L., Gaberman E., Gurevitch O., Lubzens E., McBride W. H. 2011. Fibrin microbeads loaded with mesenchymal cells support their long-term survival while sealed at room temperature. Tissue Eng. Part C-Methods 17, 745–755 10.1089/ten.tec.2010.0644 (doi:10.1089/ten.tec.2010.0644) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pamphilon D., Curnow E., Belfield H., Reems J., McMannis J., Lecchi L., Szczepiorkowski Z., McKenna D. 2011. Storage characteristics of cord blood progenitor cells: report of a multicenter study by the cellular therapies team of the Biomedical Excellence for Safer Transfusion (BEST) Collaborative. Transfusion 51, 1284–1290 10.1111/j.1537-2995.2010.02967.x (doi:10.1111/j.1537-2995.2010.02967.x) [DOI] [PubMed] [Google Scholar]
- 32.Malpique R., Ehrhart F., Katsen-Globa A., Zimmermann H., Alves P. M. 2009. Cryopreservation of adherent cells: strategies to improve cell viability and function after thawing. Tissue Eng. Part C Methods 15, 373–386 10.1089/ten.tec.2008.0410 (doi:10.1089/ten.tec.2008.0410) [DOI] [PubMed] [Google Scholar]
- 33.Ginis I., Grinblat B., Shirvan M. H. 2012. Evaluation of bone marrow-derived mesenchymal stem cells after cryopreservation and hypothermic storage in clinically safe medium. Tissue Eng. Part C Methods 18, 453–463 10.1089/ten.tec.2011.0395 (doi:10.1089/ten.tec.2011.0395) [DOI] [PubMed] [Google Scholar]
- 34.Mascotti K., McCullough J., Burger S. R. 2000. HPC viability measurement: trypan blue versus acridine orange and propidium iodide. Transfusion 40, 693–696 10.1046/j.1537-2995.2000.40060693.x (doi:10.1046/j.1537-2995.2000.40060693.x) [DOI] [PubMed] [Google Scholar]
- 35.Antonenas V., Garvin F., Webb M., Sartor M., Bradstock K., Gottlieb D. 2006. Fresh PBSC harvests, but not BM, show temperature-related loss of CD34 viability during storage and transport. Cytotherapy 8, 158–165 10.1080/14653240600620994 (doi:10.1080/14653240600620994) [DOI] [PubMed] [Google Scholar]
- 36.Kao G. S., 2011. Validation of short-term handling and storage conditions for marrow and peripheral blood stem cell products. Transfusion 51, 137–147 10.1111/j.1537-2995.2010.02758.x (doi:10.1111/j.1537-2995.2010.02758.x) [DOI] [PubMed] [Google Scholar]
- 37.Pal R., Hanwate M., Totey S. M. 2008. Effect of holding time, temperature and different parenteral solutions on viability and functionality of adult bone marrow-derived mesenchymal stem cells before transplantation. J. Tissue Eng. Regen. Med. 2, 436–444 10.1002/term.109 (doi:10.1002/term.109) [DOI] [PubMed] [Google Scholar]
- 38.Muraki K., Hirose M., Kotobuki N., Kato Y., Machida H., Takakura Y., Ohgushi H. 2006. Assessment of viability and osteogenic ability of human mesenchymal stem cells after being stored in suspension for clinical transplantation. Tissue Eng. 12, 1711–1719 10.1089/ten.2006.12.1711 (doi:10.1089/ten.2006.12.1711) [DOI] [PubMed] [Google Scholar]
- 39.Heng B. C., Cowan C. M., Basu S. 2008. Temperature and calcium ions affect aggregation of mesenchymal stem cells in phosphate buffered saline. Cytotechnology 58, 69–75 10.1007/s10616-008-9174-8 (doi:10.1007/s10616-008-9174-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jeanne M., et al. 2009. Low-oxygen and high-carbon-dioxide atmosphere improves the conservation of hematopoietic progenitors in hypothermia. Transfusion 49, 1738–1746 10.1111/j.1537-2995.2009.02191.x (doi:10.1111/j.1537-2995.2009.02191.x) [DOI] [PubMed] [Google Scholar]
- 41.Vermes I., Haanen C., Steffensnakken H., Reutelingsperger C. 1995. A novel assay for apoptosis—flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein-labeled annexin-V. J. Immunol. Methods 184, 39–51 10.1016/0022-1759(95)00072-I (doi:10.1016/0022-1759(95)00072-I) [DOI] [PubMed] [Google Scholar]
- 42.Lecoeur H., Prevost M. C., Gougeon M. L. 2001. Oncosis is associated with exposure of phosphatidylserine residues on the outside layer of the plasma membrane: a reconsideration of the specificity of the annexin V/propidium iodide assay. Cytometry 44, 65–72 10.1002/1097-0320(20010501)44:1%3C65::AIDCYTO1083%3E3.0.CO;2-Q (doi:10.1002/1097-0320(20010501)44:1<65::AIDCYTO1083>3.0.CO;2-Q) [DOI] [PubMed] [Google Scholar]
- 43.Corwin W. L., Baust J. M., Baust J. G., Van Buskirk R. G. 2011. The unfolded protein response in human corneal endothelial cells following hypothermic storage: implications of a novel stress pathway. Cryobiology 63, 46–55 10.1016/j.cryobiol.2011.04.008 (doi:10.1016/j.cryobiol.2011.04.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rauen U., Polzar B., Stephan H., Mannherz H. G., de Groot H. 1999. Cold-induced apoptosis in cultured hepatocytes and liver endothelial cells: mediation by reactive oxygen species. FASEB J. 13, 155–168 [DOI] [PubMed] [Google Scholar]
- 45.Niu X., Arthur P. G., Jeffrey G. P. 2010. Iron and oxidative stress in cold-initiated necrotic death of rat hepatocyte. Transplant Proc. 42, 1563–1568 10.1016/j.transproceed.2010.03.143 (doi:10.1016/j.transproceed.2010.03.143) [DOI] [PubMed] [Google Scholar]
- 46.Roobol A., Carden M. J., Newsam R. J., Smales C. M. 2009. Biochemical insights into the mechanisms central to the response of mammalian cells to cold stress and subsequent rewarming. FEBS J. 276, 286–302 10.1111/j.1742-4658.2008.06781.x (doi:10.1111/j.1742-4658.2008.06781.x) [DOI] [PubMed] [Google Scholar]
- 47.Liu A., Bian H., Huang L., Lee Y. 1994. Transient cold shock induces the heat-shock response upon recovery at 37 degrees C in human-cells. J. Biol. Chem. 269, 14 768–14 775 [PubMed] [Google Scholar]
- 48.Healy E., Dempsey M., Lally C., Ryan M. P. 1998. Apoptosis and necrosis: mechanisms of cell death induced by cyclosporine A in a renal proximal tubular cell line. Kidney Int. 54, 1955–1966 10.1046/j.1523-1755.1998.00202.x (doi:10.1046/j.1523-1755.1998.00202.x) [DOI] [PubMed] [Google Scholar]
- 49.Nassiri F., Cusimano M. D., Scheithauer B. W., Rotondo F., Fazio A., Yousef G. M., Syro L. V., Kovacs K., Lloyd R. V. 2011. Endoglin (CD105): a review of its role in angiogenesis and tumor diagnosis, progression and therapy. Anticancer Res. 31, 2283–2290 [PubMed] [Google Scholar]
- 50.Levi B., et al. 2011. CD105 protein depletion enhances human adipose-derived stromal cell osteogenesis through reduction of transforming growth factor beta 1 (TGF-beta 1) signaling. J. Biol. Chem. 286, 39 497–39 509 10.1074/jbc.M111.256529 (doi:10.1074/jbc.M111.256529) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eom Y. W., et al. 2011. Rapid isolation of adipose tissue-derived stem cells by the storage of lipoaspirates. Yonsei Med. J. 52, 999–1007 10.3349/ymj.2011.52.6.999 (doi:10.3349/ymj.2011.52.6.999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miyagi K., Yamazaki T., Tsujino I., Takahashi N., Koya Y., Masutani M., Sawada U., Horie T. 2001. Application of hypothermia to autologous stem cell purging. Cryobiology 42, 190–195 10.1006/cryo.2001.2322 (doi:10.1006/cryo.2001.2322) [DOI] [PubMed] [Google Scholar]
- 53.Yiu W., Cheng S. W. K., Sumpio B. E. 2007. Direct comparison of endothelial cell and smooth muscle cell response to supercooling and rewarming RID C-4704-2009 RID C-4273-2009. J. Vasc. Surg. 46, 557–564 10.1016/j.jvs.2007.04.072 (doi:10.1016/j.jvs.2007.04.072) [DOI] [PubMed] [Google Scholar]
- 54.Christophis C., Taubert I., Meseck G. R., Schubert M., Grunze M., Ho A. D., Rosenhahn A. 2011. Shear stress regulates adhesion and rolling of CD44+ leukemic and hematopoietic progenitor cells on hyaluronan. Biophys. J. 101, 585–593 10.1016/j.bpj.2011.05.045 (doi:10.1016/j.bpj.2011.05.045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee D., Li Y. J., Chang S., Zhou J., Ho H., Chiu J., Chien S. 2010. Oscillatory flow-induced proliferation of osteoblast-like cells is mediated by alpha(v)beta(3) and beta(1) integrins through synergistic interactions of focal adhesion kinase and Shc with phosphatidylinositol 3-Kinase and the Akt/mTOR/p70S6K pathway. J. Biol. Chem. 285, 30–42 10.1074/jbc.M109.010512 (doi:10.1074/jbc.M109.010512) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dardik A., Chen L. L., Frattini J., Asada H., Aziz F., Kudo F. A., Sumpio B. E. 2005. Differential effects of orbital and laminar shear stress on endothelial cells. J. Vasc. Surg. 41, 869–880 10.1016/j.jvs.2005.01.020 (doi:10.1016/j.jvs.2005.01.020) [DOI] [PubMed] [Google Scholar]
- 57.Zhu H., Mitsuhashi N., Klein A., Barsky L. W., Weinberg K., Barr M. L., Demetriou A., Wu G. D. 2006. The role of the hyaluronan receptor CD44 in mesenchymal stem cell migration in the extracellular matrix. Stem Cells 24, 928–935 10.1634/stemcells.2005-0186 (doi:10.1634/stemcells.2005-0186) [DOI] [PubMed] [Google Scholar]
- 58.Ode A., et al. 2011. Cd73 and Cd29 concurrently mediate the mechanically induced decrease of migratory capacity of mesenchymal stromal cells. Eur. Cells Mater. 22, 26–42 [DOI] [PubMed] [Google Scholar]