Abstract
Objective
To estimate the association between over-the-counter nonsteroidal anti-inflammatory drug (NSAID) exposure during the early first-trimester and risk for spontaneous abortion (gestation prior to 20 weeks) in a prospective cohort.
Methods
Women were enrolled in the Right from the Start study (2004–2010). Exposure data regarding over-the-counter NSAID use from the last menstrual period through the 6th week of pregnancy were obtained from intake and first-trimester interviews. Pregnancy outcomes were self-reported and verified by medical records. Gestational age was determined from last menstrual period. Stage of development prior to loss was determined from study ultrasound. Cox proportional hazards regression models were used to estimate the association between NSAID exposure and pregnancy outcome, taking into account candidate confounders.
Results
Among 2,780 pregnancies, 367 women (13%) experienced an spontaneous abortion. NSAID exposure was reported by 1,185 (43%) women. NSAID exposure was not associated with spontaneous abortion risk in unadjusted models (hazard ratio [HR] = 1.01, 95% confidence interval [CI] 0.82, 1.24) or models adjusted for maternal age (adjusted [aHR] = 1.00, 95% CI 0.81, 1.23).
Conclusions
Our findings suggest that use of non-prescription over-the-counter NSAIDs in early pregnancy does not put women at increased risk of spontaneous abortion.
INTRODUCTION
In the United States each year, at least 1.5 million women use nonsteroidal anti-inflammatory drugs (NSAIDs) around the time of conception, implantation, and early embryonic development,(1–3) making them the most common medication exposure reported in the first-trimester.(4) NSAIDs are primarily used to relieve pain and reduce inflammation and their effects generally result from inhibition of cyclooxygenase (COX)-2. Recently, three population-based observational cohort studies have implicated first-trimester NSAID use as a risk factor for spontaneous abortions (SAB or miscarriage) using data obtained from pharmacy records or study interviews that limited NSAID exposure to a subset of NSAIDs (e.g. ibuprofen, naproxen).(5–7) However, studies have not examined NSAID exposure and risk for adverse pregnancy outcomes considering all forms of over-the-counter formulations in early pregnancy.
Women and their care providers currently lack clear empirical evidence to inform clinical care regarding the consequences of NSAID use during pregnancy. Prior evidence linking NSAIDs to SAB risk is inconclusive due potentially to recall bias, limited power, and/or incomplete documentation of NSAID exposure for all participants. This includes lack of information about over-the-counter NSAID exposure that should more accurately represent typical NSAID use during early pregnancy. We used data from the Right from the Start (RFTS) study (2004–2010), a non-clinical, community-based pregnancy cohort, to examine NSAID use during the early first-trimester of pregnancy as it relates to risk for SAB. This study tests whether over-the-counter NSAIDs used early in the first-trimester are associated with SAB risk.
MATERIALS AND METHODS
Study Population and Data Collection Protocol
RFTS is an ongoing community-based cohort that began enrolling in 2000. Over time, RFTS has been funded in three major phases (RFTS1, 2, 3), and has enrolled participants in Galveston, TX; Memphis, Nashville, Knoxville, and Chattanooga, TN; and the Research Triangle region (Raleigh, Durham, and Chapel Hill) in NC. RFTS1 participants were excluded from the analyses (n = 1,956) because over-the-counter NSAID use was not ascertained during the interview. RFTS participants are 18 years or older and did not use assisted reproductive technologies to conceive. Consent was obtained to review all records pertaining to the study pregnancy. Direct marketing and recruitment strategies have been described.(8)
Women who were not yet pregnant but trying to conceive could pre-enroll before pregnancy and were followed until a positive pregnancy test. To avoid over-enrollment of sub-fertile women, non-pregnant participants in the study had to be attempting to get pregnant for fewer than six months (RFTS2) or fewer than three months (RFTS3). Women were eligible for up to 12 months of pre-enrollment. Our RFTS study participant median gestational age at enrollment since LMP was 42 days [interquartile range (IQR) 35 to 52 days]. Thirty-seven percent of women were preenrolled in the study and enrolled upon confirmation of positive pregnancy test. Participants completed an intake interview at enrollment and a computer assisted telephone interview at the end of the first trimester. The computer assisted telephone interview was conducted at a median gestational age since LMP of 98 days [IQR 95 to 103 days], providing information on history of bleeding or pain, medication use, and exposure to potential confounders in the time since last menstrual period (LMP). Follow-up was conducted to document outcomes. The institutional review boards (IRB) of Vanderbilt University, Nashville, TN and the University of North Carolina, Chapel Hill, NC approved this study.
Pregnancy outcomes were self-reported and abstraction of medical records was used to verify outcomes. Live births were linked to state vital records to assist in verifying the pregnancy outcomes for ongoing pregnancies. SABs were defined as a loss before 20 completed weeks’ gestation. Those without losses included both live births and stillbirths, excluding ectopic pregnancies (n = 9) and induced abortions (n = 14). Gestational age was estimated from self-reported last menstrual period (LMP). We have previously shown that the overall accuracy self-reported LMP in our cohort.(9) Women could enroll in RFTS during more than one pregnancy, but only the first enrollment was included (n = 251 subsequent pregnancies excluded). RFTS also includes information on prescription medications, including NSAIDs, in the first-trimester interview regarding prescription medications taken for pain, bleeding, and other reasons. However, number of women reporting prescription NSAID use was too small to perform statistical analyses (n = 10). We, therefore, excluded prescription NSAIDs from the analysis.
NSAID Assessment and Other Variables
Participants were queried about all medications in the intake and first-trimester interviews (Table 1). Both interviews included NSAID exposures during the periconceptional period, (e.g. from LMP through 6 weeks gestation). The primary exposure was classified as any NSAID use versus no NSAID use) based on whether the participant reported NSAID use in either interview. NSAIDs were further grouped by drug class, generic name, and brand name. The primary resource used to classify drugs was the Food and Drug Administration drug classification database.(10) Other resources also utilized include: Micromedex 2.0, Lexi-Comp ONLINE, Epocrates Online Premium, and DailyMed.(11–14) Over-the-counter drugs not reported according to store brands and that could not be classified with the previously listed resources were identified with drugstore.com.(15) We did not include acetaminophen use in the NSAID definition, with the exception of drugs that included acetaminophen and an NSAID as the active ingredients.
Table 1.
Questionnaire Medication Exposure: Primary Index Questions
| Intake Interview Primary Index Questions* | First-Trimester Interview Primary Index Questions |
|---|---|
| Menstrual “preloading” medicines taken before missed LMP: When you were expecting your last period, the one you didn’t have because you were pregnant, around [insert date], did you “preload” to prevent cramping or pain? What did you take? Anything else? How many days did you take _______________? |
Menstrual “preloading” medicines taken before missed LMP: When you were expecting your last period, the one you didn’t have because you were pregnant, around [insert date], did you “preload” to prevent cramping or pain? What did you take? Anything else? How many days did you take _______________? |
| Prescribed or non-prescribed medicines taken between LMP and 6 weeks after LMP: Other than the medicine(s) you took to “preload” around [date]. we’re also interested in pain medicine you may have taken with or without a prescription for other reasons. Since medicines are so common for things like colds, injuries, the flu, fevers, headaches and other aches and pains, we’d like to know how often you used them. So, I’d like you to think about the time between your last period on [date] and [6 weeks after LMP]. Other than the “preloading” you just told me about, did you take any medicines like aspirin, Advil or ibuprofen, Tylenol or acetaminophen, Theraflu, Alka Seltzer[ACOG1], or BC powder for other reasons like aches, pains or colds? What did you take? Anything else? How many days during that time did you take _______________? |
Prescribed or non-prescribed medicines taken between LMP and 6 weeks after LMP: Other than the medicine(s) you took to “preload” around [date] we’re also interested in pain medicine you may have taken with or without a prescription for other reasons. Since medicines are so common for things like colds, injuries, the flu, fevers, headaches and other aches and pains, we’d like to know how often you used them. So, I’d like you to think about the time between your last period on [date] and [6 weeks after LMP]. Other than the “preloading” you just told me about, did you take any medicines like Aspirin, Advil or Ibuprofen, Tylenol or acetaminophen, Theraflu, Alka Seltzer, or BC powder for other reasons like aches, pains or colds? What did you take? Anything else? How many days during that time did you take __________? |
LMP, last menstrual period; BC, Bernard and Commodore Council Powder.
There are two versions of our first-trimester interview, one for questions formatted for women who are still pregnant, and another modified for women who have already had a loss.
Maternal characteristics and obstetric history were also recorded. These included: maternal age, height, weight, body mass index (BMI), race/ethnicity, diabetes status, parity, gravidity, induced abortion history, study site, and smoking status (current or not current smokers). Information on these characteristics was obtained from either the first-trimester interview or in person during the study ultrasound visits.
Statistical Analysis
Analyses were conducted with STATA statistical software version 11.0 (StataCorp LP, College Station, TX, USA). We used Cox proportional hazards survival models with variable gestational age at study entry to characterize the rate of pregnancy loss in relation to NSAID exposure (any versus none) both unadjusted and adjusted for confounders. Allowing for variability for study participant gestational age at study entry will correctly estimate the risk of SAB conditional on the fact that each subject had not had pregnancy loss before they were recruited into the cohort.(16) Longitudinal data from each woman starts at enrollment or after women report a positive pregnancy test if they pre-enrolled in the study and continues through 20 completed weeks’ gestation, the occurrence of a pregnancy loss, or loss to follow-up. We used a two-sided alpha=0.05 significance level for all tests of statistical significance.
Candidate confounders included maternal age (years), BMI (kg/m2), race/ethnicity (Caucasian [referent], African American, Hispanic ethnicity), income (≤ $40,000, $40,000–$80,000 [referent], > $80,000), diabetes status (no diabetes [referent] versus any [type 1, type 2, gestational, or multiple]), parity (none [referent] versus ≥ 1), gravidity (none [referent] versus ≥ 1), induced abortion history (none [referent], ≥ 1), study site (North Carolina [referent], Tennessee, Texas), and smoking status (not current [referent] versus current). Candidate confounders were analyzed for independent association with both NSAID exposure and SAB outcome. Those that were independently associated with NSAID exposure and SAB outcome and that resulted in a 5% relative change in NSAID effect size estimates were retained in the model. No candidate met inclusion criteria. However, analyses adjusted for maternal age are also presented because this covariate was commonly included in multivariable models in previous studies of NSAID use and SAB risk.(5–7) Using Cox regression, we estimated adjusted hazard ratios (HR) with 95 percent confidence intervals (CI) for risk of miscarriage with NSAID exposure. Sub-analyses were performed to assess whether the effect sizes varied by gestational age at time of SAB (gestation < 10 weeks vs. ≥ 10 weeks), and NSAID class. Additional secondary analyses were performed examining the relationship between total days of NSAID use and SAB risk using Cox regression. Total days of NSAID use was calculated based on the total number of days reported across all NSAIDs an individual reported taking. Since individuals were asked the same set of questions in the intake and first trimester interviews, we analyzed using the largest number of days reported across the two sets of interviews. In order to compare our findings with those from studies that have used different definitions for NSAID exposure we also performed Cox regression analyses excluding aspirin from the NSAID exposure definition.
Finally, we examined developmental stage at ultrasound among women who had a loss in order to assess whether ultrasound characteristics differed if they were exposed versus unexposed to NSAIDs. Ultrasound characteristics examined included: the presence of fetal pole with a normal heart rate, fetal pole with an abnormal or no heart rate or an abnormal heart rate, the presence of a gestational sac or the presence of a gestational sac and yolk sac, an empty uterus with a positive pregnancy test.
RESULTS
We included a total of 2,780 women who enrolled in the study between 2004 and 2010 (Table 2). The overall prevalence of early first-trimester NSAID use among women in RFTS was 43% (n = 1,185). In the total sample, 367 had an SAB (13%). Half of the losses occurred prior to the 10th week of pregnancy. Compared to non-users, NSAID users were more likely to be Caucasian, have had no previous pregnancies, and have had no history of SAB. In bivariate analyses, increased maternal age, gravidity ≥ 1, African American race, having an income greater than $80,000, and having a history of SAB were associated with an increased risk of SAB (p < 0.05)). The most common class of NSAIDs taken were propionic acids (n = 1,020, such as ibuprofen and naproxen) with similar use among NSAID users with (83%) and without (86%) a loss (Table 3). Women also reported taking salicylates (n = 293, primarily aspirin), acetic acids (n = 3, such as indomethacin), and enolic acid derivatives (n = 1, piroxicam).
Table 2.
Study Characteristics of Participants Exposed and Unexposed to Nonsteroidal Anti-Inflammatory Drugs
Salicyclates included products with aspirin as an active ingredient as well as those that include aspirin and other medications as well as diflunisal, disalcid, and trilisate; acetic acids included Indomethacin, diclofenac, alclofenac, fenoclofenac, tolmetin, etodolac, sulindac; proprionic acid derivatives included Ibuprofen, naproxen, ketoprofen, fenoprofen, flubiprofen, suprofen, oxaprozin; enolic acid derivatives included piroxicams, tenoxicams, isoxicams, and meloxicams. Participants could have reported more than one NSAID and therefore could be included in more than one NSAID class. As a result total percentages across classes do not sum to 100%. Percentages represent total N for NSAID class divided by total number of NSAIDs by status.
| Variable | NSAID Use (n =1,185) | No NSAID Use (n =1,595) |
|---|---|---|
| Maternal age (mean yrs ± SD) | 29.7±4.6 | 29.6±4.8 |
| Gravidity | ||
| 1 | 472 (40%) | 532 (33%) |
| More than 1 | 713 (60%) | 1,063 (67%) |
| Parity | ||
| 0 | 601 (53%) | 706 (46%) |
| 1 or more | 526 (47%) | 840 (54%) |
| Body mass index (mean ± SD) | 25.7±6.1 | 25.4±5.9 |
| Underweight (less than 20) | 90 (8%) | 150 (10%) |
| Normal weight (20 or higher to 26) | 672 (58%) | 913 (58%) |
| Overweight (higher than 26 to 29 or lower) | 153 (13%) | 198 (13%) |
| Obese (higher than 29 and above) | 254 (22%) | 304 (19%) |
| Maternal race or ethnicity | ||
| Caucasian | 945 (80%) | 1,240 (78%) |
| African American | 124 (11%) | 184 (12%) |
| Hispanic | 63 (5%) | 82 (5%) |
| Other | 52 (4%) | 86 (5%) |
| Household income | ||
| $40,000 or lower | 436 (22%) | 599 (23%) |
| $40,001–$80,000 | 244 (40%) | 348 (40%) |
| Higher than $80,000 | 419 (38%) | 556 (37%) |
| Marital status | ||
| Married or cohabitating | 1,109 (94%) | 1,499 (94%) |
| Other | 76 (6%) | 96 (6%) |
| History of preterm birth | ||
| 0 | 1,050 (93%) | 1,423 (92%) |
| 1 or more | 77 (7%) | 123 (8%) |
| History of spontaneous abortions | ||
| 0 | 895 (79%) | 1,163 (75%) |
| 1 or more | 232 (21%) | 383 (25%) |
| History of induced abortions | ||
| 0 | 980 (87%) | 1,368 (88%) |
| 1 or more | 147 (13%) | 178 (12%) |
| Smoking status | ||
| Nonsmokers | 1,093 (97%) | 1,509 (98%) |
| Current | 31 (3%) | 34 (2%) |
| Diabetes | ||
| None | 1,102 (98%) | 1,498 (97%) |
| Any | 22 (2%) | 45 (3%) |
| Study site | ||
| North Carolina | 806 (68%) | 1,174 (74%) |
| Tennessee | 373 (31%) | 415 (26%) |
| Texas | 6 (1%) | 6 (<1%) |
NSAID, nonsteroidal anti-inflammatory drugs; SD, standard deviation;
Data are mean ± standard deviation and frequency in counts (n[%]).
Table 3.
Summary of NSAID Class by Spontaneous Abortion Outcome for Risk of Spontaneous Abortion
| NSAID Class | n | Spontaneous Abortion (n = 157) | Without Loss (n = 1,028) |
|---|---|---|---|
| Salicylates | 293 | 49 (31%) | 244 (24%) |
| Acetic acid derivatives | 3 | 0 (0%) | 3 (<1%) |
| Propionic acid derivatives | 1,020 | 131 (83%) | 889 (86%) |
| Enolic acid derivatives (oxicams) | 1 | 0 (0%) | 1 (<1%) |
Data are n(%) unless otherwise specified.
The unadjusted and adjusted models did not show an association between NSAID exposure and SAB risk (unadjusted HR = 1.01, 95% CI 0.82, 1.24; adjusted [aHR] = 1.00, 95% CI 0.81, 1.23) (Table 4). Analyses stratified by early and late losses showed no association between NSAID use and risk for early or late losses. We also examined SAB risk excluding salicylates and did not observe an association (HR = 0.91, 95% CI 0.72, 1.15; aHR = 0.91, 95% CI 0.72, 1.14). Additional analyses of total days of NSAID use and SAB risk did not show evidence of an association (Table 4).
Table 4.
Cox Regression Analysis of First-Trimester Nonsteroidal Anti-inflammatory Drug Exposure Analyses for Spontaneous Abortion Outcome
Ultrasound findings grouping SABs into developmental stage prior to pregnancy loss are presented. Ultrasounds were conducted at a gestational age in which normal pregnancies would be expected to have a fetal pole and normal heart rate.
| First-Trimester NSAID Exposure | Unadjusted HR | 95% CI | Adjusted (aHR) | 95% CI |
|---|---|---|---|---|
| None | 1.00 | 1.00 | ||
| Any NSAID use | 1.01 | 0.82, 1.24 | 1.00 | 0.81, 1.23 |
| Any NSAID use (losses before 10 weeks) | 0.95 | 0.72, 1.25 | 0.95 | 0.72, 1.25 |
| Any NSAID use (losses 10 weeks or after) | 1.09 | 0.80, 1.48 | 1.09 | 0.80, 1.48 |
| Salicylates NSAID users (aspirin) | 1.33 | 0.97, 1.81 | 1.31 | 0.96, 1.79 |
| Excluding salicylates NSAID users (non-aspirin) | 0.91 | 0.72, 1.15 | 0.91 | 0.72, 1.14 |
| Total days of NSAID use† | 1.01 | 0.99, 1.04 | 1.01 | 0.99, 1.04 |
| 0 | 1.00 | 1.00 | ||
| 1–2 | 0.93 | 0.72, 1.21 | 0.91 | 0.70, 1.17 |
| 3–5 | 1.05 | 0.75, 1.46 | 1.05 | 0.75, 1.46 |
| 6–7 | 1.31 | 0.63, 2.69 | 1.32 | 0.65, 2.69 |
| More than 7 | 1.05 | 0.62, 1.80 | 1.11 | 0.66, 1.89 |
| Total days of NSAID use† (losses before 10 weeks) | 1.02 | 0.99, 1.05 | 1.02 | 0.99, 1.05 |
| 0 | 1.00 | 1.00 | ||
| 1–2 | 0.87 | 0.61, 1.23 | 0.84 | 0.59, 1.19 |
| 3–5 | 0.97 | 0.61, 1.53 | 0.98 | 0.62, 1.55 |
| 6–7 | 1.65 | 0.73, 3.73 | 1.64 | 0.74, 3.67 |
| More than 7 | 1.11 | 0.55, 2.27 | 1.19 | 0.59, 2.39 |
| Total days of NSAID use† (losses 10 weeks or after) | 0.99 | 0.96, 1.03 | 0.99 | 0.96, 1.03 |
| 0 | 1.00 | 1.00 | ||
| 1–2 | 1.01 | 0.69, 1.49 | 0.99 | 0.67, 1.46 |
| 3–5 | 1.14 | 0.70, 1.86 | 1.15 | 0.71, 1.87 |
| 6–7 | 0.76 | 0.19, 3.05 | 0.76 | 0.20, 3.03 |
| More than 7 | 0.99 | 0.44, 2.24 | 1.05 | 0.47, 2.37 |
HR, hazard ratio; CI, confidence interval; aHR, adjusted HR for maternal age; NSAID, nonsteroidal anti-inflammatory drugs.
Continuous refers to using days as a continuous outcome.
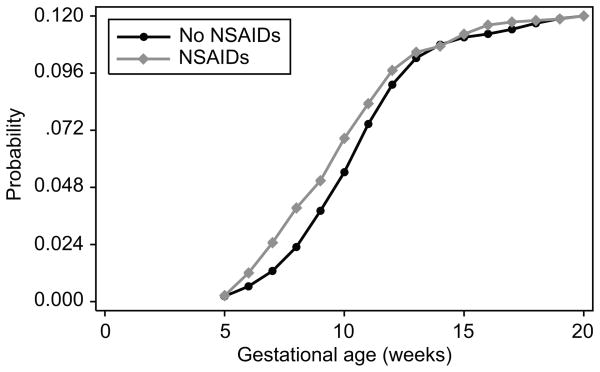
We further examined whether risk for SAB by NSAID use differed by whether the interview when NSAID data were collected was conducted prior to or after the loss occurred. All women who had interviews after their loss (n = 42) reported NSAID use. When information from ultrasound is incorporated to assess stage of pregnancy development prior to loss we observed no difference in stage of arrested development among losses across the spectrum from anembryonic gestation through normally developing early gestations (Table 5). A summary of the probability of loss by gestational age and NSAID exposure is in Figure 1.
Table 5.
Ultrasound Characteristics of Nonsteroidal Anti-inflammatory Drug-Exposed and Unexposed Women Who Had a Spontaneous Abortion
| Ultrasound Characteristic | NSAID Exposed | Unexposed | ||
|---|---|---|---|---|
|
| ||||
| Total n | % | Total n | % | |
|
| ||||
| No ultrasound | 37 | - | 40 | - |
| Fetal pole with normal heart rate | 39 | 33 | 58 | 34 |
| Fetal pole with abnormal or no heart rate | 35 | 29 | 34 | 20 |
| Gestational sac only or with yolk sac | 38 | 31 | 64 | 38 |
| Empty uterus | 8 | 7 | 14 | 4 |
NSAID, nonsteroidal anti-inflammatory drugs.
Figure 1. Cumulative probability of SAB when exposed to NSAIDs.
Figure 1 shows the probability of miscarriage by NSAID exposure. Gestational age in weeks is provided on the X-axis and the Y-axis indicates the cumulative probability of a SAB. Those unexposed to NSAIDs are labeled with a circle and those exposed to NSAIDs are labeled with a diamond.
DISCUSSION
Given the plausibility of NSAID effects on PG synthesis, embryo transport, implantation, placental development, maintenance of pregnancy, and prior evidence from epidemiologic data, we examined the relationship between periconceptional over-the-counter NSAID exposure and SAB risk in a non-clinical cohort. We found no evidence of an association between NSAID use and increased risk for SAB. Analyses stratified by NSAID class suggested that aspirin users (salicylates) were possibly at increased risk of SAB; however, this association was not statistically significant. In order to compare our results to those from other studies that examined only the effect of only non-aspirin NSAIDs, we also performed sub-analyses excluding aspirin users and did not observe and increased risk for SAB.(7) However, we had less power to detect an association with aspirin use in our cohort relative to prior studies of aspirin use and SAB risk. (7) Also, we note that a small subset of women (n = 42) were interviewed after their SAB reported. All 42 women reported taking an NSAID, which may indicate an element of recall bias but would be expected to bias results towards an finding an association.
The biological basis for suspecting a link between NSAIDs and risk of SAB rests on the multiple stages in development that involve prostaglandin (PG) synthesis, particularly during early pregnancy when PGs, such as PG E synthases (PGES), may be essential for establishing the implantation and early placentation, but may be protective later. NSAIDs inhibit COX enzymes, which catalyze the formation of PGs from arachidonic acid and thus reduce PG synthesis. PGs have important roles in both maintaining and achieving pregnancy. For example, COX-2 knockout mice (that lack COX-2) ovulate fewer oocytes have incomplete decidualization, have low fertilization rates, and fail implantation—all of which can be improved by administering PGs.(17) Altering PG levels has direct effects on conception, implantation, and maintenance of pregnancy in animal models.(18–28) Because NSAIDs inhibit PG synthesis they may influence embryo transport, implantation, placental development, and maintenance of pregnancy and have been shown to lead to embryonic demise in animal models.(29) This suggests that NSAID use during early pregnancy is a plausible biological candidate for causing a SAB.
Few studies have examined NSAID exposure and risk for SAB (Table 6). Two studies examined prescription NSAIDs only. These include a Danish study that observed an increased risk of SAB for women taking NSAIDs.(6) This study, using national healthcare and pharmacy records, considered the effect of prescription NSAID use from 30 days prior to conception through the end of pregnancy on risk of congenital anomalies, low birth weight, preterm birth, and SAB in primagravid women. One percent of the cohort had been prescribed NSAIDs. They observed a statistically significant association between NSAID use and SAB for NSAIDs taken one week and up to nine weeks before the loss.(6) However, in subsequent reexamination of results adjusting for gestational age (30) the association was no longer statistically significant (NSAIDs taken one week and up to nine weeks before SAB, OR 1.5–3.4, 95% CI 0.6–12.8). A second more recent study of prescription NSAIDs conducted using the Quebec Pregnancy Registry observed that non-aspirin NSAID use was associated with an increased risk of SAB with an OR = 2.4, (95% CI 2.1, 2.8) using NSAID prescription data available in the registry for which both indication and duration of use are likely to differ from over-the-counter. Three percent of their cohort reported exposure to non-aspirin prescription NSAIDs.(7)
Table 6.
Comparison of Association Studies of Nonsteroidal Anti-inflammatory Drug Use and Spontaneous Abortion Risk
| Study n (Country) | Median Gestational Age at Entry | Analysis Approach | NSAID | Spontaneous Abortion Definition | Effect Size | |
|---|---|---|---|---|---|---|
| Criteria | n (%) | |||||
|
Nielsen et al 2001 Nielsen et al 2004(6;30) n = 34,018 Spontaneous abortion: 4,268 Controls: 29,750 (Denmark) |
Not provided | Logistic regression (adjusted for gestational age) |
|
381 (1.12%) | Hospital discharge codes. Gestational age cutoff not indicated | Weeks before SAB NSAID taken: 1 week* OR = 3.35, 95% CI 0.88, 12.79 2 to 3 weeks OR = 1.50, 95% CI 0.58, 3.86 4 to 6 weeks OR = 1.50, 95% CI 0.91, 2.47 7 and 9 weeks OR = 1.59, 95% CI 0.93, 2.70 10 to 12 weeks OR = 0.58, 95% CI 0.18–1.85 |
|
Li et al 2003 (5) n = 1,033 SAB: 162 Controls: 871 (U.S.) |
40 days | Cox regression |
|
53 (5%) | Before 20 weeks of gestation |
Any NSAID use HR = 1.8, 95% CI 1.0, 3.2 NSAID use at conception HR = 5.6, 95% CI 2.3, 13.7 NSAID use after conception HR = 1.2, 95% CI 0.5, 2.6 |
|
Nakhai-Pour et al 2011 (7) n = 51,755 Spontaneous abortion: 4,705 Controls: 47,050 (Canada) |
Not provided | Logistic regression |
|
1,565 (3%) | Before 20 weeks of gestation |
Any NSAID use OR = 2.43, 95% CI 2.12, 2.79 |
|
Right from the Start n = 2,780 Spontaneous abortion: 376 Controls: 2,404 (U.S.) |
42 days | Cox regression |
|
1,595 (43%) | 20 weeks of gestation or before |
Any NSAID use aHR = 1.01, 95% CI 0. 82, 1.24 |
NSAID, nonsteroidal anti-inflammatory drug; OR, odds ratio; CI, confidence interval; LMP, last menstrual period.
The original dataset from Nielsen et al 2001 was reanalyzed by the authors in order to show the results of their study accounting for the length of time before spontaneous abortion when NSAIDs were used. Results presented are for the reanalysis in Nielsen et al 2004.
Only one prior study included non-prescription NSAIDs, and their analysis included adjustment for gestational age at entry. (5) This study used self-reported NSAID use, including prescription and over-the-counter NSAIDs (ibuprofen, naproxen, Advil, Motrin, and Naprosyn), with a cohort of patients from Kaiser Permanente HMO in northern California.(5) NSAID exposure included use between LMP and positive pregnancy test. The authors reported that NSAID use around the time of conception resulted in a 5.6 increase in hazard of SAB compared to no NSAID use (95% CI 2.3, 13.7). Five percent (n = 53) of the cohort reported using NSAIDs during this gestational period.
In our study we collected data on all over-the-counter pain medications including medications to prevent menstrual cramps in anticipation of menses, as well as medicine for headaches, aches, pains, and colds. We then identified all specific classes of NSAIDs in these medications. We used Cox regression and accounted for gestational age at enrollment to accurately model time at risk.(5) However, we observed much higher use of NSAIDs compared to what was reported by other studies (43%). Over-the-counter NSAIDs may be more likely to be taken at low and intermediate dosages compared to prescribed forms of NSAIDs, which may explain why the studies of prescription NSAIDs observed an increased risk for SAB while we observed a null effect. There may also be other indications for NSAID use among women who take prescribed NSAIDs (e.g., a chronic medical condition) that put them at increased risk of SAB.
We observed a much higher exposure rate of NSAIDs in our cohort than was previously observed 5% for the Li and colleagues study (2003), which also attempted to captured both prescription and non-prescription NSAID exposures.(5) This is likely due to differences in exposure assessment. We asked about any type of pain or cold medication and then determined whether it included NSAIDs while Li and colleagues (2003) limited NSAIDs to a specific subset. As a result, Li and colleagues (2003) may have missed some women exposed to NSAIDs. Another difference is the timing of the interviews across our studies. Li et al. (2003) interviewed participants immediately after pregnancy confirmation, capturing exposures between LMP and pregnancy confirmation. While in our study we obtained data from baseline screening interviews after pregnancy confirmation and from first-trimester interviews targeted at less than 13 weeks that specifically ask about use between LMP and six weeks. Finally, it may be the case that prescription NSAID use was responsible for the observed association with SAB in the previous studies. Because exposure to prescription NSAIDs was rare in our study population, we excluded the 10 women who were known to have been exposed to prescription NSAIDs.
We did not observe an association between NSAIDs and SAB risk; however, our findings have implications for women taking over-the-counter NSAID during pregnancy. In our cohort, assuming an alpha of 0.05 and a 13% prevalence of SABs we would have 81% power to detect an HR of 1.35, which is well below the risk estimate observed in prior studies. We show that typical over-the-counter NSAIDs taken by women during the first-trimester of pregnancy do not put them at increased risk of SAB. Due to limited numbers, we could not directly address the previous findings that focused on prescription-based NSAIDs, which are more likely to be at higher dosages and reflect a different risk due to NSAID exposure than what we observed in our study. It is also possible that women did not indicate if they took a prescribed NSAID or that they did not distinguish between prescribed and unprescribed forms. Our rate of miscarriage, 13%, is consistent with most epidemiological studies that are looking at SAB in the context of clinical recognized pregnancies. Further research is necessary to assess whether NSAID exposure prior to implantation or shortly after may cause an SAB prior to clinical recognition of pregnancy. Finally, further understanding the role of dose, timing in gestation, and the potential role of recall bias may help to reconcile this with previously observed associations between NSAIDs and SAB.
Acknowledgments
The field research was supported by grants from the National Institute of Child and Human Development (R01HD043883 and R01HD049675) and the American Water Works Association Research Foundation (2579). Additional funds were provided by the Building Interdisciplinary Research Careers in Women’s Health career development program (K12HD4383), supported in part by the Vanderbilt CTSA grant UL1 RR024975-01 from NCRR/NIH, and the Agency for Healthcare Research and Quality (K02HS017950). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. This research was also supported in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (i.e., support for coauthor Donna D. Baird).
Footnotes
Financial Disclosure: The authors did not report any potential conflicts of interest.
Reference List
- 1.Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. JAMA. 2002 Jan 16;287(3):337–44. doi: 10.1001/jama.287.3.337. [DOI] [PubMed] [Google Scholar]
- 2.Paulose-Ram R, Hirsch R, Dillon C, Losonczy K, Cooper M, Ostchega Y. Prescription and non-prescription analgesic use among the US adult population: results from the third National Health and Nutrition Examination Survey (NHANES III) Pharmacoepidemiol Drug Saf. 2003 Jun;12(4):315–26. doi: 10.1002/pds.755. [DOI] [PubMed] [Google Scholar]
- 3.Curhan GC, Bullock AJ, Hankinson SE, Willett WC, Speizer FE, Stampfer MJ. Frequency of use of acetaminophen, nonsteroidal anti-inflammatory drugs, and aspirin in US women. Pharmacoepidemiol Drug Saf. 2002 Dec;11(8):687–93. doi: 10.1002/pds.732. [DOI] [PubMed] [Google Scholar]
- 4.Olesen C, Steffensen FH, Nielsen GL, de Jong-van den Berg, Olsen J, Sorensen HT. Drug use in first pregnancy and lactation: a population-based survey among Danish women. The EUROMAP group. Eur J Clin Pharmacol. 1999 Apr;55(2):139–44. doi: 10.1007/s002280050608. [DOI] [PubMed] [Google Scholar]
- 5.Li DK, Liu L, Odouli R. Exposure to non-steroidal anti-inflammatory drugs during pregnancy and risk of miscarriage: population based cohort study. BMJ. 2003 Aug 16;327(7411):368. doi: 10.1136/bmj.327.7411.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nielsen GL, Sorensen HT, Larsen H, Pedersen L. Risk of adverse birth outcome and miscarriage in pregnant users of non-steroidal anti-inflammatory drugs: population based observational study and case-control study. BMJ. 2001 Feb 3;322(7281):266–70. doi: 10.1136/bmj.322.7281.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakhai-Pour HR, Broy P, Sheehy O, Berard A. Use of nonaspirin nonsteroidal anti-inflammatory drugs during pregnancy and the risk of spontaneous abortion. CMAJ. 2011 Sep 6; doi: 10.1503/cmaj.110454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Promislow JH, Makarushka CM, Gorman JR, Howards PP, Savitz DA, Hartmann KE. Recruitment for a community-based study of early pregnancy: the Right From The Start study. Paediatr Perinat Epidemiol. 2004 Mar;18(2):143–52. doi: 10.1111/j.1365-3016.2003.00546.x. [DOI] [PubMed] [Google Scholar]
- 9.Hoffman CS, Messer LC, Mendola P, Savitz DA, Herring AH, Hartmann KE. Comparison of gestational age at birth based on last menstrual period and ultrasound during the first trimester. Paediatr Perinat Epidemiol. 2008 Nov;22(6):587–96. doi: 10.1111/j.1365-3016.2008.00965.x. [DOI] [PubMed] [Google Scholar]
- 10.Drugs@FDA. Available from: URL: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm.
- 11.Micromedex 2.0. Available from: URL: http://www.thomsonhc.com/micromedex2/librarian.
- 12.LexiComp ONLINE. Available from: URL: http://online.lexi.com/crlsql/servlet/crlonline.
- 13.Epocrates Online Premium. Available from: URL: http://online.epocrates.com/noFrame.
- 14.DailyMed. Available from: URL: http://dailymed.nlm.nih.gov/
- 15.drugstore.com. Available from: URL: http://www.drugstore.com/
- 16.Dupont W. Statistical Modeling for Biomedical Researchers: A Simple Introduction to the Analysis of Complex Data. 2. Cambridge University Press; 2009. [Google Scholar]
- 17.Lim H, Paria BC, Das SK, Dinchuk JE, Langenbach R, Trzaskos JM, et al. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell. 1997 Oct 17;91(2):197–208. doi: 10.1016/s0092-8674(00)80402-x. [DOI] [PubMed] [Google Scholar]
- 18.Bergh PA, Navot D. The impact of embryonic development and endometrial maturity on the timing of implantation. Fertil Steril. 1992 Sep;58(3):537–42. doi: 10.1016/s0015-0282(16)55259-5. [DOI] [PubMed] [Google Scholar]
- 19.Duffy DM, Stouffer RL. The ovulatory gonadotrophin surge stimulates cyclooxygenase expression and prostaglandin production by the monkey follicle. Mol Hum Reprod. 2001 Aug;7(8):731–9. doi: 10.1093/molehr/7.8.731. [DOI] [PubMed] [Google Scholar]
- 20.Fazleabas AT, Kim JJ, Srinivasan S, Donnelly KM, Brudney A, Jaffe RC. Implantation in the baboon: endometrial responses. Semin Reprod Endocrinol. 1999;17(3):257–65. doi: 10.1055/s-2007-1016233. [DOI] [PubMed] [Google Scholar]
- 21.Frank GR, Brar AK, Cedars MI, Handwerger S. Prostaglandin E2 enhances human endometrial stromal cell differentiation. Endocrinology. 1994 Jan;134(1):258–63. doi: 10.1210/endo.134.1.7506205. [DOI] [PubMed] [Google Scholar]
- 22.Kelly RW, Carr GG, Elliott CL, Tulppala M, Critchley HO. Prostaglandin and cytokine release by trophoblastic villi. Hum Reprod. 1995 Dec;10(12):3289–92. doi: 10.1093/oxfordjournals.humrep.a135904. [DOI] [PubMed] [Google Scholar]
- 23.Kim JJ, Wang J, Bambra C, Das SK, Dey SK, Fazleabas AT. Expression of cyclooxygenase-1 and -2 in the baboon endometrium during the menstrual cycle and pregnancy. Endocrinology. 1999 Jun;140(6):2672–8. doi: 10.1210/endo.140.6.6716. [DOI] [PubMed] [Google Scholar]
- 24.Kistner EO, Weinberg CR. Method for using complete and incomplete trios to identify genes related to a quantitative trait. Genet Epidemiol. 2004 Jul;27(1):33–42. doi: 10.1002/gepi.20001. [DOI] [PubMed] [Google Scholar]
- 25.Norwitz ER, Schust DJ, Fisher SJ. Implantation and the survival of early pregnancy. N Engl J Med. 2001 Nov 8;345(19):1400–8. doi: 10.1056/NEJMra000763. [DOI] [PubMed] [Google Scholar]
- 26.Pall M, Friden BE, Brannstrom M. Induction of delayed follicular rupture in the human by the selective COX-2 inhibitor rofecoxib: a randomized double-blind study. Hum Reprod. 2001 Jul;16(7):1323–8. doi: 10.1093/humrep/16.7.1323. [DOI] [PubMed] [Google Scholar]
- 27.Sookvanichsilp N, Pulbutr P. Anti-implantation effects of indomethacin and celecoxib in rats. Contraception. 2002 May;65(5):373–8. doi: 10.1016/s0010-7824(01)00322-5. [DOI] [PubMed] [Google Scholar]
- 28.Wilcox AJ, Baird DD, Weinberg CR. Time of implantation of the conceptus and loss of pregnancy. N Engl J Med. 1999 Jun 10;340(23):1796–9. doi: 10.1056/NEJM199906103402304. [DOI] [PubMed] [Google Scholar]
- 29.Lala PK, Scodras JM, Graham CH, Lysiak JJ, Parhar RS. Activation of maternal killer cells in the pregnant uterus with chronic indomethacin therapy, IL-2 therapy, or a combination therapy is associated with embryonic demise. Cell Immunol. 1990 May;127(2):368–81. doi: 10.1016/0008-8749(90)90139-i. [DOI] [PubMed] [Google Scholar]
- 30.Nielsen GL, Skriver MV, Pedersen L, Sorensen HT. Danish group reanalyses miscarriage in NSAID users. BMJ. 2004 Jan 10;328(7431):109. doi: 10.1136/bmj.328.7431.109. [DOI] [PMC free article] [PubMed] [Google Scholar]