Abstract
Objective
Both the diagnosis and treatment of bipolar disorder in youth remain the subject of debate. In the Treatment of Early Age Mania (TEAM) study, risperidone was more effective than lithium or divalproex in children diagnosed with bipolar mania and highly comorbid with attention deficit/hyperactivity disorder (ADHD). We searched for treatment moderators and predictors of outcome.
Method
TEAM was a multi-site, 8-week, randomized clinical trial of risperidone, lithium, or divalproex in 279 medication-naïve patients, age 6–15 years, with a DSM-IV diagnosis of bipolar disorder currently in manic or mixed phase. Outcome measures included binary end-of-treatment responder status and change in the Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS) Mania Rating Scale (KMRS). Baseline demographics and clinical characteristics were tested as modifiers of treatment effect and as overall predictors of outcome.
Results
Moderator effects were detected for site, ADHD, and obesity. Across sites, the response ratio (RR) for risperidone vs. lithium ranged from 1.2 (95% CI 0.8, 1.7) to 8.3 (1.1, 60.8), and for risperidone vs. divalproex from 1.3 (0.8, 2.2) to 10.5 (1.4, 77.7). The RR for risperidone vs. lithium was 2.1 for patients with ADHD, but 1.0 for those without ADHD, and 2.3 (1.6, 3.3) for non-obese patients, but 1.1 (0.6, 2.0) for obese ones. Older age and less severe ADHD symptoms were associated with greater improvement on the KMRS.
Conclusions
Risperidone was more effective than lithium or divalproex across the demographics and clinical characteristics of the sample, but the magnitude of its effect was influenced by site-related characteristics and presence of ADHD.
Keywords: children, bipolar, treatment, predictors, moderators
Introduction
While the validity of the diagnosis of bipolar disorder in children remains the subject of debate and research, clinicians and families are faced with treatment decisions as to how to control the clinical manifestations of extreme mood dysregulation in youth. Monotherapy with an atypical antipsychotic, such as risperidone, or a mood stabilizer, such as lithium and divalproex, is the currently recommended first-line treatment.1 However, response to these agents is variable. Only about half of the youths receiving an antipsychotic show clinically significant improvement, and the response rate to mood stabilizers is even lower.2,3 Identification of pre-treatment variables associated with outcome across treatment conditions (i.e., predictors), or with better response to a particular medication (i.e., moderators) would help make more informed treatment decisions.4
A few studies have examined predictors and moderators in the treatment if mania. In adults, early onset of bipolar illness, history of suicide attempt, rapid cycling, anxiety, and alcohol use were associated with poorer outcome.5 In youths, early onset, severity of mania, longer duration of illness, family history of mood disorders, low socio-economic status, comorbid attention-deficit/hyperactivity disorder (ADHD), anxiety, and disruptive behavior disorders predicted poorer outcome .6–8 Psychotic symptoms predicted poorer response to divalproex, but not lithium.8 Younger age was associated with lower effectiveness of lithium,8 but did not seem to influence response to divalproex.9
The Treatment of Early Age Mania (TEAM) study was an 8-week randomized clinical trial comparing the acute effects of lithium, divalproex, and risperidone in children and adolescents diagnosed with bipolar manic or mixed state. Response rate was greater on risperidone than on lithium or divalproex.10 The superiority of risperidone was evident in both younger (6–12 years) and older (13–15 years) patients, and not influenced by concurrent stimulant treatment for ADHD or presence of psychotic symptoms. These results are consistent with the data in adults, which show that antipsychotics are more effective than mood stabilizers in the acute management of mania.11
We here report on a systematic analysis of the TEAM study database searching for possible moderators and predictors. Available demographic and clinical characteristics of the TEAM participants were evaluated for their association with outcome at the end of the 8 weeks of treatment. While these analyses were exploratory and hypothesis-generating, it was hypothesized that greater severity of mania and presence of comorbid conditions, especially conduct problems and significant suicidal ideation, would predict poorer outcome. Because later onset of mania may indicate an illness more consistent with the adult disorder, for which lithium and divalproex are effective, we expected a better response to these medications in later onset than in earlier onset mania. Given the effectiveness of antipsychotics in controlling impulsive aggression, we hypothesized that patients with conduct disorder would be more responsive to risperidone than lithium or divalproex. Other hypotheses were that patients with significant suicidal ideation would respond better to lithium, and that high level of maternal warmth would predict better outcome.12
Method
The TEAM Study Database
The design, sample, and primary results of TEAM have been reported.10 Briefly, this was an 8-week, multisite, randomized, clinical trial of lithium, divalproex, and risperidone in 6–15 year-old outpatients with a DSM-IV diagnosis of bipolar I manic or mixed phase, according to the Washington University Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS). Patients were recruited and treated at five academic sites: the Children’s National Medical Center in Washington, DC (CNMC); Johns Hopkins University Medical Institutions in Baltimore, MD (JHMI); University of Pittsburgh in Pittsburgh, PA (PITT); University of Texas, at Galveston and Dallas (UTMB/UTSW); and Washington University in St. Louis, MO (WASHU). The study was centrally organized and monitored by a coordinating site at WASHU. Prior to starting data collection, raters were trained in the study procedures and assessments, and certified for diagnostic and rating reliability if they had at least 90% agreement with the gold standard. Throughout the study, assessments were videotaped and audited by the coordinating center to ensure reliability and consistency.
While TEAM included three strata to account for previous exposure to antimanic agents, these analyses, in keeping with the primary report, were conducted on the main stratum (one), including only treatment naïve patients. Youths with IQ≤70, schizophrenia, pervasive developmental disorder, at imminent suicide risk, major medical or neurological disease, history of substance dependence, recent alcohol or substance abuse, pregnancy, or at risk for pregnancy were excluded. The sample (N=279) was 49.8% male and 72.8% white; had a mean age of 10.1 (SD 2.8) years, and frequently presented with psychosis (77.1%) and mixed mania (97.5%). There was a high rate of comorbidity with ADHD (92.8%), oppositional defiant disorder (ODD) (90.0%), conduct disorder (15.8%), and anxiety disorders (71.3%). Functioning was severely impaired (Children-Global Assessment Scale mean 39.1, SD 6.2). Youths on stable doses of stimulants for ADHD could participate, but no other psychotropic medication, including antidepressants or atomoxetine, was allowed. Treatment was not masked to treating clinicians or participants, but study outcomes were assessed by raters blind to treatment assignment. Medications were titrated based on clinical response, aiming for the therapeutic range of lithium and divalproex plasma levels. Final level was 1.09 (SD 0.34) mEq/L for lithium, and 113.6 (SD 23.0) for divalproex. Final daily dose of risperidone was 2.57 (SD 1.21) mg.
The primary outcome measure was the end of treatment Clinical Global Impressions for Bipolar Illness-Improvement Mania scale (CGI-BP-IM) score .13 A score of 1 (very much improved) or 2 (much improved) designated clinical response. The secondary outcome measure was the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children Mania Rating Scale (KMRS), a 15-item scale, which provided a continuous measure of manic symptoms.14
The premature discontinuation rate was 24.7% (15.7% on risperidone, 32.2% on lithium, and 26.0% on divalproex).10 Overall, 41.9% (n=117) of the randomized patients met response criteria. More patients responded on risperidone (68.5%) than on lithium (35.6%) or divalproex (24.0%), with no statistically significant difference between these last two.
Variables tested for possible predictor and moderator effects
Predictor was defined as a pre-randomization variable associated with response status regardless of treatment assignment, while moderator was defined as a pre-randomization variable influencing the response to a particular treatment as compared with another treatment.4
The demographic and clinical characteristics that were examined are listed in Table 1. Socio-economic status was rated on the Hollingshead Four Point Index. Prepubertal status was defined as a Tanner stage I or II on the Duke Questionnaire for Puberty Status.15 Family history of bipolar disorder was defined as parental report of child’s first-degree relative with bipolar disorder, using the Family History-Research Diagnostic Criteria.16 Severity of mania was scored on the Clinical Global Impressions for Bipolar Illness-Severity Mania scale (score of 5: markedly ill; 6: severely ill; and 7: extremely ill).13 Psychosis was considered to be present if there were hallucinations and/or manic grandiosity was deemed to be delusional by clinical raters and confirmed by the coordinating center upon review of the videotaped interviews. The severity of ADHD and ODD was measured by adding the endorsed symptom items for these disorders on the WASH-U-KSADS. Suicidality was defined by a score on the WASH-U-KSADS suicidality item of 3 or greater. Maternal warmth was scored on the item 9 of the mother-child relationship section of the Psychosocial Schedule for School-age Children-Revised, an interview-based rating instrument, with a score of 1 denoting “high warmth”. 17–18 As more than 97% of the sample was classified as having “mixed state mania” and “rapid cycling”, these baseline characteristics could not be examined as potential predictors or moderators.
Table 1.
Baseline Characteristics Assessed for Possible Predicting or Moderating Effects
| Lithium (n=90) | Risperidone (n=89) | Divalproex (n=100) | All (n=279) | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age (yr) | 9.7 | 2.8 | 11.0 | 3.0 | 9.7 | 2.4 | 10.1 | 2.8 |
| Age at mania onset (yr) | 5.0 | 2.7 | 5.7 | 2.8 | 5.0 | 2.1 | 5.2 | 2.6 |
| Severity of ADHD | 15.9 | 3.0 | 15.9 | 3.0 | 16.2 | 2.3 | 16.0 | 2.8 |
| Severity of ODD | 6.2 | 1.6 | 5.9 | 1.8 | 5.9 | 1.6 | 6.0 | 1.7 |
|
| ||||||||
| % | n | % | n | % | n | % | n | |
|
| ||||||||
| Female | 41.1 | 37 | 52.8 | 47 | 56.0 | 56 | 50.2 | 140 |
| Race/Ethnicity: | ||||||||
| White | 73.3 | 66 | 67.4 | 60 | 77.0 | 77 | 72.8 | 203 |
| African American | 18.9 | 17 | 23.6 | 21 | 16.0 | 16 | 19.3 | 54 |
| Hispanic | 3.3 | 3 | 2.2 | 2 | 1.0 | 1 | 2.2 | 6 |
| Other | 4.5 | 4 | 6.7 | 6 | 6.0 | 6 | 5.7 | 16 |
| Socio-Economic Status (SES)a | ||||||||
| 4 or 5 | 63.3 | 57 | 57.3 | 51 | 66.0 | 66 | 62.4 | 174 |
| 3 and below | 36.7 | 33 | 42.7 | 38 | 34.0 | 34 | 37.6 | 105 |
| Prepubertal Statusb | 64.4 | 58 | 56.2 | 50 | 69.0 | 69 | 63.4 | 177 |
| Referral source: | ||||||||
| Clinic | 38.9 | 35 | 41.6 | 37 | 37.0 | 37 | 39.1 | 109 |
| Advertisement | 52.2 | 47 | 47.2 | 42 | 51.0 | 51 | 50.2 | 140 |
| Other | 8.9 | 8 | 11.2 | 10 | 12.0 | 12 | 10.8 | 30 |
| Family history of Bipolar disorderc | 41.4 | 34 | 37.0 | 30 | 42.1 | 40 | 40.3 | 104 |
| Severity of mania: | ||||||||
| Score ≤5 | 14.4 | 13 | 18.0 | 16 | 23.0 | 23 | 18.6 | 52 |
| Score =6 | 64.4 | 58 | 65.2 | 58 | 59.0 | 59 | 62.7 | 175 |
| Score=7 | 21.1 | 19 | 16.9 | 15 | 18.0 | 18 | 18.6 | 52 |
| Psychosis | 71.1 | 64 | 79.8 | 71 | 80.0 | 80 | 77.1 | 215 |
| Number of comorbidities | ||||||||
| ≤1 | 18.9 | 17 | 20.2 | 18 | 24.0 | 24 | 21.2 | 59 |
| >1 | 81.1 | 73 | 79.8 | 71 | 76.0 | 76 | 78.9 | 220 |
| ADHD | 91.1 | 82 | 91.0 | 81 | 96.0 | 96 | 92.8 | 259 |
| ODD | 94.4 | 85 | 86.5 | 77 | 89.0 | 89 | 90.0 | 251 |
| Conduct disorder | 16.7 | 15 | 19.1 | 17 | 12.0 | 12 | 15.8 | 44 |
| On stimulant medication | 34.4 | 31 | 30.3 | 27 | 32.0 | 32 | 32.3 | 90 |
| History of major depression | 66.7 | 60 | 75.3 | 67 | 78.0 | 78 | 73.5 | 205 |
| Suicidality | 47.8 | 43 | 63.0 | 56 | 57.0 | 57 | 55.9 | 156 |
| Obese (BMI >95th percentile) | 18.9 | 17 | 13.5 | 12 | 17.0 | 17 | 16.5 | 46 |
| High maternal warmth | 32.2 | 29 | 40.5 | 36 | 36.0 | 36 | 36.2 | 101 |
Note: ADHD = attention-deficit/hyperactivity disorder; BMI = body mass index; ODD = oppositional defiant disorder; yr = years.
Social status scored on a scale of 1 to 5, with 5 being the highest
Pre-pubertal status was defined as a Tanner stage of I or II
Family history data were available for a total of 258 patients (82 in the lithium group, 81 in the risperidone group, and 95 in the divalproex group).
Data Analyses
Outcome Measures
Two outcome variables were examined. Consistent with the primary analyses of TEAM,10 the primary outcome was the responder status at the end of the study period, defined as a CGI-BP-IM score of 1 or 2 at week 8. Patients who prematurely discontinued study treatment and missed the final assessment were considered to be non-responders.10, 19 In sensitivity analyses, we imputed the responses for discontinued patients using the “last observation carried forward” approach.
The secondary outcome measure was the KMRS total score. As described in the primary TEAM analyses,10 the baseline and final KMRS assessments were conducted by evaluators blinded to the subjects’ treatment assignment. In addition, the KMRS was scored by the unblinded treating clinicians weekly, from week 1 through 7. We analyzed separately the baseline vs. final KMRS scores and the weekly scores. In accordance with the primary publication, for the patients who prematurely discontinued study treatment, the final score was imputed as the last available KMRS score prior to dropping out.
Analytic Approach
For the primary outcome, we calculated the probability of response (as defined by a CGI-BP-IM score of 1 or 2 at week 8) by medication status and defined the ratio of the probabilities (i.e. risk ratio, here called response ratio, or RR) as the primary effect measure using pair-wise comparison of medications. Next, in our bi-variate analyses we assessed whether the pair-wise RRs varied across levels of the covariates (i.e. effect modification analysis). The potential deviation from homogeneity of RRs across levels of the potential moderator was assessed using χ2.
Due to small sample size when stratifying on a potential moderator and the medication arm, we could evaluate effect modification for each variable separately, but not in combination with other variables. If a variable was not found to be a significant effect modifier, it was evaluated as a predictor of outcome using Poisson regression with robust standard error to estimate the RR and its 95% confidence interval (CI).20 In the regression models, we adjusted for medication and included clinical site as either moderator or predictor depending on the results of the bi-variate analyses.
For the secondary outcome measure, KMRS, we conducted 2 analyses. First, we focused on the baseline and week 8 assessments by independent evaluators. Improvement was assessed as the difference between KMRS scores at week 8 vs. baseline for each study participant. Using the improvement as the outcome in the linear regression and including an interaction term between the covariate and medication arm, we assessed each covariate as a potential effect modifier. In the absence of significant effect modification, the covariates were evaluated as predictors with a model that contained medication, baseline KMRS score, and site as either effect modifier or predictor.
Finally, we used a linear mixed effects model with random intercept to assess the average trajectory of KMRS between weeks 1 and 7 while accounting for clustering of observations within patients. The week variable was centered at 4 weeks. The random intercept was assumed to be normally distributed with a variance representing the variability of patient scores at week 4. In these models, we evaluated the potential effect modification by each covariate.
To help evaluate the impact of drop-out rates on outcome, we performed sensitivity analyses by assigning all drop-outs to responder, and then all non-responder status to assess the change in the response ratio estimates, or by dropping the sites with highest drop-outs.
Because these were exploratory, rather than hypothesis-testing, analyses, the alpha level was not controlled for multiple comparisons. While a p≤0.05 was considered of interest, attention was paid to the magnitude of effect sizes rather than to a formally defined statistical significance. All analyses were conducted in STATA 12 statistical software (StataCorp. 2011. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP.).
Results
Moderators and Predictors
Table 2 reports the interaction and main effects for the tested baseline variables with the outcome defined as binary responder status at end of treatment. The rate of response was strongly influenced by site. The superiority of risperidone over lithium was evident at UTMB/UTSW (RR=8.3, 95% CI 1.1, 60.8, p=0.037), JHMI (RR=4.2, 95% CI 1.1, 16.2, p=0.039), and WASHU (RR=2.6, 95% CI 1.1, 6.0, p=0.022), with a similar pattern emerging in the risperidone vs. divalproex comparison. At PITT and CNMC, the trend was also in favor of risperidone but with smaller and not statistically significant RRs (ranging from 1.3 to 1.5). No site interaction was detected for the comparison lithium vs. divalproex.
Table 2.
Testing for Moderators of Treatment Effects and Predictors of Outcomea
| Sites: | n | Interaction Effects | PREDICTOR EFFECTSa | ||||||
|---|---|---|---|---|---|---|---|---|---|
| RR (95%CI) Risperidone vs. Lithium | p | RR (95%CI) Risperidone vs. Divalproex | P | RR (95%CI) Lithium vs. Divalproex | p | RR (95%CI) | p | ||
| PITT | 39 | 1.2 (0.8, 1.7) | 0.369 | 1.3 (0.8, 2.2) | 0.307 | 1.1 (0.6, 1.9) | 0.736 | ||
| CNMC | 68 | 1.4 (0.9, 2.1) | 0.085 | 1.5 (1.0, 2.3) | 0.049 | 1.1 (0.6, 1.8) | 0.762 | ||
| WASHU. | 63 | 2.6 (1.1, 6.0) | 0.022 | 9.6 (2.4, 38.2) | 0.001 | 3.7 (0.8, 17.1) | 0.096 | ||
| UTMB/UTSW | 59 | 8.3 (1.1, 60.8) | 0.037 | 10.5 (1.4, 77.7) | 0.021 | 1.3 (0.1, 19.0) | 0.866 | ||
| JHMI | 50 | 4.2 (1.1, 16.2) | 0.039 | 3.1 (1.0, 9.5) | 0.043 | 0.8 (0.1, 3.9) | 0.739 | ||
| Interaction: | χ2= 8.5 | 0.076 | χ2 = 11.8 | 0.019 | χ2 = 2.5 | 0.638 | |||
| Age | |||||||||
| 6 to 12 yr | 218 | 1.8 (1.3, 2.7) | 0.001 | 2.8 (1.8, 4.3) | <0.0001 | 1.5 (0.9, 2.5) | 0.093 | 1.0 | |
| 13 yr or greater | 61 | 2.0 (1.0, 3.9) | 0.041 | 2.4 (1.1, 5.7) | 0.038 | 1.2 (0.4, 3.4) | 0.708 | 1.1 (0.9, 1.4) | 0.316 |
| Age (continuous) | Interaction | χ2 = 1.0 | 0.328 | χ2= 0.04 | 0.847 | χ2 = 0.2 | 0.687 | 1.0 (0.99, 1.07) | 0.139 |
| Age-onset | |||||||||
| Less than 8 yr | 237 | 2.2 (1.5, 3.12) | <0.0001 | 2.8 (1.9, 4.2) | <0.0001 | 1.3 (0.8, 2.1) | 0.283 | 1.0 | |
| 8 yr or above | 42 | 1.2 (0.7, 2.2) | 0.521 | 3.2 (0.9, 11.8) | 0.075 | 2.7 (0.7, 10.1) | 0.148 | 1.1 (0.8, 1.4) | 0.693 |
| Age-onset (continuous) | Interaction | χ2 = 3.2 | 0.076 | χ2 = 0.2 | 0.651 | χ2= 1.8 | 0.181 | 1.0 (1.0, 1.1) | 0.222 |
| Severity of ADHD | Interaction | χ2 =4.1 | 0.044 | χ2 =0.9 | 0.344 | χ2 =0.1 | 0.794 | 0.97 (0.95,1.00) | |
| ADHD: | |||||||||
| no | 20 | 1.0 (0.6,1.8) | 1.000 | 1.0 | |||||
| yes | 259 | 2.1 (1.5, 3.0) | <0.0001 | b | b | 0.8 (0.6, 1.2) | |||
| Interaction | χ2 = 5.0 | 0.025 | |||||||
| Severity of ODD | Interaction | χ2= 2.3 | 0.131 | χ2= 1.2 | 0.278 | χ2= 3.4 | 0.064 | 1.01 (0.95, 1.07) | 0.822 |
| ODD: | |||||||||
| no | 28 | 1.3 (0.6, 2.7) | 0.579 | 4.1 (1.1, 15.1) | 0.032 | 3.3 (0.8, 14.0) | 0.106 | 1.9 | |
| yes | 251 | 2.0 (1.4, 2.8) | <0.0001 | 2.7 (1.8, 4.1) | <0.0001 | 1.4 (0.9, 2.2) | 0.178 | 1.1 (0.8, 1.5) | 0.594 |
| χ2=1.1 | 0.293 | χ2=0.4 | 0.552 | χ2=1.3 | 0.261 | ||||
| Sex: | |||||||||
| males | 139 | 2.3 (1.5, 3.5) | <0.0001 | 3.0 (1.7, 5.1) | <0.0001 | 1.3 (0.7, 2.4) | 0.449 | −1.0 | |
| females | 140 | 1.6 (1.0, 2.5) | 0.046 | 2.7 (1.6, 4.6) | <0.0001 | 1.7 (0.9, 3.2) | 0.076 | 1.1 (0.9, 1.4) | 0.349 |
| Interaction: | χ2= 1.4 | 0.231 | χ2= 0.03 | 0.853 | χ2 = 0.5 | 0.499 | |||
| Race: | |||||||||
| White | 203 | 1.8 (1.2, 2.7) | 0.002 | 3.0 (1.9, 4.9) | <0.0001 | 1.7 (1.0, 2.9) | 0.064 | 1.1 (0.8, 1.4) | 0.629 |
| Other | 76 | 2.1 (1.2, 3.7) | 0.008 | 2.3 (1.3, 4.1) | 0.006 | 1.1 (0.5, 2.3) | 0.847 | −1.0 | |
| Interaction: | χ2 = 0.2 | 0.657 | chi-2 = 0.6 | 0.454 | chi-2 = 0.9 | 0.357 | |||
| Social Status: | |||||||||
| 4 or 5 | 174 | 2.0 (1.3, 2.9) | <0.0001 | 2.8 (1.8, 4.4) | <0.0001 | 1.4 (0.8, 2.4) | 0.188 | 1.0 | |
| 3 and below | 105 | 1.9 (1.1, 3.3) | 0.021 | 3.1 (1.5, 6.2) | 0.002 | 1.6 (0.7, 3.7) | 0.249 | 0.9 (0.7, 1.1) | 0.28 |
| Interaction: | χ2 = 0.01 | 0.909 | χ2= 0.04 | 0.841 | χ2= 0.1 | 0.804 | |||
| Puberty status: | |||||||||
| Pubertal | 102 | 1.8 (1.1, 2.8) | 0.016 | 3.2 (1.6, 6.3) | 0.001 | 1.8 (0.8, 3.9) | 0.138 | −1.0 | |
| Pre-pubertal | 177 | 2.0 (1.3, 3.1) | 0.001 | 2.7 (1.7, 4.2) | <0.0001 | 1.3 (0.8, 2.3) | 0.314 | 0.9 (0.7, 1.1) | 0.197 |
| Interaction: | χ2= 0.2 | 0.681 | χ2= 0.2 | 0.683 | χ2= 0.4 | 0.534 | |||
| Family history of bipolar disorder | |||||||||
| no | 154 | 1.7 (1.2, 2.4) | 0.006 | 3.1 (1.9, 5.1) | <0.0001 | 1.9 (1.0, 3.3) | 0.036 | 1.0 | |
| yes | 104 | 1.9 (1.1, 3.3) | 0.033 | 2.3 (1.3, 4.3) | 0.006 | 1.3 (0.6, 2.6) | 0.529 | 1.0 (0.9, 1.2) | 0.608 |
| Interaction: | χ2 = 0.1 | 0.745 | χ2= 0.4 | 0.544 | χ2= 0.6 | 0.444 | |||
| Severity of Mania | |||||||||
| 5 | 52 | 1.2 (0.6, 2.2) | 0.644 | 2.4 (1.1, 5.3) | 0.030 | 2.1 (0.9, 4.8) | 0.096 | −1.0 | |
| 6 | 175 | 2.4 (1.6, 3.7) | <0.0001 | 3.0 (1.9, 4.7) | <0.0001 | 1.2 (0.7, 2.2) | 0.502 | 0.9 (0.6, 1.1) | 0.251 |
| 7 | 52 | 1.3 (0.6, 2.8) | 0.563 | 2.8 (0.9, 9.0) | 0.084 | 2.2 (0.7, 7.3) | 0.192 | 0.8 (0.5, 1.2) | 0.297 |
| Interaction: | χ2= 4.7 | 0.095 | χ2= 0.2 | 0.895 | χ2 = 1.4 | 0.493 | |||
| Psychosis: | |||||||||
| no | 64 | 1.6 (0.9, 2.7) | 0.109 | 3.3 (1.3, 8.5) | 0.012 | 2.1 (0.8, 5.7) | 0.137 | −1.0 | |
| yes | 215 | 2.1 (1.4, 3.1) | <0.0001 | 2.8 (1.8, 4.2) | <0.0001 | 1.3 (0.8, 2.2) | 0.303 | 1.1 (0.8, 1.4) | 0.546 |
| Interaction: | χ2= 0.7 | 0.403 | χ2= 0.1 | 0.718 | χ2 = 0.7 | 0.401 | |||
| Number of comorbidities: | |||||||||
| ≤ 1 | 59 | 1.5 (0.9, 2.7) | 0.148 | 2.2 (1.1, 4.1) | 0.017 | 1.4 (0.7, 3.0) | 0.373 | −1.0 | |
| >1 | 220 | 2.1 (1.4, 3.0) | <0.0001 | 3.2 (2.0, 5.1) | <0.0001 | 1.6 (0.9, 2.7) | 0.110 | 0.9 (0.7, 1.1) | 0.266 |
| Interaction: | χ2 = 0.7 | 0.404 | χ2 = 1.0 | 0.327 | χ2 = 0.04 | 0.833 | |||
| Conduct Disorder (CD): | |||||||||
| no | 235 | 2.0 (1.4, 2.7) | <0.0001 | 2.8 (1.9, 4.1) | <0.0001 | 1.4 (0.9, 2.3) | 0.127 | −1.0 | |
| yes | 44 | 1.8 (0.7, 4.7) | 0.256 | 5.6 (0.8, 39.6) | 0.081 | 3.2 (0.4, 25.1) | 0.268 | 0.7 (0.4, 1.1) | 0.096 |
| Interaction: | χ2 = 0.04 | 0.833 | χ2 = 0.5 | 0.492 | χ2 = 0.6 | 0.454 | |||
| On stimulant medication: | |||||||||
| no | 189 | 1.8 (1.2, 2.6) | 0.002 | 2.6 (1.7, 4.0) | <0.0001 | 1.5 (0.9, 2.5) | 0.137 | −1.0 | |
| yes | 90 | 2.3 (1.2, 4.2) | 0.008 | 3.6 (1.6, 7.7) | 0.001 | 1.5 (0.6, 3.8) | 0.346 | 0.9 (0.7, 1.2) | 0.520 |
| Interaction: | χ2 = 0.5 | 0.482 | χ2= 0.5 | 0.498 | χ2 = 0.01 | 0.925 | |||
| History of Major Depression | |||||||||
| no | 74 | 2.2 (1.2, 3.9) | 0.007 | 4.0 (1.6, 10.1) | 0.003 | 1.8 (0.7, 5.1) | 0.245 | −1.0 | |
| yes | 205 | 1.8 (1.3, 2.7) | 0.001 | 2.6 (1.7, 4.0) | <0.0001 | 1.4 (0.9, 2.4) | 0.165 | 1.0 (0.8, 1.3) | 0.894 |
| Interaction: | χ2= 0.3 | 0.614 | χ2= 0.7 | 0.413 | χ2= 0.2 | 0.669 | |||
| Suicidality: | |||||||||
| not suicidal | 123 | 1.8 (1.2, 2.7) | 0.005 | 2.8 (1.6, 4.9) | <0.0001 | 1.6 (0.9, 2.9) | 0.147 | −1.0 | |
| suicidal | 156 | 2.2 (1.3, 3.6) | 0.002 | 2.9 (1.7, 4.8) | <0.0001 | 1.3 (0.7, 2.7) | 0.403 | 0.9 (0.7, 1.2) | 0.501 |
| Interaction: | χ2= 0.4 | 0.550 | χ2= 0.00 | 0.961 | χ2= 0.2 | 0.703 | |||
| Obesity (BMI>95th perc.): | |||||||||
| no | 233 | 2.3 (1.6, 3.3) | <0.0001 | 2.9 (1.9, 4.3) | <0.0001 | 1.3 (0.7, 2.1) | 0.398 | −1.0 | |
| yes | 46 | 1.1 (0.6, 2.0) | 0.664 | 2.8 (1.1, 7.3) | 0.031 | 2.5 (1.00, 6.4) | 0.058 | 1.2 (0.9, 1.6) | 0.169 |
| Interaction: | χ2 = 4.1 | 0.044 | χ2 = 0.00 | 0.988 | χ2 = 1.6 | 0.208 | |||
| High maternal warmth: | |||||||||
| no | 177 | 2.1 (1.3, 3.3) | 0.002 | 3.1 (1.8, 5.4) | <0.0001 | 1.5 (0.8, 2.9) | 0.233 | −1.0 | |
| yes | 101 | 1.6 (1.1, 2.4) | 0.014 | 2.5 (1.5, 4.1) | <0.0001 | 1.6(0.9, 2.8) | 0.139 | 1.2 (0.9, 1.5) | 0.297 |
| Interaction: | χ2= 0.7 | 0.415 | χ2 = 0.3 | 0.584 | χ2 = 0.01 | 0.923 | |||
Note: Binary outcome: responder vs. non-responder status at end of treatment based on the on the Clinical Global Impressions for Bipolar Illness-Improvement Mania scale (CGI-BP-IM) at week 8. Consistent with the primary analysis of the clinical trial, patients who dropped out were considered non-responders. The response ratio (RR) is the ratio between the response rate in one treatment group vs. another, in pair-wise comparison. ADHD = attention-deficit/hyperactivity disorder; BMI = body mass index; CI = confidence interval; CNMC = Children’s National Medical Center; JHMI = Johns Hopkins Medical Institutions; ODD = oppositional defiant disorder; PITT = University of Pittsburgh; UTMB/UTSW = University of Texas at Galveston and at Dallas; WASHU = Washington University.
The models include site by medication interaction and the variable in the corresponding row
RR cannot be computed for these comparisons as response probability in patients with ADHD receiving divalproex is 0.
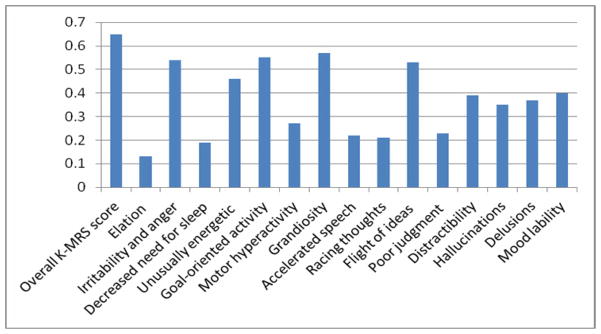
A treatment modifier effect was found also for presence of ADHD and severity of ADHD symptoms (p=0.044). Patients with ADHD had a greater probability of responding to risperidone than lithium (RR=2.1, 95% C.I. 1.5, 3.0, p<0.0001) compared with patients not meeting criteria for ADHD (RR=1.0, 0.6, 1.8, p=1.000). A comparison of the change in KMRS items on risperidone vs. lithium showed differences of effect size of d ≥ 0.50 in favor of risperidone for the total mania score and four individual symptoms (irritability and anger, goal-oriented activity, grandiosity, and flight of ideas). Moreover, differences with smaller effect size (0.30 ≤ d <.50), also in favor of risperidone, were found for five other mania symptoms (unusually energetic, distractibility, hallucinations, delusions, and mood lability) (Figure 1).
Figure 1.
Effect Size (d) for Pair-wise Comparison of Risperidone vs. Lithium on KMRS Total Score and Individual Item Scores. Note: From linear regression models of the scores of the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children Mania Rating Scale (KMRS), which was administered at baseline and week 8. Cohen’s d was calculated as a measure of pairwise comparison between risperidone and lithium according to the method by Rosnow et al.21
We could not find a moderating effect of ODD. ODD symptoms improved with treatment. Of the 251 children meeting criteria for ODD at baseline, only 85 (33.9%) still met ODD criteria at end of treatment, with a significant difference between treatment groups (24.7% in the risperidone group, 32.9% in the lithium, and 42.7% in the divalproex group; χ2=11.7, df=2, p=0.004).
Risperidone was less likely to be better than lithium for obese patients (RR=1.1, 95% C.I. 0.6, 2.0, p=0.664) than for non-obese ones (2.3, 95% CI 1.6, 3.3, p<0.0001). The average dose of risperidone was not different in obese (1.5 mg/day) compared to non-obese patients (1.7 mg/day).
Additional analyses using the change in mania symptoms on the KMRS scored by blinded raters from baseline to week 8 were consistent with the presence of a treatment moderator effect by site. The difference in improvement on the KMRS after adjustment for baseline score between risperidone and lithium was −15.5 (95% CI −23.1, −7.8; p<0.0001) at the UTMB/UTSW, −11.9 (−18.2, −5.7; p<0.0001) at JHMI −8.6 (−15.0, −7.8; p<0.009) at WASHU, −4.4 (−10.1, 1.3, p=0.131) at CNMC, and −3.9 (−11.3, −3.6, p=0.308) at PITT, with an overall interaction effect χ2=7.8, df=4, p=0.099. For the risperidone vs. divalproex comparison, the difference in improvement was −11.3 (95% CI −18.7, −3.8; p=0.003) at the UTMB/UTSW, −13.6 (−19.8, −7.5; p<0.0001) at JHMI, −13.2 (−18.0, −8.4; p<0.0001) at WASHU, −5.2 (−11.3, −0.9; p=0.098) at CNMC, and −7.8 (−16.3, − 0.6); p=0.069) at PITT, with an overall interaction effect χ2=5.5, p=0.236.
Older age predicted greater decline of KMRS score (average improvement in KMRS for each one-year increment in age= −0.5 (95% CI −1.0, −0.1; p=0.029), and more severe ADHD was associated with higher KMRS score (average increase in KMRS for one additional ADHD symptom=0.5, 95% CI: 0.1, 1.0; p=0.022). These estimates were adjusted for site, medication group, and baseline KMRS score.
Further sensitivity analyses conducted on KMRS scored by the unblinded treating clinicians from weeks 1 to 7 were also consistent with the presence of site differences in mania symptoms change over time by treatment group. Estimate of average trajectories ranged from −1.41/week (JHMI) to −2.60/week (UTMB/UTSW) for risperidone (p=0.001 for the difference in trajectories across the sites), from −1.03/week (UTMB/UTSW) to −2.87/week (PITT) for lithium (p=0.007), and from −0.98 (UTMB/UTSW) to −2.19 (CNMC) for divalproex (p<0.0001).
Changing the outcome definition in patients who dropped out of the study prematurely from non-response to the last available scores (“last observation carried forward” approach) did not change results or conclusions.
Site differences
A number of site characteristics were examined in an effort to clarify the source of the site moderator effects. Sites differed with respect to sample size, ranging from 39 to 68. There were site differences by gender (p=0.0023), race (p<0.0001), family history of bipolar disorder (p = 0.001), severity of mania (p<0.0001), severity of ODD symptoms (p= 0.045), severity of ADHD symptoms (p= 0.0004), source of referral (p=0.043), and high maternal warmth (p<0.0001). Of these variables, only severity of ADHD was found to have a moderator and predictor effect. The prevalence of ADHD was high at all the sites (89.7% at PITT, 92.7% at CNMC, 93.7% at WASHU, 98.3% at UTMB/UTSW, and 88.0% at JHMI; p=0.0004), and not associated with site response rate.
A greater proportion of patients were clinically referred, rather than recruited through media or other sources, at PITT (53.8%) and CNMC (47.1%) than at JHMI (26.0%), UTMB/UTSW (33.9%), or WASHU (36.5%). Also, the proportion of subjects with “high maternal warmth” was greater at CNMC (70.6%) and PITT (43.6%) than at other sites (16.1% at WASHU, 16.9% at UTMB/UTSW, and 32.0% at JHMI). There were no site differences in average or maximum lithium or divalproex plasma levels, or maximum risperidone dose.
The premature discontinuation (“drop-out”) rate differed across sites: 14.7% at CNMC, 15.4% at PITT, 17.5% at WASHU, 30.0% at JHMI, and 45.8% at UTMB/UTSW (χ2= 22.1, df = 4, p< 0.001) (Tables 2, 3). Sites significantly differed in both early drop-out rate (defined as dropping out in the first two weeks of study treatment) (Fisher’s exact test, p=0.012) and late drop-out rate (defined as dropping out after the first two weeks of study treatment) (Fisher’s exact test, p=0.041). There was no statistically significant interaction between site and medication on the drop-out rate (Fisher’s exact test, p=.939). Sensitivity analyses were conducted by dropping the sites with highest drop-out rates (UTMB/UTSW and JHMI): the site effect on the comparison between risperidone and lithium, which was significant (p=.014) when all sites were included, became non-significant (p=.166); the site effect on the comparison between risperidone and valproate remained significant. There was not site effect on the comparison between lithium and valproate in either analysis. Taken together, these data suggest that the inferiority of lithium over risperidone was associated with dropping out from the study treatment at two of the five sites.
Table 3.
Response and Premature Discontinuation by Study Site
| Lithium | Risperidone | Divalproex | All | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Responders % | Drop-outs % | n | Responders % | Drop-outs % | n | Responders % | Drop-outs % | n | Responders % | Drop-outsa % | |
| PITT | 15 | 73.3 | 13.3 | 15 | 86.7 | 13.3 | 9 | 66.7 | 22.2 | 39 | 76.9 | 15.4 |
| CNMC | 22 | 59.1 | 27.3 | 24 | 83.3 | 4.2 | 22 | 54.6 | 13.6 | 68 | 66.2 | 14.7 |
| WASHU | 19 | 26.3 | 15.8 | 16 | 68.8 | 12.5 | 28 | 7.1 | 21.4 | 63 | 28.6 | 17.5 |
| UTMB/UTSW | 19 | 5.3 | 57.9 | 16 | 43.8 | 31.2 | 24 | 4.2 | 45.8 | 59 | 15.3 | 45.8 |
| JHMI | 15 | 13.3 | 46.7 | 18 | 55.6 | 22.2 | 17 | 17.7 | 23.5 | 50 | 30.0 | 30.0 |
| Total | 90 | 35.6 | 32.2 | 89 | 68.5 | 15.7 | 100 | 24.0 | 26.0 | 279 | 41.9 | 24.7 |
Note: Response: a score of 1 (very much improved) or 2 (much improved) on the Clinical Global Impressions for Bipolar Illness-Improvement Mania scale (CGI-BP-IM) at week 8. CNMC = Children’s National Medical Center; JHMI = Johns Hopkins Medical Institutions;; PITT = University of Pittsburgh; UTMB/UTSW = University of Texas at Galveston and at Dallas; WASHU = Washington University.
Between site difference in drop-out rate: χ2=22.1, df=4, p<0.0002. No significant site x drug interaction in drop-out rate.
When only the completers of the 8-week trial were analyzed (n=210), site differences in response rate were still evident (77.6% at CNMC, 87.9% at PITT, 34.6% at WASHU, 42.9% at JHMI, and 28.1% at UTMB/UTSW; χ2= 21.5, df = 4, p< 0.001).
Discussion
We systematically searched for moderators of treatment effect and predictors of outcome in a randomized trial of lithium, risperidone, and divalproex with a sample of 279 children diagnosed with bipolar mania highly comorbid with ADHD and ODD. The main finding was that study site moderated the treatment effect by modifying the effect size of risperidone vs. lithium and risperidone vs. divalproex. Thus, the response ratio (RR) was substantially greater at some sites (UTMB/UTSW and JHMI) than at others (PITT and CNMC). Site was also a strong predictor of response regardless of the specific treatment assignment, with overall response rate ranging from 15.3% at UTMB/UTSW to 76.9% at PITT. These site differences were evident on both the binary response outcome, which was the primary outcome measure of the trial, and the change in the continuous mania symptoms. Results of separate analyses of KMRS scores by the blinded raters and the unblinded treating clinicians were consistent. Unfortunately, separate self-rated symptoms by patients or parents were not available in this database.
Sites differed in drop-out rate, with sites having a better response rate presenting with a lower drop-out rate. However, site differences in outcome persisted when only completers were analyzed, thus indicating that drop-out did not fully account for the observed differences in outcome. The site variability in lithium and valproate response, rather than risperidone, was especially noteworthy. These differences could not be explained by differences in medication doses or plasma levels between sites. The sites differed on a number of sample characteristics, such as sex, race, severity of mania, severity of ADHD, maternal warmth, source of patient referral, and family history of bipolar disorder. The small size of the subgroups defined by these variables at each site, however, prevented proper testing of possible interactions. The greater proportion of clinically referred study participants and of parents with high maternal warmth at the sites with better response rate suggests that differences in sample characteristics contributed to the observed differences in outcome.
Severity of ADHD modified the effect size of risperidone vs. lithium thus acting as a moderator. In particular, while the large majority of the sample met criteria for ADHD, risperidone was not superior to lithium in those patients without ADHD (RR=1.0). This finding may suggest that risperidone improved mood dysregulation and hyperactivity/ impulsivity, whereas lithium’s effect was limited to mood symptoms. Of note, the size of the non-ADHD subgroups was small: in both the risperidone and lithium groups, there were 8 patients, of whom 6 were responders. A comparison of these two medications on each of the symptoms of the KMRS shows superiority of risperidone on a variety of symptoms other than ADHD, such as irritability/anger, behavioral agitation, grandiosity, and flight of ideas.
The analyses supported only some of the a priori stated hypotheses. As expected, older age was associated with greater improvement. Contrary to expectation, severity of mania did not predict outcome, perhaps due to the fact that the sample was severely impaired. No effects were found for conduct disorder, suicidality, or maternal warmth. Unexpectedly, obesity acted as a moderator, reducing the RR of risperidone vs. lithium. This effect could not be accounted by differences in medication absolute dose, although heavier patients might have received lower mg/kg dosage than lighter ones. It might be due to a type I error.
These analyses have important limitations. They are exploratory and hypothesis-testing, exposed to considerable risk for both type I error (false positive findings), due to multiple comparisons, and type II errors (false negative findings), due to the small sample size of the subgroups. In addition, our analyses could account only for the variables captured in the database. Other factors, relative to patient characteristics, clinician skills, or their interaction, likely contributed to the differences in outcome, but could not be ascertained. In particular, the database did not included self-rated measures that could reflect the direct scoring of patients or parents.
These data, while confirming the greater efficacy of risperidone, point to study site as a major source of variability in clinical outcome in spite of the tightly controlled implementation of the protocol and ongoing central monitoring. Site effects are frequently found in multi-site trials, and site-by-treatment effects are not uncommon.22,23 For example, in the Treatment of SSRI-Resistant Depression in Adolescents (TORDIA), a four-fold variation in the response rate to the medication-only treatment was found across the sites, with a significant site x treatment interaction, in part explained by differences in patient baseline clinical characteristics.24 Thus, the site differences in TEAM cannot be considered specific to child mania, a condition for which there is considerable diagnostic uncertainty.25 The elements and processes underlying site difference remain unclear. The dropout rate tended to be lower at the sites with higher response rate. It should be noted that drop-out and response rate are not independent constructs, as they are both post-randomization variable, affected by treatment. Whether the difference in drop-out was due to a greater ability at some sites to retain participants on study protocol, or merely reflected lack of response to treatment cannot be elucidated. However, site differences in response were found also with completer-only analyses, which excluded dropouts, thus suggesting that retention did not fully account for the differences in outcome. The level of expertise in child bipolar research seems to be an unlikely explanation for the observed variance in outcome, as both sites with high or low response rate had extensive history of conducting treatment studies in child affective disorders.
These data suggest that factors other than specific treatment have a powerful influence on outcome. Some of these factors may relate to the organization of the clinical setting and/or the quality of the therapeutic alliance between the clinician and the patient/family. Other factors may reside within the family, school, or community. Context may influence the pressure to achieve rapid control of symptoms, and therefore the threshold for discontinuing treatment in the absence of improvement. Early discontinuation impacts on efficacy assessment, as it can take several weeks of treatment for improvement to emerge, especially with lithium or divalproex. As in other conditions, 26 also in child bipolar disorder the clinical outcome appears to depend on more than just a specific pharmacological treatment.
In conclusion, site was both a moderator of treatment effect and a predictor of clinical outcome in TEAM. In general, ADHD severity predicted less improvement, and lithium was less effective in the presence of comorbid ADHD. These findings point to the need to further investigate the role of site characteristics and comorbid ADHD in future clinical trials of children with mood dysregulation.
Acknowledgments
This study received funding from NIMH cooperative agreement grants U01 MH064846, U01 MH064850, U01 MH064851, U01 MH064868, U01 MH064869, U01 MH064887, and U01 MH064911.
The authors gratefully acknowledge the pivotal contributions of Barbara Geller, MD, at Washington University, in designing and overseeing this project from its inception through to its publication.
Footnotes
The opinions and assertions contained in this report are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of Health and Human Services, the National Institutes of Health (NIH), or NIMH.
Clinical trial registration information—Treatment of Early Age Mania; http://clinicaltrials.gov/; NCT00057681.
This article is discussed in an editorial by Dr. Argyris Stringaris on page xxx.
This article can be used to obtain continuing medical education (CME) category 1 credit at www.jaacap.org.
Disclosure: Dr. Riddle has served as a member of a Data and Safety Monitoring Board (DSMB) for studies sponsored by the National Institute of Child Health and Human Development. He has served on a committee of the Institute of Medicine, has provided expert witness testimony for Teva Canada, and has received aripiprazole from Bristol-Myers Squibb for an NIMH-sponsored study. Dr. Wagner has received honoraria from the American Psychiatric Association, Physicians Postgraduate Press, CMP Medica, the American Academy of Child and Adolescent Psychiatry (AACAP), Doctors Hospital at Renaissance, UBM Medica, Quantia Communications, Continuing Medical Education (CME) LLC, the Nevada Psychiatric Association, Slack Inc., Mercy, Hospital Universitario Ramón y Cajal, the Las Vegas Psychiatric Society, and Partners Healthcare. Dr. Walkup has served on the advisory board and speakers’ bureau for and has received grant or research support from the Tourette Syndrome Association. He has received honoraria and travel funding from the Tourette Syndrome Association. He has received free drug and placebo from Eli Lilly and Co., Pfizer, and Abbott for NIMH-funded studies. He has served as a consultant for Shire and has received royalties from Guilford Press and Oxford University Press. Dr. Luby has received grant or research support from NIMH, the National Alliance for Research on Schizophrenia and Depression (NARSAD), the Communities Healing Adolescent Depression and Suicide (CHADS) Coalition, and the Sidney R. Baer Foundation. She has served as a consultant for the Food and Drugs Administration (FDA) Advisory Board. Dr. Emslie has received research support from NIMH, BioMarin, Eli Lilly and Co., Forest Laboratories, GlaxoSmithKline, and Somerset. He has served as a consultant for Biobehavioral Diagnostics Inc., Bristol-Myers Squibb, Eli Lilly and Co., GlaxoSmithKline, Integrated Neuroscience Consortium (INC) Research Inc., Lundbeck, Pfizer, Seaside Therapeutics, Shire, Valeant, and Wyeth. He has served on the speakers’ bureau for Forest Laboratories. Dr. Birmaher has received research grant support from NIMH, royalties from Random House and Lippincott Williams and Wilkins, and has served as a consultant for Schering Plough. Dr. Robb has served on the boards of Eli Lilly and Co., Bristol-Myers Squibb, Lundbeck, Otsuka, Shinogi, and McNeil Pediatrics. She has served as a consultant for Lundbeck, has provided expert testimony for a case on antipsychotic use, has served on the data safety monitoring board for Otsuka, and has received grant support from Bristol-Myers Squibb, Merck, Schering Plough, GlaxoSmithKline, Janssen, Supernus, Otsuka, Johnson and Johnson, and Forest Laboratories. She has received a contract for research from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), has served as speakers’ bureaus for Bristol-Myers Squibb, Eli Lilly and Co., and McNeil Pediatrics, and has received royalties from Epocrates. She has received payment for development and education presentations from the University of Texas, AACAP, and the American Academy of Pediatrics (AAP), and has served on the program committee for the American Psychiatric Association annual meeting. Drs. Vitiello, Yenokyan, Axelson, Joshi, and Ryan, and Ms. Tillman report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McClellan J, Kowatch R, Findling RL Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(1):107–125. doi: 10.1097/01.chi.0000242240.69678.c4. [DOI] [PubMed] [Google Scholar]
- 2.Liu HY, Potter MP, Woodworth KY, Yorks DM, Petty CR, Wozniak JR, Faraone SV, Biederman J. Pharmacologic treatments for pediatric bipolar disorder: a review and meta-analysis. J Am Acad Child Adolesc Psychiatry. 2011;50(8):749–762. doi: 10.1016/j.jaac.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Correll CU, Sheridan EM, DelBello MP. Antipsychotic and mood stabilizer efficacy and tolerability in pediatric and adult patients with bipolar I mania: a comparative analysis of acute, randomized, placebo-controlled trials. Bipolar Disord. 2010;12(2):116–141. doi: 10.1111/j.1399-5618.2010.00798.x. [DOI] [PubMed] [Google Scholar]
- 4.Kraemer HC, Wilson GT, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Arch General Psychiatry. 2002;59:877–883. doi: 10.1001/archpsyc.59.10.877. [DOI] [PubMed] [Google Scholar]
- 5.Perlis RH, Ostacher MJ, Miklowitz DJ, Hay A, Nierenberg AA, Thase ME, Sachs GS. Clinical features associated with poor pharmacologic adherence in bipolar disorder: results from the STEP-BD study. J Clin Psychiatry. 2010 Mar;71(3):296–303. doi: 10.4088/JCP.09m05514yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DelBello MP, Hanseman D, Adler CM, Fleck DE, Strakowski SM. Twelve-month outcome of adolescents with bipolar disorder following first hospitalization for a manic or mixed episode. Am J Psychiatry. 2007;164:582–590. doi: 10.1176/ajp.2007.164.4.582. [DOI] [PubMed] [Google Scholar]
- 7.Birmaher B, Axelson D, Goldstein B, Strober M, Gill MK, Hunt J, Houck P, Ha W, Iyengar S, Kim E, Yen S, Hower H, Esposito-Smythers C, Goldstein T, Ryan N, Keller M. Four-year longitudinal course of children and adolescents with bipolar spectrum disorders: the Course and Outcome of Bipolar Youth (COBY) study. Am J Psychiatry. 2009;166(7):795–804. doi: 10.1176/appi.ajp.2009.08101569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masi G, Perugi G, Millepiedi S, Mucci M, Pfanner C, Berloffa S, Pari C, Gagliano A, D’Amico F, Akiskal HS. Pharmacological response in juvenile bipolar disorder subtypes: a naturalistic retrospective examination. Psychiatry Res. 2010;177:192–198. doi: 10.1016/j.psychres.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 9.Wagner KD, Redden L. Divalproex treatment effect not influenced by age (10–13 vs. 14–17) in placebo controlled trial (4 weeks) in mania. J Am Acad Child Adolesc Psychiatry. 2009;48(5):519–532. doi: 10.1097/CHI.0b013e31819c55ec. [DOI] [PubMed] [Google Scholar]
- 10.Geller B, Luby JL, Joshi P, Wagner KD, Emslie G, Walkup JT, Axelson DA, Bolhofner K, Robb A, Wolf DV, Riddle MA, Birmaher B, Nusrat N, Ryan ND, Vitiello B, Tillman R, Lavori P. A randomized controlled trial of risperidone, lithium, or divalproex for initial treatment of bipolar I disorder, manic or mixed phase, in children and adolescents. Arch Gen Psychiatry. 2012;69(5):515–28. doi: 10.1001/archgenpsychiatry.2011.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cipriani A, Barbui C, Salanti G, Rendell J, Brown R, Stockton S, Purgato M, Spinelli LM, Goodwin GM, Geddes JR. Comparative effectiveness and acceptability of antimanic drugs in acute mania: a multiple-treatments meta-analysis. Lancet. 2011;378:1306–1315. doi: 10.1016/S0140-6736(11)60873-8. [DOI] [PubMed] [Google Scholar]
- 12.Geller B, Craney JL, Bolhofner K, Nickelsburg MJ, Williams M, Zimerman B. Two-year prospective follow-up of children with a prepubertal and early adolescent bipolar disorder phenotype. Am J Psychiatry. 2002;159(6):927–933. doi: 10.1176/appi.ajp.159.6.927. [DOI] [PubMed] [Google Scholar]
- 13.Spearing MK, Post RM, Leverich GS, Brandt D, Nolen W. Modification of the Clinical Global Impressions (CGI) Scale for use in bipolar illness (BP): the CGI-BP. Psychiatry Res. 1997;73(3):159–171. doi: 10.1016/s0165-1781(97)00123-6. [DOI] [PubMed] [Google Scholar]
- 14.Axelson D, Birmaher BJ, Brent D, Wassick S, Hoover C, Bridge J, Ryan N. A preliminary study of the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children mania rating scale for children and adolescents. J Child Adolesc Psychopharmacol. 2003;13(4):463–470. doi: 10.1089/104454603322724850. [DOI] [PubMed] [Google Scholar]
- 15.Duke PM, Litt IF, Gross RT. Adolescents’ self-assessment of sexual maturation. Pediatrics. 1980;66:918–920. [PubMed] [Google Scholar]
- 16.Andreasen NC, Endicott J, Spitzer RL, Winokur G. The family history method using diagnostic criteria. Reliability and validity. Arch Gen Psychiatry. 1977;34:1229–1235. doi: 10.1001/archpsyc.1977.01770220111013. [DOI] [PubMed] [Google Scholar]
- 17.Puigh-Antich J, Lukens E, Brent D. Psychosocial Schedule for School Age Children- Revised. Pittsburgh: Western Psychiatric Institute and Clinic; 1986. [Google Scholar]
- 18.Geller B, Bolhofner K, Craney JL, Williams M, DelBello MP, Gundersen K. Psychosocial functioning in a prepubertal and early adolescent bipolar disorder phenotype. J Am Acad Child Adolesc Psychiatry. 2000;39:1543–1548. doi: 10.1097/00004583-200012000-00018. [DOI] [PubMed] [Google Scholar]
- 19.Lachin JM. Worst-rank score analysis with informatively missing observations in clinical trials. Controlled Clinical Trials. 1999;20:408–422. doi: 10.1016/s0197-2456(99)00022-7. [DOI] [PubMed] [Google Scholar]
- 20.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 21.Rosnow RL, Rosenthal R, Runin DB. Contrasts and Correlations in Effect-Size Estimation. Psychol Sci. 2000;11:446–453. doi: 10.1111/1467-9280.00287. [DOI] [PubMed] [Google Scholar]
- 22.Feaster D, Mikulich-Gilbertson S, Brincks AM. Modeling site effects in the design and analysis of multi-site trials. Am J Drug Alcohol Abuse. 2011;37:383–391. doi: 10.3109/00952990.2011.600386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kraemer HC. Pitfalls of multisite randomized clinical trials of efficacy and effectiveness. Schizophr Bull. 2000;26:533–541. doi: 10.1093/oxfordjournals.schbul.a033474. [DOI] [PubMed] [Google Scholar]
- 24.Spirito A, Abebe KZ, Iyengar S, Brent D, Vitiello B, Clarke G, Wagner KD, Asarnow J, Emslie G, Keller M. Sources of site differences in the efficacy of a multisite clinical trial: the Treatment of SSRI-Resistant Depression in Adolescents. J Consult Clin Psychol. 2009;77(3):439–450. doi: 10.1037/a0014834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leibenluft E. Severe mood dysregulation, irritability, and the diagnostic boundaries of bipolar disorder in youths. Am J Psychiatry. 2011 Feb;168(2):129–142. doi: 10.1176/appi.ajp.2010.10050766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKay KM, Imel ZE, Wampold BE. Psychiatrist effects in the psychopharmacological treatment of depression. J Affect Dis. 2006;92:287–290. doi: 10.1016/j.jad.2006.01.020. [DOI] [PubMed] [Google Scholar]