Abstract
Wildlife reintroductions select or treat individuals for good health with the expectation that these individuals will fare better than infected animals. However, these individuals, new to their environment, may also be particularly susceptible to circulating infections and this may result in high morbidity and mortality, potentially jeopardizing the goals of recovery. Here, using the reintroduction of the grey wolf (Canis lupus) into Yellowstone National Park as a case study, we address the question of how parasites invade a reintroduced population and consider the impact of these invasions on population performance. We find that several viral parasites rapidly invaded the population inside the park, likely via spillover from resident canid species, and we contrast these with the slower invasion of sarcoptic mange, caused by the mite Sarcoptes scabiei. The spatio-temporal patterns of mange invasion were largely consistent with patterns of host connectivity and density, and we demonstrate that the area of highest resource quality, supporting the greatest density of wolves, is also the region that appears most susceptible to repeated disease invasion and parasite-induced declines. The success of wolf reintroduction appears not to have been jeopardized by infectious disease, but now shows signs of regulation or limitation modulated by parasites.
Keywords: wildlife reintroduction, parasite invasion, enemy release, Sarcoptes scabiei, sarcoptic mange, canine distemper virus
1. Introduction
Habitat destruction [1], climate change [2], over harvesting [3–5] and persecution have resulted in the local extirpation of many species within parts of their natural range. In an attempt to re-establish species within their indigenous range, conservation managers have deliberately reintroduced or augmented existing populations [6]. Since 1973, a large number of threatened and endangered species have been successfully reintroduced back into their natural habitat in the United States, some of which have since been removed from listing under the United States' Endangered Species Act [6–9]. Particular success has been achieved with persecuted species when habitat quality remains high and persecution ceases [6,10]. Furthermore, reintroduced individuals are increasingly selected or treated for good health [11], often held in captivity under veterinary supervision for a period of time in an attempt to ensure that healthy individuals re-establish the population. These released individuals and their subsequent generations may initially benefit from a relative lack of infectious disease—a concept akin to the enemy release hypothesis of invasive species [12]. However, these individuals will also tend to be susceptible to circulating infections and, consequently, initial morbidity and mortality may be relatively high, potentially jeopardizing the primary objective of reintroduction. As we continue to work towards restoring ecosystems, one of the pressing questions facing wildlife managers and conservationists is: how do parasites invade a reintroduced host, and what are the risks of parasite-induced failure to reintroduction efforts?
The grey wolf (Canis lupus), historically found throughout most of the North American continent, was heavily persecuted and eventually extirpated from the majority of the lower 48 United States by the early twentieth century [13]. In 1995 and 1996, wolves were reintroduced into the Northern Rockies where they have since established and spread [10,14,15]. The release sites were characterized by excellent habitat, an abundant resource base and the existence of core protected areas [14]; these, coupled with the large number of animals released, the species' high intrinsic growth rate, and its tendency towards habitat generalization, have resulted in broad re-establishment [15]. In some areas, managers have suppressed wolf densities so as to reduce conflict and competition with ranchers and hunters [16]. However, within Yellowstone National Park, one of the core protected release sites, the unmanaged population steadily increased to high densities [17], producing a large wolf population susceptible to infections such as canine parvovirus (CPV), canine distemper virus (CDV) and sarcoptic mange. In this study, we address the questions of how diseases spread in these susceptible reintroduced populations, and how they impact population dynamics and the long-term success of reintroduction efforts.
2. Parasite acquisition and invasion in a reintroduced host: theory
Depending on the length or extent of a species' absence, a reintroduced host may return with a reduced suite of specialist parasites, having lost some of these specialists during periods of small population size [12] or through veterinary intervention. (Throughout, we will use the term ‘parasite’ in the broadest sense, referring to both microparasites, such as viruses and bacteria, and macroparasites, including organisms such as S. scabiei.) However, reintroduced individuals will almost certainly face native, and perhaps even non-native, generalist parasites that have been sustained or have invaded into reservoir hosts in their absence. A reservoir is defined as a population or community that maintains a parasite infection and is responsible for its spillover to the target species of interest [18]. Reintroduced hosts are expected to acquire and accumulate these parasites via spillover from reservoirs or itinerant conspecifics within their reintroduced range. The speed at which this occurs will be largely determined by the infection dynamics in the primary reservoir and by the frequency and type of contact necessary for transmission to occur. This process of accumulating parasites may take time and thus a reintroduced species may benefit from a temporary enemy release-like effect in the form of reduced parasite pressure. However, unlike non-native invasive hosts that are likely to encounter parasites with which they share no evolutionary history [19], reintroduced native hosts are expected to encounter parasites that are well adapted to exploiting them, and thus any parasite release is likely to be short-lived.
Aside from simply being capable of acquiring a suite of native infections, reintroduced species may be particularly vulnerable to parasite invasion and the adverse effects thereof. Reintroduced host populations may have no/low herd immunity towards native, circulating parasites, resulting in more explosive and severe infections than under endemic conditions [20]. Changes in community composition and abundance, driven by the absence of the reintroduced host, may also result in increased opportunities for pathogen spillover and parasite-mediated apparent competition [21]. One hypothetical example based on our own study system involves coyotes (Canis latrans) and wolves: coyotes, which reached much higher densities in the absence of wolves, may have been capable of supporting higher levels of endemic parasites and thus could have provided a particularly intense source of spillover to wolves during reintroduction. Small, recovering populations would be particularly vulnerable to the adverse effects of these parasite pressures.
Following a spillover event from either a reservoir or an itinerant conspecific, the likelihood of invasion is typically defined by the basic reproduction number, R0. This is essentially the product of the transmission rate and the infectious period of the parasite in the average individual case, yielding the estimated number of new infections caused by a primary infection in a completely susceptible host population. If R0 is greater than 1, the parasite is assumed capable of invasion. However, we now know that the effects of heterogeneities in contact rates, susceptibility and infectiousness are not well captured by the average metric of R0 [22–24], and that these variations can have pronounced impacts on the likelihood and course of parasite invasion. Highly connected, susceptible or infectious individuals increase the likelihood of parasite establishment and rapid spread throughout the population [23].
Once a successful chain of transmission has been established, parasite invasion is predicted to follow the dominant spatial patterns of host connectivity when all hosts are equally susceptible. Broadly, these connectivity patterns will vary with scale and, with increasing scale, will be dependent on host social behaviour, underlying resources, local host population density and landscape structure. In the case of group-living species, such as wolves, transmission within groups is expected to be much greater than between groups, a phenomenon that may, for some parasites, impede invasion [22] but ultimately improve persistence [25,26]. High resource-protected areas, often comprising the core of reintroduction sites, are likely to support the highest densities of reintroduced hosts; these, paradoxically, may also be the sites most vulnerable to invasion by parasites with density-dependent transmission. Deviations in predicted patterns of disease invasion may be explained by structural complexities within the landscape [27], heterogeneity in individual host behaviour [28], variations in the infectious dose received, individual susceptibility and parasite strains or types.
Here, we present a case study of the patterns and impacts of parasite invasion into a reintroduced population of grey wolves in Yellowstone National Park (Yellowstone), Wyoming. We demonstrate that the analogous effects of enemy release were relatively short-lived among reintroduced wolves. We focus on describing the spatio-temporal patterns of the relatively slow and visible invasion of sarcoptic mange, and to a lesser extent, the repeated invasions of CDV. We demonstrate that the best regions for wolf habitat and resource quality within Yellowstone, supporting the densest and most highly connected sub-populations of wolves, seem also to be those most susceptible to widespread parasite invasion and parasite-associated declines.
3. Wolves in Yellowstone: history of release and infection
Thirty-one wolves were captured from British Columbia and Alberta, Canada and released in groups to establish seven packs in Yellowstone in 1995 and 1996 [10,14]. All translocated wolves, as well as several early litters that were temporarily brought into holding pens for management purposes [29], were vaccinated for a suite of common canid pathogens (rabies, CDV, canine adenovirus type-1, Leptospiria sp., CPV and canine parainfluenza virus) and were treated with ivermectin, a broad-spectrum antiparasitic, prior to their release. All subsequent wolves born into the wild, which remained unvaccinated, were assumed to be completely susceptible and thus their exposure history was interpreted as a reflection of parasites acquired in Yellowstone.
Within the first year following their release, wild-born (unvaccinated) wolves acquired several viral infections that had been present in Yellowstone coyotes prior to wolf reintroduction [30,31]. By 1997, after only a year of ranging on the Yellowstone landscape, 100 per cent (18/18) of the wolves sampled across the park tested positive for exposure to CPV and 61 per cent (11/18) tested positive for canine adenovirus type-1 (CAV-1). By 1997, 63 per cent (12/19) of wolves tested positive for canine herpesvirus (CHV), although because CHV is a chronic infection and several reintroduced wolves tested positive at the time of release (Yellowstone Wolf Project 2011, unpublished data), we cannot distinguish between spillover from coyotes and its introduction with, and spread from, infected reintroduced wolves. From 1998 to 2008, each of these three viruses became endemic with high seroprevalence: 99 per cent (211/213) tested positive for CPV, 96 per cent (205/214) tested positive for CAV-1 and 91 per cent (190/208) were positive for CHV. The rapid acquisition and high seroprevalences of these pathogens are in keeping with what we know about their transmission biology. CPV is transmitted via faecal–oral contact and the exchange of oral–nasal exudates, shedding can last 30 days post-infection, and because the virus is both extremely stable in the environment (six months at 20°C) and can induce carrier states, the effective infectious period can be remarkably long and permit persistence in the host population [32]. CAV-1 is transmitted via contact with nasal or conjunctival secretions and urine or faeces; it is relatively less stable in the environment (several days at 20°C), and shedding typically lasts 8 days, but the infection can become chronic with shedding lasting six to nine months in the urine [33,34]. CHV is transmitted both vertically and through direct contact with oral, nasal or genital secretions, and induces lifelong infection with periodic recrudescence activated by stress or by other forms of immunosuppression [35]. All three of these viruses invaded the Yellowstone wolf population so rapidly and completely, possibly via multiple spillover events, that we were unable to discern any spatial patterning to their invasion [30].
CDV is a directly transmitted generalist parasite that causes acute infections and high juvenile mortality, and confers lifelong immunity in surviving individuals [36]. CDV caused epidemics within the Yellowstone coyote population prior to wolf reintroduction [37], but appears to have been absent from Yellowstone from 1995 to 1998 [30,38]. Subsequent to this, three distinct canine distemper outbreaks took place (in 1999, 2005 and 2008), resulting in 100 per cent seroprevalence among all wolves sampled in those years in the northern region of the park coupled with very high (60–90%) wolf pup mortality [30,38]. In contrast to CPV, CAV-1 and CHV, for which there were no discernible spatial patterns to invasion, CDV exposure varied in accordance with regional host density [30]. Wolves that were in packs within the less dense and poorly connected interior of the park showed some exposure during outbreak years, but seroprevalence and pup mortality rates appeared to be much lower than in the well-connected and high-density northern region of the park [30]. These patterns are consistent with a parasite that requires direct, rapid transmission and thus an abundance of well-connected susceptible hosts [38].
In contrast to the four viral parasites, the invasion of sarcoptic mange (an infection of the skin caused by the mite Sarcoptes scabiei) was relatively slow and an individual's infection status could be visually scored. State veterinarians introduced mange into the Greater Yellowstone Ecosystem in 1905 in an attempt to aid wolf eradication during the predator control era [39]. In the absence of wolves, it was thought to have persisted among regional carnivores, although curiously there were no known records of mange from animals inside Yellowstone prior to wolf reintroduction. Following reintroduction, mange was first detected in wolves outside Yellowstone in 2002 [39] and inside the park by early 2007 [40].
Mange is transmitted through direct bodily contact or via contact with mites that have dropped off their host into the environment. The mites are capable of surviving away from a host for days and sometimes up to several weeks, depending on the microclimate at the mite's drop-off site [41]. Potential sites for environmental transmission include carcass sites, bed sites and dens. The mites, which burrow into their host's epidermis, trigger an allergic/inflammatory reaction that causes severe itchiness that, in turn, causes the host to scratch and bite, resulting in thickening of the skin, hair loss and increased susceptibility to secondary infections. On the basis of experimental work among several canid species, the incubation period between infection and the onset of scratching and visible lesions ranges from two to five weeks post-exposure, depending on the infectious dose [42]. The duration of infection in wolves and coyotes appears to be highly variable, ranging from months to well over a year [39,43]. Upon recovery, there is some evidence of short-term immunity [44]. S. scabiei does appear to exhibit some host specificity, possibly even showing preference among closely related canid species [42], suggesting that, once initiated among wolves, the majority of subsequent transmission may have been intra-specific.
4. Methods
(a). Study area
Yellowstone National Park encompasses 8991 km2 of protected land in northwestern Wyoming and adjacent parts of Montana and Idaho in the western United States (44°33′ N, 110°30′ W). Yellowstone National Park is surrounded by the Greater Yellowstone Ecosystem, a 60 000 km2 area that includes Yellowstone and Grand Teton National Parks, national forests, wildlife refuges, and a mosaic of state and private lands. Yellowstone is mountainous (elevation range: 1500–3500 m), and contains varied land cover, including riparian vegetation, shrubland, grassland, alpine meadows and mixed coniferous forests.
In keeping with previous studies [30,45], we divided the park into two ecological units: the Northern Range and the Interior, based on ecological and physiographical differences. The comparatively small (1000 km2 versus 7991 km2) Northern Range of Yellowstone is characterized by lower elevations (1500–2200 m versus >2500 m), serves as prime wintering habitat for the park's ungulates [46], and consequently supports a higher density of wolves than the Interior (20–99 wolves 1000 km-2 versus 2–11 wolves 1000 km-2 [17]; minimum population count for all of Yellowstone National Park ranged between 97 and 172 wolves between 2000 and 2010 [47]).
(b). Population monitoring and disease status
Since reintroduction in 1995–1996, the National Park Service has captured and radio-collared an annual average of 25 wolves (range: 14–39) spanning all known packs in the park (mean packs sampled per year = 8; range = 6–12). Collaring efforts, which take place between December and March, generally target breeders and young of the year, with an emphasis on maintaining contact with each pack. At the time of collaring, staff record sex, weight and body condition, estimate age based on tooth wear [48], collect blood samples for genetic and serological analyses and examine the body for ectoparasites, including the clinical signs of infection with S. scabiei. The project radio-tracks individuals on a weekly to monthly basis with the goal of obtaining visual observations of entire packs. During each aerial or ground sighting, staff record location, pack size, membership, behaviour and, since it was first recorded, the prevalence and severity of mange within each pack.
An individual was recorded as being positive for infection with S. scabiei based on the presence of visible, hairless lesions and scratching behaviour. The date of the first observation of a positive individual within a pack became known as the date of first infection for that pack. The severity of infection was categorically assessed based on the percentage of an individual's body that was affected by hairless lesions: 0–5%, 6–50% and more than 50 per cent were scored as class 1, 2 and 3 mange, respectively [49]. The infection status of all radio-collared individuals has been recorded over time. For the purposes of analysis, we report and use estimates of mange prevalence at a pack level from March, July and November of every year, the first and last months of which coincide with the Wolf Project's most intensive monitoring periods.
As part of previous work [30], serum samples isolated from blood collected during 1996–2008 captures were analysed at the Cornell University's Animal Health Diagnostic Center (Ithaca, NY, USA) for specific antibodies to CPV (haemagglutination inhibition test; positive titre 20 or higher), CAV-1 (serum neutralization (SN) test; positive titre 8 or higher), CHV (SN test; positive titre 8 or higher) and CDV (SN test; positive titre higher than 12). Previous research identified three discrete outbreaks of canine distemper within Yellowstone wolves in 1999, 2005 and 2008 based on the seroconversion of pups (less than 1 year) caught during those years [30]. In the present study, we expanded upon this initial work by classifying individual packs as having been exposed to CDV in a given outbreak year if at least one individual in that pack was seropositive and was known, based on age and year of sampling, to have seroconverted during the outbreak in question (i.e. either 1999, 2005 or 2008). We chose to increase the cut-off titre used to define a positive CDV test to higher than 24 to minimize false positives. We considered this to be justified because the average titres of pups and yearlings during outbreak years were orders of magnitude higher (mean titre = 128, s.d. = 6) than during non-outbreak years (mean titre = 9, s.d. = 2). We treated non-outbreak years similarly, whereby we used data from all individuals born and sampled between known outbreaks, and alive during the year of interest, as indicators of pack exposure to CDV. This approach increased our sample size from an average of 10 (s.d. = 5) pups per year across 5 (s.d. = 2) packs to an average of 24 (s.d. = 7) mixed-age samples per year distributed across an average of 8 packs (s.d. = 2). Despite this increase in pack-level information on CDV exposure, our sampling still remained too sparse for detailed spatial analyses. Thus, we limit our discussion of spatial dynamics of CDV to regional patterns across the Northern Range versus the Interior.
(c). Spatial progression of mange
Using Hawth's analysis tools [50] in ArcGIS [51], we calculated the 95 per cent kernel density home-range estimate (smoothing parameter: h = 3000) for each pack using three months of location data before and after each date at which a new pack became infected with mange (mean number of locations per pack: 28, s.d. = 16). Using a subset of these data, including only the home-ranges of packs when they were first infected (mean number of locations per pack: 33, s.d. = 21), we calculated the new area invaded by mange with each jump to a new pack; the ratio of these values to the time between consecutive infections yielded rates of geographical disease spread. This was a simplified metric, as it did not include the territorial shifts of packs once they had become infected.
All uninfected packs were considered at risk of becoming infected. Using Hawth's analysis tools [50], we calculated the linear distance between the centroids of all uninfected packs to the centroids of their nearest infected neighbour for a given date of infection. Similarly, we calculated the percentage of territory overlap between each uninfected pack and its nearest infected neighbour (% territory overlap = (area of overlap between packs A and B)/(area of the union of packs A and B)). For each infection event, we also calculated the number of infected individuals in the nearest infected neighbouring pack as another potential predictor of infection risk. Using a Cox proportional hazards model, specified with a continuous-time study-based baseline hazard [52,53], we modelled a pack's risk of infection as a function of Euclidean distance to, percentage territory overlap with, and the number of infected individuals in, its nearest infected neighbouring pack.
(d). Demographic dynamics and disease impacts
The Yellowstone Wolf Project produces annual, end-of-year minimum wolf counts based on air and ground surveys conducted in December. These population counts include all pack counts, lone animals and individuals within small subgroups. Using end-of-year estimates of pack size, we calculated annual pack growth rates (λ = Nt/Nt − 1) for each pack within the population. Using generalized linear regression models [54], we examined the ability of disease and time since reintroduction, a proxy for declining food availability, to explain the variation observed in annual pack growth rates. Specifically, we hypothesized that both the presence of CDV and mange within a pack and increases in time since reintroduction, corresponding to a linear decline in prey abundance, would be negatively correlated with annual pack growth rates. The final model was specified as: annual pack growth rate ∼1 + (mange metric) + CDV + time. We examined several different metrics of mange infection including: (i) the presence of mange during a year; (ii) the presence of class 2 or 3 mange during the year; (iii) the maximum prevalence of mange recorded in the March, July or November survey months, and the maximum prevalence of mange class 2 or 3 recorded during the three survey months. As described earlier, packs were classified as having experienced a CDV outbreak if at least one individual was known to have seroconverted during the year in question. Serological data were unavailable for 2009 and 2010, and analyses were run once with only the available data, and once where we assumed that 2009 and 2010 were non-CDV outbreak years. All other data spanned 1997–2010. All models and statistical analyses were undertaken in program R [54].
5. Yellowstone wolves, canine distemper and mange: spatio-temporal patterns of invasion
Yellowstone's wolves experienced three discrete outbreaks of canine distemper: in 1999, 2005 and 2008 [30]. Using age-specific exposure data, we found that CDV invaded 12 of 13 packs sampled on the Northern Range (1999: 3/4; 2005: 6/6; 2008: 3/3) versus six of 11 packs sampled in the Interior (1999: 1/2; 2005: 4/7; 2008: 1/2) of Yellowstone during these outbreak years (table 1). Furthermore, we saw some evidence for CDV exposure in 2000 (2/3 packs positive) and 2006 (1/5 packs positive) on the Northern Range, although the seroprevalences during these years were much lower than those observed during the primary outbreak years (samples positive/samples tested: 1999 = 6/7 versus 2000 = 3/16; and 2005 = 14/14 versus 2006 = 1/9). All other years were confirmed as being negative for CDV exposure.
Table 1.
Wolf packs present, sampled and found to be positive for exposure to canine distemper virus on the Northern Range and Interior of Yellowstone National Park, Wyoming, from 1997 to 2010.
| Northern Range |
Interior |
|||
|---|---|---|---|---|
| year | packs present | packs positive/sampled (prevalence) | packs present | packs positive/sampled (prevalence) |
| 1997 | 3 | 0/3 (0) | 4 | 0/4 (0) |
| 1998 | 3 | 0/3 (0) | 4 | 0/3 (0) |
| 1999 | 4 | 3/4 (0.75) | 4 | 1/2 (0.5) |
| 2000 | 4 | 2/3 (0.67) | 4 | 0/4 (0) |
| 2001 | 5 | 0/4 (0) | 5 | 0/5 (0) |
| 2002 | 8 | 0/5 (0) | 6 | 0/2 (0) |
| 2003 | 9 | 0/6 (0) | 7 | 0/2 (0) |
| 2004 | 7 | 0/5 (0) | 9 | 0/5 (0) |
| 2005 | 6 | 6/6 (1) | 8 | 4/7 (0.57) |
| 2006 | 7 | 1/5 (0.2) | 6 | 0/3 (0) |
| 2007 | 6 | 0/5 (0) | 6 | 0/5 (0) |
| 2008 | 8 | 3/3 (1) | 7 | 1/2 (0.5) |
| 2009 | 6 | no data | 7 | no data |
| 2010 | 4 | no data | 7 | no data |
Sarcoptic mange has been recorded in wolves surrounding Yellowstone since 2002 [39]. By the summer of 2004, mange was recorded in the Chief Joseph pack, a pack that straddled the northwestern boundary of Yellowstone but spent the majority of its time outside the park [55]. Despite this proximity, mange failed to invade the core of Yellowstone until late 2006, when it was detected in the Mollie's pack (figures 1 and 2). (It is important to note that we assumed that the presence or absence of clinical signs of mange accurately reflected an individual's infection status. However, we cannot rule out the possibility that asymptomatic infections occurred prior to the observation of clinical signs.) Although the source of this initial infection remains unknown, mange had been present to the east of Yellowstone at that time, and thus may have been acquired either during an out-of-park foray or via infected wolves dispersing into the park.
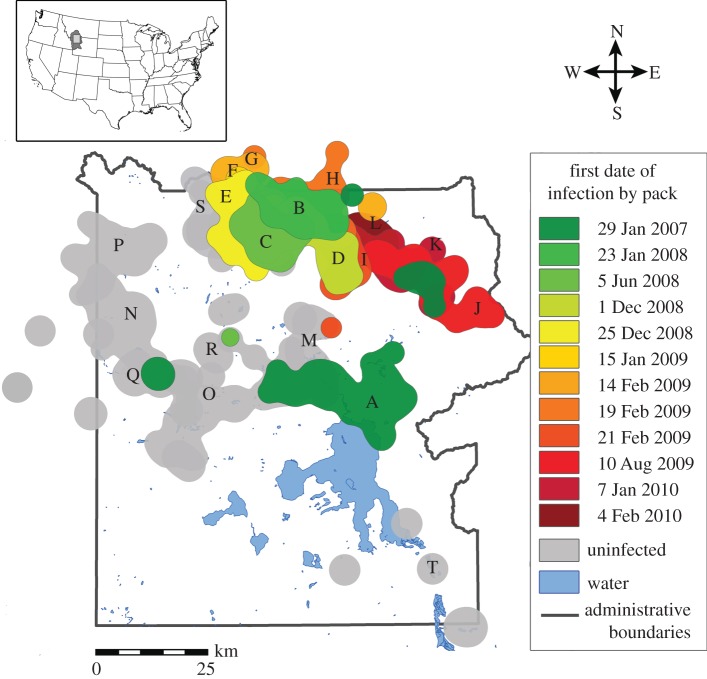
Figure 1.
A map depicting the spatial spread of mange across wolf pack territories over time (95% kernel density estimates based on ± three months location data surrounding the first date of a pack's infection). The timing of infection is represented by the colour of the pack territory, and grey pack territories are those that remained uninfected during the study. Letters correspond to pack data displayed in figures 2 and 4. The Northern Range within Yellowstone is delineated by the northern band of packs (B–L, S) on the map, and the Interior encompasses the remainder of the park to the south and west.
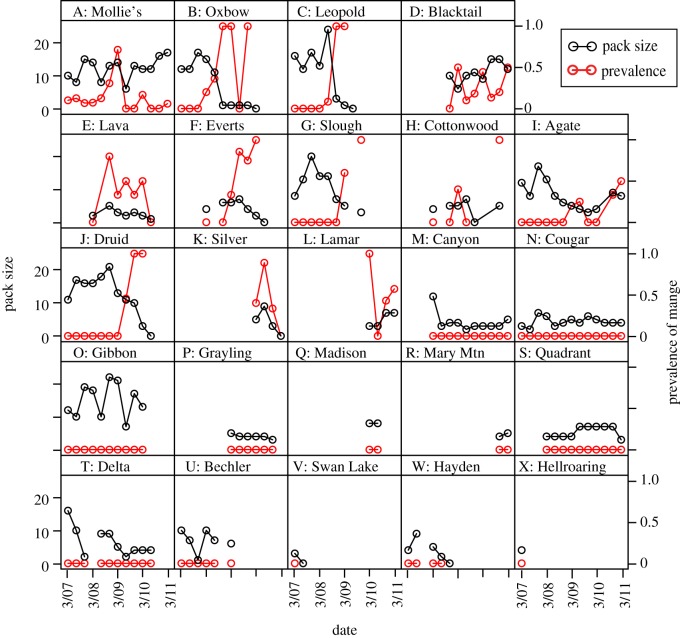
Figure 2.
Time series of pack sizes and mange prevalence (class 1, 2 or 3) corresponding to packs displayed on the map in figure 1. Pack size and prevalence estimates were conducted in March, July and November of every year since the invasion of mange. Packs U–X are not displayed on the map as they remained uninfected and had disappeared or were not located ± three months surrounding the last documented pack infection.
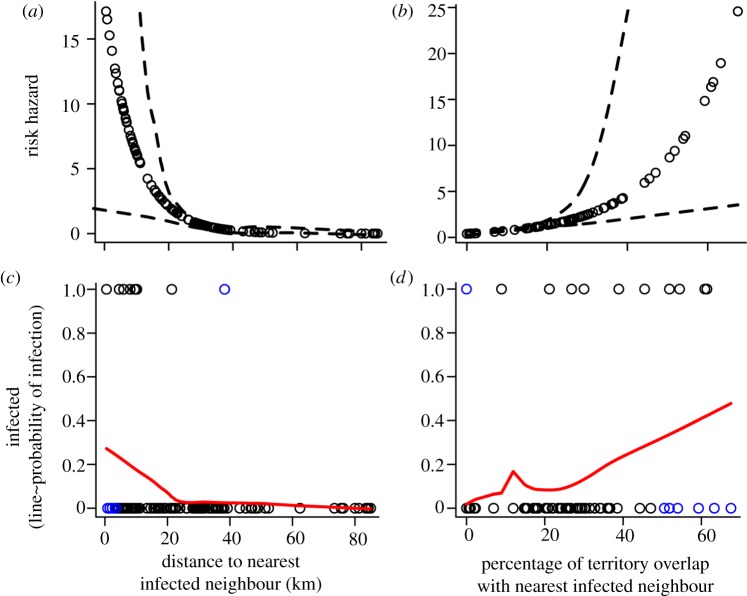
After infecting the Mollie's pack, mange spread to the northern region of the park and outward (figure 1) at an average rate of 315 ± 171 km2, or 3 ± 2 wolf packs per year. Overall, Euclidean distance and the percentage of territory overlap with a pack's nearest infected neighbour were significant predictors of infection risk (figure 3a,b). With every 10 km increase in distance to a nearest infected neighbour, uninfected packs experienced a 66 per cent decline in their relative risk of infection (β = −0.108, s.e. = 0.042, p = 0.01; figure 3a). No infections occurred at distances greater than 38 km from an infected pack. Similarly, uninfected packs experienced an 80 per cent increase in their relative risk of infection for every 10 per cent increase in territory overlap with an infected neighbour (β = 0.061 (coefficient for arcsine-transformed data), s.e. = 0.018, p = 0.001; figure 3b). The number of diseased individuals in the nearest infected neighbouring pack did not affect a pack's risk of infection (β = −0.057, s.e. = 0.136, p = 0.68).
Figure 3.
The risk of a wolf pack becoming infected with mange (a) decreases with Euclidean distance to its nearest infected neighbour and (b) increases with the percentage of territory overlap with its nearest infected neighbour (95% CIs depicted by dashed lines). Infection events are given for packs at risk in relation to (c) distance to and (d) percentage territory overlap with a pack's nearest infected neighbour. The red line depicts a Lowess fit to the data, or the approximate probability of infection given distance or proportion territory overlap. Blue dots are points that lie at the top or the bottom fifth percentile of their distributions within the subset that either became infected or remained negative. Individual points reflect actual samples for distance and territory overlap. Euclidean distances and extent of territory overlap were calculated using GIS software (ArcGIS v. 9.0), and risks of infection were estimated using a Cox proportional hazards analysis (Program R, survival package).
Despite the strong spatial pattern of mange spread in Yellowstone, there were several packs that either remained uninfected, despite geographical proximity and overlap with infected neighbours, or became infected despite large distances or no measurable territory overlap. By examining the upper and lower fifth percentile of the distributions of distances and territory overlap, the outstanding cases included Oxbow Creek, which became infected despite a distance of 38 km and no measurable territory overlap with the Mollie's pack during the six-month period surrounding the date of first infection; and the Canyon pack, which was within a distance of 3 km and at one point had 18 per cent territory overlap with Leopold, but never became infected (figure 3c,d). While not identified as an outlier by the above method, the Quadrant pack also stood out as an exception on the Northern Range; despite relatively close proximity to infected packs (approx. 5 km), it is the only pack on the Northern Range in which mange has never been recorded (figures 1 and 2).
Infected lone dispersers, while potentially very important to the local and regional transmission of mange, were very difficult to track within our system. However, we did have occasional sightings of such individuals. For example, an unknown mangy male was seen interacting with the Slough Creek and Druid Peak packs nearly two months before the first case of mange appeared on the Northern Range. Genetic data suggested that this individual may have been an infected disperser from outside the park (D. Stahler, Yellowstone Wolf Project 2011, personal communication). Furthermore, following the mange-associated dissolution of several packs (e.g. Leopold and Oxbow), the mangy remnants of these groups were seen travelling/dispersing within the Northern Range. These, as well as other undetected individuals, may have contributed to the unexplained variance in the observed patterns of infection.
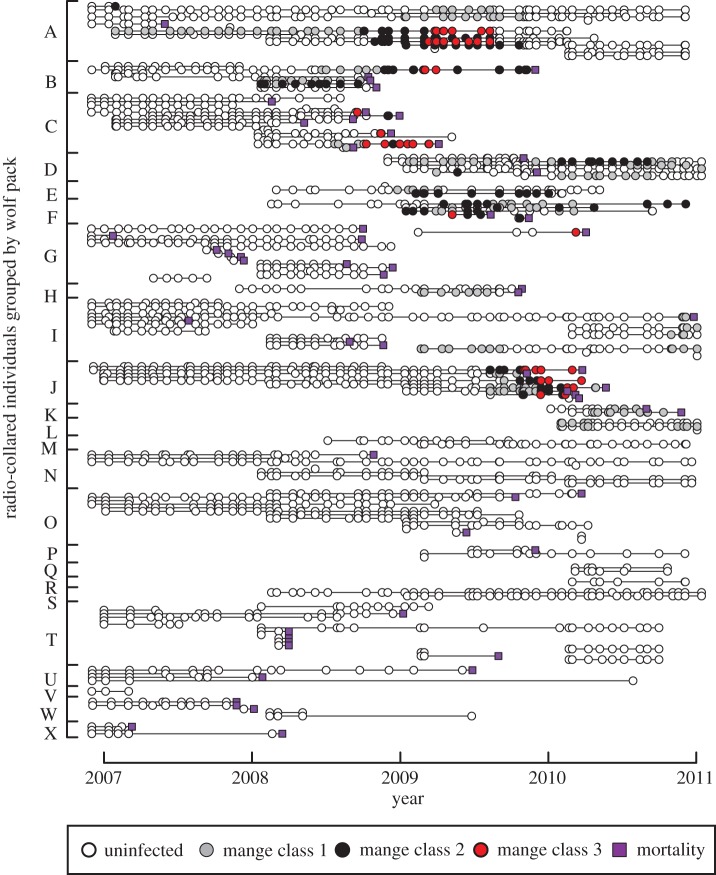
Once packs became infected, the spread and severity of clinical signs of mange among individuals within the pack appeared to be quite variable (figures 2 and 4). In some cases, the infection remained at a low prevalence within the pack, as in the case of the first year of both Agate and Mollie's infections, whereas in others, such as in the case of the Druid pack, the infection spread rapidly and infected nearly the entire pack, coinciding with the pack's extinction (figure 2).
Figure 4.
Mange status of radio-collared individuals within packs over time. Each horizontal line is an individual tracked over time with their mange status denoted each time they are observed. Individuals are grouped by pack, the letters of which correspond to the packs displayed in figures 1 and 2.
(a). Demographic dynamics and disease impacts
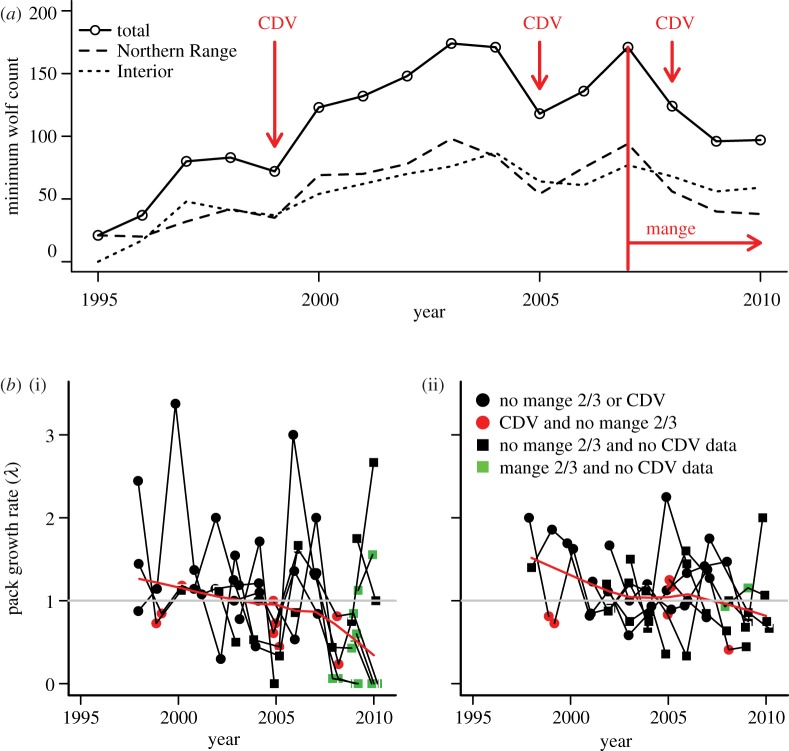
Since reintroduction, the wolf population within Yellowstone has shown phases of rapid growth (1995–2003), stabilization (2003–2007) and slight decline (2007–2010) (figure 5a). As expected, individual packs, both within the Northern Range and the Interior, are roughly at equilibrium (mean λNR = 0.99; mean λInterior = 1.10); however, Northern Range packs exhibit 2.75 times as much variance in their annual growth rates when compared with Interior packs (σNR = 0.512 versus σInterior = 0.186) (figure 5b).
Figure 5.
(a) Minimum wolf population counts within Yellowstone National Park from 1995 to 2010 with outbreaks of canine distemper and the first detection of mange noted. (b) Annual pack growth rates (λ = pack sizet/pack sizet−1) of the (i) Northern Range and (ii) Interior regions of the park, 1998–2010. Lines connect individual packs and symbols are colour-coded to denote years in which CDV and severe mange (class 2 or 3) were detected (squares represent the absence of CDV data). When λ = 0, packs went extinct. The red line is a Lowess fit to all the data showing the general trend in pack growth rates over time.
At the population level, periodic outbreaks of canine distemper and the invasion of mange have coincided with declines in population size (figure 5a). Incomplete serological data from 2009 and 2010 meant that we were unable to estimate the pack-level impacts of mange separate from the strong effects of CDV. However, if we assumed that 2009 and 2010 were non-CDV outbreak years, which is plausible given previous epidemic patterns and the high levels of herd immunity following the 2008 outbreak, both mange and CDV were found to be important predictors of pack growth rates. At the pack level, the presence (β = −0.390, s.e. = 0.181, p = 0.03) and prevalence of mange (β = −0.008, s.e. = 0.002, p < 0.001), but particularly the presence (β = −0.586, s.e. = 0.191, p < 0.01) and prevalence (β = −0.010, s.e. = 0.003, p < 0.01) of the more severe class 2 and 3 mange, were significantly associated with negative growth rates in pack size (figure 5b), and in several cases were associated with pack dissolution events. As has been shown previously [30], canine distemper outbreaks among Northern Range packs also remained a significant predictor of declines in pack growth rates (from model including presence of mange class 2 and 3: β = −0.512, s.e. = 0.158, p < 0.01). Disease had a larger, one-time effect on a pack's annual growth rate than the negative linear time trend (β = −0.034, s.e. = 0.017, p = 0.05). In a simplified model, the presence of class 2 or 3 mange, together with canine distemper outbreaks alone, explained 19 per cent of the variation in annual pack growth rate (adjusted R2 = 0.190; F = 11.91, d.f. = 93, p < 0.001), the majority of which was within the Northern Range of Yellowstone (figure 5b).
6. Discussion
The enemy release hypothesis states that invading species, particularly non-native species, may experience a release from their former specialist and generalist parasites once transported to their novel range [12]. Reintroduced native species, often selected for good health and treated to minimize infections, may also experience a brief release from their parasitic enemies, but are ultimately expected to acquire a full suite of circulating infections. During this period of invasion, the high proportion of susceptibles within the population may result in relatively intense invasions in terms of associated morbidity and mortality. Following invasion, an array of host and parasite factors determine the likelihood of pathogen persistence and whether individual or suites of parasites end up contributing to the limitation or regulation of host populations.
In examining the specific case of wolves, we found, as expected, that the population has undergone a number of parasite invasions since reintroduction. Several viral parasites invaded the population rapidly after reintroduction, perhaps reflecting their relatively high R0 values and prevalence in sympatric reservoir hosts [31,38,56]. More recently, we have found evidence that Yellowstone's wolf population has suffered negative growth rates associated with CDV and mange, and that several mange-associated declines have even continued to the point of pack extinction. However, in contrast to the North American black-footed ferret (Mustela nigripes) [57,58] and bighorn sheep (Ovis canadensis) [59–61] reintroductions, where repeated parasite invasions via spillover have posed a substantial challenge to recovery efforts, wolf recovery has remained a success despite the invasion of infectious disease. The spatial spread of mange has been largely consistent with the hypothesis of nearest neighbour, pack-to-pack spread as opposed to a hypothesis of frequent spillover events from outside the park. Furthermore, we found that the densest, most productive region of the park (the Northern Range) was also the most susceptible to CDV and mange-induced variation in annual wolf growth rates. The protection of Yellowstone that has afforded the wolf reintroduction effort such great success has also allowed us to watch the natural transition from population growth to limitation or regulation, in which parasites appear to play a significant role.
The variation in time-to-invasion observed among the various parasites that have invaded Yellowstone's wolf population may be explained in part by differences across parasites in their modes and rates of transmission as well as their distribution among reservoir hosts at the time of reintroduction. For example, CPV and CAV-1, both of which induce carrier states, are environmentally stable, and were locally present within Yellowstone's coyotes and foxes at the time of wolf reintroduction [30,31], rapidly spilled over into Yellowstone's wolves [30]. By contrast, neither CDV nor mange appeared to be locally present within Yellowstone at the time of reintroduction, which may help to explain the delay in their invasion. However, given that mange was present among wolf packs adjacent to Yellowstone by 2002, the mite took a surprisingly long time to invade the park. One explanation for this relatively long time-lag to invasion involves differences in relative densities of wolves between Yellowstone and the surrounding regions. The protected, resource-rich park has supported much higher population densities of wolves than the surrounding areas, and led to a nearly constant saturation of available territory [62]. Both genetic [63,64] and observational data suggest that, until about 10 years post-reintroduction, dispersal and migration of individuals was largely directed outwards from Yellowstone towards the less densely populated surrounding regions. This directional flow of individuals may have, in effect, created a short-term buffer against the invasion of mange. However, this same phenomenon seems to have posed no barrier to the repeated invasion of CDV. CDV may benefit from being both more infectious (i.e. higher probability of infection given contact, or higher rates of relevant contacts) and having higher rates of cross-species transmission than S. scabiei.
At the regional level, CDV [30] and mange have both tended to track patterns of host density, exhibiting a tendency towards thoroughly invading the much more dense Northern Range over the Interior region of Yellowstone. The Northern Range, which is home to the region's largest migratory elk (Cervus Canadensis) herds, supports nearly 10 times the density of wolves as the Interior (20–99 wolves 1000 km−2 versus 2–11 wolves 1000 km−2) [17]. Distances between packs on the Northern Range are much shorter and there is much more territory overlap than among packs in the Interior of the park (figure 1). The greater distances between packs in the Interior of the park may result in contacts being too infrequent for the efficient transmission of either CDV or mange.
Outbreaks of canine distemper and the presence and prevalence of mange are correlated with reduced pack growth rates. CDV has acute impacts on pup survival [30], which likely accounts for declines in pack growth rates during outbreak years. In the case of mange, pack growth rate declines may be due to reduced pup survival and increased adult morbidity (possibly resulting in lower fecundity and/or increased dispersal) and mortality, and these individual-level impacts of infection will be the subject of future study. However, the invasion of mange has also been coincident with a general decline in Northern Range elk numbers [47] and increasing evidence of food stress among Yellowstone wolves (D. Smith 2011, personal communication). We attempted to account for this decline in the resource base by including a linear time trend in our models of pack growth rates. We did in fact find a negative time trend, which suggests that from 1998 to 2010, packs have experienced a −0.41 decline (95% CI: −0.814, −0.002) in their annual growth rates, perhaps reflecting increased competition and food stress. However, the fact that variation in infection status among packs is strongly correlated with annual pack growth rates suggests that parasites are having a significant impact, even if they are, for example, interacting with increased susceptibility driven by food stress. Continued long-term data collection will hopefully improve our understanding of the relative effects of parasites and resource limitation on host population dynamics. Furthermore, future data collection will allow us to assess whether the effects of parasites are likely to change beyond the phases of reintroduction and invasion.
The success of wolf reintroduction in the Northern Rockies has been due in large part to the existence of large tracts of high-quality habitat and the removal or management of human persecution. Fortunately, parasites have not posed a threat to the success of wolf reintroduction, but rather reintroduction has presented an opportunity to study the patterns of parasite invasion and their impacts on their hosts. Within Yellowstone, the current fluctuation in population size is likely to be a sign that the population has reached a point of natural regulation—by prey and perhaps by parasites. The resources and protection offered within the park should ensure that Yellowstone wolves will serve as an important source of emigrants for surrounding areas well into the future. However, the high wolf densities afforded by protection within Yellowstone may come at the cost of some population stability; the high-density population within the Northern Range of Yellowstone suffers consistently more inter-annual variation in pack growth rates than the less dense Interior, some of which can be explained by increased vulnerability to disease invasion and associated parasite-induced declines. Awareness of this variability may be the key to regional managers tasked with setting appropriate harvest quotas as Montana and Idaho enter their second public wolf hunt since delisting.
Acknowledgements
We thank the Yellowstone Wolf Project, and in particular, R. Raymond and C. Anton, for their help with data collection. This work was funded by a Park Oriented Biological Support grant from the US Geological Survey and the National Park Service. P.J. Hudson was supported by RAPIDD.
References
- 1.Tilman D., May R. M., Lehman C. L., Nowak M. A. 1994. Habitat destruction and the extinction debt. Nature 371, 65–66 10.1038/371065a0 (doi:10.1038/371065a0) [DOI] [Google Scholar]
- 2.Maclean I. M. D., Wilson R. J. 2011. Recent ecological responses to climate change support predictions of high extinction risk. Proc. Natl Acad. Sci. USA 108, 12 337–12 342 10.1073/pnas.1017352108 (doi:10.1073/pnas.1017352108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Worm B., et al. 2009. Rebuilding global fisheries. Science 325, 578–585 10.1126/science.1173146 (doi:10.1126/science.1173146) [DOI] [PubMed] [Google Scholar]
- 4.Rosser A. M., Mainka S. A. 2002. Overexploitation and species extinctions. Conserv. Biol. 16, 584–586 10.1046/j.1523-1739.2002.01635.x (doi:10.1046/j.1523-1739.2002.01635.x) [DOI] [Google Scholar]
- 5.Cardillo M., Mace G. M., Jones K. E., Bielby J., Bininda-Emonds O. R. P., Sechrest W., Orme C. D. L., Purvis A. 2005. Multiple causes of high extinction risk in large mammal species. Science 309, 1239–1241 10.1126/science.1116030 (doi:10.1126/science.1116030) [DOI] [PubMed] [Google Scholar]
- 6.Griffith B., Scott J. M., Carpenter J. W., Reed C. 1989. Translocation as a species conservation tool: status and strategy. Science 245, 477–480 10.1126/science.245.4917.477 (doi:10.1126/science.245.4917.477) [DOI] [PubMed] [Google Scholar]
- 7.Wolf C. M., Griffith B., Reed C., Temple S. A. 1996. Avian and mammalian translocations: update and reanalysis of 1987 survey data. Conserv. Biol. 10, 1142–1154 10.1046/j.1523-1739.1996.10041142.x (doi:10.1046/j.1523-1739.1996.10041142.x) [DOI] [Google Scholar]
- 8.Wolf C. M., Garland T., Griffith B. 1998. Predictors of avian and mammalian translocation success: reanalysis with phylogenetically independent contrasts. Biol. Conserv. 86, 243–255 10.1016/S0006-3207(97)00179-1 (doi:10.1016/S0006-3207(97)00179-1) [DOI] [Google Scholar]
- 9.US Fish and Wildlife Service. 2011. U.S. Fish and Wildlife Service Endangered Species Program. See http://www.fws.gov/endangered/ [Google Scholar]
- 10.Fritts S. H., Bangs E. E., Fontaine J. A., Johnson M. R., Phillips M. K., Koch E. D., Gunson J. R. 1997. Planning and implementing a reintroduction of wolves to Yellowstone National Park and central Idaho. Restor. Ecol. 5, 7–27 10.1046/j.1526-100X.1997.09702.x (doi:10.1046/j.1526-100X.1997.09702.x) [DOI] [Google Scholar]
- 11.Griffith B., Scott J. M., Carpenter J. W., Reed C. 1993. Animal translocations and potential disease transmission. J. Zoo Wildl. Med. 24, 231–236 [Google Scholar]
- 12.Shea K., Chesson P. 2002. Community ecology theory as a framework for biological invasions. Trends Ecol. Evol. 17, 170–176 10.1016/S0169-5347(02)02495-3 (doi:10.1016/S0169-5347(02)02495-3) [DOI] [Google Scholar]
- 13.Boitani L. 2003. Wolf conservation and recovery. In Wolves: behavior, ecology, and conservation (eds Mech L. D., Boitani L.), pp. 317–340 Chicago, IL: University of Chicago Pres; s [Google Scholar]
- 14.Bangs E. E., Fritts S. H. 1996. Reintroducing the gray wolf to central Idaho and Yellowstone National Park. Wildl. Soc. Bull. 24, 402–413 [Google Scholar]
- 15.Bangs E. E., Fritts S. H., Fontaine J. A., Smith D. W., Murphy K. M., Mack C. M., Niemeyer C. C. 1998. Status of gray wolf restoration in Montana, Idaho, and Wyoming. Wildl. Soc. Bull. 26, 785–798 [Google Scholar]
- 16.Smith D. W., et al. 2010. Survival of colonizing wolves in the Northern Rocky Mountains of the United States, 1982–2004. J. Wildl. Manage. 74, 620–634 10.2193/2008-584 (doi:10.2193/2008-584) [DOI] [Google Scholar]
- 17.Smith D. W., Bangs E. E. 2009. Reintroduction of wolves to Yellowstone National Park: history, values, and ecosystem restoration. In Reintroduction of top-order predators (eds Hayward M. W., Somers M. J.), pp. 92–125, 1st edn Hoboken, NJ: Wiley-Blackwell [Google Scholar]
- 18.Haydon D. T., Cleaveland S., Taylor L. H., Laurenson M. K. 2002. Identifying reservoirs of infection: a conceptual and practical challenge. Emerg. Infect. Dis. 8, 1468–1473 10.3201/eid0812.010317 (doi:10.3201/eid0812.010317) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torchin M. E., Lafferty K. D., Dobson A. P., Mckenzie V. J., Kuris A. M. 2003. Introduced species and their missing parasites. Nature 421, 628–630 10.1038/nature01346 (doi:10.1038/nature01346) [DOI] [PubMed] [Google Scholar]
- 20.Tompkins D. M., et al. 2002. Parasites and host population dynamics. In The ecology of wildlife diseases (eds Hudson P. J., Rizzoli A., Grenfell B. T., Heesterbeek H., Dobson A. P.), pp. 45–62 New York, NY: Oxford University Press [Google Scholar]
- 21.Hudson P. J., Greenman J. 1998. Competition mediated by parasites: biological and theoretical progress. Trends Ecol. Evol. 13, 387–390 10.1016/S0169-5347(98)01475-X (doi:10.1016/S0169-5347(98)01475-X) [DOI] [PubMed] [Google Scholar]
- 22.Cross P. C., Lloyd-Smith J. O., Johnson P. L. F., Getz W. M. 2005. Duelling timescales of host movement and disease recovery determine invasion of disease in structured populations. Ecol. Lett. 8, 587–595 10.1111/j.1461-0248.2005.00760.x (doi:10.1111/j.1461-0248.2005.00760.x) [DOI] [Google Scholar]
- 23.Lloyd-Smith J. O., Schreiber S. J., Kopp P. E., Getz W. M. 2005. Superspreading and the effect of individual variation on disease emergence. Nature 438, 355–359 10.1038/nature04153 (doi:10.1038/nature04153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adler F. R. 1992. The effects of averaging on the basic reproduction ratio. Math. Biosci. 111, 89–98 10.1016/0025-5564(92)90080-G (doi:10.1016/0025-5564(92)90080-G) [DOI] [PubMed] [Google Scholar]
- 25.Swinton J., Harwood J., Grenfell B. T., Gilligan C. A. 1998. Persistence thresholds for phocine distemper virus infection in harbour seal (Phoca vitulina) metapopulations. J. Anim. Ecol. 67, 54–68 10.1046/j.1365-2656.1998.00176.x (doi:10.1046/j.1365-2656.1998.00176.x) [DOI] [Google Scholar]
- 26.Mccallum H., Dobson A. 2006. Disease and connectivity. In Connectivity conservation (eds Crooks K. R., Sanjayan M.), pp. 479–501 Cambridge, UK: Cambridge University Press [Google Scholar]
- 27.Real L. A., Biek R. 2007. Spatial dynamics and genetics of infectious diseases on heterogeneous landscapes. J. R. Soc. Interface 4, 935–948 10.1098/rsif.2007.1041 (doi:10.1098/rsif.2007.1041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyers L. A., Pourbohloul B., Newman M. E. J., Skowronski D. M., Brunham R. C. 2005. Network theory and SARS: predicting outbreak diversity. J. Theor. Biol. 232, 71–81 10.1016/j.jtbi.2004.07.026 (doi:10.1016/j.jtbi.2004.07.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phillips M. K., Smith D. W. 1997. Yellowstone Wolf Project: Biennial Report, 1995 and 1996. Yellowstone National Park: National Park Service, Yellowstone Center for Resources
- 30.Almberg E. S., Mech L. D., Smith D. W., Sheldon J. W., Crabtree R. L. 2009. A serological survey of infectious disease in Yellowstone National Park's canid community. PLoS ONE 4, e7042. 10.1371/journal.pone.0007042 (doi:10.1371/journal.pone.0007042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gese E. M., Schultz R. D., Johnson M. R., Williams E. S., Crabtree R. L., Ruff R. L. 1997. Serological survey for diseases in free-ranging coyotes (Canis latrans) in Yellowstone National Park, Wyoming. J. Wildl. Dis. 33, 47–56 [DOI] [PubMed] [Google Scholar]
- 32.Barker I. K., Parrish C. R., Williams E. S. 2001. Parvovirus infections. In Infectious diseases of wild mammals (ed. Anonymous), pp. 131–146 Ames, IA: Iowa State University Press [Google Scholar]
- 33.Woods L. W., Williams E. S., Barker I. K. 2001. Adenoviral diseases. In Infectious diseases of wild mammals (ed. Anonymous), pp. 202–211 Ames, IA: Iowa State University Press [Google Scholar]
- 34.Greene C. E. 2006. Infectious canine hepatitis and canine acidophil cell hepatitis. In Infectious diseases of the dog and cat (ed. Anonymous), pp. 41–47 London, UK: Saunders/Elsevier [Google Scholar]
- 35.Greene C. E., Carmichael L. E. 2006. Canine herpesvirus infection. In Infectious diseases of the dog and cat (ed. Anonymous), pp. 47–53 Philadelphia, PA: Saunders/Elsevier [Google Scholar]
- 36.Greene C. E., Appel M. J. 2006. Canine distemper. In Infectious diseases of the dog and cat (ed. Greene C. E.), pp. 25–41 Philadelphia, PA: Saunders/Elsevier [Google Scholar]
- 37.Murie A. 1940. Ecology of the coyote in the Yellowstone. Washington, DC: United States Government Printing Office; See http://www.nps.gov/history/history/online_books/fauna4/fauna.htm [Google Scholar]
- 38.Almberg E. S., Cross P. C., Smith D. W. 2010. Persistence of canine distemper virus in the Greater Yellowstone Ecosystem's carnivore community. Ecol. Appl. 20, 2058–2074 10.1890/09-1225.1 (doi:10.1890/09-1225.1) [DOI] [PubMed] [Google Scholar]
- 39.Jimenez M. D., Bangs E. E., Sime C., Asher V. J. 2010. Sarcoptic mange found in wolves in the Rocky Mountains in western United States. J. Wildl. Dis. 46, 1120–1125 [DOI] [PubMed] [Google Scholar]
- 40.Smith D. W., Almberg E. S. 2007. Wolf diseases in Yellowstone National Park. Yellowstone Sci. 15, 17–19 [Google Scholar]
- 41.Arlian L. G., Vyszenski-Moher D. L., Pole M. J. 1989. Survival of adults and developmental stages of Sarcoptes scabiei var. canis when off the host. Exp. Appl. Acarol. 6, 181–187 10.1007/BF01193978 (doi:10.1007/BF01193978) [DOI] [PubMed] [Google Scholar]
- 42.Samuel W. M. 1981. Attempted experimental transfer of sarcoptic mange (Sarcoptes scabiei, Acarina: Sarcoptidae) among red fox, coyote, wolf and dog. J. Wildl. Dis. 17, 343–347 [DOI] [PubMed] [Google Scholar]
- 43.Pence D. B., Windberg L. A. 1994. Impact of a sarcoptic mange epizootic on a coyote population. J. Wildl. Manage. 58, 624–633 10.2307/3809675 (doi:10.2307/3809675) [DOI] [Google Scholar]
- 44.Bornstein S., Morner T., Samuel W. M., Williams E. S., Barker I. K. 2001. Sarcoptes scabiei and sarcoptic mange. In Infectious diseases of wild mammals (ed. Anonymous), pp. 107–115 Ames, IA: Iowa State University Press [Google Scholar]
- 45.Smith D. W., Drummer T. D., Murphy K. M., Guernsey D. S., Evans S. B. 2004. Winter prey selection and estimation of wolf kill rates in Yellowstone National Park, 1995–2000. J. Wildl. Manage. 68, 153–166 10.2193/0022-541X(2004)068[0153:WPSAEO]2.0.CO;2 (doi:10.2193/0022-541X(2004)068[0153:WPSAEO]2.0.CO;2) [DOI] [Google Scholar]
- 46.Houston D. B. 1982. The northern Yellowstone elk: ecology and management. New York, NY: Macmillan [Google Scholar]
- 47.Smith D. W., et al. 2011. Yellowstone Wolf Project: Annual Report, 2010. Yellowstone National Park, Wyoming: National Park Service, Yellowstone Center for Resources
- 48.Gipson P. S., Ballard W. B., Nowak R. M., Mech L. D. 2000. Accuracy and precision of estimating age of gray wolves by tooth wear. J. Wildl. Manage. 64, 752–758 10.2307/3802745 (doi:10.2307/3802745) [DOI] [Google Scholar]
- 49.Pence D. B., Windberg L. A., Pence B. C., Sprowls R. 1983. The epizootiology and pathology of sarcoptic mange in coyotes, Canis latrans, from south Texas. J. Parasitol. 69, 1100–1115 10.2307/3280873 (doi:10.2307/3280873) [DOI] [PubMed] [Google Scholar]
- 50.Beyer H. L. 2004. Hawth's analysis tools for ArcGIS. See http://www.spatialecology.com/htools [Google Scholar]
- 51.ESRI 2009. ArcGIS 9: ArcMap version 9.3.1. Redlands, CA: Environmental Systems Research Institute [Google Scholar]
- 52.Cox D. R. 1972. Regression models and life-tables. J. R. Stat. Soc. B (Methodological) 34, 187–220 [Google Scholar]
- 53.Fieberg J., Delgiudice G. D. 2009. What time is it? Choice of time origin and scale in extended proportional hazards models. Ecology 90, 1687–1697 10.1890/08-0724.1 (doi:10.1890/08-0724.1) [DOI] [PubMed] [Google Scholar]
- 54.R Development Core Team 2011. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 55.Smith D. W., Stahler D., Guernsey D. S. 2005. Yellowstone Wolf Project: Annual Report, 2004. Yellowstone National Park: National Park Service, Yellowstone Center for Resources
- 56.Biek R., et al. 2006. Factors associated with pathogen seroprevalence and infection in Rocky Mountain cougars. J. Wildl. Dis. 42, 606–615 [DOI] [PubMed] [Google Scholar]
- 57.Antolin M. F., et al. 2002. The influence of sylvatic plague on North American wildlife at the landscape level, with special emphasis on black-footed ferret and prairie dog conservation. (ed. Rahm J.), pp. 104–127 Washington, DC: North American Wildlife and Natural Resources Conference [Google Scholar]
- 58.Thorne E. T., Williams E. S. 1988. Disease and endangered species: the black-footed ferret as a recent example. Conserv. Biol. 2, 66–74 10.1111/j.1523-1739.1988.tb00336.x (doi:10.1111/j.1523-1739.1988.tb00336.x) [DOI] [Google Scholar]
- 59.Singer F. J., Williams E., Miller M. W., Zeigenfuss L. C. 2000. Population growth, fecundity, and survivorship in recovering populations of bighorn sheep. Restor. Ecol. 8, 75–84 10.1046/j.1526-100x.2000.80067.x (doi:10.1046/j.1526-100x.2000.80067.x) [DOI] [Google Scholar]
- 60.Gross J. E., Singer F. J., Moses M. E. 2000. Effects of disease, dispersal, and area on bighorn sheep restoration. Restor. Ecol. 8, 25–37 10.1046/j.1526-100x.2000.80063.x (doi:10.1046/j.1526-100x.2000.80063.x) [DOI] [Google Scholar]
- 61.Singer F. J., Zeigenfuss L. C., Spicer L. 2001. Role of patch size, disease, and movement in rapid extinction of bighorn sheep. Conserv. Biol. 15, 1347–1354 10.1046/j.1523-1739.2001.99488.x (doi:10.1046/j.1523-1739.2001.99488.x) [DOI] [Google Scholar]
- 62.US Fish and Wildlife Service, Nez Perce Tribe, National Park Service, Montana Fish Wildlife and Parks, Blackfeet Nation, Confederated Salish and Kootenai Tribes, Idaho Fish and Game, & USDA Wildlife Services 2010. Rocky Mountain Wolf Recovery 2009 Interagency Annual Report. Helena: USFWS, Ecological Services
- 63.Vonholdt B. M., Stahler D. R., Bangs E. E., Smith D. W., Jimenez M. D., Mack C. M., Niemeyer C. C., Pollinger J. P., Wayne R. K. 2010. A novel assessment of population structure and gene flow in grey wolf populations of the Northern Rocky Mountains of the United States. Mol. Ecol. 19, 4412–4427 10.1111/j.1365-294X.2010.04769.x (doi:10.1111/j.1365-294X.2010.04769.x) [DOI] [PubMed] [Google Scholar]
- 64.Vonholdt B. M., Stahler D. R., Smith D. W., Earl D. A., Pollinger J. P., Wayne R. K. 2008. The genealogy and genetic viability of reintroduced Yellowstone grey wolves. Mol. Ecol. 17, 252–274 10.1111/j.1365-294X.2007.03468.x (doi:10.1111/j.1365-294X.2007.03468.x) [DOI] [PubMed] [Google Scholar]